Abstract
Background
Chronic idiopathic or spontaneous urticaria (CIU/CSU) impairs patients’ quality of life, and updated information on disease prevalence, treatment patterns, and disease burden is lacking.
Objectives
We aimed to estimate these figures in a large US real-world claims database via a validated algorithm.
Methods
In this retrospective cross-sectional cohort study, we identified patients with CIU/CSU, estimated disease prevalence, comorbidities, and healthcare use (medications, office visits, emergency department visits, and hospitalizations) and costs (urticaria related and all cause).
Results
We identified 6350 CIU/CSU patients in a population of just over 5.8 million: 0.11 % prevalence. Women accounted for the majority of sufferers (68.3 %) and had a greater burden of illness than men. Patients had relatively few comorbidities (mean 3.3, standard deviation 2.2). Primary care physicians and allergists were the most common providers of CIU/CSU-related care. Oral corticosteroids were the most commonly prescribed medication, used in 54.7 % of patients. Patients accumulated a mean of 15.1 office visits per year (standard deviation 12.6). The mean all-cause healthcare cost totaled over US$9000 per year.
Conclusions
Although the disease affects a relatively young population, CIU/CSU carries a substantial cost. Frequent oral corticosteroid use in CIU/CSU patients is a concern because of adverse events associated with the drug.
Electronic supplementary material
The online version of this article (doi:10.1007/s40257-015-0134-8) contains supplementary material, which is available to authorized users.
Key Points
| The prevalence of chronic idiopathic or spontaneous urticaria is 0.11 % in a commercially insured US population. |
| While urticaria-related hospitalizations are uncommon, the mean total healthcare cost for patients with chronic idiopathic or spontaneous urticaria was over US$9000 per year. |
Introduction
Urticaria is a dermatologic condition characterized by well-defined, pruritic, erythematous hives and wheals, associated with superficial swelling of the dermis [1]. While most urticaria resolves spontaneously, a small proportion of the population develops chronic symptoms (defined as lasting at least 6 weeks) [1, 2]. A minority of chronic urticaria cases may be attributed to a clear external cause such as physical stimuli; however, most cases are idiopathic and are termed chronic idiopathic or spontaneous urticaria (CIU/CSU) [2]. The average duration of CIU/CSU is 2–5 years, though CIU-related symptoms may persist beyond 5 years in nearly one-fifth of patients [2]. Angioedema, swelling of the subcutaneous and submucosal tissues, accompanies CIU/CSU in more than a third of patients [3, 4].
CIU/CSU lesions can severely impact patients’ lives; directly, through itching, and indirectly, through disturbances in sleep and work/school-related daily activities [5]. Patients with CIU/CSU have worse health-related quality of life than those with psoriasis or atopic dermatitis [6]. Their degree of impairment is comparable to that observed in patients with severe ischemic heart disease [7]. Treatment involves H1 antihistamines as a first line, often at multiples of typical doses. For those who require additional treatment, a variety of medications are used, including oral corticosteroids (OCS), leukotriene receptor antagonists (LTRA), and methotrexate, none of which has been US Food and Drug Administration (FDA) approved for use in CIU/CSU [8]. In 2014, omalizumab became the first drug to gain a FDA label to treat CIU/CSU in patients with inadequate response to H1 antihistamines [9].
Despite decades of study, many factors about the condition remain unknown. Estimates of the prevalence of CIU/CSU are generally derived from small non-representative samples, come from outside USA, or are decades old. According to a 2011 report by the Global Allergy and Asthma European Network (GA2LEN), there is a strong need for higher quality information on disease epidemiology [5]. The report further notes that, despite the existence of disease management guidelines, real-world treatment patterns are still not well studied.
One method for obtaining data on disease epidemiology in USA is through the use of insurance claims. Approximately 55 % of the US population is commercially insured [10] (compared with about 30 % of the population covered by Medicare or Medicaid). Furthermore, such claims may be a good source of medication-use data, as a claim is generated whenever a prescription is filled using insurance benefits.
Health insurance claims are coded using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnostic codes, which has no specific code for CIU/CSU. This has represented a major stumbling block to studying this condition using insurance data. However, the results of a multicenter study to validate a method of identifying CIU/CSU using combinations of ICD-9-CM codes were recently published. The published algorithm has a 90 % positive predictive value (PPV) and 71 % sensitivity for CIU/CSU [11]. In the current study, our objective was to estimate disease prevalence in the insured population and report real-world treatment patterns using that validated approach.
