Abstract
Purpose of Review
The use of the erythropoiesis stimulating agent erythropoietin (Epo) has been studied as a red cell growth factor in preterm and term infants for over 20 years. Recent studies have evaluated Darbepoetin (Darbe, a long acting ESA) for both erythropoietic effects and potential neuroprotection. We review recent clinical trials of Darbe in term and preterm infants.
Findings
Clinical studies in term and preterm infants have reported significant erythropoietic uses for Darbe as well as neuroprotective effects following ESA administration, and improved neurodevelopmental outcomes have been reported in studies of preterm infants.
Summary
Darbe shows great promise in decreasing or eliminating transfusions in neonates, and in preventing and treating brain injury in term and preterm infants.
Keywords: Anemia, Transfusions, Neuroprotection, Neurodevelopment, Darbepoetin, Erythropoiesis Stimulating Agents
Introduction
Premature infants receive a greater number of transfusions with exposure to a greater number of donors compared to term neonates. Transfusion guidelines are now used in many neonatal units; however, the search for the most appropriate transfusion guidelines continues (1), and long-term outcomes of neonates previously enrolled in transfusion studies are still being studied (2,3). Additionally, prematurity is a significant risk factor for neurodevelopmental delay. Mental retardation, cerebral palsy, and learning disabilities are neurodevelopmental deficits identified in former premature infants (4). While significant progress has been made in improving survival of extremely low birth weight infants (ELBW) infants, improved neurodevelopmental outcomes remain elusive.
The use of erythropoiesis stimulating agents (ESAs) such as erythropoietin (Epo) to improve neuroprotection has been validated in both animal and human studies (5). Both retrospective studies and prospective randomized trials have been performed in neonatal populations at greatest risk for long-term developmental abnormalities, namely ELBW infants, and term infants with hypoxic ischemic encephalopathy (HIE). This review outlines data that support darbepoetin (Darbe) as both an ESA and a neuroprotective agent, and reviews promising clinical studies that demonstrate benefit in premature infants, and safety in term infants undergoing cooling for HIE.
Clinical Studies
Infants born prematurely do not increase production of endogenous Epo in order to make new red cells, and thus are at risk for repeated transfusions (6). Numerous studies evaluating the use of recombinant human erythropoietin (rHuEpo) to prevent and treat the anemia of prematurity show that it is successful in preterm infants in stimulating erythropoiesis, and transfusion requirements are decreased (7).
Studies in newborn monkeys and sheep demonstrated that neonates have a larger volume of distribution and a more rapid elimination of Epo, necessitating the use of higher doses than required for adults (8,9). In preterm infants, the volume of distribution is three- to four-fold greater than that seen in adults, while the clearance is also three to four times greater (8,9). This was confirmed in very low birth weight (VLBW) infants (10). Although we anticipated that similar pharmacokinetics would exist with Darbe, it remained to be determined clinically.
Preliminary in vitro studies of the effects of Darbe compared to rHuEpo on fetal and neonatal erythroid progenitors showed similar responsiveness (11). Erythroid progenitor cells were isolated from 12–22 week fetal liver and marrow, and from term (37–41 weeks) and preterm (<32 weeks) cord blood. The number of burst forming units-erythroid (BFU-E) colonies derived from fetal marrow progenitor cells increased significantly with both Darbe (p<0.01, 10 vs. 50, 100, and 500 ng/mL; Figure 1) and rHuEpo (p <0.01, 0.05 vs. 0.5, 1.0, and 2 U/mL). BFU-E cell counts revealed similar numbers of normoblasts per colony between Darbe and rHuEpo, and BFU-E size increased with increasing concentrations of both growth factors. Progenitors isolated from fetal liver and from term and preterm cord blood were similarly responsive. When compared with term cord blood progenitors, preterm cord blood progenitors were more sensitive to Darbe at every concentration tested (p<0.01).
Figure 1.
