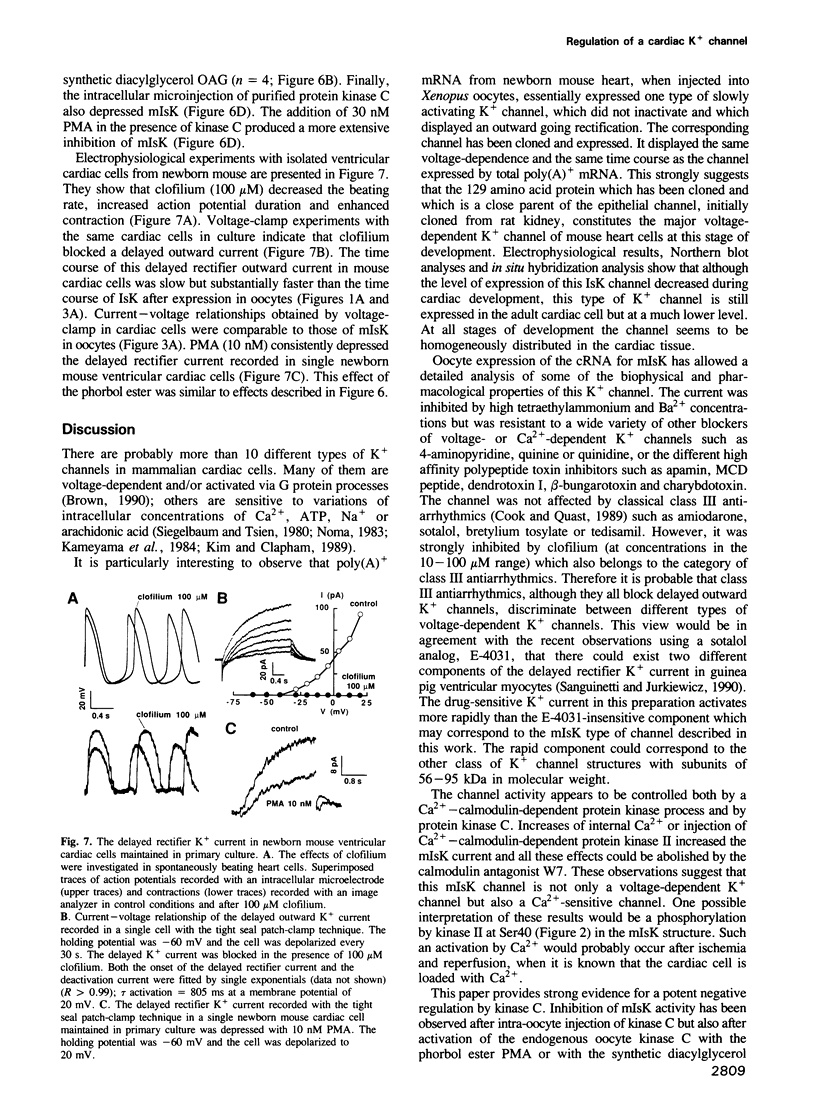
Abstract
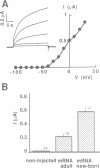
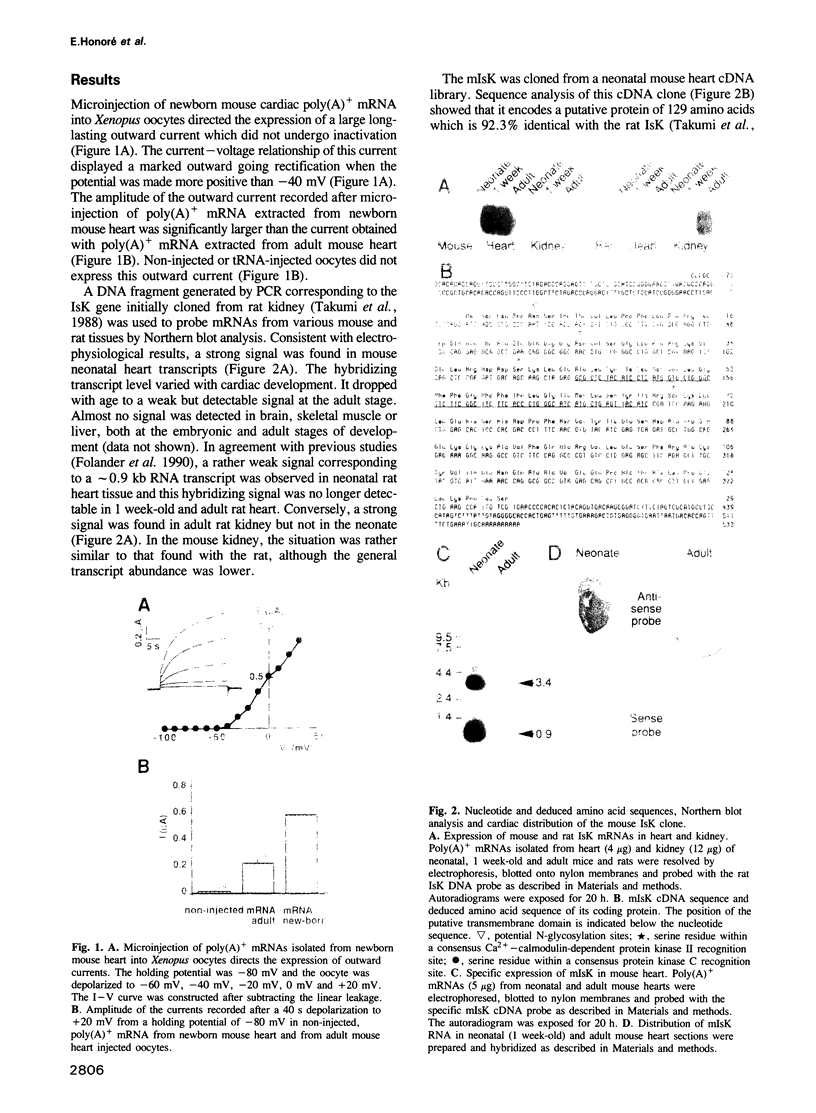
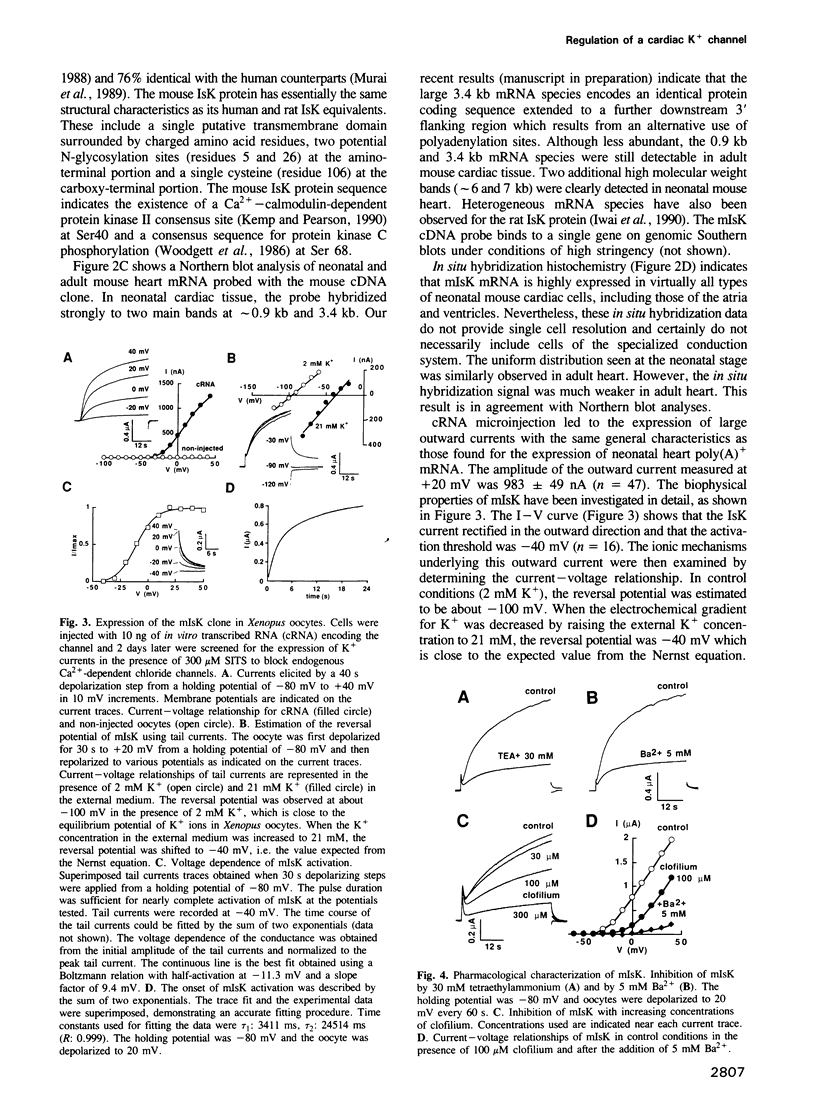
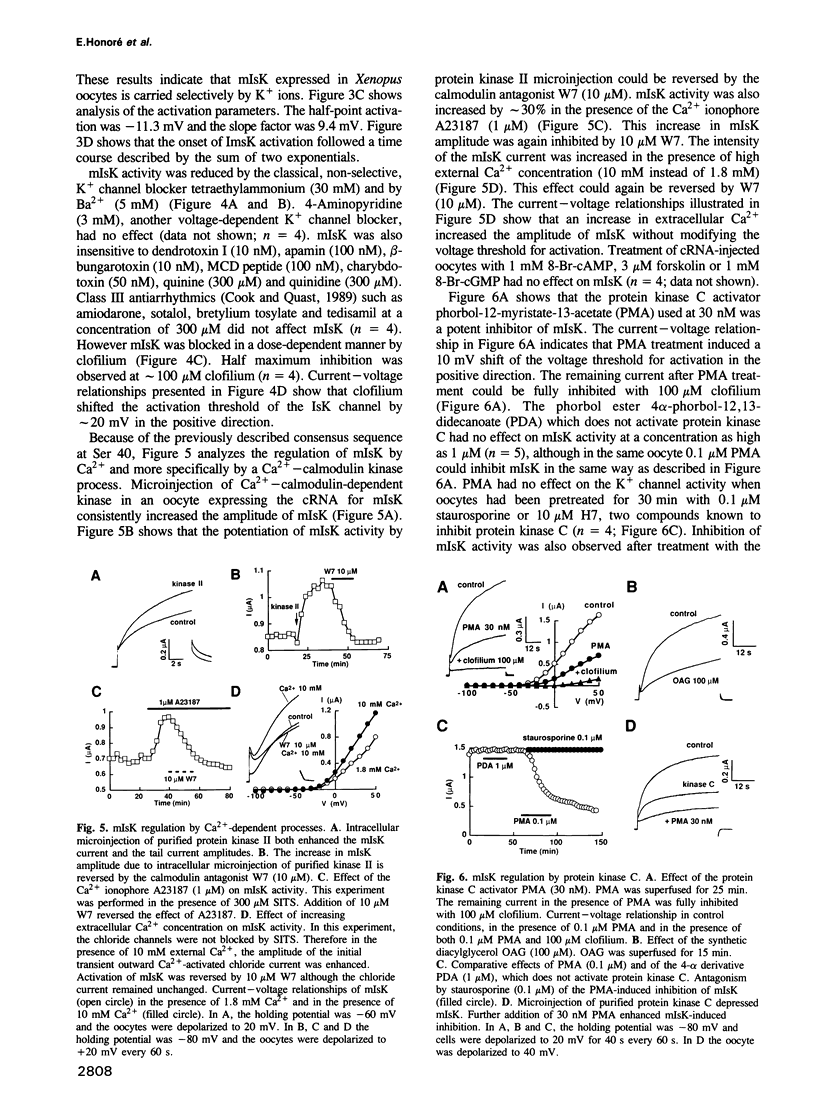
Neonatal mouse cardiac poly(A)+ mRNA microinjection into Xenopus oocytes directed the expression of a delayed rectifier K+ current. A cDNA encoding this channel, called mIsK, was cloned from a neonatal mouse heart cDNA library whose properties were studied after expression of the complementary RNA in Xenopus oocytes. Among the different known K+ channel blockers, only the class III antiarrhythmic clofilium inhibited mIsK in the 10-100 microM range. The channel was completely insensitive to other antiarrhythmics such as quinine, quinidine, sotalol or amiodarone. mIsK was enhanced by increasing intracellular Ca2+ and by microinjected Ca(2+)-calmodulin dependent protein kinase II. These stimulations were reversed by the calmodulin antagonist W7. Conversely, the phorbol ester PMA, the diacylglycerol analog OAG and microinjected purified protein kinase C inhibited mIsK. This inhibitory effect could be prevented by the protein kinase C inhibitor staurosporine. These results were consistent with the presence of consensus sequences for kinase II and kinase C in the mIsK structure. Cultured newborn mouse ventricular cardiac cells exhibited a delayed rectifier K+ current which had biophysical properties similar to those of cloned mIsK and which was inhibited by clofilium and protein kinase C activators. In situ hybridization experiments revealed that mIsK mRNA was homogeneously distributed in the cardiac tissue. Neonatal mouse heart