Abstract
Objectives
Autonomic dysfunction frequently occurs in the context of Parkinson’s disease (PD) and may precede onset of motor symptoms. Limited data exist on the prospective association of heart rate variability (HRV), a marker of autonomic function, with PD risk.
Methods
We included 12,162 participants of the Atherosclerosis Risk in Communities (ARIC) study, a community-based cohort, without a diagnosis of PD at baseline (1987-89) and with available HRV data (mean age 54, 57% women). A 2-minute electrocardiogram was used to measure HRV. Incident PD was identified through 2008 from multiple sources, and adjudicated. Multivariable Cox models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) of PD by quartiles of HRV measurements.
Results
During a mean follow-up of 18 years, we identified 78 incident PD cases. Lower values of the root mean square of successive differences in normal-to-normal R-R intervals (rMSSD) and standard deviation of normal-to-normal R-R intervals (SDNN), markers of parasympathetic activity and total variability respectively, were associated with higher PD risk during follow-up. In multivariable models, the HR (95%CI) of PD in the bottom quartiles of rMSSD and SDNN compared to the top quartiles were 2.1 (1.0-4.3) and 2.9 (1.4-6.1), respectively. Other measures of cardiac autonomic function, including mean RR interval and frequency-domain measurements, were not associated with PD risk.
Interpretation
In this prospective cohort, decreased HRV was associated with an increased risk of PD. Assessment of cardiac autonomic function may help identify individuals at risk for PD.
INTRODUCTION
In addition to its cardinal motor symptoms, Parkinson’s disease (PD) is characterized by a host of non-motor features.1 Alterations in the autonomic nervous system, including changes in heart rate variability (HRV), have been repeatedly described as typical non-motor manifestations in PD patients.2 These changes may reflect the pathological involvement of different components of the autonomic nervous system, including loss of sympathetic cardiac innervation,3 as well as deposition of Lewy bodies in sympathetic cardiac nerves4 and in the dorsal motor nucleus of the vagus.5 More importantly, alterations of the autonomic nervous system probably precede the onset of motor symptoms in PD and, therefore, may be useful in characterizing individuals at risk for PD.6 Still, to the best of our knowledge, only one prospective study has examined HRV in relation to PD risk. In the Cardiovascular Health Study (n=1587), Jain and colleagues did not find an association between HRV, measured from a 24-Holter monitoring, and PD incidence; the number of PD cases (n=44) in their analysis, however, was small.7
We evaluated whether HRV measured in middle age was associated with the risk of PD later in life in the community-based prospective Atherosclerosis Risk in Communities (ARIC) cohort. We hypothesized that individuals with lower baseline HRV, possibly a manifestation of autonomic involvement in the prodromal phase of PD, would have a higher risk of being diagnosed with PD during follow-up.
METHODS
Study sample
The ARIC study is a community-based cohort designed to investigate risk factors for atherosclerosis and cardiovascular disease in the general population. A detailed description of the cohort has been published elsewhere.8 Briefly, 15,792 men and women 45-64 years old were recruited in 1987-89 from 4 communities in the United States: Forsyth County, NC; Jackson, MS; Minneapolis suburbs, MN; and Washington County, MD. Participants were mostly white in the Minneapolis and Washington County sites, reflecting the underlying characteristics of the population. By design, only black participants were recruited in Jackson. Institutional Review Boards of participating institutions approved the study. Participants provided written informed consent.
At baseline (visit 1), participants underwent a detailed physical exam and completed questionnaires on lifestyles and cardiovascular risk factors. Additional exams were completed during follow-up visits in 1990-92 (visit 2), 1993-95 (visit 3), and 1996-98 (visit 4). Since baseline, participants have been contacted annually by phone to obtain information on their health status. Surveillance of local hospitals is conducted to identify study participants’ hospitalizations. Vital status is determined through annual follow-up calls, review of obituaries in local newspapers, and linkage with the National Death Index.
