Abstract
This paper evaluates the effects of adenoviral vector-mediated glial cell-derived neurotrophic factor (GDNF) gene delivery on survival of primary human corneal epithelial cells (PHCEC) established from limbal explants in vitro and the overexpression of GDNF gene in bioengineered human corneal constructs on substrate of corneal stromal discs followed by autograft ex vivo. In vitro, the overexpression of GDNF in the supernatant of PHCEC peaked at day 4, but lasted for at least 4 weeks after the transduction mediated by adenoviral vector. At day 10, the cell viability was 2 fold greater (P < 0.001), the number of terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling (TUNEL)-positive cells was more than 50% lower (P < 0.01) in the GDNF transduction group than the non-transduction group. 5 weeks after the transduction, the living cell population was greater in the GDNF transduction group than the non-transduction group (P < 0.01). In the ex vivo autograft of the bioengineered human corneal constructs, outgrowth of enhanced green fluorescent protein (eGFP) positive cells on the recipient corneoscleral tissue was observed. overexpression of GDNF in the supernatant peaked at day 2, but was observed for at least 4 weeks after transplantation. At day 5, immunofluorescent staining showed expression of GDNF by all layers of epithelial cells on the graft. Our findings revealed that GDNF is a survival growth factor for cultured human corneal epithelium. The use of bioengineered human corneal constructs containing GDNF-transduced epithelial cells represents a novel method for delivering of thus gene to promote survival of transplanted corneal epithelium to treat various corneal surface diseases.
Keywords: cornea, epithelium, glial cell-derived neurotrophic factor, gene therapy, adenoviral vector, bioengineering
1. INTRODUCTION
The cornea is an attractive target for gene therapy because of its immune-privileged nature, its well-defined anatomy, its transparency and its accessibility. Preclinical work in this field has concentrated on treating and preventing several acquired infections and inflammatory disorders such as herpes simplex keratitis, corneal graft rejection, corneal neovascularization and corneal haze (Klausner et al., 2007). There are many other potential applications for corneal gene therapy, including those aimed at replacing defective genes in corneal dystrophies, promoting corneal epithelial stem cell survival and treating recurrent erosions, dry eye, non healing corneal ulcers and neurotrophic keratopathy (Mohan et al., 2005;Rosenblatt and Azar, 2004).
Glial cell-derived neurotrophic factor (GDNF) is a distant member of the transforming growth factor (TGF)-ß superfamily of growth factors and a member of the GDNF family (which also includes neurturin, persephin, and artemin) (Massague et al., 1994). GDNF is widely and primarily distributed in the central and peripheral nervous systems, including the retina in the eye. Several investigators have pointed out that GDNF could be used therapeutically to provide neuroprotection and to rescue photoreceptors in the context of retinal degeneration (Frasson et al., 1999; Koeberle and Ball, 1998;Yan et al., 1999). In addition to the nervous system, the biological significance of GDNF in other cell types, such as epithelial cells was also investigated. For example, GDNF was identified as an essential growth factor supporting self-renewal of spermatogonial stem cells (Meng et al., 2000;Oatley et al., 2006). In the corneal epithelium, GDNF and its specific receptor GDNF family receptor alpha1 have been found to be expressed by a subset of basal cells in the human limbal basal epithelium, suggesting GDNF may play a vital role in maintaining corneal epithelial stem cells in the limbus (Qi et al., 2007).
The cornea is the most commonly transplanted organ. Unlike other transplantable tissues, it can be maintained in culture for several weeks which allows a window of opportunity for genetic modification for ex vivo gene therapy. Additionally, stem cells located at the corneal limbus provide a renewable source of epithelium, which allows for rapid healing in the healthy cornea. The ex vivo expansion of limbal corneal epithelial stem cells on substrates such as human amniotic membranes or fibrin-based substrates in vitro, followed by transplantation, provides a new modality for the treatment of total limbal stem cell deficiency (Ang and TAN, 2004;Koizumi et al., 2001;Pellegrini et al., 1997;Schwab et al., 2000;Tsai et al., 2000). Watanabe K and colleagues (Watanabe et al., 2007) developed a transplantable cell sheet graft that is composed of genetically modified corneal epithelial cells. As yet, progress has been slow with regard to the transplantation of cultured, genetically modified epithelium. There are no reports of utilizing transplantable limbal corneal epithelial stem cells expended in culture as a gene delivery target. In the present study, we evaluated the effects of GDNF overexpression on survival of primary human corneal epithelial cells (PHCEC) in culture and in bioengineered corneal constructs.
