Abstract
Recent neuroimaging studies suggest that the brain adapts with pain, as well as imparts risk for developing chronic pain. Within this context we revisit the concepts for nociception, acute and chronic pain, and negative moods relative to behavior selection. We redefine nociception as the mechanism protecting the organism from injury; while acute pain as failure of avoidant behavior; and a mesolimbic threshold process that gates the transformation of nociceptive activity to conscious pain. Adaptations in this threshold process are envisioned to be critical for development of chronic pain. We deconstruct chronic pain into four distinct phases, each with specific mechanisms; and outline current state of knowledge regarding these mechanisms: The limbic brain imparting risk, while mesolimbic learning processes reorganizing the neocortex into a chronic pain state. Moreover, pain and negative moods are envisioned as a continuum of aversive behavioral learning, which enhance survival by protecting against threats.
INTRODUCTION
Classically, pain has been conceptualized from the narrow viewpoint of nociceptive processing. The field has generated extensive knowledge regarding the transduction, transmission, and spinal cord processing of nociceptive signals related to acute and chronic pain; similarly, animal studies have unraveled properties of primary afferents, their spinal cord circuitry, and related specialized pathways in the brain that mediate pain-like behavior. Post-nerve injury reorganization of nociceptive afferents and spinal cord circuitry, in particular, has been extensively characterized in rodent models, with the tacit assumption that acute and chronic pain is best understood through this circuitry. In parallel, human brain imaging studies have identified nociceptive brain circuits. However, recent human brain imaging studies examining a variety of pain conditions indicate that the brain plays an active role in acute and clinical pain perception (figure 1), leading to a heated debate regarding the respective importance of peripheral afferents versus the brain’s interpretation of afferent signals. Here we review implications of these competing concepts in light of emerging evidence.
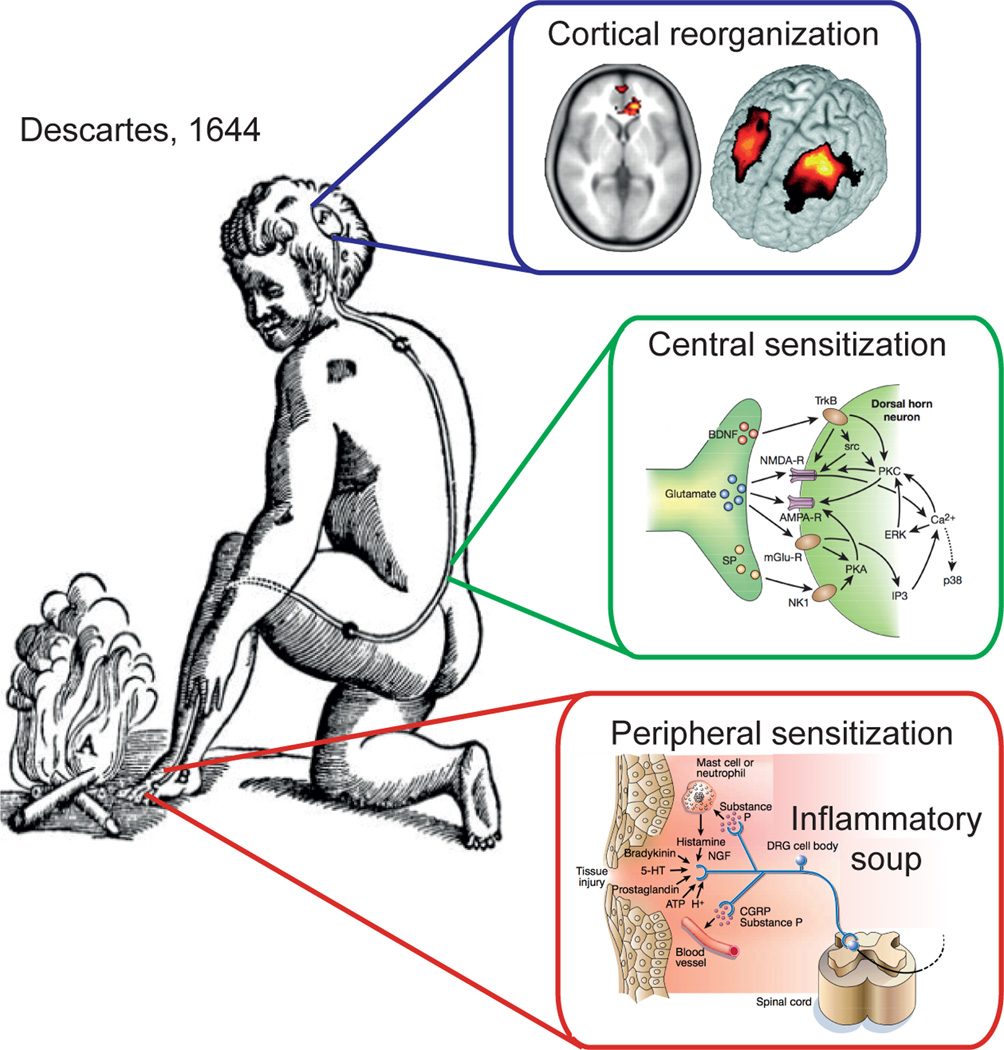
Figure 1.
Descartes’s concept of sensation illustrates the pain system. In addition reorganization of its components are superimposed based on modern rodent model physiology and human brain imaging studies. The Cartesian illustration is explicit regarding an impinging stimulus being transduced and transmitted to a specific brain region where perception takes place. The additional panels emphasize the modern evidence that all components of this system undergo reorganization following an injury that gives rise to a persistent or chronic pain state. End organ injury gives rise to changes in the local milieu, inflammatory soup, and in afferent response properties; collectively described as peripheral sensitization (adapted from (Julius and Basbaum, 2001)). Additionally, spinal cord circuitry undergoes a large number of changes resulting in central sensitization (adapted from (Scholz and Woolf, 2002)), which includes enhanced glutamatergic signaling, changes in second order messenger processes and activation of microglia. At the level of the brain, human neuroimaging studies indicate anatomical and functional reorganization.
The standard definition of pain emphasizes its subjectivity. Subjectivity, in turn, implies a conscious experience. A central goal of our perspective is to revise the understanding of conscious pain perception by incorporating nociception, acute and chronic pain, and negative moods into the unifying framework of behavior selection, where behavioral selections encompass the full range of possible actions for stimuli, whether internal or external, conscious or subconscious, and voluntary or involuntary. This viewpoint calls for a re-examination of the definitions we have inherited, as their narrow meanings have limited the types of questions posed within the field. Furthermore, we will introduce a novel interpretation of supraspinal processing of pain distinguishing between the subjectively conscious pain state and sub-, un- or pre-conscious nociceptive processes. We propose a comprehensive mechanistic model that properly incorporates acute and chronic pain, where the emotional limbic brain plays a critical role in bridging nociception and pain perception, as well as in the transition from acute to chronic pain; leading to the generalization of the functional continuity between pain and negative moods. Given that the literature supporting our model remains recent and fragmentary, we highlight important gaps in knowledge, as well as fruitful directions of inquiry.
Nociception
Sherrington coined the term nociception (Sherrington, 1900), and outlined its underlying neural structures. He viewed nociceptive reflexes and pain perception as tightly linked processes, such that “were the brain intact, [nociceptive activity] would, we may presume, evoke ‘pain’” (Woodworth and Sherrington, 1904). Since these first observations more than a hundred years of research has produced incontrovertible evidence regarding the specialized neuronal/molecular properties that define and characterize the nociceptive machinery (Basbaum et al., 2009; Woolf and Salter, 2000). Activation of nociceptors and nociceptive pathways undoubtedly can give rise to pain, and the close correspondence between nociceptor properties and human pain perception has been confirmed using a variety of experimental approaches (Marks et al., 2006).
On the other hand, ample evidence indicates that nociceptors can be active in the absence of pain perception. For example, any pain psychophysicist would agree that applying a 50 kg weight on a 1 cm area of skin would evoke excruciating pain. Yet, experienced ballerinas dance with point shoes for many hours, reporting deep positive emotional satisfaction while their toes carry the weight of their body, an activity that must massively and continuously activate their toe nociceptors. Therefore, at least ballerinas with intense training are capable of dissociating pain from nociception. The primary reason I fidget in my chair while writing this article is because nociceptors innervating my skin, muscle, and bone command that my posture needs adjustment. Whereas proprioceptors provide (conscious, but usually habituated) information about the location and position of my body in space, nociceptors provide inputs that protect my body from injury. One (especially the pain scientist) commonly forgets the fact that most humans, and perhaps many other species as well, spend most of their lives free of pain and with no obvious tissue injuries. This must be ascribed to active nociceptors, as there are no other alternative neuronal mechanisms available to continuously protect the body and subvert the potential for injury and resulting pain perception.
The necessity of nociceptive activity in the absence of stark pain is perhaps best illustrated by patients with various pain insensitivities due to leprosy, where the simple act of walking a mile gives rise to severe soft tissue and bone damage due to the lack of nociceptive afferents which render these subjects unable to modulate their gait and incapable of avoiding tissue injury (Brand, 1993). The best related clinical evidence comes from painless Charcot joints of tabes dorsalis (Sanders, 2004), and painless channelopathies (Bennett and Woods, 2014). Dissociations between nociceptive stimuli and perceived pain are also demonstrable in the lab setting. For example, repetitive painful laser stimuli induce rate-dependent modulation of evoked potentials, presumably reflecting nociceptive barrage of varying intensity, for a stable perception of pain (Mouraux et al., 2013; Wang et al., 2010). Moreover, when pain perception is assessed dynamically for complex stimulus patterns (“offset analgesia”; (Yelle et al., 2008) (Cecchi et al., 2012)), the statistical power function relating stimulus intensity to perceived magnitude of pain degrades and becomes highly nonlinear (Price, 1988; Stevens, 1957).
The electrophysiology of nociceptors and of nociceptive pathways are fully consistent with this idea, as extensive evidence shows that the majority of peripheral nociceptors can be activated with stimuli that are sub-threshold for pain perception, and a large proportion of central nociceptive neurons respond convergently to non-nociceptive stimuli as well (Kenshalo et al., 1980; Meyer et al., 2006; Willis and Coggeshall, 1978). As a result, the nociceptive control of behavior routinely occurs in the absence of consciously perceived pain, rendering it “subconscious.” An important caveat to this position relates to microneurographic studies in humans that elegantly demonstrate the existence of certain peripheral nociceptors that, when individually activated (single action potentials), can give rise to pain perception within a perceptive field that closely matches the receptive field of the stimulated nociceptor (Weidner et al., 1999). The latter establishes existence of a class of nociceptors with direct access to pain, at least in the controlled lab setting, but does not discount the existence of other nociceptors activated with various bodily postures but that usually do not give rise to pain.
The active role of subconscious nociceptors extends directly to behavior, as even the most common behavioral repertoires require nociception to avoid injury. Daily motor movements could easily produce injury and tissue damage if one exceeds their natural range of motion, such as extreme extensions of fingers, elbows, shoulders, hips, knees or ankles, or chewing on or hitting hard objects, etc., which supports the conclusion that motor behaviors are collectively inhibited by nociceptors. Surprisingly, this idea has not been studied, apart from spinal reflex behaviors. Similarly, there is no physiological evidence describing the nociceptive circuitry inhibiting motor repertoires. It is unlikely that nociceptive modulation of motor programs are mediated through the motor cortex, as only excitatory nociceptive inputs have been described in the region (Chen et al., 2011b; Frot et al., 2013). More likely it is probably mediated through dorsal striatal circuitry, where nociceptive inputs have been reported (Braz et al., 2005; Chudler et al., 1993; Newman et al., 1996) and where a large portion of the output is inhibitory to thalamocortical motor circuits.