expressed the most mIsK mRNA compared with various other rat and mouse tissues. Since this K+ channel generates a current which appears to be involved in the control of both the action potential duration and the beating rate, these results suggest an important role for the mIsK channel in cardiac cell physiology and cardiac pathology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arena J. P., Kass R. S. Block of heart potassium channels by clofilium and its tertiary analogs: relationship between drug structure and type of channel blocked. Mol Pharmacol. 1988 Jul;34(1):60–66. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi H., Bidard J. N., Fosset M., Hugues M., Mourre C., Rehm H., Romey G., Schmid-Antomarchi H., Schweitz H., de Weille J. R. Molecular properties of potassium channels. Arzneimittelforschung. 1989 Jan;39(1A):159–163. [PubMed] [Google Scholar]
- Betz H. Homology and analogy in transmembrane channel design: lessons from synaptic membrane proteins. Biochemistry. 1990 Apr 17;29(15):3591–3599. doi: 10.1021/bi00467a001. [DOI] [PubMed] [Google Scholar]
- Brown A. M. Regulation of heartbeat by G protein-coupled ion channels. Am J Physiol. 1990 Dec;259(6 Pt 2):H1621–H1628. doi: 10.1152/ajpheart.1990.259.6.H1621. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dascal N., Snutch T. P., Lübbert H., Davidson N., Lester H. A. Expression and modulation of voltage-gated calcium channels after RNA injection in Xenopus oocytes. Science. 1986 Mar 7;231(4742):1147–1150. doi: 10.1126/science.2418503. [DOI] [PubMed] [Google Scholar]