2. MATERIALS AND METHODS
2.1. Materials and Reagents
Cell culture dishes, plates, centrifuge tubes and other plastic items were purchased from Becton Dickinson (Bedford, MA; http://www.bdbiosciences.com). Optimal cutting temperature (OCT) compound and cryomolds were from Sakura Finetek USA Inc (Torrance, CA; http://www.sakura-americas.com). Nunc Lab-Tek II eight-chamber slides were from VWR International (West Chester, PA; http://www.vwr.com). Dulbecco modified Eagle medium (DMEM), Ham F-12, amphotericin B, gentamicin, 0.25%trypsin/0.03%EDTA solution and Fluorescein Alexa Fluor 488 (green) conjugated goat anti-rabbit IgG were from Invitrogen (Carlsbad, CA; http://www.invitrogen.com). Fetal bovine serum (FBS) was from Hyclone (Logan, UT; http://www.hyclone.com). Affinity-purified rabbit polyclonal antibody (pAb) against GDNF for immunofluorescent staining was purchased from Lab Vision (Fremont, CA; http://www.labvision.com). Propidium iodide (PI) was purchased from Sigma-Aldrich (St. Louis, MO; http://www.sigmaaldrich.com). MTT reagent (TA5355, TACS) and detergent reagent were from R&D Systems, Inc. (Minneapolis, MN; http://www.rndsystems.com). ApopTag® Fluorescein in Situ Apoptosis Detection Kit (S7110) was from Chemicon (Temecula, CA; http://www.chemicon.com).
2.2. Recombinant Adenoviral Vectors
The construction and characterization of the adenoviral vector carrying the rat GDNF gene under control of the Rous sarcoma virus long-terminal-repeat promoter (Adv.RSV-GDNF) was previously described (Baumgartner and Shine, 1997;Baumgartner and Shine, 1998;Zhou et al., 2003). An adenoviral vector carrying the enhanced green fluorescent protein (eGFP) gene (reporter gene) driven by the cytomegalovirus (CMV) promoter (Adv. CMV-eGFP) was used as a control.
2.3. PHCEC Preparation and Transduction in Vitro
Fresh human corneoscleral tissues from donors aged 19–67 years (less than 72 h post mortem) were obtained from the Lions Eye Bank of Texas (LEBT, Houston, TX). These tissues were preserved in Optisol™ GS (Bausch and Lomb Inc, Rochester, NY, USA) at 4°C until they were processed for culture. PHCEC was cultured from fresh limbal explants by a previously described method (Kim et al., 2004). Briefly, after carefully removing the central cornea, excess sclera, iris, corneal endothelium, conjunctiva and Tenon's capsule, the remaining limbal rim was cut into 12 pieces and cultured in a 6-well plate with the epithelial side up in SHEM for explant culture. At day 10, after renewing the culture medium, the same dosage of adenoviral vectors (multiplicity of infection =50) was delivered to the subconfluent cells (n=3) and incubated for 24 hours. After washing twice, the cultures were switched to the same amount of vector-free SHEM (2 ml) which was renewed every 48 hours. A 25 μl aliquot of cell supernatant was taken just before changing the media. Samples without gene transduction were used as negative controls. All experiments were repeated at least three times on separate tissues.
2.4. Multiplex Bead ImmunoAssay
The concentration of GDNF protein in the cell supernatant was determined at different time-points after the transduction with a sensitive, fluorescent multiplex bead immunoassay (Luminex; BioSource-Invitrogen, Carlsbad, CA; http://www.invitrogen.com). Each sample diluted with the same amount of assay diluent (25 μl) was added to the well of a 96-well plate and incubated overnight at 4°C in the dark, with 25 μl of 1x beads coupled to mouse GDNF-specific antibodies. The following day, the beads were washed and mixed with 100 μl of 1x biotinylated secondary detector antibody mixture for 1 hour at room temperature, followed by washing and subsequent incubation with 100 μl of streptavidin-phycoerythrin for 30 minutes (both steps were performed in the dark). After a final wash, the wells were resuspended in 100 μl of assay buffer. GDNF standard was reconstituted and serially diluted in 50% culture medium with 50% assay diluent to generate a standard curve. GDNF concentrations were measured with a multiplex analysis system (model 100 IS 2.3; Luminex, Austin, TX). The minimum detectable concentration of GDNF was 9.3 pg/ml.
2.5. Cell Viability Assays
MTT assay was performed per the manufacturer's instructions. Briefly, a standard curve of known cell concentrations against absorbance showed that a portion of the curve was approximately linear. This was used to derive the optimal seeding concentration of 2 × 105 cells/ml. At day 10 after the transduction, cells in each group (n=3) were seeded at this density in a volume of 100 μl per well on a 96-well plate in triplicate. The tetrazolium compound MTT (3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide) was added 24 hours after seeding. Detergent reagent was added 2 hours after addition of MTT and incubated at 37°C for a further 4 hours. The absorbance was then read at 570 nm using a reference wavelength of 650 nm.