For this post-hoc analysis of the cohort, we excluded individuals not white or black, and non-whites in the Minneapolis and Washington County sites (because of small numbers) (n = 103), those with missing information on relevant covariates (n = 165), those with no or low quality HRV data (n = 3,156), those using neuroleptics during follow-up (n = 156) and those with prevalent PD at baseline (n = 14) or a non-confirmed possible case during follow-up (n = 187). After exclusions, 12,162 participants were included in the final analysis.
Parkinson disease ascertainment and validation
Ascertainment and validation of PD cases were accomplished in a multi-stage process using a variety of data sources. In a first step, potential PD cases were ascertained through the following mechanisms: self-reported use of PD-related medications (carbidopa/levodopa, dopamine agonist, monoamine oxidase B inhibitor, amantadine, and/or anticholinergic drugs) in any of the study visits or in a follow-up phone call; presence of an ICD-9-CM 332.0 discharge code in any hospitalization during follow-up; a self-reported diagnosis of PD in study visit 4; and PD listed as a cause of death on a death certificate (ICD-9 332.0 or ICD-10 G20 codes). With this approach, 293 potential cases were identified. In a second step, these participants or their proxies, if the participant was already deceased or unable to communicate, were interviewed on the phone by trained staff using a standardized questionnaire to confirm the presence of a PD diagnosis, and to obtain information on presence of PD-related symptoms, medication use, and date of first symptoms and diagnosis. At the end of the interview, participants were asked for their permission to contact the participant’s neurologist or other physician responsible for their care. Afterwards, a letter and questionnaire were sent to the participant’s neurologist asking for confirmation of the diagnosis as well as symptoms, medications, and chronology of the disease, including date of first symptoms.
Once information from all these different sources was collected, a movement disorder specialist (Dr. Xuemei Huang) reviewed all the available data and classified potential PD cases in the following categories: (1) physician confirmed [n=42], (2) physician data not available, but confirmed by participant and consistent after review by the movement disorder specialist [n=31], (3) two or more sources indicated PD, but no confirmation data available from physician [n=21], and (4) 2 or more hospitalizations with PD listed as a discharge diagnosis or reported use of PD medication at the clinical visits and also at a follow-up phone call [n=12]. Thus, 106 of the 293 possible cases had their diagnosis confirmed. Of these, 78 occurred among eligible participants and had date of first symptoms (or diagnosis, if date of first symptoms was unknown) after baseline, while 12 cases were considered prevalent cases at visit 1 based on date of first symptoms or diagnosis, and 16 occurred among ineligible participants. Of 187 non-confirmed PD cases, 9 had their diagnosis denied or considered uncertain by the participant’s physician, 110 participants denied the diagnosis themselves, and 68 had inadequate information to include in any of the previous categories.
Assessment of heart rate variability
