Abstract
Allergic reactions to drugs are a serious public health concern. In 2013, the National Institute of Allergy and Infectious Diseases, Division of Allergy, Immunology and Transplantation, sponsored a workshop on drug allergy. International experts in the field of drug allergy with backgrounds in allergy, immunology, infectious diseases, dermatology, clinical pharmacology and pharmacogenomics discussed the current state of drug allergy research. These experts were joined by representatives from several NIH Institutes and the U.S. Food and Drug Administration (FDA). The participants identified important advances that make new research directions feasible and made suggestions for research priorities and for development of infrastructure to advance our knowledge of the mechanisms, diagnosis, management, and prevention of drug allergy. The workshop summary and recommendations are presented herein.
INTRODUCTION
On March 19, 2013, the National Institute of Allergy and Infectious Diseases (NIAID) Division of Allergy, Immunology and Transplantation convened a workshop on drug allergy. The intent of the meeting was to summarize the current state of the science and to prioritize recommendations for future research on the mechanisms, prevention, diagnosis, and treatment of immunologically mediated adverse drug reactions. The panel (table 1) consisted of an international group of experts in the field of drug allergy with backgrounds in allergy, immunology, infectious diseases, dermatology, clinical pharmacology, and pharmacogenomics. The meeting was also attended by participants from the FDA and NIH representatives from NIAID, the National Cancer Institute, National Heart Lung and Blood Institute, the National Institute of Arthritis, Muscle and Skin and the National Institute of General Medical Sciences. This meeting addressed a recent Congressional Issue Brief stating concern “about the incidence of allergic reactions to drugs for debilitating and potentially fatal diseases including cancer, HIV/AIDS, cystic fibrosis and rheumatoid arthritis” and requesting an update regarding ways “to support research on desensitization of patients who have allergic reactions to potentially life-saving medications.”
TABLE 1.
DRUG ALLERGY WORKHSHOP: PARTICIPANT LIST
| NIAID-Sponsored Expert Panel |
| Aleena Banerji, MD |
| Assistant Professor |
| Assistant Training Program Director |
| Massachusetts General Hospital |
| Boston, MA |
| Mariana Castells, MD, PhD |
| Director, Drug Hypersensitivity and Desensitization Center |
| Director, Allergy Immunology Training Program |
| Associate Professor |
| Brigham and Women's Hospital |
| Boston, MA |
| Fred D. Finkelman, MD |
| McDonald Professor of Medicine |
| University of Cincinnati |
| Cincinnati, OH |
| Gerald J. Gleich, MD |
| Professor of Dermatology and Medicine |
| University of Utah |
| Department of Dermatology, 4A330 |
| Salt Lake City, UT |
| Emma Guttman-Yassky, MD, PhD |
| Associate Professor |
| Department of Dermatology |
| Immunology Institute |
| Icahn Medical School at Mount Sinai Medical Center |
| New York, NY |
| Simon A. K. Mallal, MB, BS, FRACP, FRCPA |
| Professor of Medicine, Pathology, Immunology and Microbiology |
| Major E. B. Stahlman Professor of Infectious Diseases |
| Vanderbilt University School of Medicine |
| Nashville, TN |
| Director,Institute for Immunology and Infectious Diseases |
| Murdoch, WA, Australia |
| Dean J. Naisbitt, PhD |
| The University of Liverpool |
| MRC Centre for Drug Safety Science, Department of Pharmacology |
| Liverpool, England L693GE |
| David A. Ostrov, PhD |
| Associate Professor |
| Department of Pathology, Immunology and Laboratory Medicine |
| University of Florida College of Medicine |
| Gainesville, FL |
| Elizabeth J. Phillips, MD, FRCPC, FRACP |
| Professor of Medicine and Pharmacology |
| Division of Infectious Diseases |
| John A. Oates Chair in Clinical Research |
| Director of Personalized Immunology |
| Oates Institute for Experimental Therapeutics |
| Vanderbilt University School of Medicine |
| Nashville TN |
| Professor/Director, Centre for Clinical |
| Pharmacology & Infectious Diseases |
| Institute for Immunology and Infectious Diseases |
| Murdoch, WA, Australia |
| Werner J. Pichler, MD |
| Emeritus Professor |
| ADR-AC GmbH |
| Bern, Switzerland |
| Thomas A.E. Platts-Mills, MD, PhD, FRS |
| Oscar Swineford Jr Professor of Medicine |
| Head, Asthma and Allergic Disease Center |
| University of Virginia |
| Charlottesville, VA |
| Jean-Claude Roujeau, MD |
| Emeritus Professor |
| Université Paris-Est Créteil |
| Antony, France |
| Charlottesville, VA |
| Lawrence B. Schwartz, MD, PhD |
| Professor |
| Virginia Commonwealth University |
| Richmond, VA |
| Lauren A. Trepanier, DVM, PhD |
| Professor and Director of Clinical Research |
| School of Veterinary Medicine |
| University of Wisconsin-Madison |
| Madison, WI |
| Additional Participants and Attendees |
| Mark I. Avigan, MD, CM |
| Associate Director, Office of Surveillance & Epidemiology |
| U.S. Food and Drug Administration |
| Silver Spring, |
| Wambui Chege, MD |
| Medical Officer |
| U.S. Food and Drug Administration |
| Rockville, MD |
| Ping Chen, PhD |
| Project Officer |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Badrul A. Chowdhury, MD, PhD |
| Director, Division of Pulmonary, Allergy, and Rheumatology Products |
| U.S. Food and Drug Administration |
| Silver Spring, MD |
| Ricardo R. Cibotti, PhD |
| Program Director |
| National Institute of Arthritis and Musculoskeletal and Skin Diseases |
| Bethesda, MD |
| Susan F. Cooper, MSc |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Wendy F. Davidson, PhD |
| Program Officer |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Gang Dong, MD, PhD |
| Program Officer |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Daniel Ein, MD |
| Director, Division of Allergy |
| George Washington University School of Medicine |
| Washington, DC |
| Michelle M. Freemer, MD |
| Medical Officer |
| National Heart, Lung, and Blood Institute |
| Bethesda, MD |
| Marga Gomez, MD |
| Medical Officer |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| James J. Kiley, PhD |
| Director, Division of Lung Diseases |
| National Heart, Lung, and Blood Institute |
| Bethesda, MD |
| Lynda Lanning, DVM, DABT |
| Health Scientist |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Susan Limb, MD |
| Clinical Team Leader |
| U.S. Food and Drug Administration |
| Silver Spring, MD |
| Health Scientist |
| Li Lu, PhD |
| Visiting Associate |
| U.S. Food and Drug Administration |
| Bethesda, MD |
| David H. Margulies, MD, PhD |
| Chief, Molecular Biology Section |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| James G. McNamara, MD |
| Chief, Autoimmunity and Mucosal Immunology Branch |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Michael Minnicozzi, PhD |
| Program Officer |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Jeff Moscow, MD |
| Senior Investigator |
| National Cancer Institute |
| Rockville, MD |
| Michael A. Norcross, MD |
| Senior Investigator |
| U.S. Food and Drug Administration |
| Bethesda, MD |
| Richard T. Okita, PhD |
| Program Director |
| National Institute of General Medical Sciences |
| Bethesda, MD |
| Joy Laurienzo Panza, RN, BSN |
| Nurse Consultant/Project Manager |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Marshall Plaut, MD |
| Chief, Food Allergy, Atopic Derm and Allergic Mech Section |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Julian Poyser, MPA, MS |
| Project Manager |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Michael Rogers, PhD |
| Director, Division of Pharmacology, Physiology, and Biological Chemistry |
| National Institute of General Medical Sciences |
| Bethesda, MD |
| Patricia Rohan |
| Medical Officer |
| U.S. Food and Drug Administration |
| Rockville, MD |
| Daniel Rotrosen, MD |
| Director, Division of Allergy, Immunology and Transplantation |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Sukhminder K. Sandhu, PhD, MPH, MS |
| Epidemiologist |
| U.S. Food and Drug Administration |
| Rockville, MD |
| Julie M. Schwaninger, MS |
| Health Specialist |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Susana Serrate-Sztein, MD |
| Division Director |
| National Institute of Arthritis and Musculoskeletal and Skin Diseases |
| Bethesda, MD |
| Jui R. Shah, PhD |
| Senior Regulatory Affairs Officer |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Jay Slater, MD |
| Director, Division of Bacterial, Parasitic and Allergenic Products |
| U.S. Food and Drug Administration |
| Rockville, MD |
| Alkis Togias, MD |
| Chief, Allergy, Asthma and Airway Biology Branch |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Ramesh Venna, PhD |
| Postdoc fellow |
| U.S. Food and Drug Administration |
| Bethesda, MD |
| Lisa M. Wheatley, MD, MPH |
| Medical Officer |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
| Hao Zhang, MD |
| Program Officer |
| National Institute of Allergy and Infectious Diseases |
| Bethesda, MD |
One issue of concern for the workshop was the term “drug allergy.” Many physicians and some investigators use the term “allergy” only in the context of IgE-mediated disease. However, it is clear that many drug reactions discussed at the workshop, such as hepatic necrosis and Stevens-Johnson syndrome, are immunologically mediated but not dependent upon IgE. Several expert panel members suggested that “drug hypersensitivity” could be a better term to use than “drug allergy.” While this is a valid suggestion, drug hypersensitivity has been suggested as the term to use for a reaction that might be immunologically mediated, and “drug allergy” has been recommended as the term to use for any adverse drug reaction that has a proven immunologic mechanism and it is this definition that is used in this document. (1) However, additional discussion will be required among research disciplines to harmonize terminology. For example, reactions to taxanes are termed “toxic” by oncologists and “pseudo-allergic” by allergists, whereas they are perhaps best termed simply as “immediate” until such time as they are more completely characterized. The expert panel was tasked with evaluating and developing research agendas for all immunologic reactions where the drug or drug metabolites drive an immune response, whether IgE-mediated or not.