Methods
We conducted a retrospective cross-sectional cohort study using an existing database of commercial health insurance claims. The data were provided, for a fee, by a large, US commercial health plan. Data files provided include medical claims, pharmacy claims, and enrollment information. Data represent more than 13 million individuals living in every geographic region of the country (although they may not be distributed in equal proportions to the US population). To pay claims for covered individuals, the insurer receives information on each physician visit, medical procedure, hospitalization, drug dispensed, dates of service/prescription, number of days of medication supplied, and test performed. Each claim is coded with a member identifier, allowing patients to be followed over time and linked across files. Pharmacy claims contain a physician identifier, National Drug Code, strength, quantity and date of drug dispensed, days’ supply, and dollar amount. Medical claims are submitted for each physician encounter and coded with a physician identifier, ICD-9-CM diagnosis code, ICD-9 or CPT procedure code (if a procedure was performed), lab test name (but not results), admission or discharge date (for inpatient care), date and place of service [e.g., outpatient, inpatient, emergency department (ED)], and dollar amount. Dollar amounts are reported in the database as normalized prices. These prices are intended to approximate what would be paid by the insurer for a given claim (or drug) in a fee-for-service setting. They do not include deductibles or co-insurance. Enrollment files contain basic demographic information on each patient including age (in years), geographic region (with USA divided into four regions), and dates of eligibility (e.g., whether or not the individual was enrolled in the plan). During enrollment, all of an individuals’ claims are in the database; after disenrollment, no claims are available. Therefore, an individuals’ data will be analyzed only for the period of the enrollment. The database contains no protected health information and is compliant with the Health Insurance Portability and Accountability Act of 1996. This designation, along with the retrospective nature of the research, made it exempt from institutional review board review.
We identified prevalent CIU/CSU patients through a validated ICD-9-CM coding algorithm, shown to have a PPV of 90.4 % [12]. PPV expresses how likely patients identified with a given test are to have the condition of interest. PPVs from 85 to 89 % are considered acceptable, and PPVs from 70 to 75 % are considered moderate [13]. A study of ICD-9-CM codes for 32 conditions found a median PPV of 80.7 %, a mean of 77 %, and a range of 23–100 %. High sensitivity is important to estimate disease prevalence. There are no agreed-to standards for adequate sensitivity. In a study of more than 4000 medical records, sensitivity of ICD-9-CM codes for chart review validated conditions ranged from 9 % for weight loss to higher than 83 % for metastatic cancer [14]. In only 6 of 32 conditions was sensitivity above 70 %.
The algorithm classifies patients as having CIU/CSU when one of two criteria is met: (1) either two outpatient diagnoses of 708.1 (idiopathic urticaria), 708.8 (other specified urticaria), or 708.9 (urticaria, unspecified) at least 6 weeks apart; or (2) one outpatient diagnosis of 708.1, 708.8, or 708.9, plus one diagnosis of 995.1 (angioedema, not hereditary) ≥6 weeks distant from the 708.x diagnosis. Patients of any age meeting either criteria between 1/1/2012 and 12/31/2012 were included in the study. Those not continuously enrolled for the entire calendar year were excluded.
To estimate the prevalence of CIU/CSU in commercially insured patients, we divided the number of patients meeting the criteria by the number continuously enrolled in 2012. Age categories were chosen to provide an adequate sample within each group and to distinguish clinically meaningful groupings (e.g., young children, older children, teens). The first measure of overall health was the number of chronic conditions, which were counted using the Healthcare Cost and Utilization Project Chronic Condition Indicator. This indicator defines a chronic condition as one lasting ≥12 months and limiting self-care, independent living, and social interactions, or resulting in the need for ongoing medical intervention [15]. The second general health measure was the Deyo adaptation of the Charlson Comorbidity Index (Deyo-CCI), one of the most widely used general comorbidity measures available for claims data [16, 17]. Although there are many alternatives, the Deyo-CCI has been validated in multiple settings and no clearer superior index exists [18]. “Usual physician” specialty was determined by identifying the physician specialty with the largest number of office visits with evaluation and management services during the study year [19]. We also reported rates of atopic dermatitis, vasculitis and allergic purpura, allergic rhinitis, asthma, and other allergies.
Over-the-counter (OTC) medications, which include most H1-antihistamines, were not available in the studied database, and their use could not be analyzed. We reported rates of use of prescription medications used to treat the condition, classified by the medication’s mechanism of action (Electronic Supplementary Material). We reported the proportion of patients who filled at least one prescription and, among users, the total days of supply filled in the study year. For omalizumab, the number of doses was reported, as it is injected, rather than orally administered.
To characterize healthcare use, we reported the number of physician office visits, inpatient hospitalizations, and ED visits. To characterize cost, we calculated inpatient, outpatient, and prescription medication costs, and also summed them to give total healthcare costs. We calculated urticaria-related use and costs as follows. Outpatient claims with an ICD-9-CM code for urticaria (708.x) in any position were considered “urticaria related,” as were inpatient claims with a primary diagnosis of urticaria, as well as any of the above-mentioned medications.