In summary, we argue that nociception continuously occurs in the absence of pain perception and it is a fundamental physiological process (in the language of Christof Koch: the zombie agent of the sense of pain (Koch, 2012)) that subconsciously provides more veridical and instantaneous information that protects the organism from tissue damage. From a mechanistic viewpoint, we presume that behaviors modulated by nociception, in the absence of pain, are contingent on already established habitual repertoires. In contrast, when pain is evoked it gives rise to new peripheral and spinal cord nociceptive learning/sensitization (Basbaum et al., 2009; Ikeda et al., 2003; Woolf and Salter, 2000), as well as emotional learning that is potentiated by the salience and perceived value of the aversive event. Yet by and large nociceptive functioning and its underlying mechanisms in the absence of pain surpass current understanding and require investigation in their own right.
Acute pain
We next re-examine the definition of pain in relationship to the “subconscious” concept of nociception. We emphasize that pain is a conscious subjective experience that is most commonly driven by nociceptive activity. Yet, its threshold and magnitude can be readily modulated by mood and attention (Bushnell et al., 2013), monetary reward (Vlaev et al., 2009), simple changes in instructions e.g. (Baliki et al., 2010), and through expectations (Wiech et al., 2014). A large brain imaging literature continues to examine the dependence of acute pain perception on various cognitive and emotional modulators, with a focus on the specific brain circuitry that mediate these relationships (Apkarian et al., 2005; Bushnell et al., 2013). Despite these efforts, the specificity of these cortical modulators of pain perception—as well as their shared characteristics across other sensory modalities—remains inadequately understood.
Consistent with and complementary to the variety of factors modulating pain and the related changes seen in brain circuitry, ample evidence demonstrates that conscious acute pain perception is highly malleable and a standardized nociceptive barrage does not translate to a fixed brain activity or to a prototypical perception. Pain perception can reflect moment-to-moment shifts in value judgments regarding the self and regarding the relationship between the self and the environment. Its value can change, as seen with rodents for which the availability of food trumps pain-related escape behavior when both are simultaneously present (Foo et al., 2009). Pain perception can be delayed for hours or days, when soldiers surviving the horrors of a battlefield do not report pain or suffering. We should also mention that Pavlov was able to train his dogs to salivate for painful stimuli (Pavlov, 1926, 2003). Thus the human verbal descriptions and behavioral experience of pain (as exhibited in motor, language, and facial and bodily expressions) - for a qualia that one would presume to be subjectively common - has a broad, culture- and context- dependent, repertoire. Pain therefore reflects an interaction amongst memory, attentional, and affective brain circuitry and afferent sensory inputs.
Given that nociceptors are continuously active in everyday behavior, one must posit the existence of a threshold phenomenon that transforms nociception to conscious pain (θ in figure 2). From a classical spinal cord physiology viewpoint, this threshold may be equated to the “gate control theory” (Melzack and Wall, 1965), where the balance between innocuous and noxious afferents at the level of the spinal cord determines the nociceptive signal traveling cephalad that, upon reaching the cortex, is interpreted as pain (the more modern alternative is some variation on “central sensitization,” especially for persistent pain conditions, where the gain of the spinal cord nociceptive synapse is amplified (Woolf and Salter, 2000)). In contrast, from a behavior selection viewpoint, the nociception-pain threshold is envisioned to be generated through reverberating circuitry between the ventral tegmentum/substantia nigra and ventral striatum/nucleus accumbens; modulated by limbic and cortical inputs that reflect past experiences, values, expectations, and salience relative to the self; where the output in turn modulates striatal-cortical loops to control behavioral repertoires. The threshold phenomenon emerges from a counterbalance between reward and aversion within the context of the learned history and the instantaneous state of the self that gates conversion from subconscious nociception to conscious pain (figure 2).
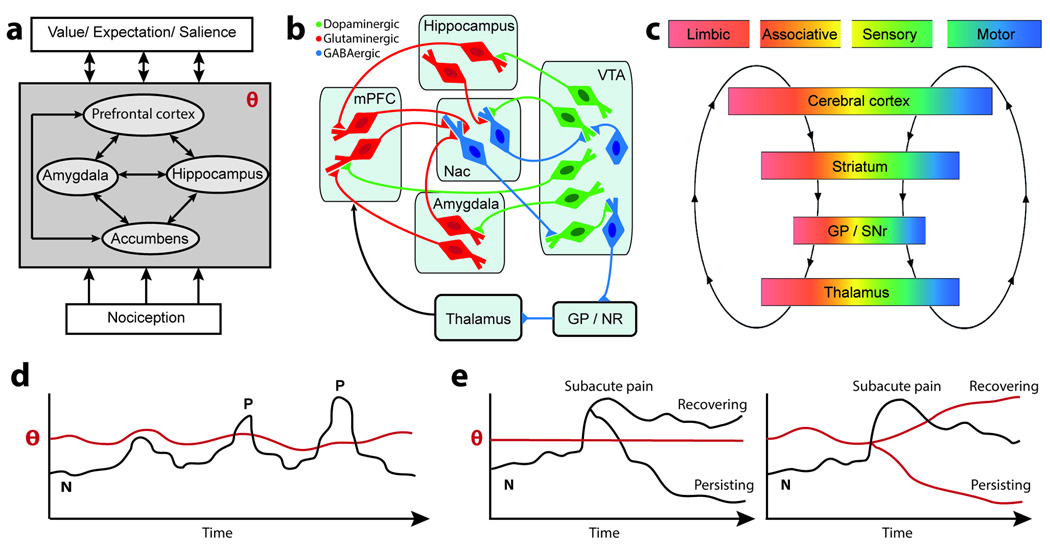
Figure 2.
Brain circuitry and temporal dynamics for the threshold phenomenon, θ, which determines conversion of nociception to conscious pain perception. a. The block diagram indicates θ is the output of the limbic brain. Internal states of the limbic brain, relative to neocortical memories determining current state of the organism (value, expectation, and salience), as well as the afferent nociceptive drive control θ. Other similar threshold processes in turn modulate the state of the organism through learning mechanisms, thus modifying values, expectations, and salience. b. More detailed circuit diagram emphasizing the interaction between limbic circuits, θ, and behavior selection. Diagram is adapted from studies of the reward/aversion circuitry, regarding striatal-cortical control loops (based on illustrations in (Luscher and Malenka, 2011; Nakanishi et al., 2014; Russo and Nestler, 2013)). Dense glutamatergic inputs from amygdala, hippocampus, and prefrontal cortex (mPFC) control the affective and motivational properties of accumbens (NAc) that responds to novel reward/aversion-related stimuli. Dopaminergic-GABAergic loops between accumbens and ventral tegmental area (VTA) provide the resultant value for θ, which through GP/SNr and thalamocortical circuits modulates behavior. Dopaminergic projections control synaptic properties and thus the affective state of the organism. c. Corticobasal ganglia-cortical loops conveying limbic, associative, and sensorimotor information. These loops are generally envisioned as a series of parallel projections. However the relay points, especially in the basal ganglia, provide opportunities for interactions between the loops. This organization enables the functional propagation of the limbic threshold phenomenon to influence goal-directed and habitual behaviors. (panle adapted from (Redgrave et al., 2010)). d. Conscious experience of acute painful events (P) depends on nociception (N) and the corticolimbic threshold, θ. e. Transitioning from subacute to chronic pain also depends on the individuals’ θ. Left panel depicts the classic viewpoint where nociceptive signal amplitude controls transition to chronic pain. Right panel is the view advanced here: For a similar injury, with equivalent nociception relayed to the brain, individuals with corticolimbic risk factors will persist to chronic pain while resilient ones will recover.
Once pain is present its salience draws attention, interferes with other thought processes, and imposes a state of negative mood. As such, the conscious perception of pain is the ultimate negative “cerebral celebrity” (Dennett, 1993) or negative “potential cerebral celebrity” (Chalmers, 1996), as it “‘perseveres’, ‘monopolizes resources long enough to achieve certain typical “symptomatic” effects – on memory, on control of behavior and so forth” (Dennett, 1993). If we view conscious perception as availability of information within the global workspace (Baars, 1988), then conscious properties of a painful state would affect even spinal nociceptive sensitivity and thus actively influence “gate control” and/or “central sensitization” spinal nociceptive processes, mediated through descending pathways as demonstrated by (Vera-Portocarrero et al., 2006).
Negative affect is an intrinsic aspect of the IASP standard definition of pain, and one commonly envisions unpleasantness as a necessary feature of this percept/qualia. It is classically explained as a consequence of transmission through medial spinocephalad pathways. If nociceptors are active in everyday behavior, and typically in the absence of pain, then transmission through both medial and lateral spinocephalad pathways must control subconscious nociceptor-driven behaviors, with or without the presence of pain. Human brain imaging studies tend to localize pain unpleasantness to specific cortical areas, most commonly either to rostral anterior cingulate (Rainville et al., 1997) or to some portion of the insula (Segerdahl et al., 2015). Yet these claims remain unconvincing. Instead, pain unpleasantness must be considered a part of the brain’s emotional repertoire that is tapped into by this particular qualia, which implies that the emotional limbic circuitry must be included in the neural network associated with pain. However, there is surprisingly little consistent evidence for activation of the amygdala, hippocampus, ventral striatum, or medial prefrontal cortex (the four most prominent nodes comprising the limbic brain) in acute pain studies (Segerdahl et al., 2015).
From the viewpoint of behavioral selection, we conclude that acute pain is not a warning signal but rather is the failure of the machinery (nociceptor activity) designed to avoid pain. As such once the conscious perception of pain occurs, aversion has failed or is imminent to fail. Thus the behavioral repertoire following pain is shifted into minimizing injury or retracting away from the environment that has the potential for injury, to protect the organism from further injury and promote healing. Therefore, here we extend the standard definition of pain to include the experiential shift as nociceptor activity breaks through from the subconscious to become a conscious unpleasant perception (figure 2d). It is unlikely that this phenomenon applies uniquely to the sense of pain (even for this sense the evidence remains minimal); rather, it likely generalizes across the sensory modalities. For example, there is extensive psychophysical evidence that large parts of the visual field do not enter into conscious visual perception, and complimentarily, visual perception is usually a coherent whole, the components of which may not even be physically present (Murray and Herrmann, 2013). Consistently there is now good evidence that activation of the primary visual cortex is not sufficient for conscious visual perception and that top down controls from the frontal cortex are necessary (Dehaene and Changeux, 2011). A similar top down control must also occur for pain. Moreover, we posit that the frontal cortical drives, across sensory modalities, are in fact embedded in corticostriatal circuits that actively control the threshold for incorporating sensory afferent inputs to cortical conscious states (figure 2a–c).
Patient H.M
There is a long and controversial history of the effects of brain injuries on pain perception (Nicholson, 2000). Unfortunately the data regarding pain insensitivity with brain lesions remains scant, localization of the site of injury is imprecise, and there is little documentation of the impact of such conditions on the everyday life of such patients.
