Abstract
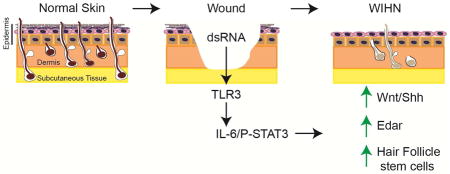
Regeneration of skin and hair follicles after wounding - a process known as wound-induced hair neogenesis (WIHN) - is a rare example of adult organogenesis in mammals. As such, WIHN provides a unique model system for deciphering mechanisms underlying mammalian regeneration. Here, we show that dsRNA, which is released from damaged skin, activates Toll-Like Receptor 3 (TLR3) and its downstream effectors IL6 and STAT3 to promote hair follicle regeneration. Conversely, TLR3-deficient animals fail to initiate WIHN. TLR3 activation promotes expression of hair follicle stem cell markers and induces elements of the core hair morphogenetic program, including EDAR and the Wnt and Shh pathways. Our results therefore show that dsRNA and TLR3 link the earliest events of mammalian skin wounding to regeneration and suggest potential therapeutic approaches for promoting hair neogenesis.
Graphical Abstract
INTRODUTION
Animals across diverse phyla can regenerate lost structures, a capacity that is considerably more limited in mammals. Several chordate species including urodele salamanders and teleost fish can regenerate appendages and solid organs, yet among mammals such adult organogenesis is rarely – if ever – observed. An important exception is wound-induced hair neogenesis (WIHN), a phenomenon in which skin, sebaceous glands, and hair follicles are regenerated following large, full thickness wounds in mice or rabbits (Breedis, 1954; Ito et al., 2007). The complete regeneration observed in WIHN is in marked contrast to the fibrotic scarring that typically results from cutaneous wound healing. Regenerated hair follicles are complex mini-organs with disparate cell types, dedicated neurovascular support, and a distinct stem cell compartment located in the bulge region. These stem cells not only repopulate hair follicles throughout life, but also aid in skin re-epithelialization after wounding, pointing to the potential therapeutic relevance of WIHN (Ito et al., 2007). As WIHN represents a rare example of adult organogenesis in mammals, understanding its mechanisms could aid in efforts to regenerate other structures.
While originally described in the 1940s, WIHN has recently been characterized in morphogenetic and molecular detail (Breedis, 1954; Gay et al., 2013; Ito et al., 2007; Kligman and Strauss, 1956; Myung et al., 2013; Nelson et al., 2013). Following complete excision of skin down to fascia, wounds on the backs of mice heal through initial contracture and then re-epithelialization. Subsequently, hair follicle morphogenesis ensues with recapitulation of events that occur during embryonic hair development. Formation and invagination of epithelial placodes in the epidermis, induction of adjacent dermal papillae, and ultimately, elaboration of distinct hair cell subtypes are observed (Ito et al., 2007). Follicle-associated structures such as sebaceous glands are also regenerated. Regenerated follicles transit through multiple hair cycles, just like neighboring hairs from unwounded skin (Ito et al., 2007). Therefore, WIHN represents functional regeneration rather than mere wound repair through scarring.
Developmental pathways required for embryonic organogenesis can be reactivated following trauma. In axolotl limb regeneration for example, Shh signaling is activated at the site of injury in the residual limb much as it is induced in the zone of polarizing activity during limb development (Torok et al., 1999). Similarly, during WIHN, signaling pathways utilized in embryonic hair formation reemerge after wounding. Activation of the canonical Wnt pathway is one of the earliest events observed in follicular morphogenesis. Wnt activation occurs around E15 in mice as the undifferentiated epithelium begins to condense into epithelial placodes at sites of future follicle formation (Millar, 2002). Similarly, after cutaneous wounding, the Wnt ligand, Wnt10b, and the Wnt effector, Lef1, are induced after re-epithelialization is complete, but prior to the emergence of new follicles (Ito et al., 2007). Wnt pathway activation is critical for hair morphogenesis during both development and regeneration, as mice deficient in Wnt signaling fail to generate hairs (Ito et al., 2007; Myung et al., 2013). Secondary to Wnt activation during follicular development, Shh signaling is induced in epithelial placodes and underlying dermal papillae. Activation of the Shh pathway contributes to subsequent hair follicle invagination and morphogenesis (St-Jacques et al., 1998). The Shh pathway is similarly induced during adult hair follicle regeneration. Other molecular details of hair regeneration are shared with hair development including expression of the hair cytokeratin Krt17 and activation of alkaline phosphatase activity in dermal papillae (Ito et al., 2007).
While downstream morphogenetic events in WIHN parallel those in hair development, the signals triggering reactivation of these programs in adult regeneration are unclear. To initiate regeneration, organisms must first sense a loss of tissue integrity. Candidate signals include molecules liberated from damaged tissues as well as mediators released by infiltrating immune cells. In newts and axolotls, activation of thrombin is a key early event in regeneration. For example, inhibition of thrombin activation abrogates lens regeneration in newts (Imokawa and Brockes, 2003). Recently, it was demonstrated that FGF9 released from γδ T cells several days after wounding promotes hair regeneration in rodents (Gay et al., 2013). However, the most proximal signals released by damaged keratinocytes to initiate regeneration in the skin remain unknown. Discovery of such damage-associated signals may explain why wound healing during WIHN proceeds through regeneration whereas most cutaneous wound healing in mammals leads to fibrotic scarring. Identifying these molecules may also suggest therapeutic approaches to promote skin and hair regeneration and reduce fibrosis.
To identify molecular events that initiate regeneration, we exploited the natural variation in follicle regenerative capacity observed in various mouse strains. Through gene expression screening of healed wounds prior to the onset of follicle regeneration, we identified the pattern recognition receptor, Toll-like Receptor 3 (TLR3), as a critical regulator of cutaneous regeneration. While TLR3 and its ligand, double stranded RNA (dsRNA), are known to be active during cutaneous wounding, their role in promoting regeneration after re-epithelialization of the skin has not been appreciated (Lin et al., 2012). We identified dsRNA released from damaged cells as a key trigger of the regeneration process through its activation of TLR3. The ensuing damage-induced signaling cascade impedes stratification and maintains keratinocytes in a less-differentiated state. Furthermore, TLR3 activation initiates molecular events in the hair morphogenetic program, with activation of canonical Wnt and Shh pathways, and EDAR resulting in augmented hair follicle neogenesis. Thus, TLR3 activation by dsRNA links damage sensing after wounding to the earliest molecular events in hair regeneration. These results uncover a role for TLR3 as a master regulator of regeneration in the skin.
RESULTS
Hair follicle regeneration after wounding recapitulates embryonic follicle development in both morphogenetic and molecular detail. However, the events that dictate whether wound healing proceeds by regeneration or fibrotic scarring remain unclear. In studies characterizing molecular mechanisms of WIHN, we and others observed significant differences in the regenerative capacity of various mouse strains when visualized by confocal scanning laser microscopy (CSLM) (Fig. 1A) (Fan et al., 2011; Ito et al., 2007; Nelson et al., 2013). To identify factors that may initiate regeneration, we compared gene expression profiles from healed wounds of mice with high and low regenerative capacity using C57BL/6 and our mixed background strain of mice (C57BL/6 x FVB x SJL). This analysis was performed at the time of wound closure but before the onset of hair morphogenesis to enrich for upstream factors in the WIHN pathway (Supp. Fig. 1A, Methods). Ingenuity Pathway Analysis identified “viral pattern recognition receptors” and “interferon-signaling” as the most significantly up-regulated pathways in highly regenerative mice.