Descriptive statistics were reported for all measures. To compare the costs and use across age group and sex, F tests and Chi-square tests (or Fisher’s exact Chi-square tests when a cell count is less than 5) were used for continuous and categorical outcomes respectively. Means and standard deviations (SDs) were reported for continuous variables, and counts and percentages for categorical variables. All data transformations and statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA).
Results
Among 5,802,466 individuals continuously enrolled in 2012, we identified 6350 as having CIU/CSU: a prevalence of 0.11 % (0.15 % in women and 0.07 % in men). CIU/CSU prevalence was 0.14 % in patients aged 11 years and younger, 0.07 % in those aged 12–24 years, 0.13 % in those aged 25–44 years, 0.12 % in those aged 45–64 years, and 0.10 % in those aged 65 years and older (Table 1). The mean age of all patients with CIU/CSU was 42.4 years, 68.3 % were female, and the mean Charlson Comorbidity Index was 0.9 (Table 2). Angioedema was coded during the study year in 23.5 % of patients. Most patients (52.0 %) received the majority of their care from primary care physicians, followed by allergists (19.1 %), and dermatologists (8.5 %). Forty-five percent of patients saw an allergist for the majority of their urticaria care (visits coded with a diagnosis of urticaria), compared with 33 % who saw a primary care physician and 15 % who saw a dermatologist.
Table 1.
Prevalence rate of CIU/CSU by age and sex
| Sex | Age group (years) | Prevalence (%) (numeratora/denominatorb) |
|---|---|---|
| Overall | ||
| All | All | 0.109 (6350/5,802,466) |
| By sex | ||
| Female | All | 0.147 (4336/2,958,556) |
| Male | All | 0.071 (2014/2,843,910) |
| By age group | ||
| All | ≤11 | 0.138 (813/590,616) |
| All | 12–24 | 0.065 (666/1,022,982) |
| All | 25–44 | 0.130 (1729/1,328,043) |
| All | 45–64 | 0.119 (1976/1,654,637) |
| All | 65+ | 0.097 (1166/1,206,188) |
| By sex and age group | ||
| Female | ≤11 | 0.143 (415/289,489) |
| Female | 12–24 | 0.093 (461/498,007) |
| Female | 25–44 | 0.196 (1298/662,756) |
| Female | 45–64 | 0.167 (1,399/838,212) |
| Female | 65+ | 0.114 (763/670,092) |
| Male | ≤11 | 0.132 (398/301,127) |
| Male | 12–24 | 0.039 (205/524,975) |
| Male | 25–44 | 0.065 (431/ 665,287) |
| Male | 45–64 | 0.071 (577/816,425) |
| Male | 65+ | 0.075 (403/536,096) |
CIU/CSU chronic idiopathic or spontaneous urticaria
aNumber of patients who met the inclusion criteria
bNumber of continuously enrolled health plan members between 1/1/2012 and 12/31/2012
Table 2.
Patient demographics, clinical characteristics, and comorbidities (n = 6350)
| Age in years, mean (SD) | 42.4 (22.1) |
| No. (%) of patients within age group (years) | |
| ≤5 | 456 (7.2) |
| 6–11 | 357 (5.6) |
| 12–17 | 326 (5.1) |
| 18–44 | 2069 (32.6) |
| 45–64 | 1976 (31.1) |
| 65+ | 1166 (18.4) |
| Female, no. (%) | 4336 (68.3) |
| Region, no. (%) | |
| Midwest | 1523 (24.0) |
| Northeast | 801 (12.6) |
| South | 2924 (46.0) |
| West | 1102 (17.4) |
| Number of chronic conditions, mean (SD) [median] | 3.3 (2.2) [3] |
| Charlson comorbidity index, mean (SD) [median] | 0.9 (1.6) [0] |
| Physicians providing usual carea, no. (%) | |
| Primary care | 3300 (52.0) |
| Allergist | 1210 (19.1) |
| Miscellaneousb | 1109 (17.5) |
| Dermatologist | 538 (8.5) |
| Unknown | 193 (3.0) |
| Angioedemac, no. (%) | 1490 (23.5) |
| Related conditions, no. (%) | |
| Allergic rhinitis | 2745 (43.2) |
| Other allergy | 1335 (21.0) |
| Asthma | 1165 (18.3) |
| Atopic dermatitis | 493 (7.8) |
| Vasculitis and allergic purpura | 27 (0.4) |
SD standard deviation
a“Usual care” is defined as the specialty accounting for the largest proportion of a given patients’ evaluation and management visits
bAll included specialties individually accounted for <2 % of patients
cPresence of an International Classification of Diseases, 9th Revision, Clinical Modification code of 995.1 (angioedema, not hereditary) during the study period
The most common prescription drugs filled by these patients were OCS: 54.7 % of patients filled at least one OCS prescription (Table 3). OTC medications were not reported in the study database; however, prescription antihistamines were filled by 24.0 %, non-sedating H1 antihistamines were used twice as often as other H1 antihistamines (11.5 vs. 5.8 %). LTRAs were used by 17.5 % of patients. Immunosuppressives were filled by 3.2 % of patients during the study year.