2.6. TUNEL Assay
The terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling (TUNEL) assay was performed as previously described (Tong et al., 2006;Yeh et al., 2003). Briefly, at day 10 after transduction, cells in each group (n=3) were seeded at the density of 2 × 104 cells/ 200μl/ well into an eight-well chamber slide overnight and the TUNEL protocol was performed. Propidium iodide (PI) was used as nuclear counterstaining. At least 400 cells were counted for each experimental group. Positive cells were identified by fluorescent imaging using the same exposure, brightness, contrast, and magnification in all experimental groups. This procedure was facilitated by overlapping fluorescent images with propidium iodide–stained images in different layers of a composite image (Photoshop, ver. 6.0; Adobe Systems Inc., San Jose, CA).
2.7. Development of Bioengineered Human Corneal Constructs with Overexpression of GDNF and Ex Vivo Autografts
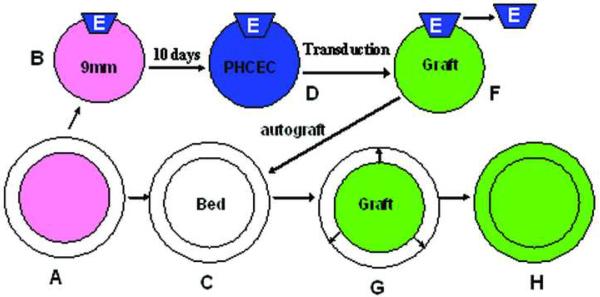
As shown by a schematic diagram in Figure 1, a de-epithelialized corneal stromal disk of 80μm thickness and 9mm diameter (B) was prepared from fresh human corneoscleral tissues (A) using the IntraLase femtosecond laser (IntraLase Corp, Irvine, California, USA). A 1 × 1.5 mm piece of human limbal explant tissue (E) obtained from another corneoscleral tissue of the same donor was placed on the top edge of Bowman's membrane side of the stromal disc (B) in submerged condition to develop a bioengineered corneal epithelial equivalent. At day 10, when the epithelial outgrowth from the limbal wedges (E) were confluent on the stromal disc (D), adenoviral vectors (MOI=50) were delivered as above (n=3). The corneal constructs (F, Graft) with the explant removed was transplanted onto its original corneal bed (C, Bed, the corneoscleral tissue was preserved in the Optisol™ GS until transplantation). The graft and recipient bed were then cultured (G) in SHEM and the concentration of GDNF protein in the supernatant was determined as described above. The graft and the recipient were photographed with a stereoscopic zoom microscope (model SMZ 1500; Nikon, Melville, NY). The fluorescence excitation was at 470 nm. A corneal constructs without transduction was used as negative control.
Figure 1.
A schematic diagram showing the procedure of development of bioengineered human corneal constructs with overexpression of GDNF or enhanced green fluorescent protein (eGFP) and ex vivo autograft. A de-epithelialized corneal stromal disk (B) was prepared from fresh human corneoscleral tissues (A) using the IntraLase femtosecond laser. A 1 ×1.5 mm piece of human limbal explant tissue (E) obtained from another corneoscleral tissue of the same donor was placed on the top edge of Bowman's membrane side of the stromal disc (B) to develop a bioengineered corneal epithelial equivalent. At day 10, when the epithelial outgrowths were confluent on the stromal disc (D), adenoviral vectors were delivered. 24h later, the corneal constructs (F, Graft) with the explant removed was transplanted into its original corneal bed (C, Bed). The graft and recipient bed were then cultured (G) for epithelial cells expanding on the surface of the recipient (H).
2.8. Immunofluorescent staining
At day 5 after transplantation, immunofluorescent staining of frozen sections of the recipient was performed to evaluate expression of GDNF using a previously described method (Chen et al., 2004). In brief, frozen sections were fixed in cold methanol:acetone (1:1) at −30°C for 3 minutes. The concentration of polyclonal anti-GDNF antibody was 2 μg/ml (1:100). The staining was wiewed with an epifluorescent microscope (Eclipse 400; Nikon) and photographed with a digital camera (model DMX 1200; Nikon).
2.9. Statistical Analysis
One-way ANOVA test was used to make comparisons among the three groups, and the Dunnett's test was further used to compare each treated group with the negative control group. A P value of ≤ 0.05 was considered significant. Data were expressed as mean±SD.
