Abstract
Adverse drug reactions (ADRs) are commonplace and occur when a drug binds to its intended pharmacological target (Type A ADR) or an unintended target (Type B ADR). Immunologically mediated Type B ADRs, such as drug hypersensitivity syndrome, drug reaction with eosinophilia and systemic symptoms (DRESS), and Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN), can be severe and result in a diverse set of clinical manifestations that include fever and rash as well as multiple organ failure (liver, kidney, lungs, and/or heart) in the case of drug hypersensitivity syndrome. There is increasing evidence that specific HLA alleles influence the risk of drug reactions. Several features of T cell–mediated ADRs are strikingly similar to those displayed by autoimmune diseases like type I diabetes, such as strong HLA association, organ-specific adaptive immune responses, viral involvement, and activation of innate immunity. There is a need to better predict patient populations at risk for developing immunologically mediated Type B ADRs. Since methods to predict type 1 diabetes using genetic and immunological biomarkers have been developed to a high level of accuracy (predicting 100% of individuals likely to progress), new research strategies based on these methods may also improve the ability to predict drug hypersensitivity.
Keywords: T cell, adverse drug reaction, autoimmunity
Introduction
The intention of this review is to compare and contrast the features of T cell–mediated adverse drug reactions (ADRs) with those of autoimmunity as they relate to disease progression, as understanding their similarities in pathogenesis will suggest future research strategies for establishing rationales to improve ADR case workups.
ADRs and autoimmunity differ in that ADRs have a relatively higher complexity of underlying immunological responses (e.g., drug reaction with eosinophilia and systemic symptoms [DRESS], Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis [SJS/TEN], erythema multiforme), which varies greatly among the different drugs, and that the pathologies underlying autoimmune diseases are better understood than those of ADRs. In autoimmunity, the understanding that adaptive immune responses are often established before the onset of disease has been taken advantage of in the clinic in terms of predicting disease progression. In a similar fashion, ADRs that stimulate T cell–mediated immune responses may benefit from new research investigating the rationale that utilizing specific immunologic and genetic tests like those currently used to predict autoimmune diseases, including HLA typing, can also predict ADR occurrences.
Similarities: Adaptive immune responses directed against self
Autoimmune diseases and drug hypersensitivity responses both involve stimulation of the adaptive immune system against self-proteins (1–3). T cell–mediated ADRs involve anatomically directed adaptive immune responses and/or systemic responses. For example, allopurinol and carbamazepine trigger SJS/TEN in which cytotoxic CD8+ T cells are thought to destroy keratinocytes at the dermal-epidermal junction using the enzyme granulysin (4), annexin (5), and FasL (6) as mechanisms for lysis. Amoxicillin clavulanate, flucloxacillin, and ximelagatran cause drug-induced liver disease (DILI) in certain individuals (7). Abacavir is an example of a drug capable of triggering systemic T cell–mediated hypersensitivity responses in HLA-B57:01–positive at-risk patients (8); these systemic symptoms can be lethal and include fever, rash, vomiting, abdominal pain, and multiple organ involvement (9). Carbamazepine stimulates both anatomically directed T cell responses and systemic responses (10).
Many autoimmune disease pathologies also involve T cell responses against self. In rheumatoid arthritis, there are self-directed autoimmune responses against connective tissue components (11). T cell responses are directed against components of the dermis in psoriasis (12). In celiac disease, exposure to gluten peptides initiates T cell–mediated autoimmune responses against transglutaminase-modified peptides expressed in self-tissues (13, 14). In type 1 diabetes, T cell–mediated autoimmune responses are thought to destroy insulin-producing beta cells in the pancreas (15, 16). Autoimmune responses and drug responses also frequently involve both T and B lymphocytes. In autoimmune diabetes, for example, patients can generate T cell responses and autoantibodies directed against insulin, glutamic acid decarboxylase (GAD), islet antigen 2, and a zinc transporter (17, 18). Systemic lupus erythematosus (SLE), an autoimmune disease in which chronic inflammation is systemic, often affects the heart, joints, skin, lungs, blood vessels, liver, kidneys, and nervous system (19, 20).
Some drugs can even act as inducible triggers of autoimmunity in an HLA-associated manner. Hydralazine and isoniazid trigger drug-induced SLE in association with HLA-DR4 (21). D-penicillamine–induced myasthenia gravis associates with HLA-DR1 (22). Thiopurines associate with pancreatitis in inflammatory bowel disease patients with the HLA-DQA1*02:01/HLA-DRB1*07:01 haplotype, showing an odds ratio (OR) of 2.6 (23).
Similarity: HLA associations
HLA associations have been found for nearly all autoimmune diseases (2). The strength of association between HLA alleles and individual diseases varies significantly (24). Some diseases including asthma (25), Crohn’s disease (26), and ulcerative colitis (27) show significant but weak HLA associations (OR < 2). HLA alleles are moderately associated (OR 2–4) with SLE (24), multiple sclerosis (28), and autoimmune thyroid diseases such as Graves’ disease and Hashimoto’s disease (29). Ankylosing spondylitis (30), celiac disease (31), rheumatoid arthritis (32), and type 1 diabetes (33) are strongly associated with HLA alleles (OR > 4). Differences in the strength of association between HLA alleles and individual diseases may reflect the variation in the role of T cell responsiveness in the context of other environmental and genetic factors influencing the pathogenesis of a given disease.