Patient H.M. (Henry Gustav Molaison), arguably the most famous and most intensively studied patient in neuroscience, for over fifty years and by hundreds of scientists, did not feel acute thermal pain applied over diverse body parts (Hebben et al., 1985): “H.M. never gave reports of pain for the most intense stimulus applied either to his forearms or chest”. Thirty years prior to this formal testing for pain sensitivity, H.M. underwent bilateral resection of the uncus, amygdala, anterior hippocampus, and parahippocampal gyrus for severe and intractable epilepsy. The authors conclude that the amygdala injury is probably the main reason for lack of pain perception. What remains incontrovertible is the fact that H.M. had normal peripheral nociceptors and an intact spinothalamic pathway and, thus unperturbed sensory encoding of noxious stimuli. Therefore, the extensive bilateral limbic resection must have dramatically increased his striatal threshold for pain perception (θ in figure 2). Even more remarkable and further consistent with the latter explanation is that unlike patients with congenital insensitivity to pain, the loss of pain perception was not coupled with any obvious tissue injury, implying that subconscious nociception was intact and protecting his body. The contrast between H.M. and subjects lacking peripheral nociceptive afferents is informative, as the former seems to have led a life free of bodily injuries whereas subjects of the latter type are unable to protect the body from injuries even after extensive training.
Localization of brain activity for acute pain
Hundreds of PET and fMRI studies now describe a long list of brain regions activated during acute thermal, mechanical, and/or chemical stimuli in humans, see (Apkarian et al., 2005; Bushnell et al., 2013). Given this large literature, meta-analytic approaches can be used to summarize reproducible trends reliably found across laboratories and scanner modalities. A particularly elegant meta-analytic tool for neuroimaging data was developed by Yarkoni and Wager (Yarkoni et al., 2011) (www.neurosynth.org), which uses text-mining and machine-learning techniques to generate mappings between search terms and brain activity. The reverse inference map for the term “pain” illustrates the confidence with which pain-related brain activity is observed in 311 studies (Figure 3A). Assuming at least 10 subjects were used in each of the studies included, the obtained map represents neural activity associated with pain in more than 3000 subjects. The regions with most robust activity are highlighted and cover more than 15% of the neocortical mantle. Thus, in contrast to the single neuron electrophysiological results that describe sparse numbers of nociceptive responsive neurons in the neocortex (Chen et al., 2011b; Kenshalo and Isensee, 1983; Mazzola et al., 2009; Vierck et al., 2013), fMRI brain activity associated with pain identifies a much larger brain network. However, the latter map of pain-related activity, often referred to as “the pain matrix”, must be interpreted with caution, given numerous methodological limitations. These issues have been debated across many pain neuroimaging studies, and it remains unclear if they can be fully resolved at the level of local regional activity.
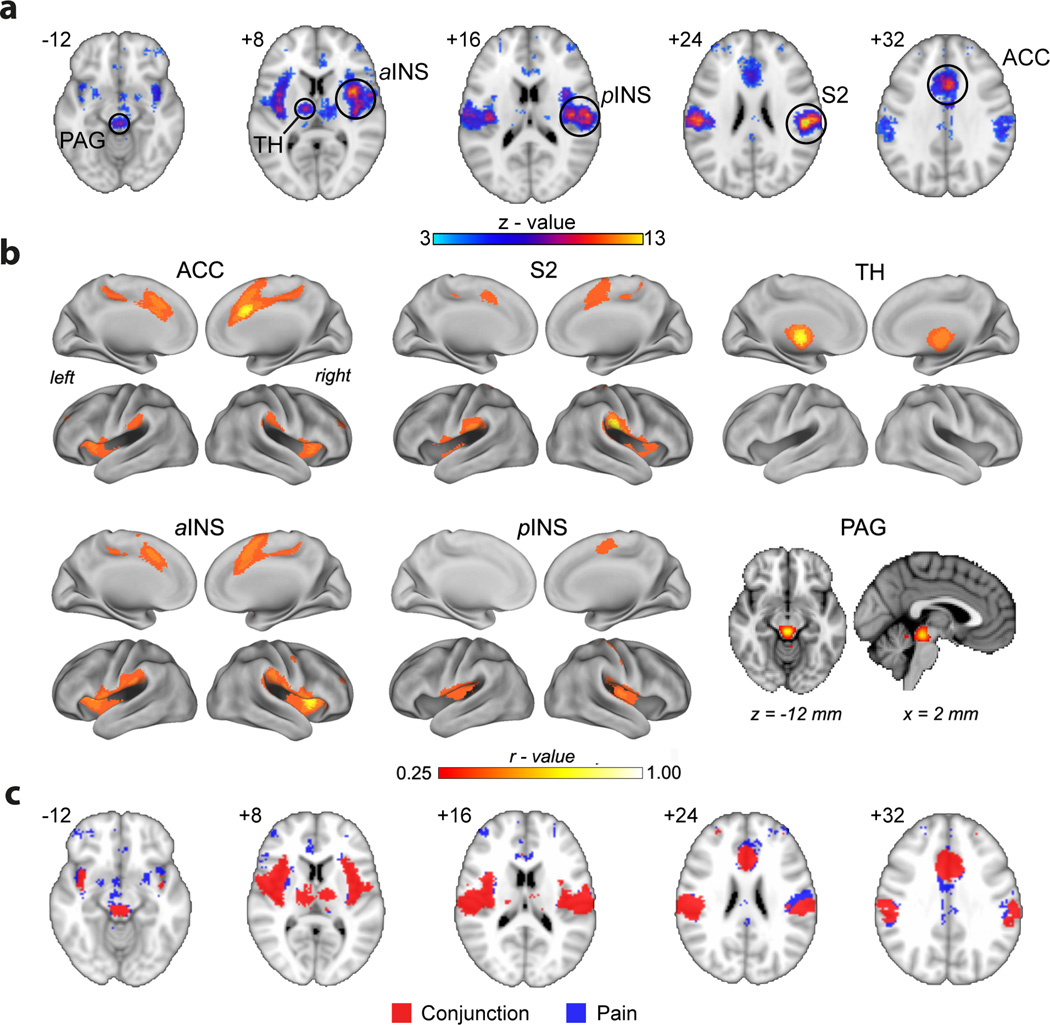
Figure 3.
Constructing the brain acute pain representation map from resting state brain activity. a. Brain regions identified for the reverse inference for the term “pain”, which identifies 311 PubMed studies in the Neurosynth meta-analysis tool (Yarkoni et al., 2011). The map is thresholded for z-values larger than 3.0. Highest confidence activations (z-values > 8.0) are localized to six brain regions: bilateral secondary somatosensory cortex (S2), anterior cingulate (ACC), bilateral anterior and posterior insula (aINS, pINS), thalamus (TH), and periaqueductal grey (PAG). b. Resting state functional connectivity networks for the six main nodes most robustly associated with the term “pain”. Functional connectivity is derived from resting state activity from 1000 subjects (Biswal et al., 2010), generated in Neurosynth (thresholded at correlation values > 0.3, approximately corresponding to > 3 standard deviations from baseline). Essentially the same network is identified when ACC, aINS, or S2 are used as seeds. The pINS seed identifies bilateral pINS as well as posterior cingulate/supplementary motor area. The TH network is limited to bilateral thalamus, and PAG seed only shows connectivity limited to itself. c. Overlap between the map for the term “pain” and sum of six resting state networks. Blue is the same map shown in panel a. Red is the sum of all functional connections identified in panel b. The overlap between red and blue maps is 72% of the blue map.
Even though we see some differentiation of properties for different brain regions activated with painful stimuli, especially when attentional and distraction-related variability is superimposed (Bushnell et al., 2013), the specificity of these regional properties remains far from clear. As a result, our ability to make inferences regarding their specific roles remains vague, see (Poldrack, 2011). In fact, the specificity of the nociceptive system or lack thereof has been the focus of a longstanding and contentious debate in pain research. Given that roughly 15% of the cortical mantle seems responsive to nociceptive stimuli, the chances that any of these regions are nociceptive specific is remote. Moreover, all brain regions shown in figure 3A also show responses to a multiplicity of other stimuli or tasks. Even at the level of the spinal cord, a large majority of nociceptive responsive neurons also respond to nonpainful tactile, proprioceptive, muscle and visceral manipulations, and this general principle seems maintained throughout the central nervous system. Furthermore, in a series of elegant studies, it has now been clearly demonstrated that almost all components of the nociceptive brain activity are not specific to nociception or to pain perception (Iannetti and Mouraux, 2010; Liang et al., 2013; Mouraux et al., 2011; Wang et al., 2010). This evidence overwhelmingly demonstrates that it is unlikely that the cortex contains neural tissue specifically responsive to nociceptive inputs or linked specifically to pain perception. Yet, the Cartesian view posits this exact hypothesis, and pain scientists continue to search for such specialized brain tissue.
Integration of brain activity for perception of acute pain
Since the work of Weber in the 18th century (see English translation (Weber, 1978)), the primary quantitative outcome measure for pain has been its subjective perceived magnitude. It is therefore proper to study the brain activity for pain in relation to the stimulus intensity and/or perception magnitudes in order to functionally differentiate components of the underlying circuitry (Porro et al., 2004). In general, somatosensory cortical areas and anterior cingulate activity seem to track stimulus intensity, whereas lateral prefrontal and posterior parietal regions do not, e.g. (Buchel et al., 2002; Coghill et al., 1999; Davis et al., 1997; Peyron et al., 1999). Brain activity related to perceived magnitude of pain is studied less systematically (Baliki et al., 2009; Johnstone et al., 2012; Loggia et al., 2012; Moulton et al., 2012). Different temporal transformations are observed between stimulus and perception in various brain regions, and the region best related to perceived pain (anterior insula) seems to also reflect perceived magnitude for visual stimuli (Baliki et al., 2009). Thus, cortical tissue specifically dedicated to perceived magnitude of pain remains unidentified.
One can relax the Cartesian interpretation to include the notion that, despite a lack of cortical regional specificity, an appropriately weighted sum of the brain regional activity implicated in nociception/pain altogether could provide specific information regarding pain states. The latter was recently demonstrated in a rather comprehensive approach (Wager et al., 2013). The authors used brain activity for acute pain (based on the maps in figure 3A) to construct a machine learning-based regression model for pain perception. The model could differentiate between tasks and predict perceived magnitude of pain, accurately across several independent data samples. The strength of the study is the authors’ claim of having derived a generally applicable brain signature for identifying pain perception magnitude. The resultant tool can be readily tested to determine extent of validity, especially in tasks that activate very similar brain regions, for example in tactile magnitude rating (Apkarian, 2013) which gives rise to an activity pattern closely matching that seen in figure 3A.
An alternative approach is to examine the local pattern of brain activity and determine whether the across-voxel ensemble pattern can differentiate between conditions. This technique, multivariate pattern analysis pioneered by Haxby (Haxby, 2012), was used to differentiate between sensory modalities (Liang et al., 2013). The study was able to establish that within-subjects, across-voxel activity patterns in the somatosensory cortex best differentiated between pain and other sensory modalities. Yet, the study also demonstrated that many other brain regions can make a similar distinction, albeit at a lower confidence level. The authors further showed that the ability to distinguish between sensory modalities is not a unique trait of the sensory cortices. These results are somewhat consistent to the observations made by (Wager et al., 2013), in the sense that both studies identify a distributed signal in the brain that can differentiate pain from other states. Both studies (Liang et al., 2013; Wager et al., 2013) remain unclear as to the extent to which the identified signals are specifically linked to the perception, or if they reflect secondary or ancillary responses due to the presence of pain.