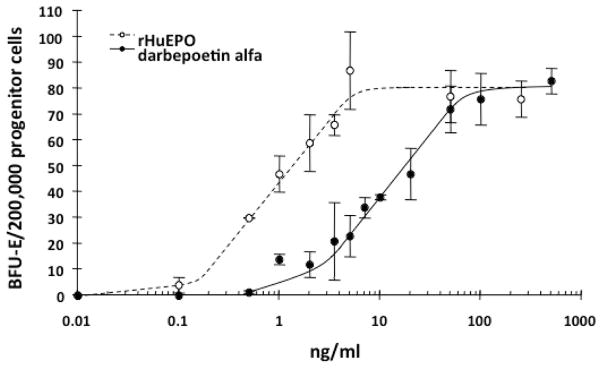
Dose response curves for rHuEpo (open circles) and Darbe (solid circles). Progenitor cells isolated from 12 to 24 week gestation fetal marrow were cultured for 10–14 days in increasing concentrations of Darbe (0–500 ng/ml) or protein equivalent concentrations of rHuEpo. The number of BFU-E increased significantly (p<0.01, 10 vs. 50, 100, and 500 ng/mL Darbe, and p <0.01, 0.05 vs. 0.5, 1.0, and 2 U/mL rHuEpo).
Darbe dosing and pharmacokinetics
Adult studies of Darbe pharmacokinetics demonstrated a half-life (t1/2) of 49 hours after a single subcutaneous dose (SC) and 25 hours after intravenous dosage (IV) (12). Table 1 (13–17) presents AUC following administration of ESAs (Darbe or Epo) in animal models and neonates. Notably, it is clear that there have been limited studies evaluating Darbe dosing and pharmacokinetics in neonates. Below, we review results from trials involving preterm infants.
Table 1.
Area under the curve (AUC) in ESA studies
| Study | Epo 1000 U/kg | Epo 2500 U/kg | Epo 3500 U/kg | Epo 5000 U/kg | Darbe 2 μg/kg | Darbe 10 μg/kg |
|---|---|---|---|---|---|---|
| Rats1 (Statler et al) | ---- | ---- | ---- | 140,331 | ---- | ---- |
| Primates2 (Traudt et al) | 94,377±5801 | ---- | 413,948±39,605 | ---- | ---- | ---- |
| ELBW infants3 (Juul et al) | 81,498±7,067 | 317,881±22,941 | ---- | ---- | ---- | |
| Term HIE4 (Wu et al) | 131,054±17,083 | 328,000±61,945 | ---- | ---- | ---- | ---- |
| Term HIE5 (Baserga et al) | ---- | ---- | ---- | ---- | 26,555 (20,049–35,029) | 180,886 (146,568–199,680) |
In a study by Warwood et al, neonates received a single SC 1 or 4 μg/kg dose of Darbe. Twelve infants <32 weeks gestation were enrolled, with birth weights 1129±245 grams, and 29.2±1.2 weeks gestation at birth. Darbe concentrations peaked at 6–12 hours after administration. A single, SC dose resulted in serum concentrations 54–308 mU/ml with a 1 μg/kg dose and 268–980 mU/kg with a 4 μg/kg dose. The t1/2 was 26 hours (range 10 to 50 hours, mean 29.6 for 1 μg/kg group and 21.5 for 4 μg/kg group). Clearance was 17.1 ml/hr/kg for the 1 μg/kg group and 20.7 μg/hr/kg for the 4 μg/kg group. Clinically, both immature (IRC) and absolute (ARC) reticulocyte counts significantly increased (12).
The same group analyzed pharmacokinetics after administration of a single, 4 μg/kg IV dose of Darbe. Ten neonates were enrolled, with gestational ages between 26 and 40 weeks (7 neonates <32 weeks, 3 neonates > 32 weeks). Doses were administered between 3 and 28 days. The t1/2 was 10.1 hours, the volume of distribution was 0.77 L/kg (range 0.180–3.05 L/kg) and clearance was 52.8 ml/hr/kg (range 22.4–158.0 ml/kg/hr). Both volume of distribution and clearance were increased in comparison to older children and adults. In comparison to SC dosing, there was a less consistent rise in both IRC and ARC (18). These studies suggested that dosing needed to be higher (μg/kg) and more frequent than that used in children and adults.