HLA alleles are also associated with an increasing number of drug hypersensitivity responses. The strongest HLA-associated drug responses are between HLA-B*57:01 and abacavir hypersensitivity syndrome in the Caucasian population, HLA-B*15:02 and carbamazepine-induced SJS/TEN in Asian populations, and HLA-B*58:01 in allopurinol hypersensitivity syndrome and SJS/TEN (OR > 500) (34). Similar to autoimmune disorders, the strength of association between drug hypersensitivity and HLA alleles ranges from weak to strong. Significant associations between drug hypersensitivity and HLA alleles suggest their potential utility in predicting at-risk individuals. For abacavir, an HIV reverse-transcriptase inhibitor, identification of the strong HLA association between abacavir hypersensitivity and HLA-B*57:01 has led to a prevention strategy currently in use (8) wherein a pharmacogenetic test is now routine in HIV clinical practice (35).
Drugs are thought to elicit ADRs in an HLA-associated manner because they may interact with HLA molecules and form drug-HLA complexes capable of being recognized by T cells. Drugs can bind HLA in at least 3 possible ways. First, drugs may act as haptens forming covalent interactions with peptides that bind HLA molecules associated with ADRs (e.g., penicilloyl-modified peptides) (36). Second, drugs may bind directly to the TCR or HLA molecules outside of the antigen-binding cleft with rapid non-covalent interactions (called the pharmacological interactions with immune receptors [pi] hypothesis) (37). Third, drugs may bind within the antigen-binding cleft of the HLA molecule, altering the repertoire of HLA-bound peptides; in this mechanism, the drug enables the presentation of self (and possibly viral) T cell epitopes to which the host is not tolerant (38, 39).
Similarity: Viral infection and heterologous immunity
A number of drug hypersensitivity reactions and HLA allele associations occur in virally infected patients. The mechanistic roles of the viruses in the initiation or perpetuation of T cell–mediated ADRs are complex and have been proposed to involve stimulation of virus-specific T cells that cross-react with the drug presented to T cells in the context of HLA (the heterologous immunity model).
Evidence that viruses play a role in ADRs include ampicillin-induced exanthema in patients with mononucleosis (expressing activated EBV). While exanthematous eruptions in mononucleosis patients are relatively uncommon (rate: 10%), they are common among ampicillin-treated patients (100% of children, 70% of adults) (40). Similarly, the incidence of severe ADRs to co-trimoxazole is significantly lower in the general population compared to HIV-infected patients (41).
Although virus infection likely influences T cell–mediated ADRs by a number of antigen non-specific mechanisms (e.g., by stimulating innate immunity), some specific viruses strongly correlate with adverse responses to certain drugs. For example, HHV-6 activation is associated with carbamazepine-induced DRESS, and reactivation is associated with slow recovery (42). Also, HIV-infected SJS patients exhibit viral reactivation (EBV, CMV) upon drug exposure (43). The clinical effects of these virus-drug interactions range from mild (EBV-linked ampicillin rash) to severe (HHV-6 reactivation and DRESS) (44). Collectively, these observations demonstrate diversity in pathogenic mechanisms for ADRs.
Cytotoxic T lymphocytes specific for viral epitopes have been found to expand in patients with drug hypersensitivity reactions (45), suggesting that the drug can stimulate T cells previously primed by virus exposure through a cross-reaction/molecular mimicry mechanism. The drug may also directly or indirectly stimulate virus reactivation and the expression of viral proteins, thus resulting in increased presentation of viral epitopes and expansion of virus-specific T cells that promote ADRs.
Data suggest that pathogen infections influence autoimmunity. In type 1 diabetes, there are strong antibody responses to coxsackievirus infections, and a similarity between the structure of the autoantigen GAD and the P2-C protein of Coxsackie B are implicated as a potential cross-reaction mechanism (46). High titers of EBV-specific autoantibodies have been reported in both multiple sclerosis (47) and SLE (48) patients. Rotavirus infections are strongly associated with celiac disease (49). In addition to coxsackievirus infections, intestinal microbiota may play a role in the development of autoimmune diabetes (50). Thus, the combination approach of defining environmental triggers (e.g., viral infection status) with identifying genetic susceptibility markers (e.g., HLA) is expected to improve the prediction and prevention of T cell–mediated ADRs.
Susceptibility genes shared between drug hypersensitivity reactions and autoimmunity
There are individuals susceptible to more than one autoimmune disease, and also individuals susceptible to multiple ADRs. HLA alleles are associated with multiple autoimmune diseases. For example, SLE (51), Adison’s disease (52), Graves’ disease 163 (53), and celiac disease (54) were associated with HLA-DR3. Autoimmune diseases can occur concomitantly in the same patient in a number of cases; for example, diabetes mellitus is present in 11.6% of SLE patients (55). Since HLA alleles are strongly associated with multiple drug hypersensitivity reactions as well as with multiple autoimmune diseases (examples of both are listed below), they too may help to inform pathological mechanisms and biomarkers to predict disease. Adverse reactions to both abacavir and flucloxacillin (drugs that are not similar in structure) strongly associate with HLA-B*57:01 (10). Adverse reactions to lumiracoxib (56) and amoxicillin (57) both associate with HLA-DRB1*15:01. For autoimmune diseases, SLE (54), Addison’s disease (55), Graves’ disease (56), and celiac disease (57) are associated with HLA-DR3. Thus, a set of HLA alleles may confer high risk for drug hypersensitivity reactions, and a partially overlapping set of alleles may confer high risk for autoimmune-related diseases. These similarities suggest shared mechanisms and potentially useful shared biomarkers for disease prediction (24).