At the baseline exam, HRV was assessed by a supine, resting 2-minute beat-to-beat heart rate recording using standardized methods during spontaneous (not paced) breathing after participants had remained comfortable for 20 minutes in the supine position. Participants were asked to refrain from smoking or ingestion of caffeine for at least one hour before the procedure.9 Variations in heart rate can be assessed by a number of measures, usually divided in time-domain measurements, calculated from direct measurements of the heart rate at a particular time or the intervals between successive normal QRS complexes, and frequency domain measurements, derived from the spectral analysis of the ECG recording.10 In the current analysis, we used the mean normal-to-normal R-R interval length, the standard deviation of normal-to-normal R-R intervals (SDNN), and the root mean square of successive differences in normal-to-normal R-R intervals (rMSSD) as time-domain measurements, and high frequency (HF) spectral power, low frequency (LF) spectral power, and the ratio LF/HF as frequency domain measurements. While SDNN reflects total variability, rMSSD primarily reflects the function of the parasympathetic nervous system. Similarly, HF spectral power, LF spectral power, and the ratio LF/HF are manifestation of parasympathetic activity, primarily sympathetic activity, and sympathetic-parasympathetic balance, respectively.11, 12 In the ARIC cohort, low HRV has been linked to higher risk for overall mortality, coronary heart disease, and hypertension.13-15
Other covariates
At baseline, information on race and cigarette smoking was self-reported by the participants. Serum uric acid was measured at baseline using the uricase-peroxidase enzymatic method. Total plasma cholesterol was determined by enzymatic methods and HDL cholesterol was measured after dextran magnesium precipitation. A previous history of coronary heart disease (CHD) was defined as a self-reported medical diagnosis of myocardial infarction, coronary bypass surgery, or angioplasty of coronary arteries, or electrocardiographic signs of a previous myocardial infarction. Coffee intake (caffeinated only, in cups/day) was measured using a validated food frequency questionnaire.16
Statistical analysis
The association of HRV measurements with incidence of PD was estimated using Cox proportional hazards regressions, with separate models for each measurement. Follow-up was defined as the time from baseline to occurrence of PD (defined as the earliest of date of first symptoms, date of diagnosis, or date of initial evidence of PD), death, loss to follow-up, or December 31, 2008, whichever occurred earlier. HRV measurements were categorized in quartiles. In an initial model, we adjusted for age, sex, and race. A subsequent model adjusted additionally for study site, smoking (never, past, current), serum uric acid (continuous), and prevalent CHD. Trends in the association between HRV and PD risk were assessed modeling the HRV measurements in 1-standard deviation units. The proportional hazards assumption was explored including time*covariate interactions in the models and examining log(-log) survival curves. We plotted cumulative incidence functions of PD by quartiles of HRV measurements considering mortality as a competing risk using the stcompet command in Stata. As sensitivity analyses, we assessed the association between HRV measurements and PD incidence fitting competing-risk regression models with death as a competing risk,17 excluding 18 cases identified during the first 5 years of follow-up to reduce the possibility of including imminent cases in the analysis, and additionally adjusting for total cholesterol, HDL cholesterol, and coffee intake, as additional potential confounders. Finally, we conducted separate analyses for the first 10 years of follow-up (33 cases) and the period afterwards (45 cases) to explore whether associations of HRV measures with PD risk changed over time.