Table 3.
Classes of medications filleda by patients with CIU/CSU
| Medication | Number | Percent | Mean (SD) days supply filled |
|---|---|---|---|
| Oral corticosteroids | 3474 | 54.7 | 32.5 (55.7) |
| Prescription antihistamineb | 1526 | 24.0 | 107.7 (115.3) |
| Prescription H1-antihistaminec | 1034 | 16.3 | 96.4 (103.4) |
| Prescription H1-antihistamine, non-sedating | 728 | 11.5 | 116.1 (102.4) |
| Prescription H1-antihistamine, other | 371 | 5.8 | 41.0 (67.6) |
| Prescription H2-antihistamined | 626 | 9.9 | 103.3 (103.0) |
| Leukotriene receptor antagonistse | 1113 | 17.5 | 133.5 (116.0) |
| Dapsone, doxepin, hydroxychloroquine, or sulfasalazine | 779 | 12.3 | 127.9 (126.6) |
| Immunosuppressive agents (cyclosporine, mycophenolate, or methotrexate) | 204 | 3.2 | 142.8 (108.5) |
| Omalizumab | 35 | 0.6 | 6.1 (3.4)f |
| Epinephrine autoinjector | 1227 | 19.3 | NA |
CIU/CSU chronic idiopathic or spontaneous urticarial, NA not applicable
aClaims data report only prescription fills, not actual medication use
bThe database did not include non-prescription antihistamines
cNon-sedating H1-antihistamines included cetirizine HCl, desloratidine, fexofenadine HCl, levocetirizine dihydrochloride, and loratidine; sedating H1-antihistamines included brompheniramine, carbinoxamine, chlorpheniramine, clemastine, cyproheptadine, dexbrompheniramine, dexchlorpheniramine, diphenhydramine, doxylamine, pheniramine, promethazine, pyrilamine, tripelennamine, triprolidine
dCimetidine, ranitidine, famotidine, nizatidine
eMontelukast, zafirlukast, zileuton
fParenteral medications do not report “days of supply” on claims, therefore the mean number of doses is reported here
CIU/CSU patients had a mean of 15.1 all-cause office visits annually, 465 (7.3 %) were hospitalized at least once for any reason, and 1012 (15.9 %) had at least one ED visit (Table 4). Patients had a mean of 3.4 urticaria-related office visits; 0.1 % of patients had at least one urticaria-related hospitalization and 1.9 % had at least one urticaria-related ED visit. Use varied with age in a statistically significant way (p < 0.001) for all outcomes except urticaria-related hospitalization (p = 0.273). The highest use (both all cause and urticaria related) was in patients aged 65+ years and the lowest in those aged <12 years (Table 4). Women had statistically significantly more office visits than men (15.9 vs. 13.3, p < 0.001 for all cause; 3.5 vs. 3.3, p = 0.015 for urticaria related), and higher rates of all-cause ED visits (17.1 vs. 13.4 %, p < 0.001). Differences between women and men in hospitalization rates were not statistically significant (7.6 vs. 6.7 %, p = 0.196 for all cause; 0.1 vs. 0.2 %, p = 0.124 for urticaria related).
Table 4.