Genes encoding proteins involved in drug metabolism have been associated with more than one ADR, including the P450 genes (CYP34A (58), CYP2C9 (59)), myeloperoxidase (60), and the glutamate-cysteine ligase catalytic subunit gene GCLC (61). For DILI, polymorphic variants of genes involved in the mitochondrial anti-defense pathway, such as Nrf-2, may play particularly informative roles (62).
Predicting autoimmunity
In contrast to autoimmune diseases in which the inciting agents are not well characterized, the triggering agents for ADRs are known. This suggests that the severity of ADRs may be lessened by early prediction of susceptible individuals based on patient biomarkers as a guide to treatment options.
Using type 1 diabetes as an example of an autoimmune disease in which susceptible individuals can reliably be identified, prediction can be achieved with a high level of sensitivity and specificity by interpreting genetic, metabolic, and immunological assays (63). The Diabetes Prevention Trial Type 1 Risk Score (DPT1RS) measures several metabolic parameters along with age and body mass index (64). When taking into account the DPT1RS score in combination with the Autoantibody Risk Score, type 1 diabetes is more accurately predicted then using either score alone (65). Among the risk factors, production of autoantibodies specific for the self-proteins GADA, IA-2A, insulin, and/or ZnT8A appears to be the most significant factor in predicting type 1 diabetes progression; indeed, generation of at least 2 of these autoantibodies has been found to correlate precisely with type 1 diabetes progression (63, 66).
The predictive value of HLA is already known for several drug hypersensitivity reactions, and both high positive predictive values and low negative predictive values have been identified. In the Han Chinese population, both the carbamazepine/HLA-B*15:02 and allopurinol/HLA-B*58:01 associations have a positive predictive value of 100% and a negative predictive value of 3% (67).
Predictive values of HLA for some autoimmune diseases are similarly high for positive predictive values and low for negative predictive values. For example, approximately 60% of type 1 diabetes patients express HLA-DQA1*0301/DQB1*0302 (DQ8) (Figure 1); in other words, the positive predictive value is 60% (63, 68–71). Since 1 out of 20 individuals expressing HLA-DQ8 progress to type 1 diabetes, the negative predictive value of HLA-DQ8 is 5% (70, 71). Despite a low level of negative predictive value, type 1 diabetes can be accurately predicted based on immunological markers (e.g., islet autoantibodies). Thus, using these data from autoimmune diseases as a rationale, the combined predictive power of several different factors, including HLA and drug recognition by B and T lymphocytes, may more accurately predict T cell–mediated ADR occurrences.
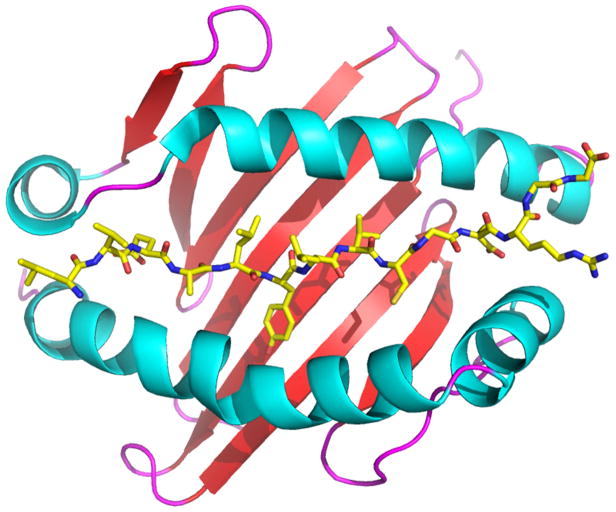
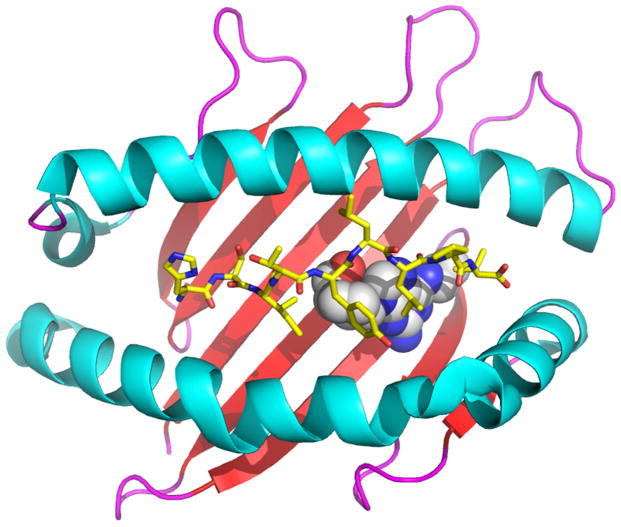
FIG 1. HLA genes strongly associated with an increasing number of ADRs encoding antigen-binding clefts with specific ligand-binding properties, a common feature of HLA molecules associated with autoimmunity.