RESULTS
Among 12,162 eligible participants, we identified and validated 78 incident PD cases during a mean (median) follow-up of 18 (20) years (incidence rate 36 per 100,000 person-years, 95% confidence interval (CI) 28-43 cases per 100,000 person-years). Table 1 provides baseline characteristics for the entire eligible cohort and by PD status during follow-up. Consistent with previous literature, individuals who developed PD were older, more likely to be male and white, and less likely to be current smokers.
Table 1.
Baseline characteristics, overall and by PD status during follow-up, ARIC Study, 1987-1989
| All | No PD | PD | |
|---|---|---|---|
| N | 12162 | 12084 | 78 |
| Age, years | 54.1 (5.7) | 54.0 (5.7) | 56.4 (5.8) |
| Women, % | 56.5 | 56.6 | 34.6 |
| Black, % | 25.3 | 25.4 | 10.3 |
| Current smoker, % | 25.7 | 25.8 | 9.0 |
| Serum urate, mg/dL | 6.0 (1.6) | 6.0 (1.6) | 6.3 (1.3) |
| Prior CHD, % | 4.5 | 4.5 | 6.4 |
| Mean RR interval, ms | 906 (137) | 906 (137) | 917 (145) |
| rMSSD, ms | 29.1 (23.4) | 29.2 (23.4) | 22.8 (13.8) |
| SDNN, ms | 37.2 (19.7) | 37.2 (19.7) | 32.2 (13.6) |
| LF, ms2 | 31.5 (52.4) | 31.5 (52.4) | 27.9 (47.4) |
| HF, ms2 | 18.3 (44.3) | 18.3 (44.5) | 12.4 (15.7) |
| LF : HF ratio | 2.7 (2.9) | 2.7 (2.9) | 3.0 (2.2) |
Values correspond to mean (standard deviation) or percentage
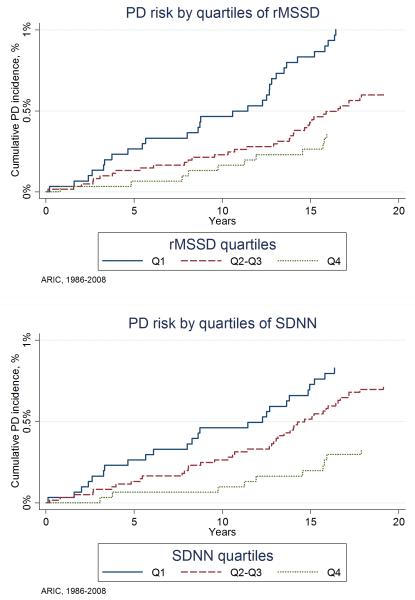
The association between quartiles of HRV measurements and PD risk is presented in Table 2. Mean RR interval, HF spectral power, LF spectral power, and the LF/HF ratio were not associated with incidence of PD. However, lower levels of rMSSD and SDNN were associated with a higher risk of PD during follow-up. In multivariable adjusted models, the hazard ratios (HR) and 95%CI of PD in the bottom quartiles of rMSSD and SDNN compared to the top quartiles were 2.1 (1.0-4.3) and 2.9 (1.4-6.1), respectively. The corresponding HRs (95%CI) for 1-standard deviation decrease in rMSSD and SDNN were 1.5 (1.0-2.2) and 1.5 (1.1-2.1). Figure 1 shows the cumulative incidence of PD by quartiles of these two variables. No evidence of violation of the proportional hazards assumption was found. The associations of rMSSD and SDNN with PD persisted after adjustment for mean RR interval [HR (95%CI) of PD comparing extreme quartiles: 2.3 (1.0-5.1) and 2.9 (1.3-6.4), respectively] and after exclusion of individuals using anti-arrhythmic or blood pressure lowering medications at baseline [HR (95%CI) of PD comparing extreme quartiles: 2.3 (1.0-5.1) and 2.7 (1.1-6.5), respectively]. Results were similar when we used competing-risk regression (Supplementary Table 1), when we excluded 18 events identified during the first 5 years of follow-up (Supplementary Table 2), when we studied associations stratified by follow-up period (first 10 years versus >10 years of follow-up, Supplementary Table 3), or when we additionally adjusted for coffee intake and plasma cholesterol levels (Supplementary Table 4). Because of the limited number of cases, we did not perform stratified analysis by sex or race.