The expert panel covered a variety of topics and made recommendations which are summarized in the sections that follow. It is the intention of the authors, in publishing this manuscript, to stimulate interest in this under-served area, because NIAID and other NIH Institutes are interested in advancing research in the field of drug allergy. This is a report of a single day workshop, and therefore does not provide a comprehensive review of the field of immunologically mediated drug reactions. Due to the limited duration of the workshop and the limited number of investigators who could be invited, it was not possible to do a comprehensive review of the field. The workshop participants prioritized discussions of topics they perceived to be most promising for supporting future research and development of critical infrastructure needed for such research.
EPIDEMIOLOGY AND PHENOTYPES
Epidemiology
Most of the epidemiologic data on adverse drug reactions (DRs) relies on clinical diagnosis with few specific diagnostic tests and physician-based assessment still remains the gold standard for phenotyping these reactions. This likely results in inaccurate characterization of drug reactions, as suggested by the low rates of positive skin and provocation tests in patients labelled as penicillin allergic. (2)While definitions and methods of ascertainment have varied, it is clear that the problem of adverse DRs affects a sizeable proportion of the population. A meta-analysis of 33 prospective studies from 1966-1996 found that 15.6 % of adult hospitalized patients either were hospitalized due to an adverse DR (4.7%), or experienced an adverse DR while hospitalized for another reason (10.9%). (3) The rates in pediatrics were somewhat lower with 2% of children being hospitalized for adverse drug reactions, and 9.5% experiencing adverse reactions during hospitalization. Of note, 39% of the adverse DRs that led to hospitalization were life threatening. (4) Drug allergy reactions represent a subset of adverse DRs.
Adverse DRs are categorized clinically as either type A or type B. Type A reactions are mediated by the known pharmacologic/toxic effects of the drug, and include overdose, side effects and drug interactions. They are often more directly related to dose and have been called “predictable.” Type B reactions are mediated by mechanisms other than pharmacologic toxicity of the drug, and may not be as reliably dose-dependent. In particular, IgE-mediated reactions occur at much lower doses than do pharmacologic effects, so type B reactions have been called “unpredictable.” Approximately 20% or less of adverse DRs are type B, and it is estimated that the majority of type B reactions have an immunological basis and hence would be allergic reactions by our definition.(5) Many of the features originally attributed to type B reactions, namely dose independence and unpredictability, are under question: some drug allergic reactions are predictable, as HLA typing can reliably identify risk groups for certain allergic reactions, and dose plays a role in at least some allergic reactions, as revealed during desensitization procedures, where small doses are tolerated. Therefore, “predictability” may reflect limitations in our current knowledge and does not necessarily relate to the mechanism of action. Only limited information is available to determine the mechanisms underlying many type B reactions, and there are diverse mechanisms in addition to immune-mediated events. For example, the initial acute respiratory reaction to aspirin and other non-selective non-steroidal anti-inflammatory agents (NSAIDs) is unpredictable with currently available clinical testing. Some of these aspirin/NSAID reactions are caused by the pharmacologic inhibition of cyclooxygenase-1 enzyme and so would not be termed allergic. However, there are also selective IgE mediated reactions to NSAIDs that can appear to have overlapping clinical phenotypes.
Some disease states are associated with an increased risk of developing drug allergy and this risk may vary across the disease course of an individual. For example, up to a third of patients in a cystic fibrosis clinic will develop an adverse reaction to antibiotics.(6) Also, the risk for drug reactions to sulfamethoxazole (SMX) is 10-fold higher in patients with HIV/AIDS than in the general population and this appears more likely when patients have uncontrolled HIV replication.(7) How much of the increase in risk in CF and HIV is due to higher exposure and how much to disease-related factors is unclear.(8)
Recent developments in the study of immediate-onset allergic drug reactions include at least two changes in the epidemiology that may have implications for diagnosis and management of these diseases. First, the frequency of IgE-mediated allergic reactions to the beta-lactam core of penicillins has decreased while the incidence of reactions to the side chains of beta-lactams has increased.(9–11) This may be because penicillin is used less frequently, is more highly purified than 50 years ago, is rarely given by intramuscular injection and is no longer in the milk supply, and also because of the increased use of later-generation β-lactam antibiotics. Second, there has been an increase in cases of IgE-mediated allergy to chemotherapeutic and biologic agents, likely due in part to the widespread use of biological agents and the greater longevity for persons with cystic fibrosis, immunodeficiency syndromes, cancer, and autoimmune diseases.(12) There may be genetic factors in immediate-allergy that need to be considered.(13)
It is important to ascertain both the accuracy of an initial diagnosis as well as the persistence of a diagnosis of allergy. About 10% of penicillin allergic patients will lose their skin test reactivity per year. (14)Therefore, within 10 years following their allergic reaction most will be penicillin tolerant. Similarly, although 8% of patients in the general population are labeled as penicillin allergic, less than 5% of the number tested will have positive results on skin testing and oral challenge.(2) Oral challenge after a negative skin test is an important component of testing. In a recent study, the majority of immediate reactors were diagnosed after a drug provocation test.(15) Testing and oral provocation may be particularly important in patients with CF, where drug allergy is thought to be common. Recent publications reporting on the results of comprehensive evaluations for beta-lactam sensitivity have documented allergy in less than 3% of children with CF.(16, 17) This means that skin testing and oral challenge are both important tools to identify individuals with a true IgE allergic phenotype as the majority are tolerant and could be treated with a penicillin. Penicillin skin testing followed by oral challenge if the skin tests are negative is safe, but not all reagents necessary to evaluate beta-lactam allergy are available.(18) Avoidance of penicillins due to an allergy label is associated with increased costs of care as well as expanded use of broad-spectrum antibiotics with attendant patient and societal risks.(19) However, it is also worth noting that patients with allergic reactions to carboplatin may rapidly lose their skin test reactivity without exhibiting tolerance to the infused drug, so a modified desensitization protocol with repeat testing after the next two infusions is advised in this situation.(20)
There are no systematic epidemiologic studies of drug allergy. ICD-9 and ICD-10 codes are neither specific nor sensitive for identifying patients with specific drug allergy phenotypes. Registries exist on specific reaction subgroups such as the severe cutaneous adverse reactions recorded by the RegiSCAR project in Europe.(21–24) In addition, consensus is lacking with regard to the definition of drug allergy phenotypes, although some steps to improve definitions have been taken in Europe with the development of standardized questionnaires and algorithms. Drug allergy specific causality scores have also been developed to help ascertain the likelihood that a specific drug is implicated in a specific immunologically mediated drug reaction.(25)
Phenotyping
Phenotyping, which may include specific testing, can substantially improve the quality of epidemiologic analysis. For example, symptoms associated with abacavir hypersensitivity cannot be easily distinguished from non-specific symptoms. It was only after the phenotyping was refined by including both clinical symptoms and a positive patch test to abacavir as co-primary endpoints in a large randomized double-blinded clinical trial, that avoiding treatment in HLA- B*57:01 positive individuals resulted in virtual elimination of abacavir hypersensitivity.(26) Phenotyping will require development of consensus criteria for each clinical syndrome, and validated methodology to measure each criterion.