Healthcare resource use by age and sex
| No. of office visits, mean (SD) | No. of hospitalized patients, no. (%) | No. of patients who visited the ED, no. (%) | ||||
|---|---|---|---|---|---|---|
| All cause (p < 0.001) | Urticaria relateda (p < 0.001) | All cause (p < 0.001) | Urticaria relateda (p = 0.073) | All cause (p < 0.001) | Urticaria relateda (p < 0.001) | |
| Age group (years) | ||||||
| ≤5 | 12.0 (7.7) | 2.8 (1.5) | 11 (2.4) | 1 (0.2) | 21 (4.6) | 3 (0.7) |
| 6–11 | 10.5 (10.6) | 3.0 (1.8) | 4 (1.1) | 0 (0.0) | 11 (3.1) | 1 (0.3) |
| 12–17 | 12.5 (11.0) | 3.5 (3.3) | 15 (4.6) | 0 (0.0) | 25 (7.7) | 2 (0.6) |
| 18–44 | 13.5 (11.7) | 3.4 (2.6) | 118 (5.7) | 1 (0.0) | 274 (13.2) | 19 (0.9) |
| 45–64 | 17.0 (14.4) | 3.7 (3.6) | 180 (9.1) | 6 (0.3) | 342 (17.3) | 32 (1.6) |
| ≥65 | 18.0 (12.3) | 3.4 (2.7) | 137 (11.7) | 1 (0.1) | 339 (29.1) | 64 (5.5) |
| No. of office visits, mean (SD) | No. of hospitalized patients, no. (%) | No. of patients who visited the ED, no. (%) | ||||
|---|---|---|---|---|---|---|
| All cause (p < 0.001) | Urticaria relateda (p < 0.015) | All cause (p < 0.196) | Urticaria relateda (p < 0.124) | All cause (p < 0.001) | Urticaria relateda (p < 0.001) | |
| Sex | ||||||
| Female | 15.9 (12.8) | 3.5 (3.0) | 330 (7.6) | 4 (0.1) | 742 (17.1) | 87 (2.0) |
| Male | 13.3 (12.0) | 3.3 (2.8) | 135 (6.7) | 5 (0.2) | 270 (13.4) | 34 (1.7) |
ED emergency department, SD standard deviation
p values: comparison across all categories
a“Urticaria-related” defined by the presence of an International Classification of Diseases, 9th Revision, Clinical Modification code of 708.x in the primary position on an inpatient claim and any position on an outpatient claim
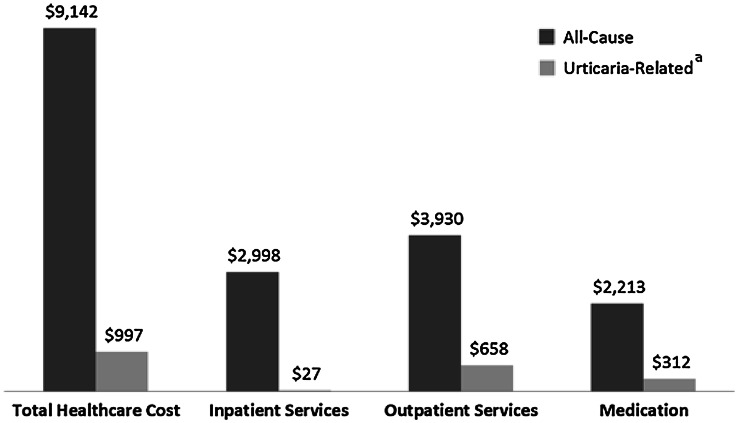
Mean all-cause healthcare costs were US$9142 (SD US$21,835, median US$3659) (Fig. 1). Outpatient services accounted for US$3930 (SD US$7490, median US$2186), or 43.0 % of total costs. Mean urticaria-related costs were US$997 (SD US$2322, median US$612). Outpatient services accounted for US$658 (SD US$1726, median US$442), or 66.0 % of urticaria-related costs and medications for US$312 (SD US$1261, median US$30) or 31.3 % of urticaria-related costs. Mean costs were US$2676 in patients aged 5 years and younger, increasing to US$14,234 in those aged 65+ years (p < 0.001). Women had higher all-cause (US$9507 vs. US$8354, p = 0.038) and urticaria-related costs (US$1048 vs. US$887, p = 0.003) than men (Table 5).
Fig. 1.
Mean healthcare cost by category in 6350 patients with chronic idiopathic or spontaneous urticarial. a “Urticaria-related” defined by the presence of an International Classification of Diseases, 9th Revision, Clinical Modification code of 708.x in the primary position on an inpatient claim and any position on an outpatient claim
Table 5.
Healthcare costs by age and sex (US$)
| Healthcare costsb | All cause (p < 0.001) | Urticaria relateda (p < 0.001) |
|---|---|---|
| Age group (years), mean (SD) | ||
| ≤5 | 2676 (2,792) | 604 (808) |
| 6–11 | 3119 (8,738) | 1109 (5,523) |
| 12–17 | 4607 (8,916) | 895 (1,022) |
| 18–44 | 7091 (19,470) | 1037 (2,073) |
| 45–64 | 11,612 (23,734) | 1181 (2,526) |
| ≥65 | 14,234 (29,059) | 762 (946) |
| Healthcare costsb | All cause (p < 0.038) | Urticaria relateda (p < 0.003) |
|---|---|---|
| Sex, mean (SD) | ||
| Female | 9507 (22,838) | 1048 (2,542) |
| Male | 8354 (19,475) | 887 (1,753) |
CIU/CSU chronic idiopathic urticaria/chronic spontaneous urticarial, SD standard deviation
p values: comparison across all categories
a“Urticaria-related” defined by the presence of an International Classification of Diseases, 9th Revision, Clinical Modification code of 708.x in the primary position on an inpatient claim and any position on an outpatient claim
bThe database did not include non-prescription (over-the-counter) antihistamines
Discussion
Our analysis identified 6350 CIU/CSU patients in a population of just over 5.8 million, a prevalence of 0.11 %. The condition is nearly twice as common in women as men, with a peak age of 25–44 years [20]. Angioedema was coded in 23.5 % of patients over the 1-year study period. Despite having a low rate of severe comorbidities, as measured by the Charlson index, CIU/CSU patients are frequent users of healthcare resources, visiting physician offices more often than once a month, on average. Urticaria-related healthcare resource use is relatively low, suggesting that CIU/CSU patients seek care frequently for reasons not coded as related to urticaria.