Panel A shows a ribbon diagram of HLA-DQ8 (Protein Data Bank code 1JK8) associated with autoimmune diabetes, which is bound to the insulin peptide B:9–23 (shown as sticks; yellow represents carbon, blue represents nitrogen, red represents oxygen). Panel B shows a ribbon diagram of HLA-B*57:01 (PDB 3UPR) associated with abacavir hypersensitivity syndrome. The ADR in response to abacavir (shown as spheres; white represents carbon, blue represents nitrogen, red represents oxygen) is the result of the drug binding to the unique portion of the HLA-B*57:01 antigen-binding cleft, facilitating presentation of peptides to which the host is not tolerant. The figure was generated with the PyMOL Molecular Graphics System, Version 1.7.4, Schrödinger, LLC.
Challenges in predicting T cell–mediated ADRs
The successful prediction of autoimmune diabetes relies in part on repository and database collections such as the Network for Pancreatic Organ Donors with Diabetes. These resources have enabled the use of a variety of genetic, biochemical, and cellular studies for prediction, including those focused on predictive biomarkers (72). Since many ADRs are rare, the most significant problem in predicting and preventing ADRs is the lack of large, relatively homogenous populations of patients to study. Thus, an international repository of ADR patient samples and a database of clinical information would be expected to facilitate significant advances in not only understanding the basic mechanisms that regulate ADRs but also developing new strategies to predict and prevent T cell–mediated pathogenesis. Indeed, ADR researchers will likely benefit from such an international repository and database that includes a sufficient number of samples in homogenous patient populations for such ADRs as DRESS, SJS/TEN, erythema multiforme, and DILI, among others. There are already several consortia focused on ADRs that currently bank clinical samples. RegiSCAR collects sample from SJS/TEN patients in Europe (73). The NIH NIDDK Drug-Induced Liver Injury Network (DILIN) collects and analyzes cases of drug-induced severe liver injury (74), and their resources allow prospective and retrospective studies of predictive biomarkers and causality. DILIN may thus serve as a model repository/database that can expand internationally to include additional patient populations, which can increase the statistical power of the data and allow researchers to gain additional insight into the ethnic differences in susceptibility to ADRs. Thorough analysis of data collected from assays relevant for understanding the pathogenesis of ADRs and autoimmunity would be expected to provide a rationale for the use of specific tests (e.g., immunological tests and HLA typing) at onset to better manage ADRs in the future.
Table I shows a list of factors associated with T cell–mediated ADRs or autoimmunity that may be assayed from patient ADR samples for research purposes. An international ADR repository with samples that permits analysis of genomic DNA can allow for the characterization of associations between factors, including HLA and other genes associated with immune disease. Based on methods established for autoimmune diseases, immunological workups that may prove informative in characterizing new ADR risk-associated factors include measurement of inducible heat shock protein 70 (HSP70i), which is associated with precipitation of vitiligo (75), and ribosomal protein L23a (RPL2A), an autoantigen recognized by B and T cells from rheumatoid arthritis patients (76).
Table I.
In vitro assays for research examining the rationale of using specific tests at the onset of ADR.
| Genetic assays |
| HLA-A (81) |
| HLA-B (82) |
| HLA-C (83) |
| HLA-DR (84) |
| HLA-DQ (85) |
| Immune-disease associated genes |
| T cell differentiation IL2-IL21 (86) |
| Signaling CTLA-4 (87) |
| Innate immunity IFIH1 (88) |
| Cytokines IL7R (24) |
| Drug metabolism genes |
| CYP34A (58) |
| CTP2C9 (59) |
| GCLC (61) |
| Nrf-2 (62) |
| Immunological assays |
|
|
| HSP70i (75) |
|
|
| RPL2A (76) |
|
|
| In vitro T cell–stimulation assays (77) |
|
|
| ELISPOT (78, 89) |
|
|
| Subset analysis |
| Th1: IL-2 |
| Th2: IL-4 |
| CD8: IFN-γ |
| CD4/CD8: IL-8 |
| Treg: IL-10 |
|
|
| Flow cytometry (90, 91) |
|
|
| Subset analysis |
| CD4 |
| CD8 |
| CTLA-4 |
| Foxp3 |
The significance of virus activation and how it may relate to specific ADRs can potentially be addressed with sufficiently large patient sample sizes. Data from assays measuring T cell responses to a given drug in vitro using peripheral blood mononuclear cells (PBMCs) are expected to generate rationales that can inform the development of new treatment options. In standard assays, suspected drugs are incubated with PBMCs (up to 14 days), and proliferation (e.g., by 3H-thymidine incorporation) is measured to detect T cell responsiveness (77) and is expressed as the stimulation index (ratio between cells incubated with and without the drug). Functional characteristics of the responding T cell population can be assayed utilizing flow cytometry (e.g., using antibodies specific for CD4 and CD8) and ELISPOT to characterize the responding T cells (e.g., by measuring the production of cytokines including IFN-γ, IL-2, and IL-4) (78).
Clinical classification of Type IV delayed-type hypersensitivity (DTH) reactions is based on the type of drug-responsive T cell: T helper 1 (Th1) for Type IVa, Th2 for Type IVb, cytotoxic T cells for Type IVc, and IL-8–producing T cells for Type IVd. Based on the similarities between ADRs and autoimmunity discussed above, new studies examining the characteristics of drug-responsive T cells may reveal additional DTH categories. For example, drugs may cause ADRs by modulating regulatory T cell (Treg) activity. Indeed, deficient Treg activity is thought to promote autoimmunity (79, 80), and drugs that inhibit Treg activity would thus be expected to increase self-reactive T cell activity and ADRs.