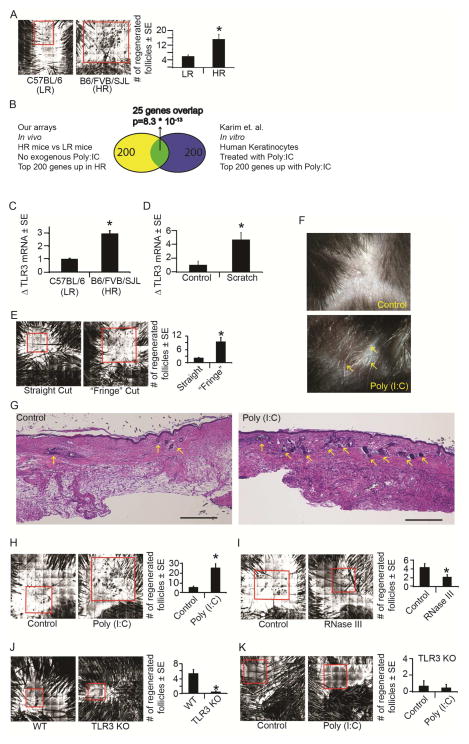
Figure 1. Tissue damage and double stranded RNA activate TLR3 to promote wound-induced hair follicle neogenesis (WIHN).
A) Confocal Scanning Laser Microscopy (CSLM) images for C57BL6J (Low Regeneration, “LR”) and Mixed B6/FVB/SJL (High Regeneration, “HR”) strains of mice. Area of WIHN shown within red box. Original image size is 4mm2.
B) Venn diagram depicting significant overlap between genes associated with high levels of follicle regeneration in mouse skin (in vivo) and human keratinocytes treated with poly (I:C) in vitro published by Karim et al., 2011 under GSE21260.
C) Mean fold change in TLR3 mRNA in healed scars at WD20-24 in LR vs. HR mice as determined by qRT-PCR and normalized to housekeeping gene β-actin.
D) Mean fold change in TLR3 mRNA four hours post scratch assay in NHEK as determined by qRT-PCR and normalized to housekeeping gene RPLP0.
E) WIHN levels in wt mice after standard straight cut or “fringe cut” to wound edge; n = 14–15 mice. Area of WIHN shown within red box. Original image size is 4mm2.
F) Regenerated hair shafts (white, arrows) in healed wounds after single injection of poly (I:C) (500ng) or control at WD3 and visualized by dissecting microscope at ~WD58-62.
G) Cross-sectional H&E histology through the middle of healed scar at WD22 after single injection of poly (I:C) as in 1F. Regenerated hair follicles are marked with arrows. Scale bar = 500um.
H) WIHN levels in wt mice after poly (I:C) (500ng) or PBS control measured by CSLM, n = 10–11 mice.
I) WIHN levels in wt mice after RNase III (15 units) or buffer control measured by CSLM, n = 17–19 mice.
J) WIHN levels in strain-matched wt control mice and TLR3 KO mice measured by CSLM n = 6 mice.
K) WIHN in TLR3 KO mice after poly (I:C) (500ng) compared to PBS control measured by CSLM; n = 9 mice.
*p < 0.05 by Student’s T-test or Single Factor ANOVA.
dsRNA released by tissue damage activate TLR3 to promote regeneration
We focused on the pattern recognition receptor TLR3, which is activated by dsRNA and known to induce interferon signaling (Uematsu and Akira, 2007). The gene expression pattern we observed in highly regenerative murine skin wounds showed strong overlap with the pattern obtained from human keratinocytes treated with the synthetic dsRNA mimic poly (I:C) (Fig. 1B) (Karim et al., 2011). Strikingly, despite the differences in species and experimental conditions, 25 of the 200 most highly up-regulated genes were common to both analyses (Supp. Fig. 1B). This observed overlap in expression of genes involved in dsRNA-sensing suggested a potentially conserved role for TLR3 in early wound healing responses. Furthermore, expression of TLR3 itself was 3-fold higher in our highly regenerative mouse strain as observed by qRT-PCR, validating the expression patterns observed in the array analyses (Fig. 1C).
In previous studies TLR3 mRNA was induced in response to dsRNA released after UVB irradiation (Bernard et al., 2012). This suggested to us that during WIHN, TLR3 may serve as a sensor of tissue damage, consistent with an upstream role in the regeneration process. To test this idea, we examined TLR3 expression following scratching of human keratinocytes in culture. TLR3 expression was nearly 5-fold higher in scratched keratinocytes compared to un-manipulated controls (Fig. 1D). Furthermore, we observed a significant increase in the number of regenerated follicles in vivo when we increased the extent of damage during wounding by placing minute perpendicular cuts at the wound edge (Fig 1E, Methods). We next explored whether augmenting the natural dsRNA release during wounding could lead to an increase in regeneration. Indeed, a single addition of the dsRNA mimic poly (I:C) into murine skin wounds led to a greater number of regenerated follicles (Fig. 1F–H) Conversely, addition of the dsRNA-specific endonuclease, RNase III, significantly decreased the number of regenerated follicles (Fig. 1I) To confirm that the effects of dsRNA on WIHN are TLR3-dependent, we next examined the extent of regeneration in TLR3 null mice. Strikingly, regeneration was almost completely abolished in these mice compared to strain-matched controls, despite their comparable re-epithelialization kinetics (Fig 1J; Supp. Fig. 1C). Moreover, the stimulatory effect of dsRNA on WIHN was abrogated in TLR3 null mice, demonstrating the necessity of TLR3 for damage-induced regeneration (Fig. 1K). The effects of dsRNA were only observed in the context of regeneration, as poly (I:C) did not affect the hair cycle in normal non-wounded murine skin (Supp. Fig 1D). Taken together, these data suggest that TLR3 activation by dsRNA released during wounding initiates regeneration.
IL-6 and pSTAT3 are induced by tissue damage and double-stranded RNA
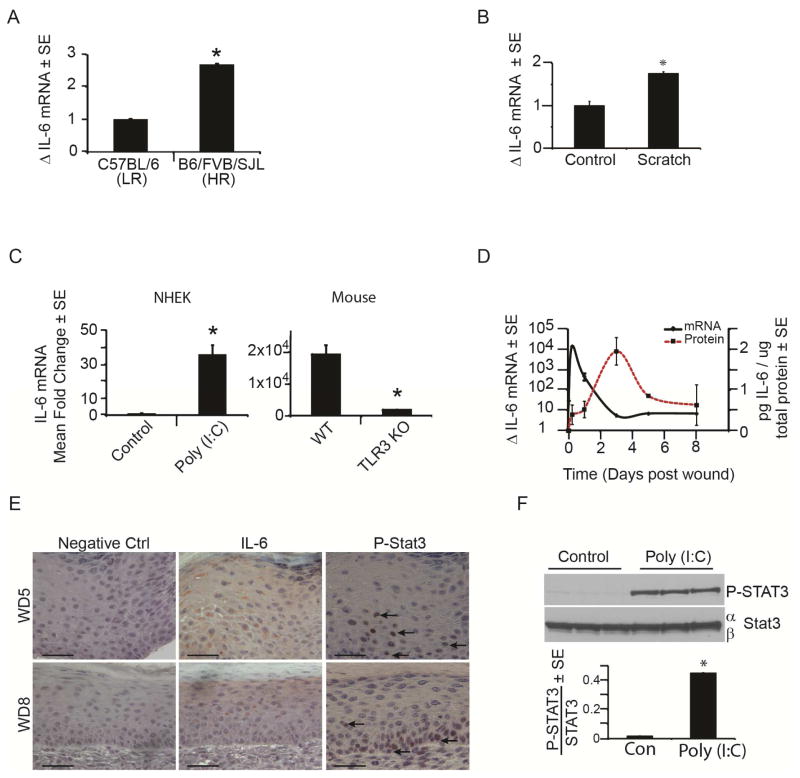
To examine the mechanism by which TLR3 promotes regeneration, we performed gene expression analysis on mice at 16 to 18 days post-wounding (WD16-18), later than the array above, and at the earliest time points at which regenerated follicles can be detected by CSLM (Supp. Fig. 2A). Gene expression from healed wound beds of animals with robust regeneration (average 49 hair follicles) were compared to those of animals that failed to regenerate hair follicles (0 hair follicles), revealing up-regulation of several interleukins and cytokines in more highly regenerative mice. Of particular interest were interleukin-6 (IL-6) and its pathway components as well as TLR3 itself, which appeared as the top upstream regulator of IL-6 in this analysis (Supp. Fig. 2B–F). Just as we had found for TLR3, mixed strain animals with high regenerative capacity had 3-fold higher levels of IL-6 compared to C57BL/6 mice with poor regeneration (Fig. 2A). These data led to the hypothesis that IL-6 may mediate the effects of TLR3 on regeneration. TLR3 has previously been demonstrated to induce IL-6 in a dsRNA dependent manner (Melkamu et al., 2013) and IL-6 is a known activator of regeneration in other contexts, particularly in response to liver damage (Galun and Rose-John, 2013; Jia, 2011). Consistent with this, just as TLR3 expression is increased in injured (scratched) keratinocytes in culture, IL-6 mRNA also increased (Fig. 2B). In keratinocytes treated with poly (I:C), we observed a greater than 30-fold induction of IL-6 mRNA (Fig. 2C), which is partially mediated through the downstream transcription factor, NFκB (Supp. Fig. 2G). This induction is TLR3-dependent as TLR3 null animals had far less IL-6 mRNA after wounding than strain-matched controls (Fig. 2C). Temporally, IL-6 mRNA and protein were sequentially up-regulated at early time points following wounding, consistent with a role for this pathway in initiating WIHN (Fig. 2D).