According to a 2011 report by GA2LEN, only three studies are available on the prevalence of CIU/CSU in the general population, and all have limited relevance for the current US population [5]. A German survey of 4093 respondents in 1999–2000 used a physical examination to confirm cases and found an annual prevalence of 0.8 % and a lifetime prevalence of 1.8 % [21]. Another frequently cited source is a Spanish population-based study that reported a prevalence of 0.6 % [22]. Finally, a 1972 study reported the prevalence of urticaria in Sweden as 0.11 % and chronic urticaria prevalence as 0.07 % [23]. We were unable to identify any additional studies of CIU/CSU prevalence. Our finding of a higher prevalence in children aged under 11 years has not been previously reported. Children visit healthcare providers more often than adults and therefore may be more likely to be given the diagnosis of urticaria, potentially biasing our results. The algorithm validation was performed in adult patients, and therefore its accuracy in children cannot be confirmed. In prior studies, angioedema has been reported as occurring in 40–50 % of patients [2, 24–26], apparently based on a chart review from the 1960s in the UK [27]. A recent clinical trial reported angioedema in 53 % of patients [28].
Using a validated ICD-9-CM algorithm in a large, recent, administrative claims database, we confirmed a prior prevalence estimate of approximately 0.1 % [29]. Our estimate is consistent with prior non-US population-based surveys but may underestimate true prevalence for two reasons. First, our data were derived only from insured patients, who may be more likely to seek care for non-life-threatening diseases than the uninsured. Second, some cases of CIU/CSU will be coded in such a way that they are not identified by our algorithm.
Antihistamines are the mainstay of chronic urticaria treatment and can control disease activity in most patients [30]. The FDA first approved loratadine, cetirizine, and fexofenadine for OTC sale in 1998. Formulations of Zyrtec® (cetirizine; Pfizer, New York City, NY, USA) and Allegra® (fexofenadine; Sanofi-Aventis, Paris, France) became available OTC in 2007 and 2011, respectively [31]. In our analysis, only 24.0 % of patients used prescription antihistamines; the majority of the remainder likely used OTC formulations. Clinical practice guidelines suggest H1 antihistamines are the initial treatment of choice, and a recent analysis from a single US center found 72 % of patients using them [32]. Other agents recommended for use in CIU/CSU include OCS, which were used by 54.7 % of patients in our study. An updated clinical practice guideline for the management of CIU, published in May 2014, has indicated that long-term use of systemic corticosteroids should be discouraged and instead, following monotherapy with a second-generation antihistamine therapy, consideration should be given to increasing the dose, adding a second antihistamine, adding an H2 antagonist or LTRA, or changing to a more potent antihistamine. If these changes are insufficient, recommendations include either adding omalizumab or cyclosporine, or (although supported by less evidence) immunomodulators (e.g., dapsone, sulfasalazine) or anti-inflammatory drugs [30].
The mean total cost of care for patients with CIU/CSU is more than US$9000 per year compared with a 2009 national estimate of US$6815 [33]. Urticaria-related cost was estimated to be US$997 annually, compared to US$1050 for hypertension and US$1160 for diabetes mellitus [34, 35]. Lacking data on OTC medications may have caused us to underestimate cost. If 72 % of the CIU/CSU population used OTC antihistamines on a daily basis, and the estimated daily cost was US$0.10, the mean annual cost would be approximately US$25 higher than what we reported. Our study confirms a prior finding that women with CIU/CSU have higher costs and use more healthcare resources than men [32], consistent with the observation that women use more healthcare resources in general [36–38]. Urticaria-related hospitalization was observed in 0.1 % of the study group. With a CIU/CSU prevalence of 0.1 %, this suggests one urticaria hospitalization per million population, consistent with the rate of 1.4 per million reported in a study using 2005 national hospital discharge data [39]. Less than 10 % of total costs were associated with a claim containing a code for urticaria, despite the fact that patients in our study appeared relatively healthy as measured by the Charlson Comorbidity Index. The current study was not designed to identify the source of non-urticaria-related costs, but such a study could be designed. A matched study, using CIU/CSU patients and disease-free controls, would be able to estimate the relative contribution of the disease to total cost.