Table 2.
Hazard ratios and 95% CI of incident Parkinson’s disease by quartiles of heart rate variability measurements, ARIC Study, 1987-2008
| Mean RR interval | |||||
| Q1 (<812.6 ms) | Q2 (812.6-<898.3 ms) | Q3 (898.3-<988.8 ms) | Q4 (≥988.8 ms) | 1-SD* | |
| PD cases [n (%)] | 21 (0.7) | 18 (0.6) | 15 (0.5) | 24 (0.8) | 78 |
| N | 3040 | 3037 | 3039 | 3046 | 12,162 |
| Age, sex, race | 1.19 (0.65-2.15) | 0.92 (0.50-1.71) | 0.69 (0.36-1.32) | 1 (ref.) | 1.04 (0.83-1.30) |
| Multivariable ** | 1.19 (0.67-2.16) | 0.93 (0.50-1.73) | 0.69 (0.36-1.31) | 1 (ref.) | 1.04 (0.83-1.30) |
|
| |||||
| rMSSD | |||||
| Q1 (<16.2 ms) | Q2 (16.2-<23.8 ms) | Q3 (23.8-<35.0 ms) | Q4 (≥35.0 ms) | 1-SD* | |
| PD cases [n (%)] | 30 (1.0) | 19 (0.6) | 18 (0.6) | 11 (0.4) | 78 |
| N | 3018 | 3039 | 3054 | 3051 | 12,162 |
| Age, sex, race | 2.23 (1.10, 4.49) | 1.43 (0.68, 3.01) | 1.50 (0.71, 3.18) | 1 (ref.) | 1.50 (1.02-2.23) |
| Multivariable ** | 2.11 (1.04, 4.27) | 1.32 (0.63, 2.80) | 1.41 (0.67, 3.00) | 1 (ref.) | 1.46 (0.99-2.16) |
|
| |||||
| SDNN | |||||
| Q1 (<24.5 ms) | Q2 (24.5-<33.3 ms) | Q3 (33.3-<45.3 ms) | Q4 (≥45.3 ms) | 1-SD* | |
| PD cases [n (%)] | 25 (0.8) | 16 (0.5) | 27 (0.9) | 10 (0.3) | 78 |
| N | 3038 | 3031 | 3042 | 3051 | 12,162 |
| Age, sex, race | 2.62 (1.25-5.50) | 1.56 (0.70-3.44) | 2.63 (1.27-5.45) | 1 (ref.) | 1.45 (1.07-1.97) |
| Multivariable ** | 2.86 (1.36-6.05) | 1.63 (0.74-3.60) | 2.68 (1.30-5.54) | 1 (ref.) | 1.51 (1.11-2.07) |
|
| |||||
| LF | |||||
| Q1 (<6.6 ms2) | Q2 (6.6-<16.2 ms2) | Q3 (16.2-<36.6 ms2) | Q4 (≥36.6 ms2) | 1-SD* | |
| PD cases [n (%)] | 15 (0.5) | 23 (0.8) | 25 (0.8) | 15 (0.5) | 78 |
| N | 3042 | 3034 | 3037 | 3049 | 12,162 |
| Age, sex, race | 1.02 (0.49-2.10) | 1.49 (0.78-2.87) | 1.49 (0.78-2.87) | 1 (ref.) | 1.10 (0.82-1.46) |
| Multivariable ** | 1.09 (0.53-2.25) | 1.57 (0.81-3.03) | 1.65 (0.87-3.13) | 1 (ref.) | 1.12 (0.83-1.51) |
|
| |||||
| HF | |||||
| Q1 (<3.6 ms2) | Q2 (3.6-<8.5 ms2) | Q3 (8.5-<18.9 ms2) | Q4 (≥18.9 ms2) | 1-SD* | |
| PD cases [n (%)] | 23 (0.8) | 25 (0.8) | 10 (0.3) | 20 (0.7) | 78 |
| N | 3038 | 3042 | 3032 | 3049 | 12,162 |
| Age, sex, race | 0.79 (0.43-1.46) | 0.94 (0.52-1.70) | 0.43 (0.20-0.93) | 1 (ref.) | 1.22 (0.75-1.99) |
| Multivariable ** | 0.77 (0.41-1.43) | 0.90 (0.49-1.63) | 0.42 (0.20-0.90) | 1 (ref.) | 1.19 (0.74-1.91) |
|
| |||||
| LF / HF ratio | |||||
| Q1 (<1.00) | Q2 (1.00-<1.88) | Q3 (1.88-<3.42) | Q4 (>=3.43) | 1-SD* | |
| PD cases [n (%)] | 13 (0.4) | 14 (0.5) | 25 (0.8) | 26 (0.9) | 78 |
| N | 3014 | 3076 | 3034 | 3038 | 12,162 |
| Age, sex, race | 0.86 (0.43-1.71) | 0.75 (0.39-1.44) | 1.20 (0.69-2.08) | 1 (ref.) | 1.07 (0.86-1.34) |
| Multivariable ** | 0.99 (0.50-1.98) | 0.81 (0.42-1.56) | 1.28 (0.73-2.22) | 1 (ref.) | 1.12 (0.88-1.41) |
Hazard ratios and 95%CIs for 1-standard deviation (SD) decrease in the corresponding variable. Values for 1-SD are 137 ms for mean RR interval, 23.4 ms for rMSSD, 19.7 ms for SDNN, 52.4 ms2 for LF, 44.3 ms2 for HF, and 2.9 for LF/HF ratio
Multivariable Cox proportional hazard model regression: adjusted for age, sex, race, study center, cigarette smoking (never, former, current), uric acid, previous history of CHD.
MSSD: mean squared successive difference in RR intervals; SDNN: standard deviation of all normal-normal RR intervals; LF: low frequency (0.04-0.15 Hz) power; HF: high frequency (0.15-0.40 Hz) power.