We previously evaluated reticulocyte responses to SC Darbe administration in preterm infants randomized in a blinded Darbe dose-response study (19). Preterm infants ≤1,500 grams and ≥10 days of age were randomized to placebo or 2.5, 5, or 10 μg/kg/dose Darbe, given once a week SC for 4 weeks. Complete blood counts, reticulocyte counts, transfusions and adverse events (AE) were recorded. Eighteen preterm infants (896±59 grams, 28.7±0.7 weeks gestation, 13±1 days of age) were enrolled (Table 2). Infants randomized to 10 μg/kg/dose achieved the highest reticulocyte counts by day 14 of the study (Figure 2, panel A; p=0.04). Infants receiving any dose of Darbe maintained hematocrits at a higher level at 14 days than infants receiving placebo (Figure 2, panel B; p=0.002). Infants receiving 5 or 10 μg/kg/dose required fewer transfusions during the study period (Table 2; p=0.006). No AEs were noted. We concluded that preterm infants respond to Darbe by increasing erythropoiesis in a dose-dependent fashion, with the greatest reticulocyte response occurring with 10 μg/kg/dose. Both the 5 and 10 μg/kg/doses were sufficient to decrease transfusions in preterm infants.
Table 2.
Characteristics of Study Infants, Darbe Dose-Response Study
| 0 μg/kg/dose | 2.5 μg/kg/dose | 5 μg/kg/dose | 10 μg/kg/dose | |
|---|---|---|---|---|
| Birth weight (grams) | 961±83 | 872±123 | 970±138 | 774±43 |
| Gestation (weeks) | 29.0±2.0 | 30.1±1.4 | 27.4±0.9 | 28.5±1.3 |
| Days of Age | 14±4 | 17±2 | 12±1 | 11±1 |
| Baseline Hematocrit (%) | 34.0±3.0 | 32.3±4.2 | 33.5±2.7 | 34.7±3.3 |
| Baseline Reticulocyte Count (x103/mL) | 50±10 | 97±23 | 95±23 | 127±61 |
| Transfusions during study (number) | 1.7±0.3 | 2.3±0.3 | 0.5±0.3* | 1.3±0.3* |
| Transfusion volume during study (mL) | 27±7 | 34±6 | 13±3 | 23±7 |
p=0.006, 5 and 10 μg/kg doses versus 0 and 2.5 μg/kg
Figure 2.
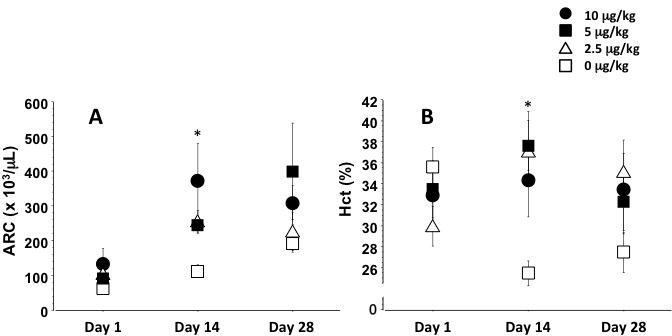
Changes in reticulocyte count (panel A) and hematocrit (panel B) in preterm infants treated with 4 weekly Darbe doses (0 ≥g/kg [placebo]: open squares; 2.5 ≥g/kg: solid triangles; 5≥g/kg: solid squares; 10 ≥g/kg: solid circles). Reticulocyte counts increased by day 14 in infants receiving either 5 or 10 ≥g/kg dosing. Infants receiving 10 ≥g/kg had the greatest reticulocyte response (p=0.04 versus placebo). Hematocrits were greater by day 14 in infants receiving any Darbe (p=0.006 versus placebo).
Preterm ESA Studies
Preterm infants have received fewer transfusions over the last several years compared to previous decades (20). This has been in part due to an increased awareness of morbidities, including transfusion-related lung injury, donor exposure, and transfusion-related intestinal injury (21). The development and adherence to transfusion guidelines, cord milking/delayed cord clamping, and reduction in phlebotomy losses have all contributed to an overall decrease in transfusions (20). Some studies have demonstrated that the more liberal use of blood transfusions may not improve outcomes (2). Interestingly, in a recent quality improvement project done across 4 newborn intensive care units (NICU), Henry and colleagues demonstrated that transfusion rates are further decreased with the use of anemia prevention guidelines. Such guidelines included initiating iron and Darbe treatment, instituting delayed cord clamping/cord milking, and introducing policies for limiting phlebotomy losses. The study found that the NICUs that adhered to such anemia preventing guidelines also had lower rates of neonatal morbidities, such as necrotizing enterocolitis (≥ Bell stage 2), retinopathy of prematurity (ROP) (≥ stage 3), or severe intraventricular hemorrhage (≥ grade 3) (20). The results of trials in which preterm infants received Darbe as an anemia preventing technique are reviewed below.