Brain resting state activity and acute pain
The discovery of the brain resting state activity (Biswal et al., 1995) has fundamentally changed modern notions of neural dynamics. In its most general form, resting state activity is a signature of neural oscillations synchronized across large scale networks that occur in the absence of external inputs (Fox and Raichle, 2007; Fox et al., 2005), persisting together with external inputs (Cabral et al., 2014), and reflecting local information integration as measured by frequency content (Baria et al., 2011; Baria et al., 2013). Properties of resting state activity are now thought to reflect an individual’s history of learning and memory that underlie perceptual variability (Lewis et al., 2009). Resting state activity is composed of a set of large-scale synchronized networks. Task related activity can be thought of either as a collection of networks that synchronize with each other (Deco and Corbetta, 2011), or components of these networks that break apart and link up with others. Figure 3B shows resting state networks identified from Neurosynth. The networks are derived for six voxels that correspond to the most pain-specific locations in figure 3A. Three of the six seeds essentially identify the same network; the thalamic seed only identifies bilateral thalamic connectivity, and the PAG seed identifies itself. The figure illustrates that any single voxel’s activity is in fact synchronized with, and thus embedded within, a large underlying brain network. Figure 3C illustrates that, with a few seed voxels, we can recapture most of the brain activity for pain. It is therefore not surprising that the properties of painful stimuli can be captured across many of the brain regions commonly identified for acute pain. More importantly, the identification of the pain-related network during (non-painful) resting state casts doubt as to the sufficiency of this network in pain perception.
Pain, vision, and their perception
There is far deeper understanding of the cortical encoding of vision compared to pain. Yet the fundamental mechanisms that transform sensory inputs to conscious perception remain equally mysterious for both senses. Here we briefly contrast the two in order to highlight their conceptual commonalities and differences.
Both electrophysiology and brain imaging studies indicate that about one-third of the primate neocortex is dedicated to visual information processing, which can be subdivided into more than thirty modules. The inter-relationship between these regions as well as their specialization has been and continues to be described in great detail (Felleman and Van Essen, 1991; Wandell and Winawer, 2011). In contrast, existence of cortical tissue specialized for processing nociceptive information remains contentious. Yet, the subjective intensity of qualia associated with each of these sensory modalities is at least equivalent, and the common saying “if you doubt reality, kick a rock with bare feet” implies that pain is often far more salient than vision. Thus, the intensity of saliency for specific qualia is not related to the amount of neocortical tissue dedicated to that sensory modality.
Additionally, although we have garnered detailed knowledge regarding the properties of visual information processed by individual neurons and information flow across the visual modules, we still lack the ability to construct the integrated visual percepts that one experiences holistically, for example see (Rokers et al., 2009). In similarity it is important to point out that existence of the meta-analytically derived map for acute pain (variations of figure 3a) with component regions preferentially encoding pain magnitude, affective characteristics, sensitivity to attention, emotion, arousal or to analgesics, is not sufficient to be concluded as the “pain matrix”/”signature”/”mechanism” for conscious perception of pain. It should be emphasized that even the scale (single molecules, single neurons, groups of neurons, networks of neurons, or the whole brain network) of the fundamental physical process of consciousness remains unresolved and mysterious. Still as our common subjective experience is comprised of qualia with access to language and influences behavior, “cerebral exuberance”, conscious experience of pain can only be properly understood within the full context of the nuances of consciousness, for which the minimal neural circuit must incorporate long-distance network interactions (Dehaene and Changeux, 2011).
Currently, the brain science of pain, in similarity to the science for all other sensations, remains correlative to psychophysics. In parallel with the recent dissociation of brain circuitry for conscious and unconscious visual stimuli (Dehaene et al., 2014), we suggest that important paradoxes regarding central pain representation can be resolved by unfolding unconscious nociception from conscious pain. Specifically, the seemingly irreconcilable evidence that pain is either localizable to specific brain sites (Garcia-Larrea and Peyron, 2013; Segerdahl et al., 2015) or it requires integrated representation across brain networks (Wager et al., 2013) may only be resolved once nociception and pain perception are delineated. Regarding an overall strategy to understand the neural signature for conscious pain we again quote Christof Koch: “We must resist the hypnotic appeal of hot spots in brain scans with their naïve phrenological interpretation: the perception of faces is computed over here, pain over there, and consciousness just yonder. Consciousness does not arise from regions but from highly networked neurons within and across regions.” (Koch, 2012).
Chronic pain
Chronic pain is an enormous health care issue with a massive price tag, yet its science has remained rudimentary. If pain persists and becomes chronic, it can lead to dramatically reduced quality of life, depression and suicide, insomnia, lowered immune function, changes in eating patterns, impaired cognitive function, maladaptive stress responses, and other long-term deleterious effects. Its prevalence has increased worldwide to affect more than 15% of the world population and 30% of the US population (Murray and Lopez, 2013). The associated health care cost in the US is a staggering $600 billion per year (Medicine, 2011). Over a third of individuals with chronic pain define their pain as severe and 40% of those suffering from chronic pain are not satisfied with their care (Breivik et al., 2006; Johannes et al., 2010). Despite considerable research on the topic, no consistently effective therapies have been identified.
Beecher first coined the term chronic pain in 1950’s. Additionally he emphasized that, in the clinical context, pain is far longer lasting and the relationship between pain and its inciting stimulus or injury remains imprecise and unpredictable, and thus that the science of pain and of analgesics must be centered on clinical trials performed in actual patients suffering from pain. The science of human neuroimaging of pain is also slowly converging to Beecher’s conclusion as accumulating evidence demonstrates that the brain in chronic pain is not equivalent to the brain experiencing prolonged acute pain.
The definition of chronic pain remains tautological, as it simply asserts that it is a long-lasting pain, or a pain persisting past the normal healing period. Over the last 30 years, studies in animal models of persistent pain have established that pain chronicity is associated with peripheral reorganization of afferent signaling, changing sensitivity for nociceptors, and perhaps also for tactile afferents (figure 1). At the level of the spinal cord, we now know of hundreds of molecular changes reorganizing neuronal circuitry and engaging glial processes, all of which give rise to heightened sensitivity of afferents and which generally conspire to give rise to “central sensitization” (Basbaum et al., 2009; Woolf and Salter, 2000). Accumulating brain imaging-based evidence now also shows that the human brain undergoes extensive reorganization in chronic pain conditions (Apkarian et al., 2011). This florid neural reorganization from the periphery to the neocortex (figure 1), which seems to uniquely manifest for different types of chronic pain, strongly agrees with Beecher’s viewpoint and affirms that, to understand chronic pain, one has to study patients suffering from its myriad clinical manifestations, comparing within and across types. The seemingly specific brain properties that are reliably linked with distinct chronic pain conditions, as well as the long-term and continued condition-specific reorganization of the brain across chronic pain diagnoses, justify the notion that chronic pain is a maladaptive neuropathological disease state (Davis and Moayedi, 2013; Tracey and Bushnell, 2009). Below we propose that chronic pain can be dissected into component phases and elaborate on the underlying mechanisms of each phase.
From a more general view, and given the model we have advanced regarding the role of striatal circuits in the conversion of nociception to acute pain, one can further expand our proposed model to chronic pain. Borrowing from the literature on mechanisms underlying addictive behavior for positive rewards (Robinson and Kolb, 2004; Schultz, 2000; Volkow et al., 2010; Willuhn et al., 2012), we affirm that long-term shifts in the threshold mechanisms that gate the conversion from nociception to pain also underlie the transition to chronic pain (figure 2e). We further propose that the threshold shift is dependent on limbic circuitry invoking synaptic learning-based reorganization (Apkarian, 2008; Apkarian et al., 2009). Taken together, these ideas can be simplified as a lowered mesolimbic threshold for the conscious perception of pain, which functionally renders the brain addicted to pain. The lowered striatal threshold is proposed to be mediated by learning mechanisms driven by limbic properties (Apkarian, 2008; Apkarian et al., 2009), which induce reorganization of neocortical memory traces (Johansen and Fields, 2004; Li et al., 2010; Xu et al., 2008).
The human brain in chronic pain – a general model
Ventral striatal circuitry links nociception, acute pain and chronic pain. This circuitry assesses salience of impending pain as well as expected reward value for relief of pain (Baliki et al., 2010). Given that its output controls motivated behavior (figure 2b,c), properties of this circuitry become critical in understanding the transition from acute to chronic pain. There is now evidence that some brain properties are candidate risk factors, while others reflect the transition to chronic pain, and the mesolimbic circuitry drives brain reorganization through synaptic learning mechanisms. These results suggest that chronification of pain may be subdivided into four temporally distinct and functional separate phases (figure 4). We presume that due to genetic and developmental forces different subjects are prone to developing chronic pain as a consequence of specific injuries. Simplistically, we assume these predisposing factors are captured by the limbic brain anatomy and physiology. Given such predispositions a specific injury initiating a large nociceptive barrage results in activating the corticostriatal circuitry into either a response that copes with the injury and in time recovers towards the healthy state, or a response that diminishes the corticostriatal threshold thus functionally amplifying the afferent signal, enhancing the gain for inducing learning, which in turn imprints novel neocortical anatomical and functional memory traces thus creating the chronic pain state (figure 2e). Recent human brain imaging data (figure 4a–h) and animal model evidence (figure 4:1–9) are consistent with this model. The model is also intentionally kept as simple as possible, because much detail remains to be uncovered. Note that it is fully consistent with observations of transient abolition of chronic pain by massive blockade of nociceptive afferents (Haroutounian et al., 2014; Vaso et al., 2014), since central amplification (by reducing mesolimbic θ) becomes immaterial in the complete absence of inputs. The model raises the more nuanced question: whether ongoing afferent nociceptive activity generated in chronic pain patients would by itself be perceived painful by healthy subjects.
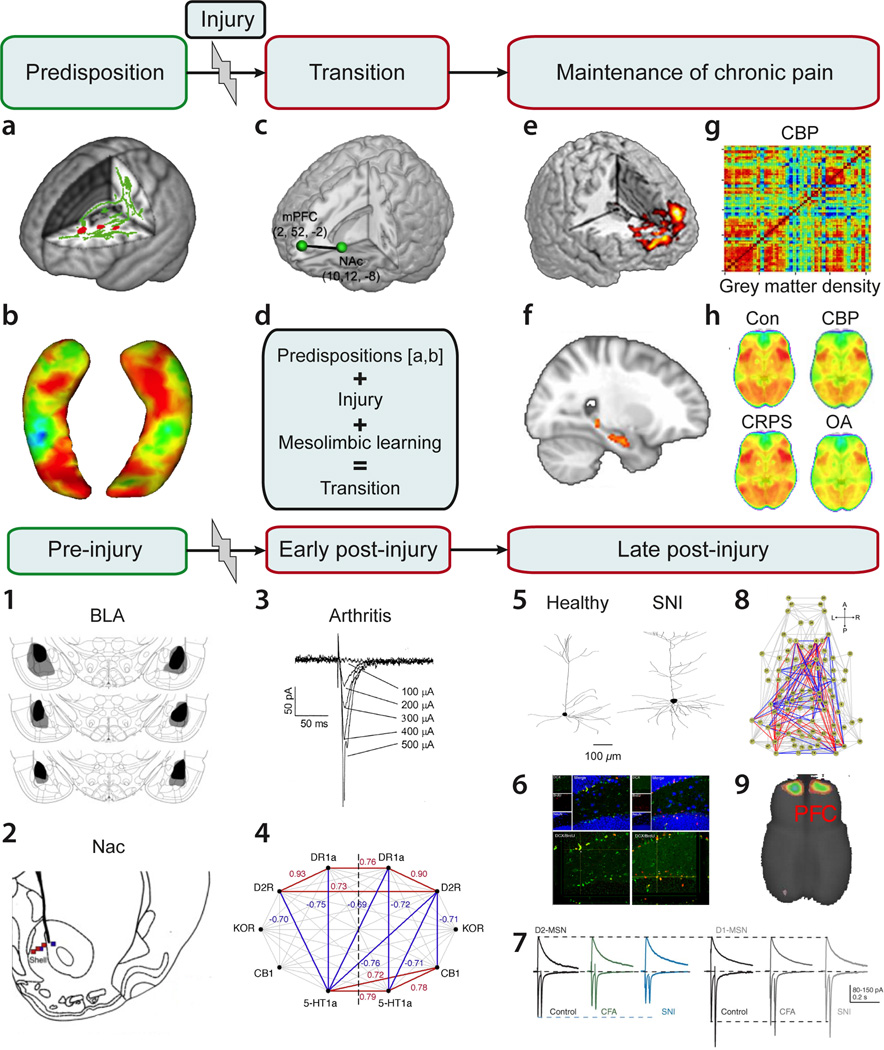
Figure 4.