Overall, establishment of an international repository/database of ADRs will likely provide the necessary set of homogenous samples to study the significance of associations between ADRs against specific drugs and a diverse set of risk factors, a strategy similar to what is already successfully used to find significant associations between some autoimmune diseases and their risk factors.
Conclusions
Autoimmune diseases and T cell–mediated ADRs are similar in the genetic and environmental factors thought to influence the onset and severity of disease progression. HLA is a prominent genetic risk factor with positive and negative predictive value. Although the roles of environmental factors are far from clear, drugs that trigger adverse immune-mediated reactions act as exogenous factors that can initiate and/or perpetuate self-destructive adaptive immune responses. The ability to predict T cell–mediated ADRs is expected to improve by combining standardized information on known variables. Thus, multivariate analysis of the following factors will likely increase the sensitivity and specificity of assays to predict drug hypersensitivity reactions: 1) factors that influence T cell immunobiology, including innate immune system activation markers; 2) metabolic markers; 3) measurement of T cell reactivity against suspected drugs; and 4) measurement of drug-specific antibody production in HLA-predisposed individuals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010 Apr 29;464(7293):1293–300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Complete sequence and gene map of a human major histocompatibility complex. The MHC sequencing consortium. Nature. 1999 Oct 28;401(6756):921–3. doi: 10.1038/44853. [DOI] [PubMed] [Google Scholar]
- 3.Phillips EJ, Chung WH, Mockenhaupt M, Roujeau JC, Mallal SA. Drug hypersensitivity: pharmacogenetics and clinical syndromes. J Allergy Clin Immunol. 2011 Mar;127(3 Suppl):S60–6. doi: 10.1016/j.jaci.2010.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008 Dec;14(12):1343–50. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 5.Saito N, Qiao H, Yanagi T, Shinkuma S, Nishimura K, Suto A, et al. An annexin A1-FPR1 interaction contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. Sci Transl Med. 2014 Jul 16;6(245):245ra95. doi: 10.1126/scitranslmed.3008227. [DOI] [PubMed] [Google Scholar]
- 6.Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998 Oct 16;282(5388):490–3. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- 7.Liss G, Rattan S, Lewis JH. Predicting and preventing acute drug-induced liver injury: what’s new in 2010? Expert Opin Drug Metab Toxicol. 2010 Sep;6(9):1047–61. doi: 10.1517/17425255.2010.503706. [DOI] [PubMed] [Google Scholar]
- 8.Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008 Feb 7;358(6):568–79. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 9.Pavlos R, Mallal S, Ostrov D, Pompeu Y, Phillips E. Fever, rash, and systemic symptoms: understanding the role of virus and HLA in severe cutaneous drug allergy. J Allergy Clin Immunol Pract. 2014 Jan-Feb;2(1):21–33. doi: 10.1016/j.jaip.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlos R, Mallal S, Ostrov D, Buus S, Metushi I, Peters B, et al. T cell-mediated hypersensitivity reactions to drugs. Annu Rev Med. 2015;66:439–54. doi: 10.1146/annurev-med-050913-022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruyssen-Witrand A, Constantin A, Cambon-Thomsen A, Thomsen M. New insights into the genetics of immune responses in rheumatoid arthritis. Tissue Antigens. 2012 Aug;80(2):105–18. doi: 10.1111/j.1399-0039.2012.01939.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Helms C, Liao W, Zaba LC, Duan S, Gardner J, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008 Mar 28;4(3):e1000041. doi: 10.1371/journal.pgen.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tollefsen S, Arentz-Hansen H, Fleckenstein B, Molberg O, Raki M, Kwok WW, et al. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J Clin Invest. 2006 Aug;116(8):2226–36. doi: 10.1172/JCI27620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tjon JM, van Bergen J, Koning F. Celiac disease: how complicated can it get? Immunogenetics. 2010 Oct;62(10):641–51. doi: 10.1007/s00251-010-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004 Feb;113(3):451–63. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebe JA, Unrath KA, Yue BB, Miyake T, Falk BA, Nepom GT. Autoreactive human T-cell receptor initiates insulitis and impaired glucose tolerance in HLA DR4 transgenic mice. J Autoimmun. 2008 Jun;30(4):197–206. doi: 10.1016/j.jaut.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sosenko JM, Skyler JS, Palmer JP, Krischer JP, Yu L, Mahon J, et al. The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care. 2013 Sep;36(9):2615–20. doi: 10.2337/dc13-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014 Jan 4;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN) Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008 Feb;40(2):204–10. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell WM. HLA and disease: guilt by association. Int J Immunogenet. 2014 Feb;41(1):1–12. doi: 10.1111/iji.12088. [DOI] [PubMed] [Google Scholar]