In a recent single center trial by Warwood et al, 20 VLBW infants were randomized to receive a single, 10 μg/kg dose of Darbe or placebo within 30 minutes of initiating a transfusion. Infants were enrolled at an average age of 34.5 to 35.1 days (corresponding with physiologic hematocrit nadir). Infants in the Darbe group had a significant increase in their absolute reticulocyte count (ARC) throughout the study period. Additionally, the Darbe group appeared to maintain higher hematocrits following the transfusion. This study was a small pilot study, but the findings suggested that Darbe treatment may abrogate the transfusion-induced suppression of erythropoiesis (21).
Based on our preliminary Darbe dose response study and previous work comparing Epo concentrations to cognitive outcome (22), we designed a multicenter, randomized, placebo controlled study of Darbe and Epo administration to preterm infants. Infants with birth weights between 500–1,250 grams and ≤48 hours of age were randomized to Darbe (10 μg/kg, 1x/week SC), Epo (400 units/kg, 3x/week SC) or placebo (sham dosing) through 35 weeks gestation. All were transfused according to protocol, and received supplemental iron, folate, and vitamin E. Transfusions (primary outcome), complete blood counts, ARC, phlebotomy losses, and AEs were recorded. Infants in the ESA groups received significantly fewer transfusions (p=0.015) and were exposed to fewer donors (p=0.044) than the placebo group (Darbe: 1.2±2.4 transfusions and 0.7±1.2 donors/infant; Epo: 1.2±1.6 transfusions and 0.8±1.0 donors/infant; placebo: 2.4±2.9 transfusions and 1.2±1.3 donors/infant). ESA-treated infants had a 50% reduction in transfusions and donor exposure than placebo-treated infants. Hematocrit and ARC were higher in the Darbe and Epo groups compared to placebo (p=0.001, Darbe and Epo versus placebo for both hematocrit and ARC). Morbidities were similar among groups, including the incidence of ROP. We concluded that infants receiving ESAs received fewer transfusions and fewer donor exposures, and fewer injections were given to Darbe recipients (23). Importantly, greater than 50% of VLBW infants in the ESA group remained transfusion free during their NICU stay.
Preterm neuroprotection
We were able to complete follow up on 80 (29 Epo, 27 Darbe, 24 placebo) of the 99 infants enrolled and evaluated during the hospital course in the above mentioned study (23,24) (Table 3). After adjusting for gender, analysis demonstrated infants who had received Darbe or Epo had significantly higher cognitive scores (96.2±7.3 and 97.9±14; mean±standard deviation) in comparison to placebo recipients (88.7±13.5; p=0.01 vs. ESA recipients). The ESA group also scored significantly higher on object permanence, an early test of executive function (p=0.05). We found no cerebral palsy among recipients of Darbe or Epo, while 5 cases of cerebral palsy were identified in the placebo group (p<0.001). The incidence of neurodevelopmental impairment in the ESA group was significantly lower compared to placebo (Odds Ratio 0.18; 95% Confidence Interval 0.05 to 0.63) (24). There were no differences in visual or hearing impairment among groups. The significance of improved neurodevelopmental outcomes in preterm infants demonstrated by this study (when confirmed by a larger trial) would support the use of Darbe in this population, especially given Darbe’s weekly dosing schedule.
Table 3.