Figure 2. IL-6 and pSTAT3 are induced by tissue damage and double stranded RNA.
A) Mean fold change in IL-6 mRNA in healed scars at WD20-24 in HR vs. LR strains of mice as determined by qRT-PCR and normalized to housekeeping gene β-actin.
B) Mean fold change in IL-6 mRNA 24 hours post scratch assay in NHEK as determined by qRT-PCR and normalized to housekeeping gene, RPLP0.
C) Mean fold change in IL-6 mRNA after poly (I:C) addition (20μg/mL) to NHEK for 6 hours or in strain matched wt and TLR3 KO mice (n = 3) 6 hours after wounding as determined by qRT-PCR and normalized to housekeeping gene RPLP0 (NHEK) or β-actin (mouse).
D) Time course of IL-6 mRNA and protein expression throughout early stage wound healing in wt mice, as determined by qRT-PCR and ELISA, respectively. N = 3 mice/time point
E) IL-6 (middle) and P-STAT3 (right; arrows) immunohistochemistry of healing scars at WD5 (top) and WD8 (bottom) in wt mice. Scale bar = 50 μm.
F) P-STAT 3 levels in NHEKs after poly (I:C) (20ug/mL) for 24 hours compared to vehicle control as measured by western blot and normalized to STAT3.
*p < 0.05 by Student’s T-test or Single Factor ANOVA.
IL-6 receptor engagement is known to cause phosphorylation of STAT3 (pSTAT3), leading to its nuclear translocation and transcriptional activation (Heinrich et al., 2003). Consistent with a role for TLR3 and IL-6 signaling in WIHN initiation, we observed increased IL-6 and pSTAT3 protein in murine keratinocytes at WD5 and 8 (Fig. 2E, Supp. Fig. 3) as well as increased pSTAT3 protein expression in human keratinocytes following poly (I:C) treatment (Fig. 2F).
TLR3 effects on regeneration are mediated by IL-6 and pSTAT3
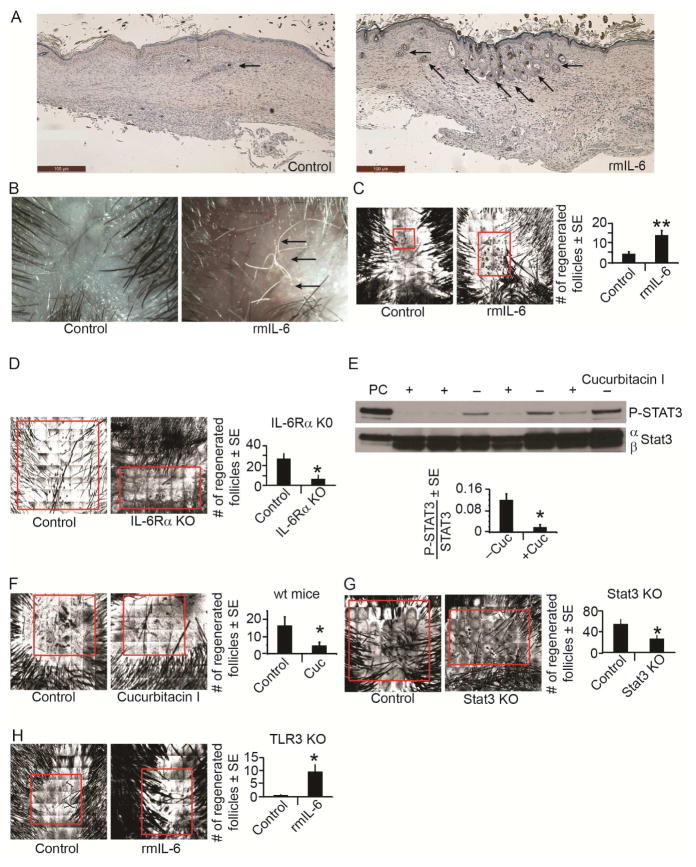
To test the functional consequences of IL-6 on follicle regeneration, we injected recombinant IL-6 protein (rmIL-6) into mice following wounding and examined subsequent hair follicle regeneration. Compared to vehicle injected controls, mice receiving IL-6 had a nearly 3-fold increase in the number of regenerated follicles (Fig. 3A–C). The increase in follicle numbers upon activation of the IL-6/STAT3 axis occurred only in the context of WIHN, as dsRNA does not stimulate anagen onset during follicular cycling in unwounded skin (Supp. Fig. 1D). We also noted a marked reduction in WIHN in mice whose keratinocytes lacked the IL-6 receptor alpha (K14CreERT2-IL-6Rαfl/fl) (Fig. 3D), highlighting the importance of the IL-6 signaling pathway during WIHN.
Figure 3. IL-6 and pSTAT3 mediate TLR3 effects on WIHN.
A) Cross-sectional H&E histology of healed scar at WD22 after a single injection of IL-6 (25ng) or PBS control at WD7. Regenerated hair follicles are marked with arrows. Scale bar=100 μm.
B) Regenerated hair shafts (white, arrows) at ~WD58-62 as visualized by dissecting microscope.
C) WIHN in wt mice after single dose of IL-6 (25ng) compared to PBS control as measured by CSLM; n = 30 mice.
D) WIHN in keratinocyte-specific knockout of IL-6Receptorα compared to control mice measured by CSLM; n = 3–6 mice.
E) P-Stat 3 levels in the presence of cucurbitacin I (+) compared to control (−) in wt mice as measured by western blot and normalized to Stat3. PC = P-STAT3 positive control cell lysate.
F) WIHN levels in wt mice after cucurbitacin I (2mg/kg) or control as measured by CSLM; n = 10–14 mice.
G) WIHN in keratinocyte-specific knockout of Stat3 compared to control mice measured by CSLM; n = 10–15 mice.
H) WIHN in TLR3 KO mice after single dose of rmIL-6 (500ng) compared to PBS control measured by CSLM; n = 8–10 mice.