Careful clinical phenotyping of individuals that includes an appropriate drug causality assessment is required not only for epidemiologic studies, but also for mechanistic studies that can define the relationship of immunologic responses to the clinical manifestations in affected patients. In turn, mechanistic studies can further refine clinical phenotypes or allow, via an iterative process, classification into endotypes. International collaboration is of essence. Phenotyping projects in the US could spring from and expand the work that has already taken place within the European Network for Drug Allergy, the European Academy of Allergy and Clinical Immunology and the RegiSCAR network.(27) The development of specific phenotype algorithms for drug allergy syndromes that could be applied along with a drug causality algorithm within the electronic health record would be helpful for large epidemiologic studies.
Workshop Recommendations
Epidemiology and phenotyping of allergic drug reactions
Harmonize terminology to improve communication, including communication between medical disciplines.
Develop a new classification of adverse DR based on current knowledge.
Develop consensus criteria with validated and standardized methodology to phenotype drug allergic reactions.
Ascribe causality to a specific drug that can be applied to prospectively defined cohorts, clinical trials and large datasets within an electronic health record.
Undertake systematic, population-based, epidemiologic surveys of drug allergy after phenotypes are established.
CLINICAL MANIFESTATIONS AND MECHANISMS: IMMEDIATE-ONSET DRUG ALLERGY
IgE-mediated allergic drug reactions manifest themselves within minutes to hours of exposure to the allergen by a set of symptoms ranging from urticaria to anaphylaxis. A recent change in the clinical paradigm is that two distinct presentations of IgE-mediated drug allergy reactions have emerged: “classical” reactions that occur after multiple doses of the drug and reactions that occur with exposure to the first dose. IgE-mediated reactions to penicillin or carboplatin, for example, present in the “classical” fashion, whereas allergy to the monoclonal antibody (MAb) cetuximab manifests on the first dose. This is because some patients have pre-existing IgE antibodies to a carbohydrate determinant, galactose-alpha-1,3-galactose, present on the humanized antibody. Bites by certain ticks induce an IgE antibody response to galactose-alpha-1,3-galactose and individuals who have been sensitized through tick bites react to cetuximab.(28) Prior to the clarification of the mechanism of cetuximab allergy, IgE antibodies to carbohydrates were not considered important in drug allergy, and were associated with non-pathogenic cross-reactivity between allergens.(29) Cetuximab also represents an example of a first dose reaction where exposure to an antigen other than a related drug has led to pre-existing IgE against the drug in question. This mechanism of drug allergy has yet to be assigned to other agents, but it raises a paradigm that is worth considering in the investigation of allergic drug reactions that occur in the absence of prior dosing. For example, reactions to taxanes typically occur at first exposure in a population of cancer patients sensitized to other allergens, raising the question of sensitization through shared epitopes.(30)
Our understanding of immediate onset drug allergy is limited because, in many cases, the underlying mechanisms are not known. For example, patients with reactions to platinum salts or to some MAbs have positive skin tests to the specific agent, suggestive of IgE-mediated reactions, while for reactions to taxanes and other MAbs, the involvement of IgE has not been demonstrated, and in many cases, such as with rituximab, the reaction occurs with the first infusion.(31, 32) We now know that reactions to the first infusion may be IgE-mediated, such as reactions to cetuximab, but it is also clear that IgE is not required, as in the majority of cases of reactions to radiocontrast media, vancomycin or opioids which are commonly associated with non-IgE mediated histamine release.(33, 34) A recent study showed that specific drugs, such as flouroquinolones and neuromuscular blocking agents, can activate mast cells through interaction with a specific G-coupled protein receptor. This is a new pathway which may potentially account for many non-IgE-mediated reactions.(35)
Understanding the role of IgE in immediate onset drug allergy is further complicated by the lack of appropriate reagents and the lack of reliable tests to detect drug-specific IgE antibodies. The timing of the reactions and elevated serum tryptase, in some cases, are consistent with mast cell activation; however, in the absence of tryptase elevation, basophils may be implicated. These cells secrete comparable levels of vasoactive histamine and LTC4, associated with anaphylaxis, but are distinguished from mast cells by not producing vasoactive PGD2 and containing much less tryptase. Activation of mast cells or basophils could be by either IgE or non-IgE mediated pathways. Even when circulating levels of IgE are below the detectable range, it is possible that sufficient IgE might be bound to mast cells or basophils and leads to their activation upon encounter with the drug.
Workshop Recommendations
Research Directions on Immediate-Onset Drug Allergy
Develop and validate multivalent skin test reagents or other in vitro tests for drugs where an IgE mechanism is likely but skin testing is either not currently available or has not been validated
Increase the sensitivity and specificity of currently available tests. Determine the predictive value of existing tests for IgE-mediated reactions to drugs including chemotherapeutic agents, monoclonal antibodies, neuromuscular blockade agents and antibiotics.
Define the mechanisms of immediate-onset reactions where specific IgE is not detectable including whether these reactions are mast cell or basophil mediated.
CLINICAL MANIFESTATIONS AND MECHANISMS: DELAYED-ONSET DRUG ALLERGY
In delayed-onset drug allergy, the reaction usually occurs days to weeks after exposure to the allergen. These reactions have heterogeneous clinical manifestations including cutaneous reactions, which range from a mild exanthem to a severe life-threatening disease such as toxic epidermal necrolysis, and other syndromes including hepatic damage or a lupus-like presentation. The majority of delayed-onset adverse drug reactions include cutaneous manifestations, and the majority of these reactions are uncomplicated exanthema. However, there are rare but severe drug reactions with cutaneous manifestations including Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS or DIHS for Drug Induced Hypersensitivity Syndrome) and acute generalized exanthematous pustulosis (AGEP).(5)
Among the most severe cutaneous allergic drug reactions are Stevens Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN). SJS and TEN are rare conditions (1–2 cases per 1 million population per year); they involve the same pathologic process with SJS representing the mildest cases (epidermal detachment of 1– 10%) and TEN the severe cases (epidermal detachment over 30% of body surface area) with an overlap syndrome between these extremes. SJS/TEN is drug-induced in 70% of cases and this risk of being drug-induced is even higher in adults with the severe spectrum of disease. The risk of SJS/TEN is higher in persons with HIV, cancer, graft versus host disease, or systemic lupus erythematosus than in the general population. Mortality rates for SJS/TEN average around 30%.(36) Of survivors, 50–90% will have persistent sequelae, frequently involving significant scarring of the ocular surface and eyelids.(37) There are no treatments of proven benefit, but literature reviews have suggested that mortality is reduced by early admission to a specialized unit with aggressive supportive care.(38) There has been progress in understanding the pathophysiology of SJS/TEN. Skin sloughing occurs due to massive keratinocyte death with roles for Fas mediated cell death, and for release of cytotoxic peptides such as granulysin, and annexin A1.(39–41)
As with SJS/TEN, the mechanisms for most delayed-onset reactions are only partially understood. This is due, at least in part, to the absence of model systems. Contact hypersensitivity is a T cell-mediated reaction that shares characteristics with some drug allergies, including the requirement for hapten formation to induce an immune response, a dose-response effect of hapten exposure, and an HLA association for some allergens. Patch testing has proven useful in some drug allergic reactions, such as those induced by abacavir in HIV infection or carbamazepine in DRESS(26, 42), but the broad utility of contact hypersensitivity as a model of delayed-onset drug allergy is unclear. Current animal models do not adequately reflect human drug allergy.