Transition to chronic pain may be deconstructed to four component phases: Predisposition, injury or inciting event, a transition period, and a maintenance phase. Brain circuitry and their interactions across the phases are illustrated in human brain imaging studies, a–h. a. Specific brain white matter regional properties (red) impart risk for developing chronic pain following an acute episode of back pain (Mansour et al., 2013). b. Limbic brain structural properties may also impart risk for pain chronification (e.g. shape and/or size of the hippocampus) (Mutso et al., 2012). c. In the transition phase, strength of information exchange between the prefrontal cortex and accumbens, after an end organ injury, determines long-term pain chronification (Baliki et al., 2012). d. The transition process is the influence of predisposing brain factors in combination with the injury-induced nociceptive signals that control mesolimbic learning mechanisms, altogether determining extent of prefrontal-accumbens information exchange (modulating θ in figure 2). Chronification of pain gives rise to: e. condition specific subjective pain-related brain activity patterns (Baliki et al., 2006; Hashmi et al., 2013; Parks et al., 2011), f. increased information exchange within the hippocampus and between the hippocampus and the cortex (Mutso et al., 2013), g. reorganization of brain grey matter regional similarity (Baliki et al., 2011), and h. distortions in information sharing in resting state brain activity, specifically brain activity phase relationship between the default mode network and the rest of the brain shows chronic pain type-specific patterns (Baliki et al., 2014b).
In rodent models for persistent pain, the four phases are better conceptualized as pre-injury manipulations that influence post-injury pain-like behavior, and early and late post-injury consequences. Supraspinal circuits implicated in the rodent four phases of pain persistence are highlighted in 1–9: 1. Bilateral lesion of the rat basolateral amygdala (BLA) diminishes post-injury tactile allodynia for 28 days after neuropathy (Li et al., 2013). 2. Lidocaine infusion within accumbens in the rat diminishes post-injury tactile allodynia for the duration of infusion (14 days), after a neuropathic injury (spared nerve injury, SNI) (Chang et al., 2014). 3. Hours following induction of an arthritis model in the rat, amygdala neurons become hyperexcitable (Neugebauer et al., 2003). 4. Five days after SNI neuropathy in the rat, accumbens covariance of receptor gene expression is upregulated (Chang et al., 2014), and 5. dendritic size and branchings of prefrontal pyramidal neurons are expanded (Metz et al., 2009). 6. Fifteen days after SNI neuropathy adult hippocampal neurogenesis is downregulated (Mutso et al., 2012). 7. Accumbens medium spiny neurons with dopamine D2 receptors show decreased AMPA/NMDA ratio in neuropathic injured rodents (Schwartz et al., 2014). 8. Resting state whole-brain functional network in the anesthetized rat shows increased (red) and decreased (blue) functional connections 28 days after SNI neuropathy relative to sham injury (Baliki et al., 2014a). 9. Six months following neuropathic injury prefrontal (PFC) cortical grey matter volume is decreased in the rat (Seminowicz et al., 2009).
Overall, the human data illustrates brain risk factors for, and brain reorganization with, chronification of pain. The rodent results show persistent painlike behavior is dependent on, and in turn reorganizes, limbic brain circuitry.
Our model posits that chronic pain is dependent on the interplay between the brain threshold phenomenon and the injury-related sensory input. The injury in most cases is envisioned to be a disturbance in nociceptive afferent input but in some conditions there may also be central drivers, such as in central pain or phantom pain conditions. The pain research community has long debated the relative contribution of the end organ (injured bodily structure) in relation to brain or gene predispositions (Robinson, 2009). The proposed model is a combination of both and it is envisioned that the relative weights of each component will be condition specific. For example, fibromyalgia seems to be mostly driven by central predispositions (Phillips and Clauw, 2011), even though hyper-excitable nociceptors were recently described in such patients (Serra et al., 2014). On the other hand, osteoarthritis may have a larger peripheral nociceptor contribution, as evidenced by the success rate of pain relief with joint replacement surgery (Buchbinder et al., 2014). The model also suggests that the rate of transition to chronic pain is condition specific and dependent on limbic brain properties. We foresee that unraveling the specifics of this circuitry for various types of chronic pain conditions will pave the way for the development of novel treatment and/or prevention therapies. As this model assumes that brain properties are the primary determinants of risk for chronic pain, chronic pain is more dominantly defined as a neurological disease and to a lesser extent a nociceptive abnormality.
The human brain in chronic pain – Anatomy
About ten years ago we discovered regional anatomical brain abnormalities that correlated with intensity and duration in chronic back pain (CBP) patients (Apkarian et al., 2004). This initial observation is now replicated across many clinical pain conditions that primarily show regional decreases in grey matter density, although there is also some evidence for increased density in a subset of pain populations, as well (May, 2008); see recent meta-analyses (Cauda et al., 2014; Smallwood et al., 2013). A direct comparison between different clinical pain conditions indicates partially overlapping maps of anatomical aberrations (Baliki et al., 2011). Mechanisms and processes underlying such changes remain unclear and speculative. Growing evidence confirms that these regional gray matter decreases can partially renormalize following successful amelioration of chronic pain (Ceko et al., 2015; Gwilym et al., 2010; Moayedi et al., 2011; Rodriguez-Raecke et al., 2009; Seminowicz et al., 2011). The simplest hypothesis regarding physiological processes that control these anatomical changes is the notion that voxel-wise gray matter properties (average profile of millions of neurons) reflects local variations in synaptic density, although neuronal atrophy may also be occurring as some of these changes persist over decades (Baliki et al., 2011).
There is also evidence that these regional gray matter changes are actually a reflection of a more pervasive reorganization of the inter-relationship of the anatomy of the neocortical mantle (based on whole-brain gray matter self-similarity analyses (Baliki et al., 2011)), which is highly distinguishable between clinical conditions and which suggests a reorganization of information shared across the neocortex. A meta-analysis of brain gray matter properties for multiple pain conditions when analyzed from the viewpoint of resting state networks, identifies a core set of networks (mainly the salience and attentional networks) as the main circuits commonly affected, while sensory regions seem to show more condition specific reorganization (Cauda et al., 2014). In our longitudinal study, where sub-acute back (SBP) patients were tracked over a year in their transition either to recovery (SBPr) or persistence to pain chronification (SPBp) indicated that gray matter decreases occurs only in the SBPp, with changes starting within the first few months, in proportion to functional connectivity changes, and in proportion to the intensity of back pain (Baliki et al., 2012). All of which implies strongly that this anatomical reorganization is part of the process of the transition to chronic pain.
Brain white matter abnormalities have also been observed in chronic pain conditions. The first such report suggested that white matter regional fractional anisotropy (reflecting myelin properties) may be linked to the grey matter changes (Geha et al., 2008), implying shared mechanisms between the two processes. A number of studies now show regional white matter abnormalities in diverse chronic pain conditions (Chen et al., 2011a; Ellingson et al., 2013; Khan et al., 2014). On the other hand, in our longitudinal study of SBP, it was observed that white matter fractional anisotropy differences between SBPp and SBPr were present in the earliest brain scan and persisted with no further changes over a one-year period (Mansour et al., 2013). The latter study concluded that specific white matter deviations from the norm are most likely preexisting risk factors that put subjects at risk to developing chronic back pain.
Perception related brain activity in chronic pain
The Cartesian expectation for brain activity in chronic pain would be a state of continued, or enhanced, activity in brain regions identified for acute pain (all or a subset of the regions seen in figure 3a). However, when brain activity is determined for subjective report of spontaneous fluctuations in the magnitude of perceived pain (Baliki et al., 2006), one observes specific brain activity for chronic pain distinct from that for acute pain, where chronic pain conditions activate more limbic and emotional brain regions, and that different chronic pain conditions engage specific patterns (Apkarian et al., 2011). Results from our longitudinal study, in fact, demonstrates how brain activity in relation to subjective pain shifts dynamically away from sensory brain regions to emotional/limbic regions (Hashmi et al., 2013). In early SBP (10–15 weeks after start of back pain), brain activity for back pain closely corresponds the activity for acute pain. However, as subjects transition to either SBPp or SBPr (one year later), brain activity diverges between the groups, with SBPr showing minimal brain activity (below detection threshold) while SBPp shows decreased activity in sensory regions and increased activity in the medial prefrontal cortex and amygdala. The latter spatial shift seems to occur even though these subjects judge their back pain as essentially unchanged over the one-year monitoring period. Thus, it seems that chronification of pain, accompanied by gray matter and functional connectivity reorganization, and also accompanied with reduced capacity to activate central opioid neurotransmission (Martikainen et al., 2013), renders the pain to become more subjective/intrapersonal and more emotional.
Chronic pain and resting state brain activity
If chronic pain underlies neocortical anatomical reorganization and functional connectivity changes, then it should be reflected in the properties of resting state activity. Indeed, there is growing reproducible evidence that resting state activity in many chronic pain conditions show a variety of abnormalities (Baliki et al., 2008; Baliki et al., 2014b; Bolwerk et al., 2013; Cauda et al., 2010; Cauda et al., 2009; Gupta et al., 2015; Loggia et al., 2013; Malinen et al., 2010). The most consistent result is a disruption of the default mode network, specifically a dissociation of its prefrontal component in many different types of chronic pain. There is also consistent evidence of increased high frequency oscillations, mainly in the prefrontal cortex, as well as disruption of insular cortex functional connectivity. How similar are these changes across chronic pain types remains unknown, and how these changes are related to the myriad other changes observed in the neocortex remains to be uncovered.
Resting state activity technology promises to become a dominant modality with which brain information exchange properties can be probed for chronic pain as it allows to probe the properties of both local and global functional properties in the naturalistic setting of unperturbed state of chronic pain. It should be emphasized that only by studying large groups of chronic pain patients we will be able to gain more comprehensive insight into underlying mechanisms. Such endeavors are complicated and hard to accomplish within single research labs, and requires collaborative effort across centers. The first such consortium has been ongoing for the study of pelvic pain and initial multi-center results are just being published (Farmer et al., 2015; Kairys et al., 2015; Kilpatrick et al., 2014).
Predicting transition from acute to chronic pain
The extent to which transition to chronic pain can be predicted from brain properties is a very important issue as it paves the way for personalized evidence-based medicine (Denk et al., 2014). Brain white matter properties seem one such predictor (Mansour et al., 2013), which when measured within weeks after the inciting event, predict SBPp and SBPr one year later at 80–100% accuracy. Another predictor identified from the same longitudinal study (Baliki et al., 2012) is the corticostriatal functional connectivity. The latter is constant and stronger in SBPp than SBPr over a one-year period, and at time of entry into the study could predict chronification of pain at about 80% accuracy. These results indicate that limbic brain properties and its responses to the injury is the primary determinant (they explain almost all of the variance of the outcome parameter) for transition to chronic pain, at least for back pain. It should be noted, however, this study remains one of a kind and thus awaits replication in other chronic pain conditions.