- 21.Batchelor JR, Welsh KI, Tinoco RM, Dollery CT, Hughes GR, Bernstein R, et al. Hydralazine-induced systemic lupus erythematosus: influence of HLA-DR and sex on susceptibility. Lancet. 1980 May 24;1(8178):1107–9. doi: 10.1016/s0140-6736(80)91554-8. [DOI] [PubMed] [Google Scholar]
- 22.Garlepp MJ, Dawkins RL, Christiansen FT. HLA antigens and acetylcholine receptor antibodies in penicillamine induced myasthenia gravis. Br Med J (Clin Res Ed) 1983 Jan 29;286(6362):338–40. doi: 10.1136/bmj.286.6362.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heap GA, Weedon MN, Bewshea CM, Singh A, Chen M, Satchwell JB, et al. HLA-DQA1-HLA-DRB1 variants confer susceptibility to pancreatitis induced by thiopurine immunosuppressants. Nat Genet. 2014 Oct;46(10):1131–4. doi: 10.1038/ng.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet. 2009 Jan;10(1):43–55. doi: 10.1038/nrg2489. [DOI] [PubMed] [Google Scholar]
- 25.Munthe-Kaas MC, Carlsen KL, Carlsen KH, Egeland T, Haland G, Devulapalli CS, et al. HLA Dr-Dq haplotypes and the TNFA-308 polymorphism: associations with asthma and allergy. Allergy. 2007 Sep;62(9):991–8. doi: 10.1111/j.1398-9995.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- 26.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008 Aug;40(8):955–62. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernando MM, Stevens CR, Walsh EC, De Jager PL, Goyette P, Plenge RM, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008 Apr 25;4(4):e1000024. doi: 10.1371/journal.pgen.1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Multiple Sclerosis Genetics Consortium. Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007 Aug 30;357(9):851–62. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J Autoimmun. 2008 Feb-Mar;30(1–2):58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wellcome Trust Case Control Consortium, Australo-Anglo-American Spondylitis Consortium (TASC) Burton PR, Clayton DG, Cardon LR, Craddock N, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007 Nov;39(11):1329–37. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt KA, Zhernakova A, Turner G, Heap GA, Franke L, Bruinenberg M, et al. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet. 2008 Apr;40(4):395–402. doi: 10.1038/ng.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowes J, Barton A. Recent advances in the genetics of RA susceptibility. Rheumatology (Oxford) 2008 Apr;47(4):399–402. doi: 10.1093/rheumatology/ken005. [DOI] [PubMed] [Google Scholar]
- 33.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007 Jun 7;447(7145):661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bharadwaj M, Illing P, Theodossis A, Purcell AW, Rossjohn J, McCluskey J. Drug Hypersensitivity and Human Leukocyte Antigens of the Major Histocompatibility Complex. Annu Rev Pharmacol Toxicol. 2011 Jan 17; doi: 10.1146/annurev-pharmtox-010611-134701. [DOI] [PubMed] [Google Scholar]
- 35.Phillips E, Mallal S. Successful translation of pharmacogenetics into the clinic: the abacavir example. Mol Diagn Ther. 2009;13(1):1–9. doi: 10.1007/BF03256308. [DOI] [PubMed] [Google Scholar]
- 36.Faulkner L, Meng X, Park BK, Naisbitt DJ. The importance of hapten-protein complex formation in the development of drug allergy. Curr Opin Allergy Clin Immunol. 2014 Aug;14(4):293–300. doi: 10.1097/ACI.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 37.Pichler WJ. The p-i Concept: Pharmacological Interaction of Drugs With Immune Receptors. World Allergy Organ J. 2008 Jun;1(6):96–102. doi: 10.1097/WOX.0b013e3181778282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A. 2012 May 29; doi: 10.1073/pnas.1207934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012 Jun 28;486(7404):554–8. doi: 10.1038/nature11147. [DOI] [PubMed] [Google Scholar]
- 40.Shiohara T, Kano Y. A complex interaction between drug allergy and viral infection. Clin Rev Allergy Immunol. 2007 Oct;33(1–2):124–33. doi: 10.1007/s12016-007-8010-9. [DOI] [PubMed] [Google Scholar]
- 41.Eliaszewicz M, Flahault A, Roujeau JC, Fillet AM, Challine D, Mansouri S, et al. Prospective evaluation of risk factors of cutaneous drug reactions to sulfonamides in patients with AIDS. J Am Acad Dermatol. 2002 Jul;47(1):40–6. doi: 10.1067/mjd.2002.120468. [DOI] [PubMed] [Google Scholar]
- 42.Aihara Y, Ito SI, Kobayashi Y, Yamakawa Y, Aihara M, Yokota S. Carbamazepine-induced hypersensitivity syndrome associated with transient hypogammaglobulinaemia and reactivation of human herpesvirus 6 infection demonstrated by real-time quantitative polymerase chain reaction. Br J Dermatol. 2003 Jul;149(1):165–9. doi: 10.1046/j.1365-2133.2003.05368.x. [DOI] [PubMed] [Google Scholar]
- 43.Smith KJ, Skelton HG, Yeager J, Ledsky R, Ng TH, Wagner KF. Increased drug reactions in HIV-1-positive patients: a possible explanation based on patterns of immune dysregulation seen in HIV-1 disease. The Military Medical Consortium for the Advancement of Retroviral Research (MMCARR) Clin Exp Dermatol. 1997 May;22(3):118–23. [PubMed] [Google Scholar]
- 44.Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International Consensus on drug allergy. Allergy. 2014 Apr;69(4):420–37. doi: 10.1111/all.12350. [DOI] [PubMed] [Google Scholar]