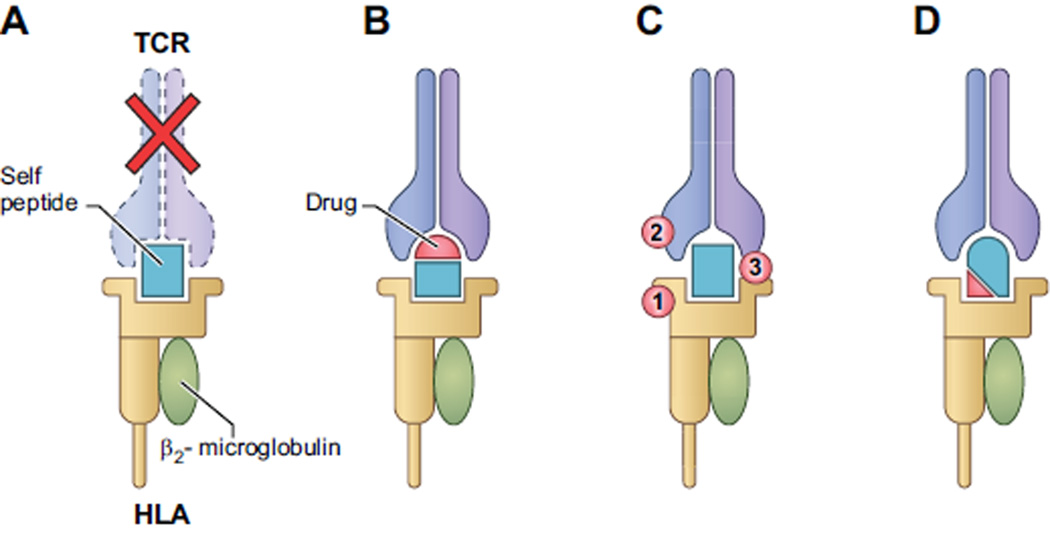
Cell-mediated immune reactions and activation of T cells are proposed to occur by three mechanisms that are not mutually exclusive (see Figure 1).(43) 1) The “hapten/ prohapten hypothesis” posits that reactive metabolites of the administered drug bind covalently to proteins to form a complete antigen, which in turn is processed by antigen presenting cells and bound to the major histocompatibility complex (MHC), which are the human leukocyte antigens (HLA). Data in contact dermatitis models imply that activation of the innate immune system, such as activation of dendritic cells, is a requirement.(44) 2) The “pharmacological interaction with immune receptor” model (p-i) states that the administered drug binds directly to the immune receptor, either the T cell receptor or MHC. Binding to either the TCR (p-i TCR) or MHC (p-i HLA) results in T cell activation, providing there is TCR interaction with peptide-MHC complex. 3) The “altered-peptide repertoire hypothesis” states that low molecular weight drugs bind non-covalently to regions of the HLA class I molecules within the antigen binding cleft. This binding alters the shape of the antigen binding cleft and, as a result, alters the repertoire of peptides that are presented, including self-peptides.(45–47) The individual is not necessarily tolerant to the new repertoire of peptides presented in the context of altered HLA molecules initiating a T cell response.
Figure 1. Mechanisms that mediate HLA or TCR associated drug hypersensitivity.
A. Under normal circumstances, HLA bound self-peptides that bind with high affinity to a T cell-receptor result in the deletion of T cells bearing the auto-reactive receptor.
B. In the Pro-hapten/ Hapten hypothesis, a reactive drug metabolite (a hapten) is generated and binds covalently to a self-peptide, generating a new composite antigen, which can bind to HLA and be presented to TCR and active T-cells. This presentation is processing dependent and requires activation of the innate immune system.
C. The Pharmacological Interaction hypothesis posits that a drug (shown in red) binds noncovalently either to 1) HLA, 2) TCR, or, rarely, 3) both simultaneously, facilitating interaction and resulting in T cell activation. This interaction with HLA or TCR is rapid and independent of metabolism or processing. Binding of the drug results in a change in the HLA-peptide-TCR complex and does not require any change in the peptide that is bound, differentiating this from both the hapten (B) and the altered repertoire (D) models.
D. In the Altered Peptide Repertoire hypothesis, a drug binds non-covalently to the peptide-binding groove of the HLA molecule, altering the repertoire of self-peptides that bind in the groove. Since the combination of HLA and self-peptide are new, the circulating T cells are not tolerant to the neo-antigen, thus stimulating T cell activation. This pathway is processing dependent.
(Note: In the figure, only class I HLA(brown) with beta2-microglobulin (gray) is depicted, but class II molecules are involved in some cases.)
Evidence for the Prohapten/ Hapten and P-I Hypotheses in Delayed-onset Drug Allergy
In vitro studies on the antigenicity of antibiotics have demonstrated the potential relevance of the first two categories in drug allergy, the hapten and the p-i model. For example, in patients who are allergic to piperacillin, the drug acts as a hapten to form immunogenic piperacillin-albumin conjugates which stimulate drug reactive T cells.(48, 49) In allergy to sulfamethoxazole (SMX), both the hapten and the p-i hypotheses are potentially relevant. In sulfonamide-allergic patients, lymphocyte proliferation can be induced in peripheral blood mononuclear cells (PBMCs) by the metabolite nitroso-SMX, which binds covalently to non-HLA proteins (a hapten) and/ or by the parent drug or its metabolites binding directly and reversibly to HLA.(50) Studies of sulfamethoxazole have also demonstrated that sulfamethoxazole may interact directly with the second complementary determining region of Vβ20-1 TCR and thereby alter the binding affinity of the TCR to the peptide-MHC complex.(51)
Investigations of other MHC related antibiotic drug reactions have also suggested that both the hapten and p-i hypotheses may play roles. In investigating flucloxacillin-induced liver injury, one group found that flucloxacillin was presented via the hapten mechanism with multiple HLA types, but was also presented in a non-proteasome dependent and labile manner restricted to HLA-B* 57:01 resulting in immediate CD8+ T cell activation, suggesting that the p-i mechanism is involved in this HLA-B* 57:01 associated drug reaction resulting in liver damage.(52, 53) However, another study found that activation of CD8+ T cells was restricted by HLA-B*57:01 and the closely related HLA-B*58:01 and that activation was processing dependent and correlated with flucloxacillin binding to albumin.(54) In studies of reactions to nevaripine, HLA I associations were found for severe skin manifestations in multiple populations, but HLA II was associated with hepatic events in Caucasians suggesting that different mechanisms of presentation may explain why some drugs induce a variety of different immunologically mediated adverse reactions. (55)
Evidence for the Altered Peptide hypothesis in Delayed-Onset Drug Allergy
Studies of the mechanisms underlying other HLA-restricted drug reactions have generated the altered peptide repertoire hypothesis. There are multiple examples where susceptibility for an allergic reaction to specific drugs is dramatically increased if the patient expresses a particular HLA type.(56) The most striking example is the anti-retroviral drug, abacavir, which binds to a specific pocket of the HLA-B* 57:01 molecule, thereby changing the ability of the pocket to bind large aromatic residues and, consequently, alters the preference for peptides that bind to the HLA molecule. The individual may not be tolerant to some of these peptides, because they may not have been previously presented in the context of this MHC molecule in the thymus. Self-peptides that bind with HLA-B*57:01 with higher affinities to MHC molecules in the presence of abacavir have been demonstrated in HLA B*-57:01 positive individuals and a set of the peptide-HLA complexes were recognized by T cells of abacavir-allergic patients. (45–47)
Evidence for the roles of viral infection and TCR restriction in Delayed-onset Drug Allergy
It is noteworthy that only 55% of HLA-B*57:01 individuals exposed to abacavir become allergic. Abacavir naïve patients expressing HLA-B*57:01 have CD8+ T cell responses to abacavir in vitro, and preliminary data suggests the potential role of a cross-reactive memory T cell response to a persistent pathogens that have co-evolved with humans such as those within the Human Herpes Virus family.(57, 58) In allergic individuals, activation of the immune system may have occurred prior to drug exposure resulting in expansion of activated T cells. These activated T cells also react to the newly presented self-peptides in HLA-B*57:01. This suggests that allergy may require both T cell recognition due to altered peptide presentation and pre-existing T cell expansion. Alternatively, the abacavir altered MHC-peptide complex takes on attributes of HLA-B*58:01 and stimulates an alloimmune-like reaction. Five percent of abacavir –induced T cells react with HLA*B58:01.(59)
Another paradigm of T cell-mediated drug allergy derives from observations in HLA-B*15:02 positive Han Chinese patients, 3–7.7% of whom develop SJS/TEN reactions to carbamazepine. (56) In 84% of the allergic patients, stimulation with carbamazepine results in an expansion of CD8+ T cells with a restricted pattern of T cell receptor V beta usage (VB-11-ISGY).(60) This V-beta usage is absent in non-allergic controls. Carbamazepine-specific cytotoxicity could be primed in vitro in the PBMCs of healthy subjects with no history of carbamazepine exposure who carried both HLA B*15:02 and the restricted V beta clonotype. Furthermore, cellular cytotoxicity could be blocked by an antibody to the V-beta clonotype. Thus, HLA-associated drug allergy may require not only a specific HLA type, but also the expression of specific V beta or other TCR genes. It is also possible that there is shared TCR V-beta usage between individuals with a common phenotype of a specific drug allergy but who do not share the same HLA Class I or II risk alleles.