Animal studies regarding chronic pain
Animal studies have failed to show critical controllers regarding transition to persistent pain-like behavior. There may be multiple technical reasons for this failure. However, from the viewpoint of the current perspective, we can assert that these studies generally have not differentiated between nociception and pain, and also ignored much of the rest of the brain, especially the limbic brain. More recent literature is now filling these gaps (figure 4:1–9). There is now good evidence of the critical role of the amygdala in multiple animal models of pain, where its properties seem to modulate even spinal cord central sensitization processes (Li and Neugebauer, 2004), and influences prefrontal activity (Ji and Neugebauer, 2011). Additionally, the dendritic size and spine density of pyramidal neurons in the medial prefrontal cortex change within days after a peripheral nerve injury (Metz et al., 2009), and long-term neuropathic pain decreases prefrontal cortical grey matter density in the rodent (Seminowicz et al., 2009), just as in humans. Hippocampus volume in humans with chronic pain is smaller (Mutso et al., 2012), and with transition to chronic pain shows changes in information exchange within the hippocampus as well as between the hippocampus and the neocortex (Mutso et al., 2013). Consistently, following a peripheral nerve injury rodents show deficits on hippocampal-dependent memory extinction tasks and exhibit abnormalities in information processing at the level of single neuron electrophysiology (Mutso et al., 2012; Ren et al., 2011). Very recent studies also show that peripheral nerve injury modulates functional connectivity of NAc and decreases expression of dopaminergic receptors in the ventral striatum (Pei-Ching Chang, 2014), and disturbs glutamatergic information processing and long term depression of ventral striatal neurons, resulting in decreased motivated behavior (Schwartz et al., 2014).
These recent animal are providing evidence that parallel the human brain imaging studies. Additionally, the rodent studies begin they are unraveling the detailed brain circuit properties associated with transition to chronic pain, pointing to potential novel therapies (Centeno et al., 2009; Millecamps et al., 2006; Schwartz et al., 2014). From the viewpoint of the model for transition to chronic pain the rodent results emphasize more the reorganization of the limbic brain with transition to chronic pain-like behavior. Yet, these are relatively early studies and much remains to be done in the field.
Mechanistic parallels between stress, anxiety, depression and chronic pain
Ample evidence supports the notion that the most prevalent clinical manifestations of negative emotion, anxiety and depression, reflect a common spectrum of symptoms with overlapping mechanisms (Watson, 2005). Chronic stress, in particular, has emerged as a dominant and common underlying factor. Here we propose that our framework regarding nociception and pain relative to behavior selection (figure 5a,b) can be extended to incorporate negative moods (see (Coenen et al., 2011) for a somewhat different formulation for interpreting depression as a type of pain).
Figure 5.
Nociception, pain, and negative moods constitute a continuum imparting inhibition of behavior through negative affect, based on expected or apparent inputs across varying spatial and temporal dimensions. The four landscapes (a–d) illustrate negative emotional value assignment relative to the individual (the contemplative Cartesian self). Zero on this space-time plane represents either the body in relation to sensory inputs, or equivalently the self within the arena or the global neural workspace of consciousness, where accumulated or experienced aversiveness is assigned for varying space-time relationships that dictate behavioral selection. Hot colored valleys represent negative affective states or valuations, blue-white undulations signify emotionally more neutral states. a. In the absence of an experienced or expected threat (e.g. while kneeling to smell the roses) nociception in the absence of negative affect subconsciously protects the organism from injury by constraining behavioral repertoires (delimiting bodily positions or postures). b. Failure of nociception results in conscious pain (burning the skin of the Cartesian self by the fire), associated with a rapid withdrawal from the environment. Thus, pain evokes conscious negative affect and behavioral modification at the scale of the immediate body vicinity (aversion at zero space-time). c. When the threat is a learnt association, and is expected to be encountered at a distance or time removed from the body, then the subject experiences anxiety or stress. d. If instead the threat is experienced as, or expected to be, pervasive the associated negative mood is more abstract, described as depression, and the behavioral inhibition is generalized across scales of time and space. As pain is a primary reinforcer, its presence or persistence can rapidly become associated with expanded aversive landscapes, incorporating various combinations of the landscapes b–d, which is complimentary to the imprecision model recently proposed for chronic pain (Moseley and Vlaeyen, 2015). In this framework, we posit that the four phases of transition to chronic pain (illustrated in figure 3) also apply to chronification of negative moods. Both specific chronic pain conditions and the variety of types of chronic negative moods are expected to have unique limbic predisposition signatures and long-term brain adaptations. Computations needed for constructing these cognitive aversiveness maps are variants of Sutton and Barto’s (Sutton and Barto, 1981) temporal difference algorithm, applied to dopaminergic activity for assimilating reward prediction error to induce approach behavior (Schultz et al., 1997), which can also be recast as a Bayesian inference that optimizes energy based on model evidence (Friston et al., 2014).
Just as pain motivates the avoidance of further bodily injury and promotes behaviors that enhance healing (figure 5b), anxiety can be recast as an emotional state, sustained by sympathetic arousal that promotes behaviors that diminish anticipated danger within one’s immediate physical space and at relatively short future time scales (figure 5c). Moreover, depression can be conceptualized as a more global generalization of perceived aversiveness to one’s environment. In such a case, perceived or anticipated danger reflects a more abstract level of cognition that results in constraining personal space (through social isolation, reduced physical activity, and diminished motivated behavior) (figure 5d). Thus, just as nociception and pain protect against bodily injury by limiting behavior, negative moods minimize exposure to danger and promote survival by inhibiting behavior as well. Moreover, in similarity to chronic pain, persistence of negative moods becomes a maladaptive process, at least partially maintained by neuropathological mechanisms.
Within this framework, brain mechanisms underlying the transition from acute to more persistent negative mood states should parallel those we describe for chronification of pain. In fact both animal model studies and human brain imaging studies show strong similarities between mood disorders and chronic pain, and both conditions critically involve limbic brain circuits. Most importantly, the structural and functional alterations in the ventral tegmental-ventral striatal circuitry associated with anhedonia (Russo and Nestler, 2013) is consistent with the threshold phenomenon we have discussed for pain. Just as chronic pain conditions are associated with decreased hippocampal volume (Khan et al., 2014; Mutso et al., 2012), a rich parallel literature indicates that depression is associated with hippocampal volume decrease and decreased synaptic and glial density, based on brain imaging and post-mortem evidence (Brown et al., 2014; Campbell et al., 2004; Czeh and Lucassen, 2007). More equivocal evidence shows decreased volume of the amygdala (Hickie et al., 2007; Whittle et al., 2014) and medial prefrontal cortex (Caetano et al., 2006; Drevets et al., 1997; Rajkowska, 2000) in humans with depression. Major depression in the adolescent is now tightly related with decreased information sharing between the amygdala and the hippocampus (Cullen et al., 2014), whereas decreased information sharing is seen between the hippocampus and the neocortex with chronic pain (Mutso et al., 2013). Moreover, medial prefrontal cortex connectivity to the nucleus accumbens has become a primary neurosurgical stimulation target for treating intractable depression (Mayberg et al., 2005; Ressler and Mayberg, 2007) with the intention of modulating properties of the corticostriatal circuit. Decreases in hippocampus and amygdala volumes have also been described in post-traumatic stress (PTSD) (Chao et al., 2013; Chao et al., 2014; Gilbertson et al., 2002; Starcevic et al., 2014), and white matter microstructural predispositions in PTSD indicate that such structural differences reflect long-term vulnerability (Sekiguchi et al., 2014), as also observed for chronic back pain (Mansour et al., 2013). In humans, amygdala response properties seem to indicate risk for developing PTSD (McLaughlin et al., 2014), and in rodents, susceptibility to stress response is dependent on hippocampal volume and functional processing (Nalloor et al., 2014; Tse et al., 2014). Chronic tinnitus, a persistent unpleasant sensation of ringing or buzzing in the ear, is also now characterized as a dysregulation of the limbic network, mainly due to hyperactivity of the ventral striatum coupled with decreased grey matter in the medial prefrontal cortex (Leaver et al., 2011). The latter result is highly consistent with our notion that would explain tinnitus too as a shift in mesolimbic threshold for conscious perception of painful/unpleasant sounds mediated through either a peripheral injury (rock concert or rave) or central events (stress), and coupled with limbic predispositions. The configuration of these predisposing factors, the inciting event(s) (i.e., bodily injury, traumatic experiences), arousal-enhanced aversive learning, and long-term maintenance of these maladaptive limbic memory traces likely contribute to the broad range of phenotypic expressions that are used to differentiate clinical diagnoses.
Overall, there seems to be a remarkable overlap between the brain structures that either impart vulnerability or are affected by pain chronification and pathological negative moods. It is therefore not surprising that these conditions are often comorbid, and indeed, there is now a small but emerging literature regarding the interaction between negative moods and acute and chronic pain (Jensen et al., 2012; Lopez-Sola et al., 2010; Mutschler et al., 2012; Rodriguez-Raecke et al., 2014; Schweinhardt et al., 2008; Strigo et al., 2013). So far, the most compelling evidence is the observation that, in pessimistic subjects, ventral striatal activity for anticipation of pain relief (Leknes et al., 2011) corresponds with the abnormal phasic activity observed for expected pain relief in the same brain region in chronic back pain patients (Baliki et al., 2010). Thus, the conceptual contiguity between pain and negative moods illustrated in figure 5 may also be extended to their corresponding persistent pathological states.
Despite their broad overlap, there is danger in oversimplifying the mechanistic parallels between pain and negative moods; we suspect that limbic brain properties will be differentially configured with chronic pain conditions in contrast to different types of persistent negative moods. For example, the deep brain stimulation site used for treating depression is more orbitally located than the medial prefrontal cortex that correlates with subjective fluctuations in chronic back pain and that also predicts pain chronification. There is also good evidence that negative moods and chronic pain can co-exist, without interacting with one another (Jensen et al., 2010).
Conclusions
The pain research community has made extensive efforts to establish the presence and identify properties of the nociceptive system. This effort has been quite successful, yet in the process, the contribution of the emotional brain on pain perception has received little serious attention. The general notion that pain can be understood in the context of behavior selection and its underlying mechanisms are grounded in limbic brain properties is not a new idea. In fact, the initial formulation of the concept of the limbic brain by MacLean (MacLean, 1955), which first characterized the main components of the brain that generate emotional responses to the environment, stated that pain expression was dependent on this circuitry. Subsequently Melzack and Casey (Melzack and Casey, 1968) expanding on the gate control theory put forward the “motivational and central control” model for pain, and stated “that pain is comprised of both sensory and affective dimensions was clear to Sherrington,” and quote Sherrington’s assertion that “… affective tone is an attribute of all sensation, and among the attribute tones of skin sensation is skin pain” (Sherrington, 1900, p. 974). Melzack and Casey concluded that pain must engage limbic brain, and specifically the hippocampus and amygdala. Even though this conceptually seminal paper has been cited more than 1300 times in the literature, its basic concepts regarding the role of limbic circuitry in pain has failed to advance until the advent of studies discussed here. For example, in a model of pain proposed about 20 years later, the author simply puts a question mark next to the contribution of the limbic brain to the affective and motivational aspects of pain (Price, 1992).
We have provided a broad range of evidence that nociception, pain and negative mood states can be viewed as a single continuum of aversion, within the framework of behavior selection. Further we suggest that the chronification of such states can be conceptualized as being composed of four distinct phases that are contingent on limbic predispositions; the precise mechanistic details, especially regarding pain, remain to be unraveled. Our synthesis of this rapidly accumulating evidence, both in human brain imaging studies and in rodent models, provides compelling evidence that pain perception, as distinguished from nociception, is part of a continuum of aversive behavioral learning that manifests as pain, anxiety, or depression over time, based on preexisting vulnerabilities dictated by emotional learning and the physical proximity of the perceived source of danger.