*p < 0.05 by Student’s T-test or Single Factor ANOVA.
We next blocked the IL-6/STAT3 signaling axis with cucurbitacin I, a highly selective pharmacological inhibitor of STAT3 (Blaskovich et al., 2003). Cucurbitacin I strongly suppressed STAT3 phosphorylation in vivo (Fig. 3E) and wild type mice injected with cucurbitacin I had a greater than 3-fold decrease in the number of regenerated follicles (Fig. 3F). Similarly, WIHN in keratinocyte-specific Stat3 KO mice (K5CreERT2-Stat3fl/fl) was significantly decreased compared to control mice (Fig. 3G). Remarkably, the previously observed attenuation of WIHN in TLR3 KO mice was rescued by a single injection of recombinant IL-6 protein (Fig. 3H), implying that IL-6 functions downstream of TLR3 in follicle regeneration. In aggregate, these data suggest that TLR3 activation during wounding promotes IL-6 production and STAT3 phosphorylation resulting in higher regeneration.
TLR3 activation alters keratinocyte differentiation and induces markers of hair follicle progenitors
Previous studies revealed that keratinocytes outside of the bulge region-- which ordinarily differentiate into corneocytes during stratification-- contribute to regenerated hair follicles during WIHN (Ito et al., 2007; Snippert et al., 2010). This finding implies that the normal stratification program is altered during hair follicle regeneration. We hypothesized that TLR3 activation during WIHN may counter stratification of keratinocytes.
To test this idea, we first injected mice with IL-6 and assessed epidermal thickness as an index of stratification. Ordinarily, stratification causes thickening of the epidermis due to the accumulation of differentiated keratinocytes. However, mice treated with IL-6 had a 2-fold reduction in epidermal thickness compared to strain matched controls, implying decreased stratification, (Fig. 4A) since no alterations in apoptosis or proliferation were observed with IL-6 treatment (Supp. Fig. 4A). Consistent with our findings in vivo, direct TLR3 activation with poly (I:C) in cultured keratinocytes led to a nearly complete loss of expression of markers of keratinocyte differentiation such as keratin 1 (KRT1) and filaggrin (FLG) (Fig. 4B). This effect was TLR3-dependent as inhibition of TLR3 through siRNA mediated depletion or direct small molecule-based antagonism abrogated the loss of KRT1 (Fig. 4C–D; Supp. Fig. 4B). Similarly, keratinocytes treated with IL-6 had a profound decrease in KRT1 expression, an effect that was reversed by the addition of cucurbitacin I (Fig. 4E). These data suggest that induction of the TLR3/IL-6 axis during wounding prevents keratinocyte differentiation. Interestingly, the morphology of poly (I:C) treated keratinocytes was also altered and came to resemble that of keratinocytes in healing human skin wounds (Supp. Fig. 4C–H) (Yan et al., 2010).
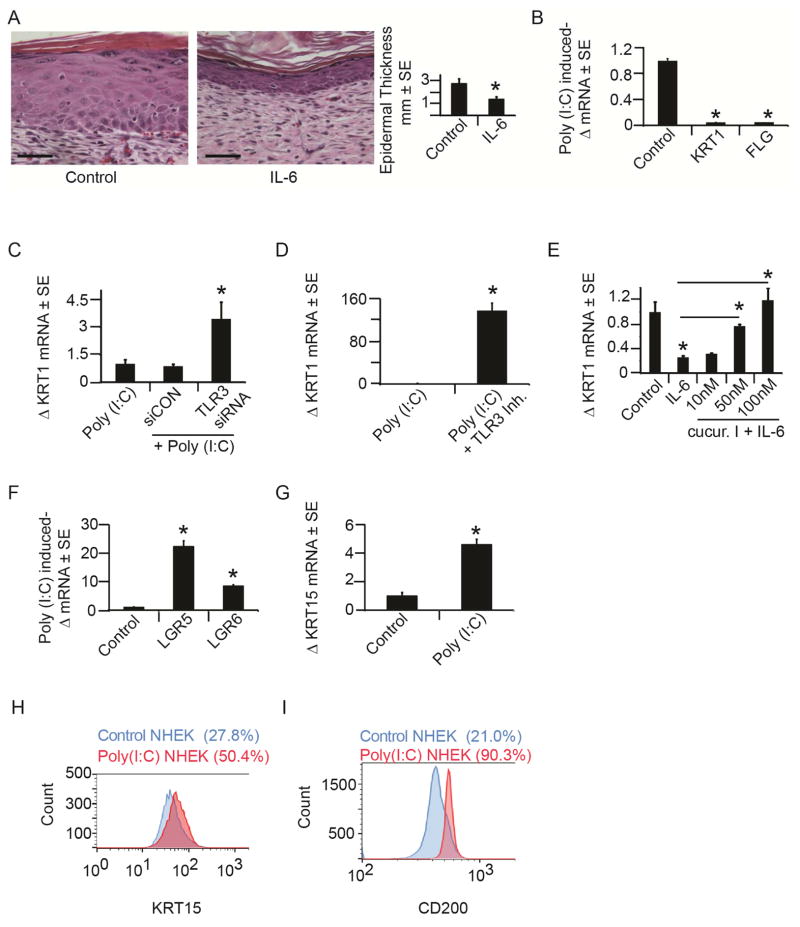
Figure 4. TLR3 activation alters keratinocyte differentiation and induces markers of hair follicle progenitors.
A) Cross-sectional H&E histology through healed scars treated with IL-6 (25ng) or control (PBS) at WD7. Scale bar = 100 μm. Quantification of healed epidermal thickness in healed scars after control or IL-6 addition.
B) Mean fold change in KRT1 and FLG mRNA after poly (I:C) (20μg/mL) addition to NHEK for 24 hours as determined by qRT-PCR and normalized to housekeeping gene, RPLP0.
C) Mean fold change in KRT1 mRNA with TLR3-specific or scrambled control siRNA in the presence of poly (I:C) (20μg/mL) in NHEK as determined by qRT-PCR and normalized as in 4B.
D) Mean fold change in KRT1 mRNA with TLR3-specific inhibitor or control in the presence of poly (I:C) (20μg/mL) in NHEK as determined by qRT-PCR and normalized as in 4B.
E) Mean fold change in KRT1 mRNA after IL-6 (50ng/mL) +/− cucurbitacin I in NHEK for 24 hours as determined by qRT-PCR and normalized as in 4B.
F) Mean fold change in Wnt pathway genes, Lgr5 and Lgr6, mRNA after 6 days of continuous exposure to poly (I:C) determined by qRT-PCR and normalized to housekeeping gene, RPLP0.
G) Mean fold change in KRT15 mRNA 72 hours after 24 hours of poly (I:C) (20μg/mL) treatment to NHEK as determined by qRT-PCR normalized to housekeeping gene, RPLP0.
H) Flow cytometry analysis of KRT15 protein expression of NHEKs treated as in 4G.
I) Flow cytometry analysis of CD200 protein expression of NHEKs treated as in 4G
*p < 0.05 by Student’s T-test or Single Factor ANOVA.