- 45.Picard D, Janela B, Descamps V, D’Incan M, Courville P, Jacquot S, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. 2010 Aug 25;2(46):46ra62. doi: 10.1126/scitranslmed.3001116. [DOI] [PubMed] [Google Scholar]
- 46.Kukreja A, Maclaren NK. Current cases in which epitope mimicry is considered as a component cause of autoimmune disease: immune-mediated (type 1) diabetes. Cell Mol Life Sci. 2000 Apr;57(4):534–41. doi: 10.1007/PL00000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ascherio A, Munger KL, Lennette ET, Spiegelman D, Hernan MA, Olek MJ, et al. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA. 2001 Dec 26;286(24):3083–8. doi: 10.1001/jama.286.24.3083. [DOI] [PubMed] [Google Scholar]
- 48.James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997 Dec 15;100(12):3019–26. doi: 10.1172/JCI119856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stene LC, Honeyman MC, Hoffenberg EJ, Haas JE, Sokol RJ, Emery L, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006 Oct;101(10):2333–40. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 50.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008 Oct 23;455(7216):1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deshmukh US, Sim DL, Dai C, Kannapell CJ, Gaskin F, Rajagopalan G, et al. HLA-DR3 restricted T cell epitope mimicry in induction of autoimmune response to lupus-associated antigen SmD. J Autoimmun. 2011 Nov;37(3):254–62. doi: 10.1016/j.jaut.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baker PR, Baschal EE, Fain PR, Triolo TM, Nanduri P, Siebert JC, et al. Haplotype analysis discriminates genetic risk for DR3-associated endocrine autoimmunity and helps define extreme risk for Addison’s disease. J Clin Endocrinol Metab. 2010 Oct;95(10):E263–70. doi: 10.1210/jc.2010-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flynn JC, Gardas A, Wan Q, Gora M, Alsharabi G, Wei WZ, et al. Superiority of thyroid peroxidase DNA over protein immunization in replicating human thyroid autoimmunity in HLA-DRB1*0301 (DR3) transgenic mice. Clin Exp Immunol. 2004 Sep;137(3):503–12. doi: 10.1111/j.1365-2249.2004.02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall MA, Lanchbury JS, Bolsover WJ, Welsh KI, Ciclitira PJ. Celiac disease is associated with an extended HLA-DR3 haplotype which includes HLA-DPw1. Hum Immunol. 1990 Mar;27(3):220–8. doi: 10.1016/0198-8859(90)90052-q. [DOI] [PubMed] [Google Scholar]
- 55.Molina MJ, Mayor AM, Franco AE, Morell CA, Lopez MA, Vila LM. Prevalence of systemic lupus erythematosus and associated comorbidities in Puerto Rico. J Clin Rheumatol. 2007 Aug;13(4):202–4. doi: 10.1097/RHU.0b013e318124a8af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singer JB, Lewitzky S, Leroy E, Yang F, Zhao X, Klickstein L, et al. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat Genet. 2010 Aug;42(8):711–4. doi: 10.1038/ng.632. [DOI] [PubMed] [Google Scholar]
- 57.Daly AK, Day CP. Genetic association studies in drug-induced liver injury. Semin Liver Dis. 2009 Nov;29(4):400–11. doi: 10.1055/s-0029-1240009. [DOI] [PubMed] [Google Scholar]
- 58.Denisov IG, Grinkova YV, Baylon JL, Tajkhorshid E, Sligar SG. Mechanism of Drug-Drug Interactions Mediated by Human Cytochrome P450 CYP3A4 Monomer. Biochemistry. 2015 Mar 17; doi: 10.1021/acs.biochem.5b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ota T, Kamada Y, Hayashida M, Iwao-Koizumi K, Murata S, Kinoshita K. Combination analysis in genetic polymorphisms of drug-metabolizing enzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A5 in the Japanese population. Int J Med Sci. 2015 Jan 1;12(1):78–82. doi: 10.7150/ijms.10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogese MO, Jenkins RE, Maggs JL, Meng X, Whitaker P, Peckham D, et al. Characterization of Peroxidases Expressed in Human Antigen Presenting Cells and Analysis of the Covalent Binding of Nitroso Sulfamethoxazole to Myeloperoxidase. Chem Res Toxicol. 2015 Jan 7; doi: 10.1021/tx500458k. [DOI] [PubMed] [Google Scholar]
- 61.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013 May;1830(5):3143–53. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang W, Jiang YF, Ponnusamy M, Diallo M. Role of Nrf2 in chronic liver disease. World J Gastroenterol. 2014 Sep 28;20(36):13079–87. doi: 10.3748/wjg.v20.i36.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simmons K, Michels AW. Lessons from type 1 diabetes for understanding natural history and prevention of autoimmune disease. Rheum Dis Clin North Am. 2014 Nov;40(4):797–811. doi: 10.1016/j.rdc.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sosenko JM, Krischer JP, Palmer JP, Mahon J, Cowie C, Greenbaum CJ, et al. A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care. 2008 Mar;31(3):528–33. doi: 10.2337/dc07-1459. [DOI] [PubMed] [Google Scholar]