Figure 1.
Cumulative incidence of PD by quartiles of rMSSD (top panel) and SDNN (bottom panel) with death considered a competing risk, ARIC study, 1986-2008.
DISCUSSION
We found that low HRV assessed by time-domain measures is associated with increased risk of PD over a 20-year period in a community-based prospective cohort. No association was observed between frequency-domain measures of HRV from 2-minute recordings and PD risk. Overall, our results suggest that alterations in the cardiac autonomic system can precede the onset of motor symptoms and subsequent diagnosis of PD, and that information on HRV may help identify individuals at higher risk of developing PD.
Impairment of the cardiac autonomic system in individuals with PD has been described in a number of cross-sectional studies. Overall, these studies found reduced HRV—using both time- and frequency-domain measures—in PD patients compared to controls.18-21 The evidence that cardiac dysautonomia is present before the motor onset of PD is more limited. The Cardiovascular Health Study, a community-based cohort of men and women age 65 and older, measured HRV in 24-hour Holter records on 1587 individuals free of PD, 44 of whom developed PD during 14 years of follow-up. PD was identified from a variety of sources, but not clinically validated. In that cohort, and in contrast to our findings in the ARIC study, HRV time- and frequency-domain measurements were not associated with risk of developing PD,7 although the Cardiovascular Health Study included a small number of cases, and limited statistical power is a plausible alternative interpretation.
In the ARIC study, time-domain (rMSSD, SDNN) but not frequency-domain measures (HF, LF, LF/HF ratio) of HRV were associated with the risk of PD. One potential explanation for our results is that time-domain measures of HRV are affected early in the pathogenesis of PD while alteration in frequency-domain measures may occur later in the course of the disease, once motor symptoms are evident. Consistent with this hypothesis, Haapaniemi and colleagues found LF and the LF/HF ratio to be inversely associated with measures of disease severity among 54 PD patients.18 Similarly, Maetzler and colleagues showed time-domain HRV measures, but not frequency-domain measures, to be affected at early PD stages, while frequency-domain measures were lower in more advanced stages among 45 PD patients compared to 26 controls.21 Future studies should conduct repeated assessments of HRV to provide better understanding of the temporal progression of cardiac dysautonomia. An alternative interpretation to consider is the limited ability to extract frequency-domain signal from short records such as the ones used in our study. Although our record length meets the minimum standards set out for frequency-domain measures,10 2 minutes is suboptimal for this purpose.
The nature of the association between reduced HRV and PD risk and its mechanisms are unknown. Deposition of Lewy bodies and Lewy neurites in the dorsal motor nucleus of the vagus and in cardiac sympathetic innervation could be one of the culprits for the changes in HRV observed in PD patients and in the prodromal stages. The dorsal motor nucleus of the vagal nerve has been hypothesized to be one of the first areas to be affected in the pathological progression of PD.5 Efferent fibers from this nucleus are responsible for providing parasympathetic innervation to the gastrointestinal system and, to a lesser extent, to the heart. Pathological involvement of the vagal nerve is responsible for typical prodromal manifestations of PD, such as constipation, but possibly also for decreased HRV: rMSDD, associated with PD risk in the ARIC cohort, has a strong parasympathetic component.10 In addition, numerous studies have found reductions in cardiac sympathetic denervation among PD patients and in individuals with incidental Lewy body disease, which can explain the lower levels of some HRV measurements in patients with PD.2, 22
The findings in the ARIC cohort support the concept that the onset of pathological changes observed in PD occurs years prior to the manifestation of detectable motor symptoms. Though HRV measurements alone are unlikely to identify individuals who will develop PD, information from cardiac autonomic function, together with other prodromal signs and symptoms, could contribute to recognition of those with a higher risk of the disease.