We next examined whether in response to TLR3 activation, keratinocytes adopt a less-differentiated state that is permissive for subsequent hair follicle differentiation. Following treatment with poly (I:C), keratinocytes expressed markers associated with hair progenitors including leucine-rich-repeat containing G proteins (LGR) 5 and 6 (Fig 4F) (Tadeu and Horsley, 2014). Induction of LGR6 is particularly notable, as previous lineage tracing experiments demonstrated that LGR6 expressing cells contribute to regenerating hair follicles during WIHN (Snippert et al., 2010). Further, we noticed induction of genes associated with hair follicle stem cells upon TLR3 activation. Hair follicle stem cells reside in the bulge region of the follicle and express keratin 15 (KRT15), which is considered the most reliable marker of this population (Liu et al., 2003) and CD200, which is associated with hair follicle progenitor cells (Garza et al., 2011). We found significantly increased expression of KRT15 mRNA in keratinocytes upon activation of TLR3 with poly (I:C) (Fig. 4G). Addition of poly (I:C) nearly doubled the percentage of KRT15 expressing cells and led to a 5-fold increase in number of cells expressing CD200 as assessed by FACS (Fig. 4H–I). Taken together, these data suggest that TLR3 pathway activation maintains keratinocytes in a less-differentiated state and induces the expression of genes associated with hair follicle progenitors.
Hair follicle morphogenetic pathways are induced by TLR3 signaling
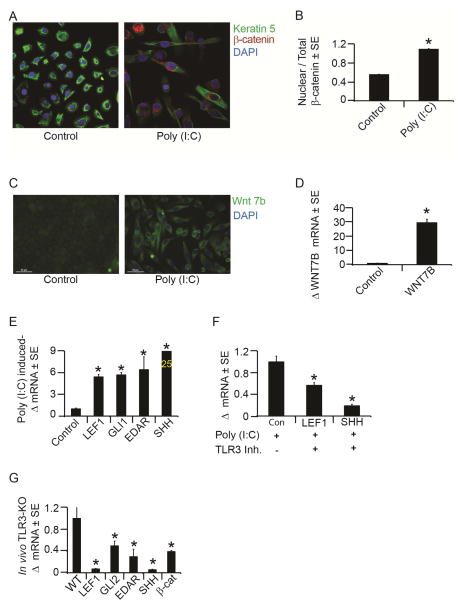
We investigated whether these TLR3 activated keratinocytes – with their increased expression of hair progenitor markers – would be poised for subsequent activation of the hair follicle morphogenetic program. Core to this program are the Shh and Wnt pathways, which are activated during both embryonic hair follicle formation and in regeneration following wounding (Ito et al., 2007). First, we examined β-catenin translocation to the nucleus, one of the earliest events in canonical Wnt signaling (Barker, 2008). TLR3 activation with poly (I:C) induced peri-nuclear accumulation and also doubled the amount of nuclear β-catenin in keratinocytes, consistent with activation of the Wnt pathway (Fig. 5A–B). In addition, expression of the Wnt ligand, Wnt 7b, and the downstream Wnt effector/target gene, LEF1, were up-regulated following poly (I:C) treatment of keratinocytes (Fig. 5C–E) (Barker and Clevers, 2010; Tadeu and Horsley, 2014). Similarly, expression of Shh pathway components SHH and GLI1 was increased following poly (I:C) addition, as was the expression of EDAR, another gene active in skin appendage formation (Fig. 5E). These pathways were stably induced for several days despite a transient 24 hour treatment of keratinocytes with poly (I:C), suggesting that TLR3 activation may prime keratinocytes toward a hair follicle or appendage fate. Importantly, the pathway activation we observed is TLR3-dependent as pretreatment of cells with a specific TLR3 small molecule antagonist markedly reduced the expression of both LEF1 and SHH (Fig. 5F). A similar dependence on TLR3 was observed in vivo where TLR3 KO mice had decreased expression of β-catenin, Lef1, Gli2, Shh and Edar following wounding when compared to strain-matched controls (Fig. 5G).
Figure 5. TLR3 activation induces hair follicle morphogenic program markers.
A) β-catenin immunofluorescence staining in NHEK after 72 hours of 24 hour treatment with poly (I:C) (20μg/mL) or control.
B) Quantitation of nuclear β-catenin to total levels of β-catenin in NHEK as in 5A.
C) WNT7b immunofluorescence staining (green) after 7 days of continuous poly (I:C)
(20mg/mL) or vehicle control treatment to NHEK. Scale bar = 50 μm; original magnification = 40X.
D) Mean fold change in Wnt7b mRNA after 6 days of continuous exposure to poly (I:C) (20mg/mL) determined by qRT-PCR and normalized to housekeeping gene, RPLP0.
E) Mean fold change in LEF1, GLI1, SHH and EDAR mRNA after poly (I:C) treatment by qRT-PCR as in 5A.
F) Mean fold change in LEF1 and SHH mRNA with TLR3-specific inhibitor or control in the presence of poly (I:C) in NHEK as determined by qRT-PCR as in 5A.
G) Mean fold change in Lef1, Edar, Gli2, Shh and β-catenin mRNA in TLR3 KO mouse wounds compared to strain-matched control mice as determined by qRT-PCR.
*p < 0.05 by Student’s T-test or Single Factor ANOVA.
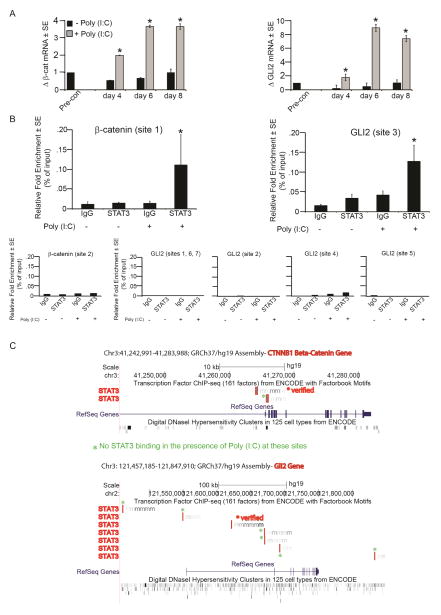
Finally, we explored a mechanism by which TLR3 pathway activation promotes induction of the hair morphogenetic program. Expression of β-catenin and GLI2 mRNA was significantly increased in keratinocytes upon poly (I:C) addition (Fig 6A). Since our data demonstrated that stimulation of TLR3 promotes STAT3 activation, we examined whether STAT3 binding sites are present in the promoters of these Wnt and Shh pathway genes. We identified several consensus binding sites. Next, using ChIP-qPCR, we demonstrated a significant increase in STAT3 occupancy at sites in both the β-catenin and GLI2 promoters upon poly (I:C) treatment in keratinocytes (Fig. 6B–C). This data suggests that TLR3 pathway activation during wounding may lead to direct transcriptional activation of genes involved in hair follicle morphogenesis.
Figure 6. TLR3 activation increases STAT3 occupancy of β-catenin and GLI2 promoters.
A) Mean fold change in β-catenin and GLI2 mRNA after continuous exposure to poly (I:C) at indicated time points as determined by qRT-PCR and normalized to housekeeping gene, RPLP0. Fold changes in mRNA expression relative to pre-confluent keratinocytes.
B) Relative fold enrichment of STAT3 occupation of β-catenin and GLI2 promoter sites after poly (I:C) treatment of keratinocytes. Negative binding sites also included below. Data representative of 5 independent experiments, p < 0.05.
C) Graphical representation of verified positive and negative STAT3 binding on β-catenin and GLI2 after poly (I:C) treatment of keratinocytes. Images obtained from ENCODE database.
*p < 0.05 by Student’s T-test or Single Factor ANOVA.
DISCUSSION
dsRNAs are damage-associated signals that promote regeneration
While a capacity for regeneration is observed in representatives of almost all animal phyla, its distribution is far from uniform, with some species demonstrating regeneration of multiple body parts while closely related species fail to do so (Brockes et al., 2001). Urodele salamanders, for example, are well known to regenerate their limbs, yet among 24 urodele species examined, four failed to reconstitute limbs after amputation (Brockes et al., 2001). Even within a single species, differences in genetic background can lead to marked differences in regenerative ability. We and others found that the capacity to regenerate skin and hair follicles, a process termed wound-induced hair neogenesis (WIHN), varies greatly among different strains of mus musculus (Fig. 1) (Ito et al., 2007; Nelson et al., 2013). While large variation between strains is challenging and requires very careful strain-matched and transgenic controls for all experiments, it is also an opportunity to explore the causes of this biological variation. We harnessed this variation and examined early time points following wounding to search for early, pivotal events that link tissue damage to regeneration.