3. RESULTS
3.1. Overexpression of GDNF in the Supernatant of PHCEC In Vitro
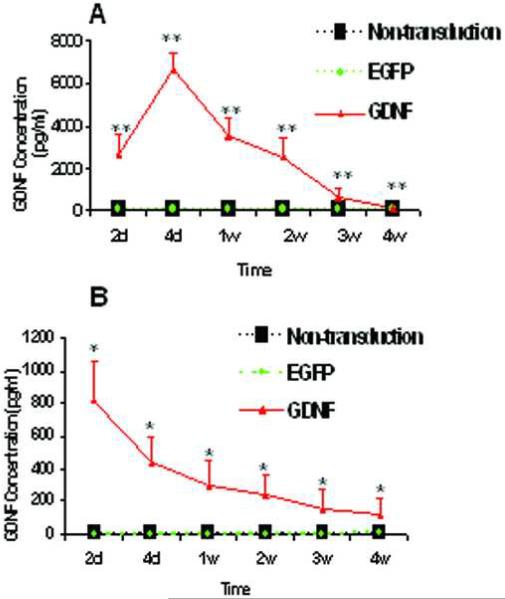
The overexpression of GDNF was detected in the supernatants of PHCEC only in the GDNF gene transduction group, evaluated by multiplex bead immunoassay (Figure 2A). GDNF concentrations were significantly different among the three groups (one-way ANOVA, P < 0.0001). The GDNF concentration peaked at 6646.67±832.91 pg/ml at day 4, compared with undetectable levels in the non-transduction group at all time points (Dunnett's test, P < 0.01). GDNF expression continuously declined after day 4 but was detectable for at least 4 weeks, when the supernatant concentration of GDNF was 82.07±4.45 pg/ml (Dunnett's test, P < 0.01, compared with the non-transduction group). There was no significant difference observed between the non-transduction and the reporter gene eGFP transduction groups at any time point (Dunnett's test, P > 0.05).
Figure 2.
The results of the GDNF expression by multiplex bead immunoassay in supernatants of GDNF transduced PHCEC in vitro (A) and in the supernatants of bioengineered human corneal epithelial constructs (B). The GDNF concentration was significantly higher in the GDNF transduction group (Dunnett's test, n = 3, *, P < 0.05; **, P < 0.01, compared with undetectable levels in the non-transduction group at all time points). There was no significant difference observed between the non-transduction group and the reporter gene eGFP transduction groups at the determined time point both in vitro and ex vivo (Dunnett's test, P > 0.05).
3.2. Effect of the Overexpression of GDNF on PHCEC Survival In Vitro
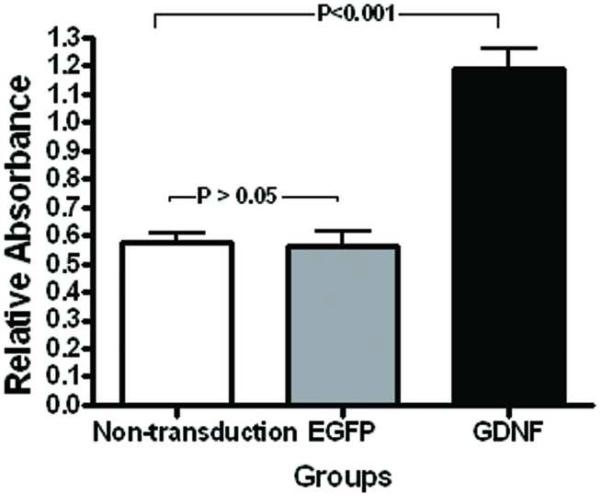
Evaluated by a MTT assay, the viability of PHCEC was significantly higher in the GDNF gene transduction group than the other two control groups (Figure 3, one-way ANOVA, P < 0.0001). At day 10 post-transduction, the relative absorbance was 2 fold greater (Dunnett's test, P < 0.001) in the GDNF transduction group (1.19±0.28) than the non-transduction group (0.57±0.11). The difference observed between the non-transduction and the eGFP transduction groups was not statistically significant (Dunnett's test, P > 0.05).
Figure 3.
MTT assay showing viability of PHCEC. A significantly greater number of cells were noted in the GDNF transduced group compared with the non-transduction group at day 10 after transduction (Dunnett's test, P < 0.001). The difference between the non-transduction and the eGFP transduction groups was not statistically significant (Dunnett's test, P > 0.05).
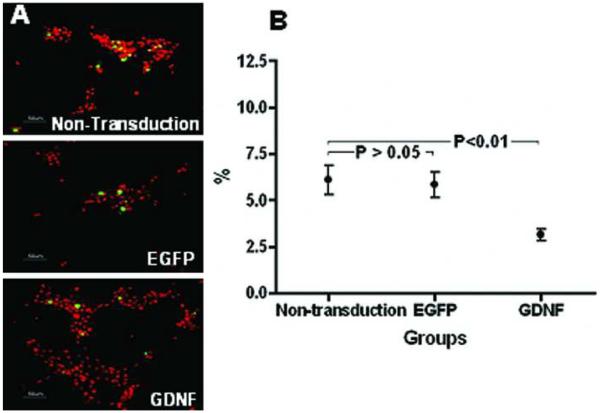
Assessed by TUNEL staining, the percentage of apoptotic PHCEC was significantly lower in the GDNF gene transduction group than the other two control groups (Fig. 4 A, B, oneway ANOVA, P < 0.01). At day 10 post-transduction, the number of TUNEL-positive cells decreased more than 50% (Dunnett's test, P < 0.01) in the GDNF transduction group (2.83±1.22%), compared with the non-transduction group (6.40±3.19%). There were no significant differences between the non-transduction and the eGFP transduction groups (Dunnett's test, P > 0.05).