Infant characteristics and neurodevelopmental outcomes from ESA RCT
| Darbe (n=27) | Epo (n=29) | Placebo (n=24) | p | Adjusted Odds Ratio | p | |
|---|---|---|---|---|---|---|
| Birth Weight (grams) | 938.3 ± 176.5 | 947.2 ± 212.7 | 953.0 ± 210.0 | NS | ||
| Gestation (weeks) | 28.1 ± 1.8 | 27.8 ± 1.9 | 27.8 ± 1.6 | NS | ||
| Corrected age at follow up (months) | 20.5 ± 1.1 | 21.2 ± 2.0 | 20.6 ± 1.9 | NS | ||
| Composite cognitive | 96.2±7.5 | 97.9±14.0 | 88.7±13.3 | 0.01 | ||
| Composite language | 92.4±12.8 | 89.9±17.4 | 83.6±13.9 | 0.05 | ||
| Object permanence | 2.8±0.4 | 2.4±0.8 | 2.2±1.0 | 0.05 | ||
|
| ||||||
| Neurodevelopmental Impairment: | 3 (11%) | 2 (7%) | 9 (38%) | 0.18 (0.05 to 0.63) | 0.01 | |
| Cerebral Palsy (%) | 0 (0) | 0 (0) | 5 (21) | 0.002 | 0 (0 to 0) | <0.001 |
| Visual deficit (%) | 1 (4) | 0 (0) | 1 (4) | 0.09 | 0.23 (0.04 to 1.54) | NS |
| Hearing deficit (%) | 0 | 1 (3) | 1 (4) | 0.33 | 0.54 (0.11 to 2.65) | NS |
| Composite Cognitive Score<85 | 0 | 3 (10.3%) | 6 (25%) | 0.02 | 0.18 (0.04 to 0.78) | 0.01 |
| Composite Cognitive Score<70 | 0 | 1 (3.4%) | 2 (8.3%) | 0.29 | ||
Odds Ratios adjusted for gender. Values represent mean±SD or number (percent). P values represent three way comparisons among groups.
Importantly, our group did not observe any differences in the incidence of any stage of ROP during the hospital phase, and no differences in visual impairment at 18–22 months corrected age. Hesitancy in the use of ESAs is linked to a 2006 meta-analysis, which suggested an association between early (first week of life) Epo administration and ROP>stage 2 (6) compared with late Epo administration (25). However, this meta analysis reflected a misclassification of a single center Epo study by Romagnoli et al into the early Epo administration group (26). When this study was correctly grouped with other studies of late Epo use, the revised meta analysis showed no significant difference in ROP with the use of early or late Epo. Evaluation for ROP continues to be a priority in most clinical trials of ESA use for preterm infants.
Term neuroprotection studies
Based on the previous studies a number of multicenter clinical trials evaluating ESAs in conjunction with hypothermia in term infants with HIE are being planned. Only one study to date has evaluated Darbe as a potential neuroprotective agent in term infants undergoing hypothermia as treatment for HIE. The DANCE study (Darbepoetin administered to neonates undergoing cooling for encephalopathy) lead by Baserga (17) enrolled 30 infants (≥36 weeks gestation) with moderate to severe HIE. Infants were randomized to placebo (n=10), 2 μg/kg Darbe (n=10) or 10 μg/kg Darbe (n=10) IV. The first dose was administered before 12 hours of life and the second on day 7. AEs and serious AEs were documented for 1 month. Serum samples were obtained at specific time points to determine pharmacokinetics. At 2 and 10 μg/kg Darbe, t1/2 was 24 and 32 hours, and the area under the curve extrapolated to infinity (AUCinf) was 26,555 (interquartile range (IRQ) 20,049–35,029) and 180,886 mU/mL (IRQ 146–568–199,680), respectively (17). The investigators concluded that the 10 μg/kg dose achieved an AUC in the neuroprotective range, and a terminal t1/2 of 53.4 hours (5) when compared to the 2 μg/kg dose. No side effects attributable to Darbe were reported. These infants are still being followed for long term developmental outcomes. If these outcomes are similar to infants treated with Epo, Darbe’s longer t1/2 and fewer number of doses needed may convey a clinical benefit.
The t1/2 of the 10 μg/kg Darbe dose reported in the DANCE study was approximately 3 times the t1/2 of Epo reported in the Neonatal Erythropoietin in Asphyxiated Term Newborns (NEAT) Trial (16). In the NEAT Trial Yvonne Wu and colleagues randomized 24 infants with moderate to severe HIE to an open label dose escalation trial of Epo (NEAT Trial: Neonatal Erythropoietin in Asphyxiated Term Newborns, NCT00719407). Infants received either 250, 500, 1,000, or 2,500 units/kg of Epo administered IV every other day for up to 6 doses. Pharmacokinetics were performed with the first, second and last doses, and infants were monitored closely for any adverse effects. Epo followed non-linear pharmacokinetics, but did not accumulate with multiple dosing. The area under the curve was 50,306, 131,054 and 328,002 unit*hour/L for 500, 1,000 and 2,500 units/kg, respectively. No serious adverse effects or deaths were reported. They concluded that 1,000 units/kg Epo administered intravenously in conjunction with hypothermia was well tolerated and produced plasma concentrations that in previous studies were neuroprotective in animals.