As noted earlier, immune responses to viruses may predispose to HLA-restricted drug allergy. Additional data suggest that viruses may also play a role in non-HLA restricted drug allergy. For example, EBV infection is the prototypical co-stimulator of the exanthema seen with aminopenicillins. Recently, evidence of reactivation of EBV or human herpes viruses 6 or 7 was found in 76% of patients with DRESS. Expanded populations of CD8+ T cells isolated from blood, liver, skin and lungs of DRESS patients shared complementarity determining region 3 sequences on the beta chain of the TCR that were homologous to EBV-specific CD8 T cell receptors. The authors proposed that the drugs allow re-activation of dormant viruses and it is the virus-driven selection of CD8+ T cells that is responsible for the organ damage.(61) However it might be that a cross-reactive memory T cell response is present in the absence of reactivation and that this is sufficient to cause organ damage.
Even for a set of drugs acting through similar pathways, the clinical manifestations of drug allergic reactions may vary. For example, abacavir, carbamazepine, and allopurinol all produce effects via T lymphocytes interacting with a combination of the drug and specific HLA molecules. However, carbamazepine and allopurinol may induce SJS/TEN, while abacavir causes a unique clinical syndrome not including SJS/TEN. In addition, the variability in the timing of the onset of drug allergic reactions, between 2 days and 3 weeks after initiation of the drug, is not accounted for by MHC binding. Other genetic factors, such as those relating to metabolism that may delay clearance or elevate levels of metabolites, are important and may determine whether reactions occur in persons carrying HLA risk alleles.(62, 63)
Workshop Recommendations
Research Directions on Delayed-Onset Drug Allergy
Establish the major types of HLA-restricted allergies to drugs and standardize and validate the methods through which these drug reactions can be identified.
Identify reaction mechanisms for delayed-onset allergy to drugs that do not have HLA-associations. or where delayed-onset allergy is associated with more than one HLA allele
Explain the mechanisms through which only a small proportion of individuals with an HLA-risk allele develop a specific delayed-onset allergy
Determine the value of contact allergy as a model system for delayed-onset drug allergy.
Develop in vitro cellular platforms to investigate the mechanisms of drug allergy utilizing state-of-the-art immunologic methods.
Develop animal models, including humanized mice and non-human primates.
Standardize and then validate utility of patch testing and prick/intradermal skin testing with delayed readings in different T-cell mediated drug reactions.
PREVENTION OF DRUG ALLERGIC REACTIONS IN SENSITIZED PATIENTS
For IgE-mediated drug allergy, rapid desensitization is a proven approach that successfully allows the administration of a drug to a patient who has a history of allergy to that drug or a cross-reactive drug.(31, 32, 64–67) The treatment involves administering a low dose of drug, with gradual dose escalation every 15– 30 minutes, until the therapeutic dose is reached. The drug is then administered at regular intervals for the recommended duration of treatment, but, if there is a gap in treatment, a desensitization protocol must be repeated prior to resuming therapeutic dosing. Desensitization reduces sensitivity only to the antigen used in the protocol and is not suppressive of reactions to unrelated antigens, as demonstrated by immediate skin tests(68) and in vitro basophil activation tests.(69) Effective desensitization does not lead to an increase of the mast cell-specific molecule, serum tryptase.
It had previously been thought that desensitization would be effective only for IgE-mediated reactions. However, multi-step desensitization protocols for immediate reactions have been shown to be effective in both IgE-mediated reactions (e.g. various antibiotics, platinum salts, MAbs and drug reactions where specific IgE involvement has not been demonstrated (e.g. taxanes).(70) There may be a role for desensitization in non-immediate reactions such as the accelerated reactions to antibiotics commonly seen in patients with cystic fibrosis and in patients with delayed reactions not accompanied by severe skin or mucosal involvement, fever or internal organ damage.(32, 71)
Although drug desensitization has a risk of inducing an allergic reaction, it is the only currently available approach that appears to provide clinical benefit. Castells et al have data suggesting that repeated desensitization in the same patient during a course of chemotherapy may result in fewer allergic reactions to subsequent desensitizations compared to the first desensitization. (70)
Workshop Recommendations
Improving Drug Desensitization
Conduct multi-center studies to develop optimal desensitization protocols.
Develop risk mitigation strategies for patients requiring frequent desensitization (i.e., carboplatin in ovarian cancer patients)
Evaluate the use of omalizumab or other pretreatment agents for improving the safety and efficacy of desensitization in the setting of repeated and predictable requirement for treatments (such as chemotherapy).
Determine the precise mechanisms by which desensitization works in both IgE-mediated and non-IgE-mediated immediate-onset reactions.
Determine whether desensitization or slow reintroduction drug protocols are effective for preventing delayed-onset, skin limited reactions, not including SJS/TEN, such as those to SMX and allopurinol.