Figure 4.
Representative images showing TUNEL staining (green) with propidium iodide nuclear counterstaining (red) in the three groups (A) at day 10 after transduction. The number of TUNEL-positive cells was 50% less in the GDNF transduction group compared with the nontransduction group (B, Dunnett's test, P < 0.01). There were no significant differences between the non-transduction and the eGFP transduction groups (B, Dunnett's test, P > 0.05). Scale bars, 50 μm.
Five weeks after the transduction, much more survival PHCEC were observed in the GDNF gene transduction group compared with the non-transduction and eGFP transduction groups, in which only 1/8 of the original cell population was viable in one of 3 culture plates while all cells had died in the other 2 plates. The cells in these two groups were large and vacuolated. In contrast, 1/3, 1/4 and 1/3 of the original cell populations were viable in the 3 dishes in the GDNF transduction group (Dunnett's test, P < 0.01, compared with the non-transduction group). The cells in the GDNF transduction group were small and primitive.
3.3. GDNF Expression in Ex Vivo Autografts of Bioengineered Human Corneal Constructs
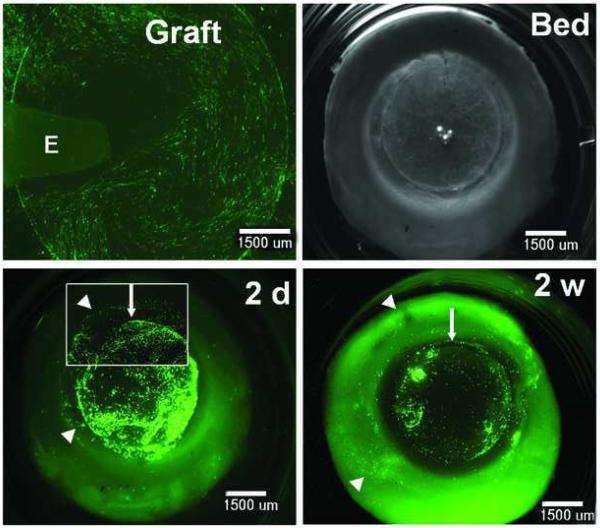
As shown by the expression of the eGFP (green) in Figure 5, PHCEC grew on the disc of human corneal stroma (Graft). After removing the explant, the graft was transplanted into its original corneal bed (Bed). The epithelial cells expanded on the surface of the recipient by day 2 after the transplantation (2d). By 2 weeks, the cell outgrowth was larger, but eGFP expression had declined (2w).
Figure 5.
Ex vivo autograft of bioengineered human corneal constructs shown by the expression of eGFP reporter (green). PHCEC (established from fresh limbal explants-E) were cultured on the substrate of human corneal stromal disk with 80μm thickness and 9mm diameter prepared from fresh human corneoscleral tissue using the IntraLase femtosecond laser. 24h after the transduction, the corneal constructs (graft) with the explant removed was placed back onto its original bed (Bed, the corneoscleral tissue was preserved in the Optisol™ GS until reimplatation). The epithelial cells expanded on the surface of the recipient by day 2 after the transplantation (2d, arrow head showing the margin of expanded epithelia on the surface of recipient bed). By 2 weeks (2w), the outgrowth was larger (arrow head showing the margin of greater expanded epithelia), but eGFP expression declined (weak). Arrow showing the margin of the graft. Inset, higher power magnification, ×75. Scale bars, 1500 μm.
The overexpression of GDNF was detected in the supernatants of the ex vivo autografts only in the GDNF gene transduction group, evaluated by multiplex bead immunoassay (Figure 2B). The GDNF concentration was significantly higher in the GDNF transduction group, peaking at 822.58±232.21 pg/ml at day 2 after the transplantation, compared with undetectable levels in the non-transduction group at all time points (Dunnett's test, P < 0.05). Its expression decreased to 299.38±145.21 at week 1 and continuously declined over the 4 week observation when the supernatant concentration of GDNF was measured at 181.03±97.45 pg/ml (Dunnett's test, P < 0.05, compared with undetectable levels in the non-transduction group). There was no significant difference observed between the non-transduction and the eGFP transduction groups at the determined time point (Dunnett's test, P > 0.05).