A number of adult studies have evaluated neuroprotective properties of ESAs. The pathology of neuronal disease and patient characteristics are different; however, information on mechanism of neuroprotection may be useful in the study of neonatal neuronal injury. A study by Messe and colleagues evaluating the effects of Darbe on adults undergoing aortic surgery (27) was terminated early when the US Food and Drug Association placed a hold on all ESA studies of neuroprotection after Epo recipients in a European multicenter study of stroke patients were found to have increased mortality (28). Only 9 adults in Messe’s study received 1 mg/kg Darbe immediately prior to surgery. An additional 9, untreated adult patients were added as a comparison cohort. Although this small number of enrolled patients prevented appropriate analysis, results demonstrated primary outcome of death or neurologic impairment between groups was non-significant (1/9, 11% in the Darbe group and 3/9, 33% in control group, p=0.58). Significantly higher concentrations of cerebral spinal fluid biomarkers (S100-beta and glial fibrillary acidic protein (GFAP)) were found in patients with perioperative neurologic ischemia; however, there were no differences in these biomarkers between the Darbe and control groups (S100-beta: 214 versus 260 ng/mL, p=0.69; GFAP: 22 versus 580 pg/mL, p=0.45 (27).
Summary
Clinical studies in preterm and term infants have shown Darbe to be safe, and evidence of neuroprotection is growing. Preterm infants administered Darbe during their NICU stay show fewer need for transfusions, higher cognitive scores and a lower incidence of neurodevelopmental impairment. Darbe is consequently being used as an ESA across several NICUs in the country. Studies of Darbe administration in term infants with HIE being cooled for encephalopathy are ongoing. Developmental follow up of those term infants is progressing. ESAs hold great promise in providing neuroprotection and improving neurodevelopmental outcomes of preterm neonates.
Key Points.
Darbe, a long acting ESA, is being studied as an anemia prevention treatment for preterm infants.
Darbe is also promising as an agent for neuroprotection in both term and preterm infants.
Further research is needed to evaluate long term and neurodevelopmental outcomes in term and preterm infants treated with Darbe.
Footnotes
Disclosure Statement: Dr. Ohls’ research sited in this work was funded by the Thrasher Research fund and by the NIH (R01HD059856).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited from
- 1.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 2.McCoy TE, Conrad AL, Richman LC, et al. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol. 2011;17:347–67. doi: 10.1080/09297049.2010.544647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whyte RK, Kirplani H, Aszatalos EV, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123:207–13. doi: 10.1542/peds.2008-0338. [DOI] [PubMed] [Google Scholar]
- 4.Stephens BE, Vohr BR. Protein intake and neurodevelopmental outcomes. Clin Perinatol. 2014;41:323–9. doi: 10.1016/j.clp.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Juul S. Neuroprotective role of erythropoietin in neonates. J Matern Fetal Neonatal Med. 2012;25 (Suppl 4):105–7. doi: 10.3109/14767058.2012.715025. [DOI] [PubMed] [Google Scholar]
- 6.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006:CD004863. doi: 10.1002/14651858.CD004863.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Bishara N, Ohls RK. Current controversies in the management of the anemia of prematurity. Semin Perinatol. 2009;33:29–34. doi: 10.1053/j.semperi.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Widness JA, Veng-Pedersen P, Peters C, et al. Erythropoietin pharmacokinetics in premature infants: developmental, nonlinearity, and treatment effects. J Appl Physiol (1985) 1996;80:140–8. doi: 10.1152/jappl.1996.80.1.140. [DOI] [PubMed] [Google Scholar]