- Folander K., Smith J. S., Antanavage J., Bennett C., Stein R. B., Swanson R. Cloning and expression of the delayed-rectifier IsK channel from neonatal rat heart and diethylstilbestrol-primed rat uterus. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2975–2979. doi: 10.1073/pnas.87.8.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A. E., HUTTER O. F., NOBLE D. Current-voltage relations of Purkinje fibres in sodium-deficient solutions. J Physiol. 1963 Apr;166:225–240. doi: 10.1113/jphysiol.1963.sp007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Iwai M., Masu M., Tsuchida K., Mori T., Ohkubo H., Nakanishi S. Characterization of gene organization and generation of heterogeneous mRNA species of rat ISK protein. J Biochem. 1990 Aug;108(2):200–206. doi: 10.1093/oxfordjournals.jbchem.a123181. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. How might the diversity of potassium channels be generated? Trends Neurosci. 1990 Oct;13(10):415–419. doi: 10.1016/0166-2236(90)90123-r. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Kuret J., Schulman H. Purification and characterization of a Ca2+/calmodulin-dependent protein kinase from rat brain. Biochemistry. 1984 Nov 6;23(23):5495–5504. doi: 10.1021/bi00318a018. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. The potassium current underlying delayed rectification in cat ventricular muscle. J Physiol. 1978 Jan;274:217–246. doi: 10.1113/jphysiol.1978.sp012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Haghani Z., Brady J., Bush L., McBride W., Buja L. M., Willerson J. T. Differences in myocardial alpha- and beta-adrenergic receptor numbers in different species. Am J Physiol. 1983 Dec;245(6):H957–H961. doi: 10.1152/ajpheart.1983.245.6.H957. [DOI] [PubMed] [Google Scholar]