There were several limitations of our study. First, there is no single ICD-9-CM diagnosis for CIU/CSU. While the algorithm we used has a PPV of 90.4 % and sensitivity of 71.1 %, the validation study involved 149 patients at four centers, and our ability to make claims on the performance of specific patient subgroups, such as children, is limited [12]. Furthermore, the reliance on an algorithm, rather than a single code, meant we could not attribute healthcare resources specifically to CIU/CSU (as the algorithm could not be applied to individual visits). Instead, we identified claims associated with any urticaria (708.x) as being “urticaria related.” OTC medications are not submitted for insurance payment, thus no claim for these medications was available in our database, a particularly important limitation for examining treatment patterns in a disease treated with antihistamines. Other study designs, including surveys and chart reviews, could be used to overcome this limitation, as well as the prior one. Undercoding of angioedema may explain the lower rate reported in this study compared with others; claims studies often suffer this limitation, particularly for less common conditions. As it comprises a privately insured population, the claims database includes more young people and fewer people aged over 65 years than the general population [20]. Other limitations of the database include under- and over-representation of various geographic regions and lack of data on the uninsured populations.
Conclusions
The prevalence of CIU/CSU is 0.11 % in a commercially insured US population. The mean age of CIU/CSU patients was 42.4 years. A claim for non-hereditary angioedema was seen in 23.5 % of patients. More than half used OCS during the year of observation. Seven percent of patients were hospitalized at least once during the study, and 15.9 % were seen in the ED. The mean annual healthcare cost for a patient with CIU/CSU was over US$9000.
Electronic supplementary material
Acknowledgments
The authors thank Gordon H. Sun, MD, MS for his contributions to study design and drafting of the initial manuscript.
Conflict of interest
K. Raimundo is employed by Genentech, Inc. and holds stock options in the company. E. Antonova is employed by Genentech, Inc. M.S. Broder and E. Chang are employed by Partnership for Health Analytic Research, LLC, a health services research company paid by Genentech, Inc. to conduct this research.
References
- 1.Schaefer P. Urticaria: evaluation and treatment. Am Fam Physician. 2011;83:1078–1084. [PubMed] [Google Scholar]
- 2.Saini SS. Chronic spontaneous urticaria: etiology and pathogenesis. Immunol Allergy Clin North Am. 2014;34(1):33–52. doi: 10.1016/j.iac.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurer M, Rosén K, Hsieh JH, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan AP. Clinical practice. Chronic urticaria and angioedema. N Engl J Med. 2002;346(3):175–179. doi: 10.1056/NEJMcp011186. [DOI] [PubMed] [Google Scholar]
- 5.Maurer M, Weller K, Bindslev-Jensen C, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66(3):317–330. doi: 10.1111/j.1398-9995.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 6.Grob JJ, Revuz J, Ortonne JP, Auquier P, Lorette G. Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br J Dermatol. 2005;152:289–295. doi: 10.1111/j.1365-2133.2005.06385.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell BF. Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin North Am. 2014;34(1):89–104. doi: 10.1016/j.iac.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM, Grattan CEH, Kapp A, Maurer M, Merk HF, Rogala B, Saini S, Sánchez-Borges M, Schmid-Grendelmeier P, Schünemann H, Staubach P, Vena GA, Wedi B. EAACI/GA2LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009;64:1427–1443. [DOI] [PubMed]
- 9.Genentech, Inc. Omalizumab (Xolair®) packaging insert. http://www.gene.com/download/pdf/xolair_prescribing.pdf. Last revised Sept 2014. Accessed 11 Nov 2014.
- 10.Health Insurance Coverage of the Total Population. The Henry J. Kaiser family foundation. http://kff.org/other/state-indicator/total-population. Accessed 4 Apr 2015.
- 11.Cherepanov D, Raimundo K, Chang E, Eagan M, Zazzali JL, Solari PG, DeCotiis B, Hussain I, Rehman SM, Shahab N, Tilles SA, Broder MS. Validation of an ICD-9-based claims algorithm for identifying patients with chronic idiopathic/spontaneous urticaria. Ann Allergy Asthma Immunol. 2015. doi: 10.1016/j.anai.2015.02.003 (Epub ahead of print). [DOI] [PubMed]
- 12.Raimundo K, DeCotiis B, Hussain I, et al. Validation of an ICD-9-based claims algorithm for identifying patients with chronic idiopathic/spontaneous urticaria. Poster presented at: International Society for Pharmacoeconomics and Outcomes Research 19th annual international meeting, May 31–June 4, 2014, Montreal, QC, Canada.