- 9.George JW, Bracco CA, Shannon KM, et al. Age-related differences in erythropoietic response to recombinant human erythropoietin: comparison in adult and infant rhesus monkeys. Pediatr Res. 1990;28:567–71. doi: 10.1203/00006450-199012000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Ohls RK, Veerman MW, Christensen RD. Pharmacokinetics and effectiveness of recombinant erythropoietin administered to preterm infants by continuous infusion in parenteral nutrition solution. J Pediatr. 1996;128:518–523. doi: 10.1016/s0022-3476(96)70363-3. [DOI] [PubMed] [Google Scholar]
- 11.Ohls RK, Dai A. Long-acting erythropoietin: clinical studies and potential uses in neonates. Clin Perinatol. 2004;31:77–89. doi: 10.1016/j.clp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Warwood TL, Ohls RK, Wiedmeier SE, et al. Single-dose darbepoetin administration to anemic preterm neonates. J Perinatol. 2005;25:725–30. doi: 10.1038/sj.jp.7211387. [DOI] [PubMed] [Google Scholar]
- 13.Statler PA, McPherson RJ, Bauer LA, et al. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr Res. 2007;61:671–5. doi: 10.1203/pdr.0b013e31805341dc. [DOI] [PubMed] [Google Scholar]
- 14.Traudt CM, McPherson RJ, Bauer LA, et al. Concurrent erythropoietin and hypothermia treatment improve outcomes in a term nonhuman primate model of perinatal asphyxia. Dev Neurosci. 2013;35:491–503. doi: 10.1159/000355460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juul SE, McPherson RJ, Bauer LA, et al. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122:383–91. doi: 10.1542/peds.2007-2711. [DOI] [PubMed] [Google Scholar]
- 16.Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130:683–91. doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baserga MC, Beachy JC, Roberts JK, et al. DANCE (darbe administration in newborns undergoing cooling for encephalopathy): a safety and pharmacokinetics trial. Pediatric Research. 2015 doi: 10.1038/pr.2015.101. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warwood TL, Ohls RK, Lambert DK, et al. Intravenous administration of darbepoetin to NICU patients. J Perinatol. 2006;26:296–300. doi: 10.1038/sj.jp.7211498. [DOI] [PubMed] [Google Scholar]
- 19.Roohi M, Peceny MC, Ohls RK. A randomized, masked, dose response study of darbepoetin administered to preterm infants [abstract 5899.3). Programs and abstracts of the Pediatric Academic Society Annual Meeting; Toronto. 2007; EPAS2007: 5899.3. [Google Scholar]
- 20.Henry E, Christensen RD, Sheffield MJ, et al. Why do four NICUs using identical RBC transfusion guidelines have different gestational age-adjusted RBC transfusion rates? J Perinatol. 2015;35:132–6. doi: 10.1038/jp.2014.171. [DOI] [PubMed] [Google Scholar]
- 21.Warwood TL, Lambert DK, Henry E, et al. Very low birth weight infants qualifying for a ‘late’ erythrocyte transfusion: does giving darbepoetin along with the transfusion counteract the transfusion’s erythropoietic suppression? J Perinatol. 2011;31:S17–21. doi: 10.1038/jp.2010.165. [DOI] [PubMed] [Google Scholar]
- 22.Bierer R, Peceny MC, Harenberger CH, et al. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006;118:e635–40. doi: 10.1542/peds.2005-3186. [DOI] [PubMed] [Google Scholar]
- 23.Ohls RK, Christensen RD, Kamath-Rayne BD, et al. A randomized, masked, placebocontrolled study of darbepoetin alfa in preterm infants. Pediatrics. 2013;132:e119–27. doi: 10.1542/peds.2013-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohls RK, Kamath-Rayne BD, Christensen RD, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133:1023–30. doi: 10.1542/peds.2013-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aher S, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006:CD004868. doi: 10.1002/14651858.CD004868.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Romagnoli C, Zecca E, Gallini F, et al. Do recombinant human erythropoietin and iron supplementation increase the risk of retinopathy of prematurity? Eur J Pediatr. 2000;159:627–8. doi: 10.1007/pl00008390. [DOI] [PubMed] [Google Scholar]
- 27.Messe SR, McGarvey ML, Bavaria JE, et al. A pilot study of darbepoetin alfa for prophylactic neuroprotection in aortic surgery. Neurocrit Care. 2013;18:75–80. doi: 10.1007/s12028-012-9710-4. [DOI] [PubMed] [Google Scholar]
- 28.Ehrenreich H, Wessenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–56. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]