ACKNOWLEDGEMENTS
We gratefully acknowledge extensive discussions with all members of the Apkarian Lab, especially suggestions and comments on previous drafts by Melissa Farmer, Etienne Vachon-Presseau, Pascal Tetreault, Sara Berger, and Thomas J. Schnitzer. We thank Etienne Vachon-Presseau and Bogdan Petre for help in constructing the figures. We are also delighted for the suggestions proposed and historical notes provided by Ken Casey. This work was supported by National Institutes of Health funding from NINDS, NIDDK, NIDCR, NIDA and NCCIH (formerly NCCAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Apkarian AV. Pain perception in relation to emotional learning. Curr.Opin.Neurobiol. 2008;18:464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV. A brain signature for acute pain. Trends in cognitive sciences. 2013;17:309–310. doi: 10.1016/j.tics.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog.Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:s49–s64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RE, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars BJ. A cognitive theory of consciousness. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Baliki MN, Chang PC, Baria AT, Centeno MV, Apkarian AV. Resting-sate functional reorganization of the rat limbic system following neuropathic injury. Scientific reports. 2014a;4:6186. doi: 10.1038/srep06186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J.Neurophysiol. 2009;101:875–887. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional Reorganization of the Default Mode Network across Chronic Pain Conditions. PloS one. 2014b;9:e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nature neuroscience. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PloS one. 2011;6:e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baria AT, Mansour A, Huang L, Baliki MN, Cecchi GA, Mesulam MM, Apkarian AV. Linking human brain local activity fluctuations to structural and functional network architectures. NeuroImage. 2013;73:144–155. doi: 10.1016/j.neuroimage.2013.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Woods CG. Painful and painless channelopathies. The Lancet. Neurology. 2014;13:587–599. doi: 10.1016/S1474-4422(14)70024-9. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson.Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwerk A, Seifert F, Maihofner C. Altered resting-state functional connectivity in complex regional pain syndrome. The journal of pain : official journal of the American Pain Society. 2013;14:1107–1115. e1108. doi: 10.1016/j.jpain.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Brand PYP. Pain: the gift nobody wants. London: Harper-Colins; 1993. [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Brown ES, Hughes CW, McColl R, Peshock R, King KS, Rush AJ. Association of depressive symptoms with hippocampal volume in 1936 adults. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:770–779. doi: 10.1038/npp.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder R, Richards B, Harris I. Knee osteoarthritis and role for surgical intervention: lessons learned from randomized clinical trials and population-based cohorts. Current opinion in rheumatology. 2014;26:138–144. doi: 10.1097/BOR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J Neurosci. 2002;22:970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nature reviews. Neuroscience. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral J, Kringelbach ML, Deco G. Exploring the network dynamics underlying brain activity during rest. Progress in neurobiology. 2014;114:102–131. doi: 10.1016/j.pneurobio.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Kaur S, Brambilla P, Nicoletti M, Hatch JP, Sassi RB, Mallinger AG, Keshavan MS, Kupfer DJ, Frank E, Soares JC. Smaller cingulate volumes in unipolar depressed patients. Biological psychiatry. 2006;59:702–706. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. The American journal of psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Cocito D, Paolasso I, Isoardo G, Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 2010;81:806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, Torta DM. Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. NeuroImage. Clinical. 2014;4:676–686. doi: 10.1016/j.nicl.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PloS one. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi GA, Huang L, Hashmi JA, Baliki M, Centeno MV, Rish I, Apkarian AV. Predictive dynamics of human pain perception. PLoS computational biology. 2012;8:e1002719. doi: 10.1371/journal.pcbi.1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Human brain mapping. 2015;36:2075–2092. doi: 10.1002/hbm.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno MV, Mutso A, Millecamps M, Apkarian AV. Prefrontal cortex and spinal cord mediated anti-neuropathy and analgesia induced by sarcosine, a glycine-T1 transporter inhibitor. Pain. 2009;145:176–183. doi: 10.1016/j.pain.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DJ. The conscious mind: In search of a fundamental theory. London: Oxford University Press; 1996. [Google Scholar]
- Chang PC, Pollema-Mays SL, Centeno MV, Procissi D, Contini M, Baria AT, Martina M, Apkarian AV. Role of nucleus accumbens in neuropathic pain: linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain. 2014;155:1128–1139. doi: 10.1016/j.pain.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L, Weiner M, Neylan T. Regional cerebral volumes in veterans with current versus remitted posttraumatic stress disorder. Psychiatry research. 2013;213:193–201. doi: 10.1016/j.pscychresns.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Chao LL, Yaffe K, Samuelson K, Neylan TC. Hippocampal volume is inversely related to PTSD duration. Psychiatry research. 2014;222:119–123. doi: 10.1016/j.pscychresns.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain research. 2011a;1392:121–131. doi: 10.1016/j.brainres.2011.03.069. [DOI] [PubMed] [Google Scholar]
- Chen LM, Dillenburger BC, Wang F, Friedman RM, Avison MJ. High-resolution functional magnetic resonance imaging mapping of noxious heat and tactile activations along the central sulcus in New World monkeys. Pain. 2011b;152:522–532. doi: 10.1016/j.pain.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudler EH, Sugiyama K, Dong WK. Nociceptive responses in the neostriatum and globus pallidus of the anesthetized rat. Journal of neurophysiology. 1993;69:1890–1903. doi: 10.1152/jn.1993.69.6.1890. [DOI] [PubMed] [Google Scholar]
- Coenen VA, Schlaepfer TE, Maedler B, Panksepp J. Cross-species affective functions of the medial forebrain bundle-implications for the treatment of affective pain and depression in humans. Neuroscience and biobehavioral reviews. 2011;35:1971–1981. doi: 10.1016/j.neubiorev.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO. Abnormal Amygdala Resting-State Functional Connectivity in Adolescent Depression. JAMA psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? European archives of psychiatry and clinical neuroscience. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Davis KD, Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:518–534. doi: 10.1007/s11481-012-9386-8. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J.Neurophysiol. 1997;77:3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- Deco G, Corbetta M. The dynamical balance of the brain at rest. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2011;17:107–123. doi: 10.1177/1073858409354384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Charles L, King JR, Marti S. Toward a computational theory of conscious processing. Current opinion in neurobiology. 2014;25:76–84. doi: 10.1016/j.conb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nature neuroscience. 2014;17:192–200. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- Dennett DC. The message is: There is no medium. Philosopht and Phenomenological Research. 1993;53:919–931. [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Ellingson BM, Mayer E, Harris RJ, Ashe-McNally C, Naliboff BD, Labus JS, Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013;154:1528–1541. doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer MA, Huang L, Martucci K, Yang CC, Maravilla KR, Harris RE, Clauw DJ, Mackey S, Ellingson BM, Mayer EA, et al. Brain White Matter Abnormalities in Female Interstitial Cystitis/Bladder Pain Syndrome: A MAPP Network Neuroimaging Study. The Journal of urology. 2015 doi: 10.1016/j.juro.2015.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Foo H, Crabtree K, Thrasher A, Mason P. Eating is a protected behavior even in the face of persistent pain in male rats. Physiology & behavior. 2009;97:426–429. doi: 10.1016/j.physbeh.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews. Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc.Natl.Acad.Sci.U.S.A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Schwartenbeck P, FitzGerald T, Moutoussis M, Behrens T, Dolan RJ. The anatomy of choice: dopamine and decision-making. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2014:369. doi: 10.1098/rstb.2013.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frot M, Magnin M, Mauguiere F, Garcia-Larrea L. Cortical representation of pain in primary sensory-motor areas (S1/M1)--a study using intracortical recordings in humans. Human brain mapping. 2013;34:2655–2668. doi: 10.1002/hbm.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain. 2013;1(154 Suppl):S29–S43. doi: 10.1016/j.pain.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Rapkin AJ, Gill Z, Kilpatrick L, Fling C, Stains J, Masghati S, Tillisch K, Mayer EA, Labus JS. Disease-related differences in resting-state networks: a comparison between localized provoked vulvodynia, irritable bowel syndrome, and healthy control subjects. Pain. 2015;156:809–819. doi: 10.1097/01.j.pain.0000461289.65571.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwilym SE, Fillipini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty; a longitudinal voxel-based-morphometric study. Arthritis and rheumatism. 2010 doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrom JB, Jensen TS. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain. 2014;155:1272–1279. doi: 10.1016/j.pain.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain : a journal of neurology. 2013;136:2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV. Multivariate pattern analysis of fMRI: the early beginnings. NeuroImage. 2012;62:852–855. doi: 10.1016/j.neuroimage.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behavioral neuroscience. 1985;99:1031–1039. doi: 10.1037//0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Naismith SL, Ward PB, Scott EM, Mitchell PB, Schofield PR, Scimone A, Wilhelm K, Parker G. Serotonin transporter gene status predicts caudate nucleus but not amygdala or hippocampal volumes in older persons with major depression. Journal of affective disorders. 2007;98:137–142. doi: 10.1016/j.jad.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back) Experimental brain research. 2010;205:1–12. doi: 10.1007/s00221-010-2340-1. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. 2012;153:1495–1503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Gracely R, Ingvar M, Kosek E. Anxiety and depressive symptoms in fibromyalgia are related to poor perception of health but not to pain sensitivity or cerebral processing of pain. Arthritis and rheumatism. 2010;62:3488–3495. doi: 10.1002/art.27649. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. Journal of neurophysiology. 2011;106:2642–2652. doi: 10.1152/jn.00461.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat.Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Salomons TV, Backonja MM, Davidson RJ. Turning on the alarm: the neural mechanisms of the transition from innocuous to painful sensation. NeuroImage. 2012;59:1594–1601. doi: 10.1016/j.neuroimage.2011.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, Farmer MA, Ness TJ, Deutsch G, Mayer EA, et al. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. The Journal of urology. 2015;193:131–137. doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenshalo DR, Jr, Giesler GJ, Jr, Leonard RB, Willis WD. Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol. 1980;43:1594–1614. doi: 10.1152/jn.1980.43.6.1594. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Jr, Isensee O. Responses of primate SI cortical neurons to noxious stimuli. J Neurophysiol. 1983;50:1479–1496. doi: 10.1152/jn.1983.50.6.1479. [DOI] [PubMed] [Google Scholar]
- Khan SA, Keaser ML, Meiller TF, Seminowicz DA. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain. 2014;155:1472–1480. doi: 10.1016/j.pain.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, et al. Alterations in Resting State Oscillations and Connectivity in Sensory and Motor Networks in Women with Interstitial Cystitis/Painful Bladder Syndrome. The Journal of urology. 2014 doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. Consciousness: confessions of a romantic reductionist. Cambridge, Massachusetts: NIT press; 2012. [Google Scholar]