- 13.Kim SY, Servi A, Polinski JM, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441. [DOI] [PMC free article] [PubMed]
- 15.HCUP Chronic Condition Indicator. Healthcare Cost and Utilization Project (HCUP). 2015. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed 30 Jan 2015.
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Yurkovich M, Avina-Zubieta JA, Thomas J, Gorenchtein M, Lacaille D. A systematic review identifies valid comorbidity indices derived from administrative health data. J Clin Epidemiol. 2015;68(1):3–14. doi: 10.1016/j.jclinepi.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 19.O’Malley AS, Pham HH, Schrag D, Wu B, Bach PB. Potentially avoidable hospitalizations for COPD and pneumonia: the role of physician and practice characteristics. Med Care. 2007;45:562–570. doi: 10.1097/MLR.0b013e3180408df8. [DOI] [PubMed] [Google Scholar]
- 20.Howden LM, Meyer JA. Age and sex composition: 2010. In 2010 census briefs. 2011. http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. Accessed 4 Apr 2015.
- 21.Zuberbier T, Balke M, Worm M, Edenharter G, Maurer M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin Exp Dermatol. 2010;35(8):869–873. doi: 10.1111/j.1365-2230.2010.03840.x. [DOI] [PubMed] [Google Scholar]
- 22.Gaig P, Olona M, Muñoz Lejarazu D, et al. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004;14(3):214–220. [PubMed] [Google Scholar]
- 23.Hellgren L. The prevalence of urticaria in the total population. Acta Allergol. 1972;27(3):236–240. doi: 10.1111/j.1398-9995.1972.tb01420.x. [DOI] [PubMed] [Google Scholar]
- 24.Brodell LA, Beck LA. Differential diagnosis of chronic urticaria. Ann Allergy Asthma Immunol. 2008;100(3):181–8 (quiz 188–90, 215). [DOI] [PubMed]
- 25.Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105(4):664–672. doi: 10.1067/mai.2000.105706. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114(3):465–74 (quiz 475). [DOI] [PubMed]
- 27.Champion RH, Roberts SO, Carpenter RG, Roger JH. Urticaria and angio-oedema. A review of 554 patients. Br J Dermatol. 1969;81(8):588–597. doi: 10.1111/j.1365-2133.1969.tb16041.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, Veith J, Kamath N, Staubach P, Jakob T, Stirling RG, Kuna P, Berger W, Maurer M, Rosén K. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132(1):101–109. doi: 10.1016/j.jaci.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Zazzali JL, Broder MS, Chang E, Chiu MW, Hogan DJ. Cost, utilization, and patterns of medication use associated with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2012;108:98–102. doi: 10.1016/j.anai.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270–1277. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration. Prescription to over-the-counter (OTC) switch list. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106378.htm. Last updated 25 July 2014. Accessed 7 2014.
- 32.Delong LK, Culler SD, Saini SS, Beck LA, Chen SC. Annual direct and indirect health care costs of chronic idiopathic urticaria: a cost analysis of 50 nonimmunosuppressed patients. Arch Dermatol. 2008;144(1):35–39. doi: 10.1001/archdermatol.2007.5. [DOI] [PubMed] [Google Scholar]
- 33.Health Care Expenditures per Capita by State of Residence. The Henry J. Kaiser family foundation. http://kff.org/other/state-indicator/health-spending-per-capita-by-service. Accessed 30 Oct 2014.
- 34.Expenditures for hypertension among adults age 18 and older, 2010: estimates for the US civilian noninstitutionalized population. Agency for Healthcare Research and Quality. http://meps.ahrq.gov/data_files/publications/st404/stat404.shtml. Accessed 4 Apr 2015. [PubMed]
- 35.Trends in use and expenditures for diabetes among adults 18 and older, US Civilian Noninstitutionalized Population, 1996 and 2007. Agency for Healthcare Research and Quality. http://meps.ahrq.gov/mepsweb/data_files/publications/st304/stat304.shtml. Accessed 4 Apr 2015.
- 36.Bertakis KD, Azari R, Helms LJ, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract. 2000;49(2):147–152. [PubMed] [Google Scholar]
- 37.Manteuffel M, Williams S, Chen W, et al. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health (Larchmt). 2014;23(2):112–119. doi: 10.1089/jwh.2012.3972. [DOI] [PubMed] [Google Scholar]
- 38.Owens GM. Gender differences in health care expenditures, resource utilization, and quality of care. J Manag Care Pharm. 2008;14(3 Suppl):2–6. doi: 10.18553/jmcp.2008.14.S6-A.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol. 2008;101(2):185–192. doi: 10.1016/S1081-1206(10)60208-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.