- 65.Sosenko JM, Skyler JS, Palmer JP, Krischer JP, Yu L, Mahon J, et al. The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care. 2013 Sep;36(9):2615–20. doi: 10.2337/dc13-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013 Jun 19;309(23):2473–9. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phillips EJ, Chung WH, Mockenhaupt M, Roujeau JC, Mallal SA. Drug hypersensitivity: pharmacogenetics and clinical syndromes. J Allergy Clin Immunol. 2011 Mar;127(3 Suppl):S60–6. doi: 10.1016/j.jaci.2010.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Concannon P, Onengut-Gumuscu S, Todd JA, Smyth DJ, Pociot F, Bergholdt R, et al. A human type 1 diabetes susceptibility locus maps to chromosome 21q22.3. Diabetes. 2008 Oct;57(10):2858–61. doi: 10.2337/db08-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Concannon P, Onengut-Gumuscu S, Todd JA, Smyth DJ, Pociot F, Bergholdt R, et al. A human type 1 diabetes susceptibility locus maps to chromosome 21q22.3. Diabetes. 2008 Oct;57(10):2858–61. doi: 10.2337/db08-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009 Apr 16;360(16):1646–54. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 71.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009 Jun;41(6):703–7. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pugliese A, Yang M, Kusmarteva I, Heiple T, Vendrame F, Wasserfall C, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes. 2014 Feb;15(1):1–9. doi: 10.1111/pedi.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013 Nov;169(5):1071–80. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 74.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32(1):55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med. 2013 Feb 27;5(174):174ra28. doi: 10.1126/scitranslmed.3005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ito Y, Hashimoto M, Hirota K, Ohkura N, Morikawa H, Nishikawa H, et al. Detection of T cell responses to a ubiquitous cellular protein in autoimmune disease. Science. 2014 Oct 17;346(6207):363–8. doi: 10.1126/science.1259077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thong BY, Mirakian R, Castells M, Pichler W, Romano A, Bonadonna P, et al. A world allergy organization international survey on diagnostic procedures and therapies in drug allergy/hypersensitivity. World Allergy Organ J. 2011 Dec;4(12):257–70. doi: 10.1097/WOX.0b013e31823dc02c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lucas A, Lucas M, Strhyn A, Keane NM, McKinnon E, Pavlos R, et al. Abacavir-reactive memory T cells are present in drug naive individuals. PLoS One. 2015 Feb 12;10(2):e0117160. doi: 10.1371/journal.pone.0117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kinnunen T, Chamberlain N, Morbach H, Choi J, Kim S, Craft J, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013 Feb 28;121(9):1595–603. doi: 10.1182/blood-2012-09-457465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang P, Zheng SG. Regulatory T cells and B cells: implication on autoimmune diseases. Int J Clin Exp Pathol. 2013 Nov 15;6(12):2668–74. [PMC free article] [PubMed] [Google Scholar]
- 81.Genin E, Chen DP, Hung SI, Sekula P, Schumacher M, Chang PY, et al. HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: an international study and meta-analysis. Pharmacogenomics J. 2014 Jun;14(3):281–8. doi: 10.1038/tpj.2013.40. [DOI] [PubMed] [Google Scholar]
- 82.Bharadwaj M, Illing P, Theodossis A, Purcell AW, Rossjohn J, McCluskey J. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol. 2012 Feb 10;52:401–31. doi: 10.1146/annurev-pharmtox-010611-134701. [DOI] [PubMed] [Google Scholar]
- 83.Cornejo Castro EM, Carr DF, Jorgensen AL, Alfirevic A, Pirmohamed M. HLA-allelotype associations with nevirapine-induced hypersensitivity reactions and hepatotoxicity: a systematic review of the literature and meta-analysis. Pharmacogenet Genomics. 2015 Apr;25(4):186–98. doi: 10.1097/FPC.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 84.Vitezica ZG, Milpied B, Lonjou C, Borot N, Ledger TN, Lefebvre A, et al. HLA-DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008 Feb 19;22(4):540–1. doi: 10.1097/QAD.0b013e3282f37812. [DOI] [PubMed] [Google Scholar]
- 85.Kindmark A, Jawaid A, Harbron CG, Barratt BJ, Bengtsson OF, Andersson TB, et al. Genome-wide pharmacogenetic investigation of a hepatic adverse event without clinical signs of immunopathology suggests an underlying immune pathogenesis. Pharmacogenomics J. 2008 Jun;8(3):186–95. doi: 10.1038/sj.tpj.6500458. [DOI] [PubMed] [Google Scholar]
- 86.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007 Jun 7;447(7145):661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003 May 29;423(6939):506–11. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 88.Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006 Jun;38(6):617–9. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 89.Torres MJ, Mayorga C, Blanca-Lopez N, Blanca M. Hypersensitivity reactions to beta-lactams. EXS. 2014;104:165–84. doi: 10.1007/978-3-0348-0726-5_11. [DOI] [PubMed] [Google Scholar]
- 90.Chessman D, Kostenko L, Lethborg T, Purcell AW, Williamson NA, Chen Z, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008 Jun;28(6):822–32. doi: 10.1016/j.immuni.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 91.Keane NM, Roberts SG, Almeida CA, Krishnan T, Chopra A, Demaine E, et al. High-avidity, high-IFNgamma-producing CD8 T-cell responses following immune selection during HIV-1 infection. Immunol Cell Biol. 2012 Feb;90(2):224–34. doi: 10.1038/icb.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]