- Murai T., Kakizuka A., Takumi T., Ohkubo H., Nakanishi S. Molecular cloning and sequence analysis of human genomic DNA encoding a novel membrane protein which exhibits a slowly activating potassium channel activity. Biochem Biophys Res Commun. 1989 May 30;161(1):176–181. doi: 10.1016/0006-291x(89)91577-5. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Pragnell M., Snay K. J., Trimmer J. S., MacLusky N. J., Naftolin F., Kaczmarek L. K., Boyle M. B. Estrogen induction of a small, putative K+ channel mRNA in rat uterus. Neuron. 1990 May;4(5):807–812. doi: 10.1016/0896-6273(90)90207-v. [DOI] [PubMed] [Google Scholar]
- Reuter H. Ion channels in cardiac cell membranes. Annu Rev Physiol. 1984;46:473–484. doi: 10.1146/annurev.ph.46.030184.002353. [DOI] [PubMed] [Google Scholar]
- Roberds S. L., Tamkun M. M. Cloning and tissue-specific expression of five voltage-gated potassium channel cDNAs expressed in rat heart. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1798–1802. doi: 10.1073/pnas.88.5.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romey G., Garcia L., Rieger F., Lazdunski M. Targets for calcium channel blockers in mammalian skeletal muscle and their respective functions in excitation-contraction coupling. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1324–1332. doi: 10.1016/s0006-291x(88)80777-0. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990 Jul;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubeita H. E., McDonough P. M., Harris A. N., Knowlton K. U., Glembotski C. C., Brown J. H., Chien K. R. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes. A paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990 Nov 25;265(33):20555–20562. [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. Ion channels. Making a submicroscopic hole in one. Nature. 1991 Feb 21;349(6311):657–658. doi: 10.1038/349657a0. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séquier J. M., Brennand J., Barhanin J., Lazdunski M. Regional expression of a MCD-peptide and dendrotoxin I-sensitive voltage-dependent potassium channel in rat brain. FEBS Lett. 1990 Apr 9;263(1):163–165. doi: 10.1016/0014-5793(90)80729-3. [DOI] [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Tamkun M. M., Knoth K. M., Walbridge J. A., Kroemer H., Roden D. M., Glover D. M. Molecular cloning and characterization of two voltage-gated K+ channel cDNAs from human ventricle. FASEB J. 1991 Mar 1;5(3):331–337. doi: 10.1096/fasebj.5.3.2001794. [DOI] [PubMed] [Google Scholar]
- Tseng-Crank J. C., Tseng G. N., Schwartz A., Tanouye M. A. Molecular cloning and functional expression of a potassium channel cDNA isolated from a rat cardiac library. FEBS Lett. 1990 Jul 30;268(1):63–68. doi: 10.1016/0014-5793(90)80973-m. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Kass R. S. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988 Oct 7;242(4875):67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Bertics P. J., Hudson L. G., Vedvick T. S., Gill G. N. A three-step purification procedure for protein kinase C: characterization of the purified enzyme. Anal Biochem. 1987 Mar;161(2):425–437. doi: 10.1016/0003-2697(87)90471-4. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986 Nov 17;161(1):177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]