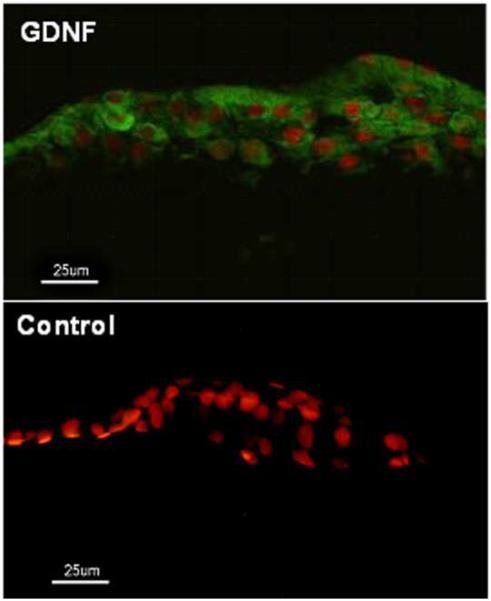
Immunofluorescent staining showed that there were 2–6 layers of epithelial cells cover the stroma of the graft at day 5 after the autograft ex vivo. GDNF was expressed by all epithelial cells layers (Fig 6, green). There was no GDNF expression in the epithelium of the graft in the non-transduction group (Fig 6, control) and in the EGPF transduction group (data not shown).
Figure 6.
Immunofluorescent staining for GDNF in the bioengineered human corneal construct at day 5 after autograft ex vivo. Propidium iodide (PI) was used as nuclear counterstaining (red). There were 2–6 layers of epithelial cells covering the stroma of the graft (red). GDNF was expressed by all layers of the epithelial cells (green) in the GDNF transduction group (GDNF). There was no GDNF expression in the epithelium in the non-transduction group (control). Scale bars, 25 μm.
4. DISCUSSION
In addition to its traditional neuroprotection function, GDNF has demonstrated essential biological significance in the self-renewal of spermatogonial stem cells (Meng et al., 2000;Oatley et al., 2006) and the colony formation of corneal epithelial cells (You et al., 2001). In the present study, the GDNF overexpression promoted cell survival, increased cell viability and decreased cell apoptosis in PHCEC. This study further verified that GDNF could also serve as a survival growth factor for human corneal epithelium and be used as a candidate therapeutic gene to modify corneal epithelial cells by gene therapy.
Corneal epithelium is comprised of distinct populations of cells that divide at various rates, from the undifferentiated slowly dividing putative stem cells at the limbus to transient amplifying cells in the peripheral cornea and post-mitotic cells in the superficial central cornea. For the purpose of treating corneal epithelial disorders using gene therapy, the choice of gene transfer technique is especially important. The choice of vector must, therefore, rely on the therapeutic need. Recombinant adenoviral vector is the most common vector tested for the corneal gene therapy (Borras et al., 1996;Borras et al., 2001;Fehervari et al., 1997;Hoffman et al., 1997;Mashhour et al., 1994;Araki-Sasaki et al., 1995). The adenoviral vector has numerous features that make it suitable for somatic gene therapy (Tsubota et al., 1998). It is replication deficient, accepts large exogenous sequences of DNA, has a high infection rate for most classes of epithelial cells, and shows no possibility of causing human neoplasms. However, adenoviral vector also have limitations. Given the episomal location of the adenoviral DNA following infection, there will be an early peak of expression followed by an exponential decline in transgene expression. In this study, the peak expression of GDNF was detected in the cell supernatant shortly after the transduction (at day 4 in vitro and day 2 ex vivo). The expression declined over 50 % by 1 week post-transduction. Chronic expression over the course of weeks to years will require gene transfer to slowly cycling stem cells in the corneal limbus, which can only be achieved using viral vectors such as retrovirus, lentivirus, and adeno-associated virus (AAV). But in the case of acute processes such as recurrent erosions or persistent epithelial defects, transient expression of adenoviral vector would be preferable (Rosenblatt and Azar, 2004).
The ability to harvest, store, and transplant corneal tissues offers an opportunity to use ex vivo methods for corneal epithelial gene therapy. However, the lack of efficacy for epithelial gene transfer of corneal grafts is in sharp contrast to those obtained for the endothelium, where efficient transfer with potent biologic activity has been demonstrated (Fibbe et al., 1989;Klebe et al., 2001). Ex vivo application of an array of adenoviral, AAV, and lentiviral vectors to corneal buttons prior to transplantation has shown lack of success in full thickness grafts because the vectors do not readily penetrate corneal epithelium (Fehervari et al., 1997;Wang et al., 2000). Therefore, transferring genes to keratolimbal explants prior to transplantation appears to be a promising option for corneal epithelium gene therapy (Bradshaw et al., 1999). Our previous study shown that similar to in vivo, PHCEC expanded ex vivo from a limbal explant also contain a heterogeneous population of cells at different stages, including putative stem cells, transient amplifying cells and post-mitotic cells (Kim et al., 2004). Because only a small limbal biopsy (approximately 1.5 × 1 mm2) is required in this technique, the use of autologus cultivated limbal stem cell transplantation not only overcomes the problem of immunologic rejection, but also minimizes the risk of surgically induced limbal stem cells deficiency on the donor's other eye. In addition, ex vivo gene transfer followed by transplantation presents another advantage. In the case of in vivo gene transfer as noted above, following topical application of viral vectors to treat corneal epithelial defects, gene expression may be observed in other non-epithelial ocular tissues. Ex vivo gene transfer limits the transfer of genes specifically to the cultured epithelium and therefore results in epithelial specific gene expression.