PREVENTIVE SCREENING FOR DRUG ALLERGIC REACTIONS
After a mechanism for a drug allergy reaction is proposed and there is experimental evidence supporting plausibility, the next goal is to develop a valid, rapid, inexpensive and easily interpretable laboratory test. Potential laboratory tests must be evaluated and validated in an accurately phenotyped population. For primary prevention of hypersensitivity in drug naïve patients, such programs should be undertaken only if the following pre-requisites for translational success are met. 1) The medications in question have an otherwise acceptable safety profile, 2) alternative drugs are unavailable, more costly or have inferior efficacy and/or worse safety profiles, 3) the reactions are sufficiently severe to warrant prevention, 4) the frequency of the reactions is relatively high, and 5) the test has a high negative predictive value and a favorable positive predictive value so that the number needed to test in order to prevent a treatment limiting allergic reaction is feasible.(72)
There have been successful attempts to transform mechanistic studies into valuable clinical tests. (72) For example, if a particular HLA type is associated with a high risk of a specific drug reaction, HLA screening to exclude drug treatment of susceptible individuals is clinically appropriate. However, the value of currently available HLA screening tests varies, and usually applies only to a specific ancestral group. Prevention by screening individuals for particular HLA types and drug avoidance for those at risk is the guideline based approach currently in widespread routine clinical practice for abacavir. Selective screening for HLA-B*15:02 has been recommended for high-risk populations such as Southeast Asians prior to prescribing carbamazepine. Single allele HLA-B*58:01 typing also exists for allopurinol and cost-effectiveness analyses have also proposed its use in clinical practice.(73)
In the case of abacavir, excluding HLA-B*57:01 positive individuals eliminates specific abacavir hypersensitivity reactions. However, excluding these individuals will not prevent all types of suspected abacavir reactions, only the systemic hypersensitivity syndrome, which is characterized by fever, malaise, gastrointestinal, respiratory symptoms and skin rash occurring within the first 3 weeks of first dosing and which has been associated with a positive abacavir patch test.(26) In a study of clinically diagnosed abacavir hypersensitivity reactions, 33.7% were confirmed by patch test as immunologic reactions (all of whom were HLA B*57:01 positive). In addition, the positive predictive value of HLA B*57:01 is only 55%, so that the screening test results in restricting the drug from all HLA-B*57:01 patients, half of whom might have benefitted from abacavir use, making the case for developing a screening test with higher specificity. In addition, abacavir testing is cost-effective in Caucasians where HLA B*57:01 is relatively common, but potentially not in African populations where population admixture is uncommon and the carriage rate of HLA-B*57:01 is <1%.(74)
HLA screening is recommended for people of Asian descent prior to administration of carbamazepine. HLA B*15:02 is a common allele among some Asian groups. Over 90% of Chinese people are Han Chinese, and the frequency of HLA B*15:02 in this ethnic group is 10– 11%. The same allele has a frequency of 5% among people of Asian descent in North America. The positive predictive value of the presence of HLA B*15:02 in predicting SJS/TEN when using carbamazepine is in the range of 3 to 7.7%. Avoiding the use of carbamazepine in HLA-B*15:02 positive individuals prevents SJS/TEN. However, this screening excludes over 90% of HLA-B*15:02 patients who would have tolerated and benefited from carbamazepine. HLA screening to prevent reactions to carbamazepine is useful only in Asians because HLA-B*15:02 is relatively common in this group. In addition, screening for HLA-B*15:02 is specific only for SJS/TEN and not other drug reactions to carbamazepine, such as DRESS. An even more extreme example is flucloxacillin-associated liver injury, where 85% of affected patients carry HLA-B*57:01 but only 1 in 500–1,000 carriers of HLA-B*57:01 will develop disease when treated with flucloxacillin. (53)
Screening for antibodies to galactose-alpha-1,3-galactose may also be of benefit prior to administration of cetuximab, in order to prevent the risk of anaphylaxis. However, testing is probably indicated only for patients who have lived in or visited geographic regions where those species of ticks associated with production of IgE antibodies to galactose-alpha-1,3-galactose are found, such as the southeastern United States and parts of Europe and Australia.(75–77)
Workshop Recommendations
Improve Screening Programs to Prevent Drug Allergy
Focus on mechanisms of drug allergies with emphasis on translational program that will facilitate the development of additional screening tests to prevent drug allergic reactions.
Develop approaches to both explain and increase the positive predictive value of screening tests for HLA risk alleles in drug allergic reactions with HLA involvement.
STRATEGIES FOR ESTABLISHING INFRASTRUCTURE AND EXPANDING RESEARCH IN THE FIELD
While immunologically mediated drug reactions are common, the wide variation of clinical manifestations, immunologic mechanisms, and specific drugs means that for the severest of reactions there are few patients with the same reaction to the same drug in the same clinical context in any one institution. Therefore, multi-center clinical networks need to be established to support studies adequately powered to draw conclusions about the pathophysiology of drug allergy.
The workshop participants suggested several strategies that could be adopted to advance drug allergy research. To achieve the necessary progress, it is important for five groups interested in improving the treatment and prevention of drug allergy to work together: (1) investigators from the basic and clinical research community, (2) the NIH and other research funding agencies, (3) the FDA and other regulatory agencies, (4) the pharmaceutical industry, and (5) patient advocacy groups. An important goal of any such program should include initial and ongoing education of medical students, clinicians, researchers, regulators, consumers and government funders in drug allergy to ensure the investment and engagement necessary to foster growth of research programs and adoption by clinicians and other health professionals. Advances in this field will depend on the cooperative formulation of a coherent and feasible plan, and have the potential for a very positive impact on the quality of life for affected patients. The pharmaceutical industry and patient advocacy groups should be engaged in future initiatives. It is hoped that there will be interest by industry in allowing investigators to have access to adverse drug reaction data (notably from post-marketing studies) and by regulatory agencies in facilitating the transfer of information, which would greatly enhance the likelihood of making advances in drug allergy research.
Workshop Recommendations
Establish Infrastructure and Foster Future Research
- Establish a US cooperative group including multidisciplinary clinical and basic scientists, government, industry, and patient advocates. This group would, in turn:
-
◦Seek funding sources and facilitate the establishment of a network of investigators. It is anticipated that such a network would engage multiple sites in the United States.
-
◦Involve investigators with expertise in the integration of basic and clinical studies, including mechanistic studies, and with broad expertise in areas such as clinical phenotyping and drug causality assessment, genetics and epigenetics, pharmacogenomics, cellular immunology, and structural biology.
-
◦The tasks of this network would include:
-
▪Developing standardized methodology for identifying drug allergy phenotypes across large datasets including phenotype replication in distinct patient populations.
-
▪Establishing the frequency of drug allergic reactions and of specific drug allergy phenotypes in various clinical settings and conditions.
-
▪Performing studies on the pathophysiology of drug allergy and the mechanism(s) of drug desensitization.
-
▪Developing models of in vitro immune stimulation using cells from drug naïve individuals combined with immunogenic forms of a drug
-
▪Developing and conducting clinical studies focusing on preventive testing, as well as on drug desensitization and challenge procedures.
-
▪Engaging in collaborations with other national or international networks.
-
▪
-
◦
- Establish guidelines for the diagnosis and management of drug allergy that will encourage widespread use of standard protocols for diagnosis and management. Potential components of guidelines development include:
-
◦An independent organization to conduct literature reviews.
-
◦A diverse group of professionals (including clinical investigators, practicing clinicians, nurses and others) to form an expert panel. The panelists would write the guidelines based on the literature reviews and on expert opinion when the literature is not definitive. The panelists could also identify promising research directions.
-
◦An advisory group consisting of diverse professional organizations, government agencies, and patient advocacy groups.