For regeneration to occur, three interrelated events must take place: (1) organisms must sense loss of tissue integrity, (2) precursor cells must be mobilized to reconstitute missing structures, and (3) these cells must be directed along appropriate morphogenetic pathways (Brockes et al., 2001). While the latter two processes have been extensively examined in studies of regeneration, less is known about how organisms sense damage and transduce this information to trigger a regenerative response. In hydra, the peptide head activator (HA) is secreted at sites of tissue damage and is required for regeneration (Sanchez Alvarado, 2006). In salamanders and newts, an unidentified, thrombin-activated serum factor initiates regeneration of both the limb and lens (Brockes et al., 2001; Imokawa and Brockes, 2003). No such triggers had been discovered in the rare examples of mammalian epimorphic regeneration.
In the context of WIHN, we identified dsRNA released by damaged cells as early molecular signals triggering regeneration. Several lines of evidence support this: dsRNA responsive pathways are up-regulated in mice with a high capacity for regeneration, addition of exogenous dsRNA increases the number of regenerated follicles, and degradation of endogenous dsRNA inhibits regeneration (Fig. 1). In previous work, dsRNA was shown to be released upon UV-induced damage to keratinocytes (Bernard et al., 2012). Further, dsRNAs have been shown to accelerate re-epithelialization in small wounds of both mice and humans (Lin et al., 2012) suggesting that they play an important, early role in the response to cutaneous wounding. Also, TLR3 has been shown to increase pro-inflammatory cytokine accumulation after wounding in a manner counterbalanced by cutaneous microflora (Lai et al., 2009). While these studies demonstrate that the dsRNA-TLR3 pathway is active early in wound healing, but its consequences for events after wound closure have not been examined. Our results demonstrate that dsRNA initiates key events in the regeneration process following re-epithelialization.
A major receptor for dsRNA in mammalian cells is TLR3. While originally identified for its role in dorsal-ventral patterning in drosophila and its response to viral pathogens, recent evidence has emerged that TLR3 plays a role in cutaneous wound healing. TLR3-defeicient animals have a decreased inflammatory response to wounding (Lai et al., 2009; Lebre et al., 2007). TLR3 is activated by mRNAs released from dying cells, linking its activation to tissue damage (Kariko et al., 2004). We find that TLR3 is activated in response to cutaneous wounding in mice as TLR3 mRNA is strongly induced, an effect that can be augmented by administration of exogenous dsRNA. Downstream TLR3 pathway components including IL-6 are also strongly indcued by dsRNA. The activation of IL-6 is critical as it can rescue the defects in hair regeneration observed in TLR3 deficient animals. The early and strong induction of TLR3 and its pathway components upon wounding, coupled with the role of dsRNA in stimulating hair follicle neogenesis, suggest that TLR3 may relay information about tissue damage to activate regeneration. Of note, healed wounds of TLR3 KO mice also have significantly fewer γδ Tcells than wildtype mice (Supp. Fig. 5A). These cells have been demonstrated to augment WIHN (Gay et al., 2013), though we find WIHN does occur in mice lacking T- and B-cells suggesting that they are not absolutely necessary (Supp. Fig 5B). Intriguingly, downstream events induced by TLR3-– including possibly recruitment of γδ Tcells-– appear to differ between humans and mice (Lundberg et al., 2007). It will be interesting to examine whether differences in TLR3 responses account for the greater regeneration of skin wounds in mice compared to humans.
TLR3 activation increases markers of hair follicle progenitors
In response to damage, organisms must recruit precursor cells to rebuild lost structures. During both physiologic hair cycling and WIHN, KRT15 expressing stem cells of the bulge are mobilized and differentiate into multiple constituent cells of hair follicles. However, during WIHN cells of the interfollicular epidermis, including those expressing Lgr5 and Lgr6, also contribute to regenerated hair follicles(Ito et al., 2007; Snippert et al., 2010; Tadeu and Horsley, 2014). We find an increase in markers of both types of hair progenitor cells upon activation of the TLR3 pathway. In keratinocytes isolated form interfollicular skin, we observed induction of Lgr6 and KRT15 following TLR3 pathway activation with dsRNA.
These results are in accord with recent findings on the roles of TLR3 and the IL6/pSTAT3 axis in hair follicle and stem cell biology. For example, the induction of hair follicle progenitor markers by TLR3 we observe is consistent with the correlation of increased KRT15 positive hair follicle stem cells to keratinocyte pSTAT3 during aging (Doles et al., 2012); this and another report of drug inhibition of JAK activity promoting anagen might instead be through inhibition of hematopoietic cells(Xing et al., 2014). In further support for our findings, TLR3 has been implicated in the reprogramming of fibroblasts to IPS cells using virally-encoded reprogramming factors (Lee et al., 2012). Activation of TLR3 by dsRNA during wounding may similarly promote the conversion of keratinocytes destined to form stratified epidermis into cells with increased capacity for hair morphogenesis.
TLR3 activation initiates hair morphogenesis
The final event in regeneration is the reactivation of embryonic morphogenetic programs to direct mobilized stem cells to form missing structures. Hair follicle morphogenesis in the developing embryo proceeds through epithelial-mesenchymal crosstalk between the undifferentiated epithelium and the underlying dermis. Our data provide the first physiologic role for TLR3 in Wnt and Shh pathway activation during regeneration, likely through promoting this crosstalk. As with Wnt and Shh signaling, we find that EDAR pathway components are also activated in response to TLR3 signaling in vitro and in vivo. Activation of these appendage specification signals by dsRNA is TLR3 dependent since TLR3 chemical inhibition in vitro or TLR3 gene deletion in vivo blunt the Wnt and Shh pathway. Finally, the IL-6/STAT3 axis directly links TLR3 and these pathways since dsRNA increases occupancy of STAT3 at the promoters of β-Catenin and Gli2. Given the importance in hair development of in vivo epithelial-mesenchymal crosstalk to amplify EDAR, Wnt, and Shh signaling (Millar, 2002), it is notable that we can detect induction of these pathways with keratinocytes alone. We hypothesize these signals will be enhanced in the presence of competent fibroblasts. These findings for TLR3 initiating morphogenesis are consistent with the original description of Toll receptors as regulators of dorsal ventral patterning in drosophila (Anderson et al., 1985). Together with our findings, this suggests that Toll receptors have an equally important role in tissue specification in addition to their more well-known roles in innate immune activation.
In summary we identified the activation of TLR3 by damage induced dsRNA as the linchpin of the regenerative response to murine skin wounds. Strikingly, TLR3 plays a role in all three aspects of regeneration – damage sensing, precursor recruitment, and activation of hair follicle morphogenesis. As such, TLR3 agonists may be powerful therapeutics to decrease fibrosis and promote cutaneous regeneration.
EXPERIMENTAL PROCEDURES
Wound Induced Hair Neogenesis (WIHN)
All animal protocols are approved by the Johns Hopkins University Animal Care and Use Committee. C57BL/6J, B6;129SF2/J, TLR3 null mice (B6;129S1-Tlr3tm1Flv/J and B6N.129S1-Tlr3tm1Flv/J), Nod-Scid-Gamma (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ), IL-6Rαfl/fl (B6;SJL-Il6ratm1.1Drew/J) were obtained from The Jackson Laboratory. K5-Ert2-Cre and K14-Ert2-Cre mice were provided by Pierre Chambon. Stat3fl/fl mice were kindly provided by Cynthia Sears (Johns Hopkins University; Stat3tm2Aki(Takeda et al., 1998)) and mixed strain (C57BL/6J x FVB/N x SJL/J) animals were provided by Dr. Jean Richa (University of Pennsylvania).