The reported substrates for ex vivo expansion of limbal epithelial stem cells includes human amniotic membrane, fibrin-based substrates or carrier-free transplantable corneal epithelial cell sheet (Nishida et al., 2004). Donated human corneoscleral tissue is an excellent source, not only for whole or lamellar corneal transplantation, for isolation of PHCEC for corneal cell therapy, but also for stromal substrate tissue engineering. In the present study, we chose fresh human corneal stroma prepared from donated human corneoscleral tissue using the IntraLase femtosecond laser as a substrate for bioengineered human corneal construct. The IntraLase femtosecond laser is capable of cutting the substance with a variety of diameter and thickness according to surgical demands. In conjunction with a variety of transplant techniques, including penetrating keratoplasty, lamellar keratoplasty, and limbal transplantation, this ex vivo techniques with genetically modified corneal epithelial cells may present another novel method for gene therapy of various corneal surface diseases.
In this study, we revealed that Adv-mediated GDNF gene delivery enhanced survival of human corneal epithelium in vitro, we also showed its delivery in bioengineered constructs. This kind of graft with autologous cultured stem cells may thus provide a safer and more effective treatment option for treating total limbal stem cell deficiency. In vivo model need to be further studied to verify its effects.
ACKNOWLEDGEMENT
The authors thank the Lions Eye Bank of Texas for their kindly providing human corneoscleral tissues.
This study was supported by NIH Grants, EY11915 (SCP), NS35280 (HDS) and EY014553 (DQL), National Institutes of Health, Bethesda, MD; Department of Defense Congressionally Directed Medical Research Programs (CDMRP) PRMRP FY06 PR064719 (DQL); a grant from Lions Eye Bank Foundation (HQ); an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation and the William Stamps Farish Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Commercial relationship: None
REFERENCES
- Ang LP, Tan DT. Ocular surface stem cells and disease: current concepts and clinical applications. Ann. Acad. Med. Singapore. 2004;33:576–580. [PubMed] [Google Scholar]
- Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol. Vis. Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- Baumgartner BJ, Shine HD. Targeted transduction of CNS neurons with adenoviral vectors carrying neurotrophic factor genes confers neuroprotection that exceeds the transduced population. J. Neurosci. 1997;17:6504–6511. doi: 10.1523/JNEUROSCI.17-17-06504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner BJ, Shine HD. Neuroprotection of spinal motoneurons following targeted transduction with an adenoviral vector carrying the gene for glial cell line-derived neurotrophic factor. Exp. Neurol. 1998;153:102–112. doi: 10.1006/exnr.1998.6878. [DOI] [PubMed] [Google Scholar]
- Borras T, Gabelt BT, Klintworth GK, Peterson JC, Kaufman PL. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J. Gene Med. 2001;3:437–449. doi: 10.1002/jgm.210. [DOI] [PubMed] [Google Scholar]
- Borras T, Tamm ER, Zigler JS., Jr. Ocular adenovirus gene transfer varies in efficiency and inflammatory response. Invest Ophthalmol. Vis. Sci. 1996;37:1282–1293. [PubMed] [Google Scholar]
- Bradshaw JJ, Obritsch WF, Cho BJ, Gregerson DS, Holland EJ. Ex vivo transduction of corneal epithelial progenitor cells using a retroviral vector. Invest Ophthalmol. Vis. Sci. 1999;40:230–235. [PubMed] [Google Scholar]
- Chen Z, De Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li D-Q. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehervari Z, Rayner SA, Oral HB, George AJ, Larkin DF. Gene transfer to ex vivo stored corneas. Cornea. 1997;16:459–464. [PubMed] [Google Scholar]
- Fibbe WE, Daha MR, Hiemstra PS, Duinkerken N, Lurvink E, Ralph P, Altrock BW, Kaushansky K, Willemze R, Falkenburg JH. Interleukin 1 and poly(rI).poly(rC) induce production of granulocyte CSF, macrophage CSF, and granulocyte-macrophage CSF by human endothelial cells. Exp. Hematol. 1989;17:229–234. [PubMed] [Google Scholar]
- Frasson M, Picaud S, Leveillard T, Simonutti M, Mohand-Said S, Dreyfus H, Hicks D, Sabel J. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol. Vis. Sci. 1999;40:2724–2734. [PubMed] [Google Scholar]
- Hoffman LM, Maguire AM, Bennett J. Cell-mediated immune response and stability of intraocular transgene expression after adenovirus-mediated delivery. Invest Ophthalmol. Vis. Sci. 1997;38:2224–2233. [PubMed] [Google Scholar]