-
◦
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International Consensus on drug allergy. Allergy. 2014;69(4):420–437. doi: 10.1111/all.12350. [DOI] [PubMed] [Google Scholar]
- 2.Macy E. Penicillin and beta-lactam allergy: epidemiology and diagnosis. Current allergy and asthma reports. 2014;14(11):476. doi: 10.1007/s11882-014-0476-y. [DOI] [PubMed] [Google Scholar]
- 3.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA : the journal of the American Medical Association. 1998;279(15):1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 4.Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. British journal of clinical pharmacology. 2001;52(1):77–83. doi: 10.1046/j.0306-5251.2001.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uetrecht J, Naisbitt DJ. Idiosyncratic adverse drug reactions: current concepts. Pharmacological reviews. 2013;65(2):779–808. doi: 10.1124/pr.113.007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pleasants RA, Walker TR, Samuelson WM. Allergic reactions to parenteral beta-lactam antibiotics in patients with cystic fibrosis. Chest. 1994;106(4):1124–1128. doi: 10.1378/chest.106.4.1124. [DOI] [PubMed] [Google Scholar]
- 7.Coopman SA, Johnson RA, Platt R, Stern RS. Cutaneous disease and drug reactions in HIV infection. The New England journal of medicine. 1993;328(23):1670–1674. doi: 10.1056/NEJM199306103282304. [DOI] [PubMed] [Google Scholar]
- 8.Milpied-Homsi B, Moran EM, Phillips EJ. Antiviral drug allergy. Immunology and allergy clinics of North America. 2014;34(3):645–662. doi: 10.1016/j.iac.2014.04.011. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. The Permanente journal. 2009;13(2):12–18. doi: 10.7812/tpp/08-073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antunez C, Blanca-Lopez N, Torres MJ, Mayorga C, Perez-Inestrosa E, Montanez MI, et al. Immediate allergic reactions to cephalosporins: evaluation of cross-reactivity with a panel of penicillins and cephalosporins. The Journal of allergy and clinical immunology. 2006;117(2):404–410. doi: 10.1016/j.jaci.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Bourke J, Pavlos R, James I, Phillips E. Improving the Effectiveness of Penicillin Allergy Delabeling. The journal of allergy and clinical immunology In practice. 2015 doi: 10.1016/j.jaip.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Kadoyama K, Kuwahara A, Yamamori M, Brown JB, Sakaeda T, Okuno Y. Hypersensitivity reactions to anticancer agents: data mining of the public version of the FDA adverse event reporting system, AERS. Journal of experimental & clinical cancer research : CR. 2011;30:93. doi: 10.1186/1756-9966-30-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bursztejn AC, Romano A, Gueant-Rodriguez RM, Cornejo JA, Oussalah A, Chery C, et al. Allergy to betalactams and nucleotide-binding oligomerization domain (NOD) gene polymorphisms. Allergy. 2013;68(8):1076–1080. doi: 10.1111/all.12196. [DOI] [PubMed] [Google Scholar]
- 14.Blanca M, Torres MJ, Garcia JJ, Romano A, Mayorga C, de Ramon E, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. The Journal of allergy and clinical immunology. 1999;103(5 Pt 1):918–924. doi: 10.1016/s0091-6749(99)70439-2. [DOI] [PubMed] [Google Scholar]
- 15.Zambonino MA, Corzo JL, Munoz C, Requena G, Ariza A, Mayorga C, et al. Diagnostic evaluation of hypersensitivity reactions to beta-lactam antibiotics in a large population of children. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2014;25(1):80–87. doi: 10.1111/pai.12155. [DOI] [PubMed] [Google Scholar]
- 16.Matar R, Le Bourgeois M, Scheinmann P, de Blic J, Ponvert C. Beta-lactam hypersensitivity in children with cystic fibrosis: a study in a specialized pediatric center for cystic fibrosis and drug allergy. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2014;25(1):88–93. doi: 10.1111/pai.12154. [DOI] [PubMed] [Google Scholar]
- 17.Caimmi S, Sanfiorenzo C, Caimmi D, Bousquet PJ, Chiron R, Demoly P. Comprehensive allergy work-up is mandatory in cystic fibrosis patients who report a history suggestive of drug allergy to beta-lactam antibiotics. Clinical and translational allergy. 2012;2(1):10. doi: 10.1186/2045-7022-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox SJ, Park MA. Penicillin skin testing is a safe and effective tool for evaluating penicillin allergy in the pediatric population. The journal of allergy and clinical immunology In practice. 2014;2(4):439–444. doi: 10.1016/j.jaip.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Solensky R. The time for penicillin skin testing is here. The journal of allergy and clinical immunology In practice. 2013;1(3):264–265. doi: 10.1016/j.jaip.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Patil SU, Long AA, Ling M, Wilson MT, Hesterberg P, Wong JT, et al. A protocol for risk stratification of patients with carboplatin-induced hypersensitivity reactions. The Journal of allergy and clinical immunology. 2012;129(2):443–447. doi: 10.1016/j.jaci.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Halevy S, Kardaun SH, Davidovici B, Wechsler J, EuroScar, Regi Ssg. The spectrum of histopathological features in acute generalized exanthematous pustulosis: a study of 102 cases. The British journal of dermatology. 2010;163(6):1245–1252. doi: 10.1111/j.1365-2133.2010.09967.x. [DOI] [PubMed] [Google Scholar]
- 22.Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. The British journal of dermatology. 2013;169(5):1071–1080. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 23.Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenetics and genomics. 2008;18(2):99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- 24.Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. The Journal of investigative dermatology. 2013;133(5):1197–1204. doi: 10.1038/jid.2012.510. [DOI] [PubMed] [Google Scholar]
- 25.Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clinical pharmacology and therapeutics. 2010;88(1):60–68. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 26.Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 screening for hypersensitivity to abacavir. The New England journal of medicine. 2008;358(6):568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 27.Bousquet PJ, Demoly P, Romano A, Aberer W, Bircher A, Blanca M, et al. Pharmacovigilance of drug allergy and hypersensitivity using the ENDA-DAHD database and the GALEN platform. The Galenda project. Allergy. 2009;64(2):194–203. doi: 10.1111/j.1398-9995.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- 28.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. The New England journal of medicine. 2008;358(11):1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aalberse RC, Akkerdaas J, van Ree R. Cross-reactivity of IgE antibodies to allergens. Allergy. 2001;56(6):478–490. doi: 10.1034/j.1398-9995.2001.056006478.x. [DOI] [PubMed] [Google Scholar]
- 30.Grosen E, Siitari E, Larrison E, Tiggelaar C, Roecker E. Paclitaxel hypersensitivity reactions related to bee-sting allergy. Lancet. 2000;355(9200):288–289. doi: 10.1016/S0140-6736(99)04306-8. [DOI] [PubMed] [Google Scholar]
- 31.Hong DI, Bankova L, Cahill KN, Kyin T, Castells MC. Allergy to monoclonal antibodies: cutting-edge desensitization methods for cutting-edge therapies. Expert review of clinical immunology. 2012;8(1):43–52. doi: 10.1586/eci.11.75. quiz 3–4. [DOI] [PubMed] [Google Scholar]
- 32.Liu A, Fanning L, Chong H, Fernandez J, Sloane D, Sancho-Serra M, et al. Desensitization regimens for drug allergy: state of the art in the 21st century. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2011;41(12):1679–1689. doi: 10.1111/j.1365-2222.2011.03825.x. [DOI] [PubMed] [Google Scholar]
- 33.Brockow K. Immediate and delayed cutaneous reactions to radiocontrast media. Chemical immunology and allergy. 2012;97:180–190. doi: 10.1159/000335631. [DOI] [PubMed] [Google Scholar]
- 34.Veien M, Szlam F, Holden JT, Yamaguchi K, Denson DD, Levy JH. Mechanisms of nonimmunological histamine and tryptase release from human cutaneous mast cells. Anesthesiology. 2000;92(4):1074–1081. doi: 10.1097/00000542-200004000-00026. [DOI] [PubMed] [Google Scholar]
- 35.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cellspecific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519(7542):237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. Journal of the American Academy of Dermatology. 2007;56(2):181–200. doi: 10.1016/j.jaad.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. Journal of the American Academy of Dermatology. 2013;69(2):187, e1–e16. doi: 10.1016/j.jaad.2013.05.002. quiz 203-4. [DOI] [PubMed] [Google Scholar]
- 38.McGee T, Munster A. Toxic epidermal necrolysis syndrome: mortality rate reduced with early referral to regional burn center. Plastic and reconstructive surgery. 1998;102(4):1018–1022. doi: 10.1097/00006534-199809040-00014. [DOI] [PubMed] [Google Scholar]