A 1 cm2 excisional full-thickness wound to the level of skeletal muscle on the backs of 21-day old male and female mice was performed as previously described (Ito et al., 2007; Nelson et al., 2013). Numbers of regenerated hair follicles were quantified in the re-epithelialized skin by non-invasive confocal scanning laser microscopy (CSLM) as published (Fan et al., 2011). For all experiments, 50μL of “intervention” was injected into healing wound (under scab) or applied topically into open wound as shown in the Table 1.
Table 1.
Mouse strain, genotype, number, intervention and schedule used for WIHN trials.
| Experiment | Mouse Strain | # of Mice | Intervention | Day of Intervention | Day of CSLM Assessment |
|---|---|---|---|---|---|
| High vs Low Gene Expression-Early; High vs Low Gene Expression-Late | C57 vs. C57 x FVB x SJL C57 x FVB x SJL | 4 mice per strain; 3 mice/group | none | ----- | wound closure; ~WD20-24 |
| Standard WIHN vs Fringe Cuts | C57BL/6J | 14–15 mice/group | 10 cuts/side | WD0 | ~WD20-24 |
| Exogeneous Poly (I:C) addition | C57 x FVB x SJL; B6;129S1-Tlr3tm1Flv/J | 10–11 mice/group; 9 mice/group | 500ng Poly IC injected into wound | WD3 | ~WD20-24 |
| Rnase III addition | C57 x FVB x SJL | 17–19 mice/group | 15 units Rnase III injected into wound | WD3 | ~WD20-24 |
| WIHN in TLR3 KO | B6;129S1-Tlr3tm1Flv/J; B6;129SF2/J | 6 mice/group | none | ----- | ~WD20-24 |
| Exogeneous IL-6 addition | C57BL/6J B6N.129S1-Tlr3tm1Flv/J | 30 mice/group 8–10 mice/group | 25 ng rmIL-6 protein injected into wound 500ng rmIL-6 protein injected into wound | WD7 WD7 |
~WD20-24 |
| Importance of IL-6Rα | K14-ERT2-Cre x IL-6Ralphafl/fl (both C57BL/6) | 3–6 mice/group | I.P. tamoxifin every other day | WD5-WD14 | ~WD20-24 |
| Cucurbitacin I | C57 x FVB x SJL | 10–14 mice/group | 2mg/kg cucurbitacin I injected into wound | WD7 | ~WD20-24 |
| Importance of Stat3 in WIHN | K5-ERT2-Cre x Stat3 fl/fl (both C57BL/6) | 10–15 mice/group | I.P. tamoxifin every other day | WD0-WD14 | ~WD20-24 |
| Role of T- and B- cells | NOD.Cg- PrkdcscidIl2rgtm1Wjl (NOD/ShiLt) | 5 mice | none | ----- | ~WD20-24 |
Cell Culture
Neo-natal human epidermal keratinocytes (Lonza, Walkersville, MD) or lab-isolated foreskin keratinocytes were cultured in keratinocyte medium with added supplements (KGM-GOLD). Treatment with recombinant IL-6 protein (50 ng/mL), cucurbitacin I (10–100 nM), poly (I:C) (20 μg/mL) and TLR3 pharmacological inhibitor (80 μM; EMD Millipore, Billerica, MA) was applied in basal medium containing transferrin, hydrocortisone, and antibiotics for up to 24 hours. After 24 hours, treatment medium was replaced with KGM-GOLD and isolation of RNA as indicated. In some experiments, poly (I:C) was applied for up to a week, with replenishment of poly (I:C) and medium every other day.
Nucleofection
Nucleofection with siGENOME SMARTpool Human TLR3, REL-A, and siCONTROL siRNA duplex oligonucleotides (Dharmacon-ThermoFischer Scientific) was performed in NHEK using the Amaxa 4D-Nucleofector according to manufacturer’s instruction. Plated cells were treated with recombinant human IL-6 (50ng/mL) protein or poly(I:C) (20μg/mL) for 24 hours. Afterwards, treatment medium was removed and replaced with KGM-GOLD complete medium for the duration of the experiment. Levels of appropriate gene expression were assessed by qRT-PCR using inventoried TaqMan reagents in three independent experiments.
Gene Expression Analysis
RNA from immediately re-epithelialized skin at ~12 days after wounding (early stage) or after the earliest time point of hair follicle detection by CSLM (late stage; ~16 days) was submitted to the JHMI Deep Sequencing & Microarray core for Affymetrix® Mouse Exon 1.0ST microarray chips according to manufacturer’s protocols. Raw gene expression signals in the form of Affymetrix CEL files were extracted and normalized with Partek® Genomics Suite™ software using the Robust Multichip Analysis (RMA) algorithm (Irizarry et al., 2003). The Student’s t-test ANOVA was used to detect genes with significantly different expression. These analyses have been submitted to the Gene Expression Omnibus database (under GSE50418 and GSE50419; http://www.ncbi.nlm.nih.gov/geo/).
Quantitative real-time PCR (qRT-PCR)
Mouse skin was harvested prior to wounding and throughout wounding as described (Nelson et al., 2013). RNA was isolated from NHEK with RNeasy Mini Kit (Qiagen, Valencia, CA) with DNase I digestion followed by conversion to cDNA using the High Capacity RNA-to-cDNA kit (Life Technologies, MD). qRT-PCR was performed for genes of interest and 18S or ribosomal protein, large P0 (RPLP0) (housekeeping genes) using inventoried TaqMan reagents. Differences in gene expression were assessed by comparative ΔΔCT values with fold change calculations.
ELISA
IL-6 protein levels were assayed by ELISA (R&D Systems, Minneapolis, MN) from non-wounded and from wounded skin or healed mouse scars at times indicated. A minimum of three independent mice were used for each time point.
Immunohistochemistry, immunocytochemistry and histology
Immunohistochemistry was performed on formalin-fixed paraffin-embedded mouse skin samples using the avidin-biotin complex method and AEC development (Vector Laboratories). Indicated antibodies were applied overnight. Sections were counterstained with hematoxylin. Images were captured at 40X magnification using a Nikon Optiphot microscope and Nikon Elements F software. Histology was assessed by H&E after IL-6 addition. The epidermal thickness from the basal layer keratinocytes to beginning of stratum corneum in three locations per healed mouse wound in multiple histology sections was measured by Image J software.
Immunocytochemistry was performed on NHEKs plated on plated on collagen-coated coverslips and treated with 20 μg/ml poly (I:C) as above. Fixed cells were incubated with primary antibodies overnight, appropriate Alexa Fluor secondary antibodies and counterstained using VectaShield DAPI mounting medium (Vector Labs, Burlingame, CA). Slides were imaged at 60x magnification using the Nikon C1si True Spectral Imaging Confocal Laser Scanning Microscope system (Cell Imaging Core Facility, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins, Baltimore, MD). Cell morphology and beta-catenin nuclear localization were quantified using the CellProfiler image analysis software (www.cellprofiler.org) (Carpenter et al., 2006) from confocal images of nuclei.
Flow Cytometry
Keratinocytes and fibroblasts were fixed, permeabilized (BD Cytofix/Cytoperm kit), and stained with antibodies against human vimentin (BD Pharmingen clone RV202), KRT15 (Abam clone LHK15) labeled with a chromophore preconjugated to Fab (Zenon mouse IgG labeling kit) or human CD200 (BD Biosciences; 552475). Data was collected on a dual-laser flow cytometer (BD FACSCalibur) followed by FlowJo 10 (TreeStar) software analysis.