- Kim HS, Jun S, X, De Paiva CS, Chen Z, Pflugfelder SC, Li D-Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp. Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner EA, Peer D, Chapman RL, Multack RF, Andurkar SV. Corneal gene therapy. J. Control Release. 2007;124:107–133. doi: 10.1016/j.jconrel.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Klebe S, Sykes PJ, Coster DJ, Krishnan R, Williams KA. Prolongation of sheep corneal allograft survival by ex vivo transfer of the gene encoding interleukin-10. Transplantation. 2001;71:1214–1220. doi: 10.1097/00007890-200105150-00006. [DOI] [PubMed] [Google Scholar]
- Koeberle PD, Ball AK. Effects of GDNF on retinal ganglion cell survival following axotomy. Vision Res. 1998;38:1505–1515. doi: 10.1016/s0042-6989(97)00364-7. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108:1569–1574. doi: 10.1016/s0161-6420(01)00694-7. [DOI] [PubMed] [Google Scholar]
- Mashhour B, Couton D, Perricaudet M, Briand P. In vivo adenovirus-mediated gene transfer into ocular tissues. Gene Ther. 1994;1:122–126. [PubMed] [Google Scholar]
- Massague J, Attisano L, Wrana JL. The TGF-beta family and its composite receptors. Trends Cell Biol. 1994;4:172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, De Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- MohaN RR, Sharma A, Netto MV, SinhA S, Wilson SE. Gene therapy in the cornea. Prog. Retin. Eye Res. 2005;24:537–559. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Nishida K, Yamato M, Hayashida Y, Watanabe K, Maeda N, Watanabe H, Yamamoto K, Nagai S, Kikuchi A, Tano Y, Okano T. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77:379–385. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- OatleY JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- Qi H, Chuang EY, Yoon KC, de Paiva CS, Shine HD, Jones DB, Pflugfelder SC, LI D-Q. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Mol. Vis. 2007;13:1934–1941. [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt MI, Azar DT. Gene therapy of the corneal epithelium. Int. Ophthalmol. Clin. 2004;44:81–90. doi: 10.1097/00004397-200404430-00010. [DOI] [PubMed] [Google Scholar]
- Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–426. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Tong L, Chen Z, De Paiva CS, Beuerman R, Li D-Q, Pflugfelder SC. Transglutaminase participates in UVB-induced cell death pathways in human corneal epithelial cells. Invest Ophthalmol. Vis. Sci. 2006;47:4295–4301. doi: 10.1167/iovs.06-0412. [DOI] [PubMed] [Google Scholar]
- Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N. Engl. J. Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Inoue H, Ando K, Ono M, Yoshino K, Saito I. Adenovirus-mediated gene transfer to the ocular surface epithelium. Exp. Eye Res. 1998;67:531–538. doi: 10.1006/exer.1998.0557. [DOI] [PubMed] [Google Scholar]
- Wang X, Appukuttan B, Ott S, Patel R, Irvine J, Song J, Park JH, Smith R, Stout JT. Efficient and sustained transgene expression in human corneal cells mediated by a lentiviral vector. Gene Ther. 2000;7:196–200. doi: 10.1038/sj.gt.3301075. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yamato M, Hayashida Y, Yang J, Kikuchi A, Okano T, Tano Y, Nishida K. Development of transplantable genetically modified corneal epithelial cell sheets for gene therapy. Biomaterials. 2007;28:745–749. doi: 10.1016/j.biomaterials.2006.09.043. [DOI] [PubMed] [Google Scholar]
- Yan Q, Wang J, Matheson CR, Urich JL. Glial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: comparison to and combination with brain-derived neurotrophic factor (BDNF) J. Neurobiol. 1999;38:382–390. doi: 10.1002/(sici)1097-4695(19990215)38:3<382::aid-neu7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Yeh S, Song XJ, Farley W, Li D-Q, Stern ME, Pflugfelder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol. Vis. Sci. 2003;44:124–129. doi: 10.1167/iovs.02-0581. [DOI] [PubMed] [Google Scholar]
- You L, Ebner S, Kruse FE. Glial cell-derived neurotrophic factor (GDNF)-induced migration and signal transduction in corneal epithelial cells. Invest Ophthalmol. Vis. Sci. 2001;42:2496–2504. [PubMed] [Google Scholar]
- Zhou L, Shine HD. Neurotrophic factors expressed in both cortex and spinal cord induce axonal plasticity after spinal cord injury. J. Neurosci. Res. 2003;74:221–226. doi: 10.1002/jnr.10718. [DOI] [PubMed] [Google Scholar]