- 39.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nature medicine. 2008;14(12):1343–1350. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 40.Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282(5388):490–493. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- 41.Saito N, Qiao H, Yanagi T, Shinkuma S, Nishimura K, Suto A, et al. An annexin A1-FPR1 interaction contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. Science translational medicine. 2014;6(245):245ra95. doi: 10.1126/scitranslmed.3008227. [DOI] [PubMed] [Google Scholar]
- 42.Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. The British journal of dermatology. 2013;168(3):555–562. doi: 10.1111/bjd.12125. [DOI] [PubMed] [Google Scholar]
- 43.Adam J, Pichler WJ, Yerly D. Delayed drug hypersensitivity: models of T-cell stimulation. British journal of clinical pharmacology. 2011;71(5):701–707. doi: 10.1111/j.1365-2125.2010.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin SF, Dudda JC, Bachtanian E, Lembo A, Liller S, Durr C, et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. The Journal of experimental medicine. 2008;205(9):2151–2162. doi: 10.1084/jem.20070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(25):9959–9964. doi: 10.1073/pnas.1207934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486(7404):554–558. doi: 10.1038/nature11147. [DOI] [PubMed] [Google Scholar]
- 47.Norcross MA, Luo S, Lu L, Boyne MT, Gomarteli M, Rennels AD, et al. Abacavir induces loading of novel self-peptides into HLA-B*57: 01: an autoimmune model for HLA-associated drug hypersensitivity. Aids. 2012;26(11):F21–F29. doi: 10.1097/QAD.0b013e328355fe8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Ghaiesh S, Monshi MM, Whitaker P, Jenkins R, Meng X, Farrell J, et al. Characterization of the antigen specificity of T-cell clones from piperacillin-hypersensitive patients with cystic fibrosis. The Journal of pharmacology and experimental therapeutics. 2012;341(3):597–610. doi: 10.1124/jpet.111.190900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitaker P, Meng X, Lavergne SN, El-Ghaiesh S, Monshi M, Earnshaw C, et al. Mass spectrometric characterization of circulating and functional antigens derived from piperacillin in patients with cystic fibrosis. Journal of immunology. 2011;187(1):200–211. doi: 10.4049/jimmunol.1100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castrejon JL, Berry N, El-Ghaiesh S, Gerber B, Pichler WJ, Park BK, et al. Stimulation of human T cells with sulfonamides and sulfonamide metabolites. The Journal of allergy and clinical immunology. 2010;125(2):411–418. e4. doi: 10.1016/j.jaci.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 51.Watkins S, Pichler WJ. Sulfamethoxazole induces a switch mechanism in T cell receptors containing TCRVbeta20-1, altering pHLA recognition. PloS one. 2013;8(10):e76211. doi: 10.1371/journal.pone.0076211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nature genetics. 2009;41(7):816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 53.Wuillemin N, Adam J, Fontana S, Krahenbuhl S, Pichler WJ, Yerly D. HLA haplotype determines hapten or p-i T cell reactivity to flucloxacillin. Journal of immunology. 2013;190(10):4956–4964. doi: 10.4049/jimmunol.1202949. [DOI] [PubMed] [Google Scholar]
- 54.Monshi MM, Faulkner L, Gibson A, Jenkins RE, Farrell J, Earnshaw CJ, et al. Human leukocyte antigen (HLA)-B*57: 01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology. 2013;57(2):727–739. doi: 10.1002/hep.26077. [DOI] [PubMed] [Google Scholar]
- 55.Yuan J, Guo S, Hall D, Cammett AM, Jayadev S, Distel M, et al. Toxicogenomics of nevirapineassociated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. Aids. 2011;25(10):1271–1280. doi: 10.1097/QAD.0b013e32834779df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pavlos R, Mallal S, Ostrov D, Pompeu Y, Phillips E. Fever, rash, and systemic symptoms: understanding the role of virus and HLA in severe cutaneous drug allergy. The journal of allergy and clinical immunology In practice. 2014;2(1):21–33. doi: 10.1016/j.jaip.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavlos R, Mallal S, Ostrov D, Buus S, Metushi I, Peters B, et al. T Cell-Mediated Hypersensitivity Reactions to Drugs. Annu Rev Med. 2015;66:20.1–20.16. doi: 10.1146/annurev-med-050913-022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucas A, Lucas M, Stryn A, Keane N, McKinnon E, Pavlos R, et al. Abacavir-reactive memory T cells are present in drug naive individuals. PloS one. 2014 doi: 10.1371/journal.pone.0117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adam J, Wuillemin N, Watkins S, Jamin H, Eriksson KK, Villiger P, et al. Abacavir induced T cell reactivity from drug naive individuals shares features of allo-immune responses. PloS one. 2014;(4):e95339. doi: 10.1371/journal.pone.0095339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ko TM, Chung WH, Wei CY, Shih HY, Chen JK, Lin CH, et al. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. The Journal of allergy and clinical immunology. 2011;128(6):1266–1276. e11. doi: 10.1016/j.jaci.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Picard D, Janela B, Descamps V, D'Incan M, Courville P, Jacquot S, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Science translational medicine. 2010;2(46):46ra62. doi: 10.1126/scitranslmed.3001116. [DOI] [PubMed] [Google Scholar]
- 62.Chung WH, Chang WC, Lee YS, Wu YY, Yang CH, Ho HC, et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA : the journal of the American Medical Association. 2014;312(5):525–534. doi: 10.1001/jama.2014.7859. [DOI] [PubMed] [Google Scholar]
- 63.Chung WH, Chang WC, Stocker SL, Juo CG, Graham GG, Lee MH, et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Annals of the rheumatic diseases. 2014 doi: 10.1136/annrheumdis-2014-205577. [DOI] [PubMed] [Google Scholar]
- 64.Burrows JA, Toon M, Bell SC. Antibiotic desensitization in adults with cystic fibrosis. Respirology. 2003;8(3):359–364. doi: 10.1046/j.1440-1843.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 65.Stark BJ, Earl HS, Gross GN, Lumry WR, Goodman EL, Sullivan TJ. Acute and chronic desensitization of penicillin-allergic patients using oral penicillin. The Journal of allergy and clinical immunology. 1987;79(3):523–532. doi: 10.1016/0091-6749(87)90371-x. [DOI] [PubMed] [Google Scholar]
- 66.Stark BJ, Wendel GD, Sullivan TJ. Oral desensitization for penicillin sensitivity. JAMA : the journal of the American Medical Association. 1987;257(11):1474. [PubMed] [Google Scholar]
- 67.Wong JT, Ling M, Patil S, Banerji A, Long A. Oxaliplatin hypersensitivity: evaluation, implications of skin testing, and desensitization. The journal of allergy and clinical immunology In practice. 2014;2(1):40–45. doi: 10.1016/j.jaip.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Sullivan TJ. Antigen-specific desensitization of patients allergic to penicillin. The Journal of allergy and clinical immunology. 1982;69(6):500–508. doi: 10.1016/0091-6749(82)90174-9. [DOI] [PubMed] [Google Scholar]
- 69.Sancho-Serra Mdel C, Simarro M, Castells M. Rapid IgE desensitization is antigen specific and impairs early and late mast cell responses targeting FcepsilonRI internalization. European journal of immunology. 2011;41(4):1004–1013. doi: 10.1002/eji.201040810. [DOI] [PubMed] [Google Scholar]
- 70.Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. The Journal of allergy and clinical immunology. 2008;122(3):574–580. doi: 10.1016/j.jaci.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 71.Scherer K, Brockow K, Aberer W, Gooi JH, Demoly P, Romano A, et al. Desensitization in delayed drug hypersensitivity reactions -- an EAACI position paper of the Drug Allergy Interest Group. Allergy. 2013;68(7):844–852. doi: 10.1111/all.12161. [DOI] [PubMed] [Google Scholar]
- 72.Phillips E, Mallal S. Successful translation of pharmacogenetics into the clinic: the abacavir example. Molecular diagnosis & therapy. 2009;13(1):1–9. doi: 10.1007/BF03256308. [DOI] [PubMed] [Google Scholar]
- 73.Yeo SI. HLA-B*5801: utility and cost-effectiveness in the Asia-Pacific Region. International journal of rheumatic diseases. 2013;16(3):254–257. doi: 10.1111/1756-185X.12050. [DOI] [PubMed] [Google Scholar]
- 74.Orkin C, Sadiq ST, Rice L, Jackson F team UE. Prospective epidemiological study of the prevalence of human leukocyte antigen (HLA)-B*5701 in HIV-1-infected UK subjects. HIV medicine. 2010;11(3):187–192. doi: 10.1111/j.1468-1293.2009.00762.x. [DOI] [PubMed] [Google Scholar]
- 75.Hamsten C, Tran TA, Starkhammar M, Brauner A, Commins SP, Platts-Mills TA, et al. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. The Journal of allergy and clinical immunology. 2013;132(6):1431–1434. doi: 10.1016/j.jaci.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. The Journal of allergy and clinical immunology. 2011;127(5):1286–1293. e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Nunen SA, O'Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. The Medical journal of Australia. 2009;190(9):510–511. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]