To measure TCRγδ expression, healed wounds (~WD20) from wild type and TLRKO3 mice were minced and digested at 37 C in a buffer containing RPMI 1640, 1.67 collagenase3 Wunsch units/mL Liberase TL (Roche Life Sciences, Indianapolis, IN) and .01% DNAse (Sigma-Aldrich, St Louis, MO) for 75 minutes. Following digestion, samples were washed and filtered (40μm) to obtain a single cell suspension. Cells were stained with propidium iodide (Miltenyi Biotec, San Diego, CA) and TCRγδ (GL3) antibody (Miltenyi Biotec, San Diego, CA) followed by analysis with MACSQuant cytometer and FlowJo software.
Chromatin Immunoprecipitation
Poly(I:C) treated and control keratinocytes were crosslinked in 1% formaldehyde for 10 minutes, followed by addition of glycine for 5 minutes to quench unreacted formaldehyde. Cells were processed with EZ-ChIP Kit (Millipore) according to the manufacturer’s instructions. Cross-linked protein-DNA complexes were captured with rabbit anti-Stat3 or normal rabbit IgG (sc-482X; sc-2027X, SCBT) antibodies. qRT-PCR was performed to determine the relative abundance of the promoter DNA sequence, associated with Stat3. Primers are detailed in Expanded Experimental Methods. Primers and graphics were designed based on ENCODE data (UCSC Genome Browser).
Statistical Analysis
Each experiment was repeated with at least 3 independent litters of animals or keratinocyte cultures. Data was analyzed using Student’s t-test or ANOVA Single Factor. Statistical significance was considered at *p < 0.05.
Supplementary Material
Highlights.
dsRNA released after skin injury triggers skin regeneration via TLR3
IL-6 and Stat3 signaling, downstream mediators of TLR3, are key to regeneration
Loss of TLR3, IL-6Ra or Stat3 reduces hair neogenesis after wounding
TLR3 induces core hair morphogenetic programs and hair follicle markers
Acknowledgments
The authors thank Conover Talbot Jr. (JHU Microarray Core) for assistance with microarray analysis; Lillian Dasko-Vincent, Cell Imaging Core Facility for assistance with immunocytochemistry analysis and Dr. Pierre Coulombe and his laboratory for critical discussions. The authors thank Pierre Chambon for use of the K14-Ert2-Cre and K5-Ert2-Cre mouse lines.
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number F32AR062932 to AMN and R01AR064297 to LAG. This work was also supported by the Department of Defense, Armed Forces Institute of Regenerative Medicine, Extremities Regeneration (AFIRM2-ER11), Northrop Grumman Electronic Systems and Alliance for Veterans Support, Inc. (Veteran/Amputee Skin Regeneration Program Initiative) as well as the Thomas Provost, MD Young Faculty Development Fund of Johns Hopkins Dermatology to LAG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42:791–798. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
- Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–1696. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, Martinez L, Greidinger EL, Yu BD, Gallo RL. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012 doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- Breedis C. Regeneration of hair follicles and sebaceous glands from the epithelium of scars in the rabbit. Cancer Res. 1954;14:575–579. [PubMed] [Google Scholar]
- Brockes JP, Kumar A, Velloso CP. Regeneration as an evolutionary variable. Journal of anatomy. 2001;199:3–11. doi: 10.1046/j.1469-7580.2001.19910003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome biology. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doles J, Storer M, Cozzuto L, Roma G, Keyes WM. Age-associated inflammation inhibits epidermal stem cell function. Genes Dev. 2012;26:2144–2153. doi: 10.1101/gad.192294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Luedtke MA, Prouty SM, Burrows M, Kollias N, Cotsarelis G. Characterization and quantification of wound-induced hair follicle neogenesis using in vivo confocal scanning laser microscopy. Skin Res Technol. 2011;17:387–397. doi: 10.1111/j.1600-0846.2011.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galun E, Rose-John S. The regenerative activity of interleukin-6. Methods Mol Biol. 2013;982:59–77. doi: 10.1007/978-1-62703-308-4_4. [DOI] [PubMed] [Google Scholar]
- Garza LA, Yang CC, Zhao T, Blatt HB, Lee M, He H, Stanton DC, Carrasco L, Spiegel JH, Tobias JW, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. The Journal of clinical investigation. 2011;121:613–622. doi: 10.1172/JCI44478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa Y, Brockes JP. Selective activation of thrombin is a critical determinant for vertebrate lens regeneration. Current biology: CB. 2003;13:877–881. doi: 10.1016/s0960-9822(03)00294-x. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Jia C. Advances in the regulation of liver regeneration. Expert Rev Gastroenterol Hepatol. 2011;5:105–121. doi: 10.1586/egh.10.87. [DOI] [PubMed] [Google Scholar]
- Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Karim R, Meyers C, Backendorf C, Ludigs K, Offringa R, van Ommen GJ, Melief CJ, van der Burg SH, Boer JM. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One. 2011;6:e17848. doi: 10.1371/journal.pone.0017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman AM, Strauss JS. The formation of vellus hair follicles from human adult epidermis. J Invest Dermatol. 1956;27:19–23. doi: 10.1038/jid.1956.71. [DOI] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu ZR, Hooper LV, Schmidt RR, von Aulock S, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, de Jong EC. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–558. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Wang L, Lin Y, Liu X, Ren X, Wen S, Du X, Lu T, Su SY, Yang X, et al. Toll-like receptor 3 ligand polyinosinic:polycytidylic acid promotes wound healing in human and murine skin. J Invest Dermatol. 2012;132:2085–2092. doi: 10.1038/jid.2012.120. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 Promoter Targets Putative Epithelial Stem Cells in the Hair Follicle Bulge. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- Lundberg AM, Drexler SK, Monaco C, Williams LM, Sacre SM, Feldmann M, Foxwell BM. Key differences in TLR3/poly I:C signaling and cytokine induction by human primary cells: a phenomenon absent from murine cell systems. Blood. 2007;110:3245–3252. doi: 10.1182/blood-2007-02-072934. [DOI] [PubMed] [Google Scholar]
- Melkamu T, Kita H, O’Grady SM. TLR3 activation evokes IL-6 secretion, autocrine regulation of Stat3 signaling and TLR2 expression in human bronchial epithelial cells. J Cell Commun Signal. 2013;7:109–118. doi: 10.1007/s12079-012-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. The Journal of investigative dermatology. 2013;133:31–41. doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AM, Loy DE, Lawson JA, Katseff AS, Fitzgerald GA, Garza LA. Prostaglandin D(2) Inhibits Wound-Induced Hair Follicle Neogenesis through the Receptor, Gpr44. The Journal of investigative dermatology. 2013;133:881–889. doi: 10.1038/jid.2012.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alvarado A. Planarian regeneration: its end is its beginning. Cell. 2006;124:241–245. doi: 10.1016/j.cell.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Current biology: CB. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Tadeu AM, Horsley V. Epithelial stem cells in adult skin. Current topics in developmental biology. 2014;107:109–131. doi: 10.1016/B978-0-12-416022-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- Torok MA, Gardiner DM, Izpisua-Belmonte JC, Bryant SV. Sonic hedgehog (shh) expression in developing and regenerating axolotl limbs. The Journal of experimental zoology. 1999;284:197–206. [PubMed] [Google Scholar]
- Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282:15319–15323. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, de Jong A, Harel S, DeStefano GM, Rothman L, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Grimm WA, Garner WL, Qin L, Travis T, Tan N, Han YP. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol. 2010;176:2247–2258. doi: 10.2353/ajpath.2010.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.