Our study has noteworthy strengths, including the prospective assessment of HRV, the availability of information on potential confounders, and the excellent retention in the ARIC cohort. Limitations also exist. Although different sources were used in our study to identify and validate PD diagnosis, our approach probably missed some cases of incident PD. Also, HRV measurements were obtained from a 2-minute electrocardiogram, which is insufficient to characterize adequately low and very low frequency variability.10 Finally, these results should be interpreted cautiously given the limited number of PD cases and the multiple comparisons. The small number of cases also precluded performing stratified analysis by sex, race, or other characteristics.
In conclusion, lower HRV was associated with increased risk of PD in a large community-based cohort. The implications of this observation for understanding the autonomic system involvement in PD and for the identification of individuals at higher risk of PD remain to be explored.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This study was supported in part by the Intramural Program of NIH, the National Institute of Environmental Health Sciences (Z01-ES101986). Dr. Xuemei Huang is currently supported by NIH grants NS060722, ES019672, and NS082151, as well as a K23 award (AG21491) at the conception of this work.
REFERENCES
- 1.Chaudhuri KR, Schapira AHV. Non-motor symptoms of Parkinson’s disease: dopaminerginc pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 2.Jain S, Goldstein DS. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis. 2012;46:572–580. doi: 10.1016/j.nbd.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amino T, Orimo S, Itoh Y, Takahashi A, Uchihara T, Mizusawa H. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol. 2005;15:29–34. doi: 10.1111/j.1750-3639.2005.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwanaga K, Wakabayashi K, Yoshimoto M, et al. Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology. 1999;52:1269–1271. doi: 10.1212/wnl.52.6.1269. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K, Rüb U, de Vos R, A. I, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 6.Tolosa E, Gaig C, Santamaria J, Compta Y. Diagnosis and the premotor phase of Parkinson disease. Neurology. 2009;72:S12–S20. doi: 10.1212/WNL.0b013e318198db11. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Ton TG, Perera S, et al. Cardiovascular physiology in premotor Parkinson’s disease: a neuroepidemiologic study. Mov Disord. 2012;27:988–995. doi: 10.1002/mds.24979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 9.Liao D, Barnes RW, Chambless LE, Heiss G. A computer algorithm to impute interrupted heart rate data for the spectral analysis of heart rate variability--the ARIC study. Comput Biomed Res. 1996;29:140–151. doi: 10.1006/cbmr.1996.0012. [DOI] [PubMed] [Google Scholar]
- 10.Task Force of the European Society of Cardiology. the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 11.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 12.Lombardi F, Malliani A, Pagani M, Cerutti S. Heart rate variability and its sympatho-vagal modulation. Cardiovasc Res. 1996;32:208–216. doi: 10.1016/0008-6363(96)00116-2. [DOI] [PubMed] [Google Scholar]
- 13.Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 14.Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes. The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes. 2002;51:3524–3531. doi: 10.2337/diabetes.51.12.3524. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability. The Atherosclerosis Risk in Communities (ARIC) Study. Hypertension. 2003;42:1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Haapaniemi TH, Pursiainen V, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllylä VV. Ambulatory ECG and analysis of heart rate variability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70 doi: 10.1136/jnnp.70.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauvageot N, Vaillant M, Diederich NJ. Reduced sympathetically driven heart rate variability during sleep in Parkinson’s disease: a case-control polysomnography-based study. Mov Disord. 2011;26:234–240. doi: 10.1002/mds.23479. [DOI] [PubMed] [Google Scholar]
- 20.Trachani E, Constantoyannis C, Sakellaropoulos GC, Stavrinou ML, Nikiforidis G, Chroni E. Heart rate variability in Parkinson’s disease unaffected by deep brain stimulation. Acta Neurol Scand. 2012;126:56–61. doi: 10.1111/j.1600-0404.2011.1605.x. [DOI] [PubMed] [Google Scholar]
- 21.Maetzler W, Karam M, Berger MF, et al. Time- and frequency-domain parameters of heart rate variability and sympathetic skin response in Parkinson’s disease. J Neural Transm. 2014 doi: 10.1007/s00702-014-1276-1. (in press.) [DOI] [PubMed] [Google Scholar]
- 22.Orimo S, Uchihara T, Nakamura A, et al. Axonal α-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131:642–650. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.