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69:33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: hedonic and neural responses to safety from pain. PloS one. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain. 2004;110:112–122. doi: 10.1016/j.pain.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330:1400–1404. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang J, Chen L, Zhang M, Wan Y. Basolateral amygdala lesion inhibits the development of pain chronicity in neuropathic pain rats. PloS one. 2013;8:e70921. doi: 10.1371/journal.pone.0070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Mouraux A, Hu L, Iannetti GD. Primary sensory cortices contain distinguishable spatial patterns of activity for each sense. Nature communications. 2013;4:1979. doi: 10.1038/ncomms2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Edwards RR, Kim J, Vangel MG, Wasan AD, Gollub RL, Harris RE, Park K, Napadow V. Disentangling linear and nonlinear brain responses to evoked deep tissue pain. Pain. 2012;153:2140–2151. doi: 10.1016/j.pain.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sola M, Pujol J, Hernandez-Ribas R, Harrison BJ, Contreras-Rodriguez O, Soriano-Mas C, Deus J, Ortiz H, Menchon JM, Vallejo J, Cardoner N. Effects of duloxetine treatment on brain response to painful stimulation in major depressive disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2305–2317. doi: 10.1038/npp.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. The limbic system ("visceral brain") in relation to the central gray and reticulum of the brain stem. Psychosomatic Medicine. 1955;17:355–366. doi: 10.1097/00006842-195509000-00003. [DOI] [PubMed] [Google Scholar]
- Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6493–6497. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Brain white matter structural properties predict transition to chronic pain. Pain. 2013;154:2160–2168. doi: 10.1016/j.pain.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks LE, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. London: Elsevier; 2006. pp. 3–34. [Google Scholar]
- Martikainen IK, Pecina M, Love TM, Nuechterlein EB, Cummiford CM, Green CR, Harris RE, Stohler CS, Zubieta JK. Alterations in endogenous opioid functional measures in chronic back pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:14729–14737. doi: 10.1523/JNEUROSCI.1400-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Peyron R, Guenot M, Mauguiere F. Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain. 2009;146:99–104. doi: 10.1016/j.pain.2009.07.014. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA. Amygdala Response to Negative Stimuli Predicts Ptsd Symptom Onset Following a Terrorist Attack. Depression and anxiety. 2014 doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine Io. In: Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Medicine Io., editor. Washington (D.C.): 2011. [Google Scholar]
- Melzack R, Casey K. The skin senses. Springfield: Charles C. Thomas; 1968. Sensory, motivational, and central contol determinants of pain; pp. 423–443. [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. London: Elsevier; 2006. [Google Scholar]
- Millecamps M, Centeno MV, Berra HH, Rudick CN, Lavarello S, Tkatch T, Apkarian AV. D-cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain. 2006 doi: 10.1016/j.pain.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayedi M, Weissman-Fogel I, Crawley AP, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Contribution of chronic pain and neuroticism to abnormal forebrain gray matter in patients with temporomandibular disorder. NeuroImage. 2011;55:277–286. doi: 10.1016/j.neuroimage.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Vlaeyen JW. Beyond nociception: the imprecision hypothesis of chronic pain. Pain. 2015;156:35–38. doi: 10.1016/j.pain.0000000000000014. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Pendse G, Becerra LR, Borsook D. BOLD responses in somatosensory cortices better reflect heat sensation than pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6024–6031. doi: 10.1523/JNEUROSCI.0006-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, De Paepe AL, Marot E, Plaghki L, Iannetti GD, Legrain V. Unmasking the obligatory components of nociceptive event-related brain potentials. Journal of neurophysiology. 2013;110:2312–2324. doi: 10.1152/jn.00137.2013. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the "pain matrix". NeuroImage. 2011;54:2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Measuring the global burden of disease. The New England journal of medicine. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- Murray MM, Herrmann CS. Illusory contours: a window onto the neurophysiology of constructing perception. Trends in cognitive sciences. 2013;17:471–481. doi: 10.1016/j.tics.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neuroscience letters. 2012;520:204–209. doi: 10.1016/j.neulet.2012.03.095. [DOI] [PubMed] [Google Scholar]
- Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann K, Schnitzer TJ, Apkarian AV. Reorganization of Hippocampal Functional Connectivity with Transition to Chronic Back Pain. Journal of neurophysiology. 2013 doi: 10.1152/jn.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Hikida T, Yawata S. Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neuroscience. 2014;282C:49–59. doi: 10.1016/j.neuroscience.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Nalloor R, Bunting KM, Vazdarjanova A. Altered hippocampal function before emotional trauma in rats susceptible to PTSD-like behaviors. Neurobiology of learning and memory. 2014;112:158–167. doi: 10.1016/j.nlm.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJHK, HG JM. Some ascending pathways in the brain stem reticular formation of the cat. In: Jasper HHPLD, editor. Reticular Formation of the Brain. Boston: Little, Brown; 1958. pp. 3–30. [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci. 2003;23:52–63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman HM, Stevens RT, Apkarian AV. Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. J Comp Neurol. 1996;365:640–658. doi: 10.1002/(SICI)1096-9861(19960219)365:4<640::AID-CNE10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Nicholson K. An overview of pain problems associated with lesions, disorder or dysfunction of the central nervous system. NeuroRehabilitation. 2000;14:3–13. [PubMed] [Google Scholar]
- Parks EL, Geha PY, Baliki MN, Katz J, Schnitzer TJ, Apkarian AV. Brain activity for chronic knee osteoarthritis: Dissociating evoked pain from spontaneous pain. Eur J Pain. 2011 doi: 10.1016/j.ejpain.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. New York: Dover publications; 1926, 2003. [Google Scholar]
- Pei-Ching Chang PCSLP-MSL, Maria Virginia Centeno MV, Procissi D, Contini M, Baria AT, Martina M, Apkarian AV. Role of Nucleus Accumbens in Neuropathic Pain: Linked Multi-Scale Evidence in the Rat Transitioning to Neuropathic Pain. Pain. 2014 doi: 10.1016/j.pain.2014.02.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain : a journal of neurology. 1999;122(Pt 9):1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best practice & research. Clinical rheumatology. 2011;25:141–154. doi: 10.1016/j.berh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro CA, Lui F, Facchin P, Maieron M, Baraldi P. Percept-related activity in the human somatosensory system: functional magnetic resonance imaging studies. Magnetic resonance imaging. 2004;22:1539–1548. doi: 10.1016/j.mri.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanims of pain. New York: Raven Press; 1988. [Google Scholar]
- Price DDHSW. The affective-motivational dimension of pain. American Pain Society. 1992;1:229–239. [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biological psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nature reviews. Neuroscience. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, et al. Peripheral Nerve Injury Leads to Working Memory Deficits and Dysfunction of the Hippocampus by Upregulation of TNF-alpha in Rodents. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:979–992. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat.Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JPAAV. Low back pain. In: Mayer EABMC, editor. Functional pain syndromes: presentation and pathophysiology. Seattle: International association for the study of pain; 2009. [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;1(47 Suppl):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Ihle K, Ritter C, Muhtz C, Otte C, May A. Neuronal differences between chronic low back pain and depression regarding long-term habituation to pain. Eur J Pain. 2014;18:701–711. doi: 10.1002/j.1532-2149.2013.00407.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokers B, Cormack LK, Huk AC. Disparity- and velocity-based signals for three-dimensional motion perception in human MT+ Nature neuroscience. 2009;12:1050–1055. doi: 10.1038/nn.2343. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews. Neuroscience. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LJ. The Charcot foot: historical perspective 1827–2003. Diabetes/metabolism research and reviews. 2004;(20 Suppl 1):S4–S8. doi: 10.1002/dmrr.451. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. Can we conquer pain? Nat.Neurosci. 2002;(5 Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat.Rev.Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC. Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science. 2014;345:535–542. doi: 10.1126/science.1253994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P, Kalk N, Wartolowska K, Chessell I, Wordsworth P, Tracey I. Investigation into the neural correlates of emotional augmentation of clinical pain. NeuroImage. 2008;40:759–766. doi: 10.1016/j.neuroimage.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nature neuroscience. 2015;18:499–500. doi: 10.1038/nn.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi A, Sugiura M, Taki Y, Kotozaki Y, Nouchi R, Takeuchi H, Araki T, Hanawa S, Nakagawa S, Miyauchi CM, et al. White matter microstructural changes as vulnerability factors and acquired signs of post-earthquake distress. PloS one. 2014;9:e83967. doi: 10.1371/journal.pone.0083967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. NeuroImage. 2009;47:1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Collado A, Sola R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H. Hyperexcitable C nociceptors in fibromyalgia. Annals of neurology. 2014;75:196–208. doi: 10.1002/ana.24065. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Textbook of physiology (Edinburgh) 1900. [Google Scholar]
- Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. The journal of pain : official journal of the American Pain Society. 2013;14:663–675. doi: 10.1016/j.jpain.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcevic A, Postic S, Radojicic Z, Starcevic B, Milovanovic S, Ilankovic A, Dimitrijevic I, Damjanovic A, Aksic M, Radonjic V. Volumetric analysis of amygdala, hippocampus, and prefrontal cortex in therapy-naive PTSD participants. BioMed research international. 2014;2014:968495. doi: 10.1155/2014/968495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SS. On the psychophysical law. Psychol.Rev. 1957;64:153–181. doi: 10.1037/h0046162. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Matthews SC, Simmons AN. Decreased frontal regulation during pain anticipation in unmedicated subjects with major depressive disorder. Translational psychiatry. 2013;3:e239. doi: 10.1038/tp.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Toward a modern theory of adaptive networks: expectation and prediction. Psychological review. 1981;88:135–170. [PubMed] [Google Scholar]
- Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? The journal of pain : official journal of the American Pain Society. 2009;10:1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Tse YC, Montoya I, Wong AS, Mathieu A, Lissemore J, Lagace DC, Wong TP. A longitudinal study of stress-induced hippocampal volume changes in mice that are susceptible or resilient to chronic social defeat. Hippocampus. 2014;24:1120–1128. doi: 10.1002/hipo.22296. [DOI] [PubMed] [Google Scholar]
- Vaso A, Adahan HM, Gjika A, Zahaj S, Zhurda T, Vyshka G, Devor M. Peripheral nervous system origin of phantom limb pain. Pain. 2014;155:1384–1391. doi: 10.1016/j.pain.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006;140:1311–1320. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Whitsel BL, Favorov OV, Brown AW, Tommerdahl M. Role of primary somatosensory cortex in the coding of pain. Pain. 2013;154:334–344. doi: 10.1016/j.pain.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaev I, Seymour B, Dolan RJ, Chater N. The price of pain and the value of suffering. Psychol Sci. 2009;20:309–317. doi: 10.1111/j.1467-9280.2009.02304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. The New England journal of medicine. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Winawer J. Imaging retinotopic maps in the human brain. Vision research. 2011;51:718–737. doi: 10.1016/j.visres.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Mouraux A, Liang M, Iannetti G. Stimulus novelty and not neural refractoriness explains the repetition suppression of laser-evoked potentials (LEPs) Journal of neurophysiology. 2010 doi: 10.1152/jn.01088.2009. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. Journal of abnormal psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weber EH. In: The sense of touch. Ross HE, Murray DJ, editors. London: Academic Press; 1978. [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, Simmons JG, Yucel M, Pantelis C, McGorry P, Allen NB. Structural brain development and depression onset during adolescence: a prospective longitudinal study. The American journal of psychiatry. 2014;171:564–571. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
- Wiech K, Vandekerckhove J, Zaman J, Tuerlinckx F, Vlaeyen JW, Tracey I. Influence of prior information on pain involves biased perceptual decision-making. Current biology : CB. 2014;24:R679–R681. doi: 10.1016/j.cub.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord. New York: Plenum press; 1978. [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth RS, Sherrington CS. A pseudaffective reflex and its spinal path. The Journal of physiology. 1904;31:234–243. doi: 10.1113/jphysiol.1904.sp001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7445–7453. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelle MD, Rogers JM, Coghill RC. Offset analgesia: a temporal contrast mechanism for nociceptive information. Pain. 2008;134:174–186. doi: 10.1016/j.pain.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]