Abstract
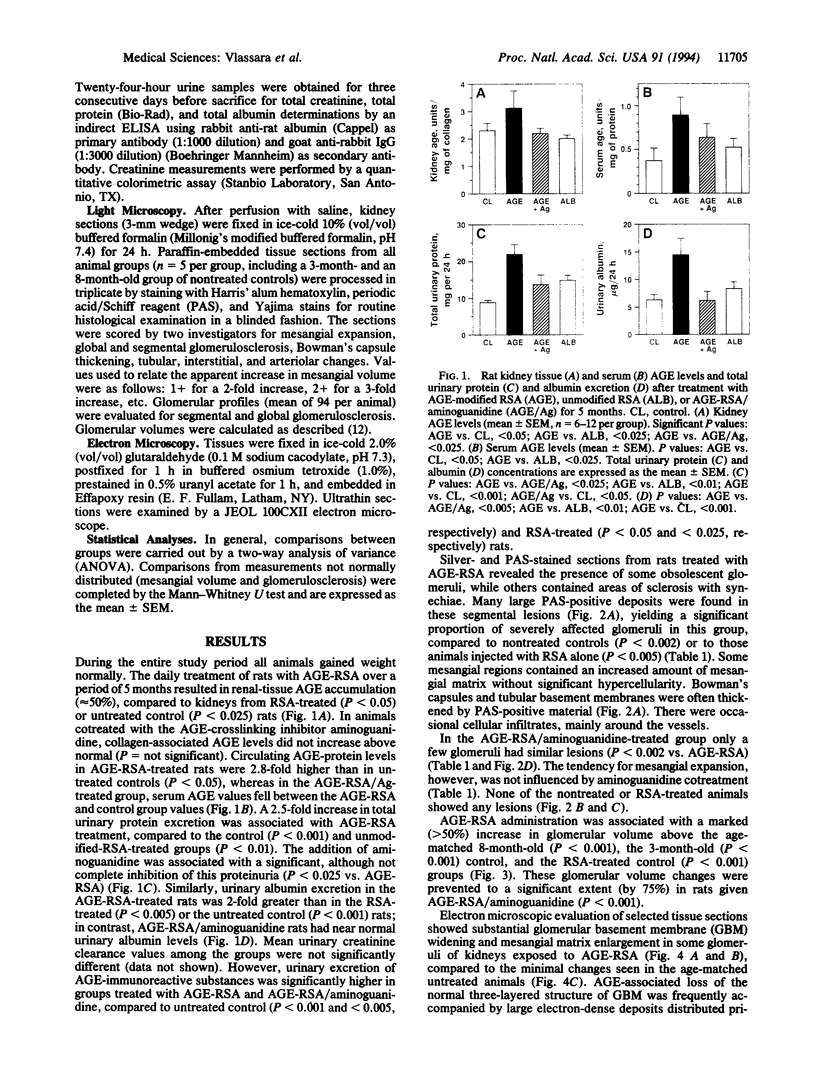
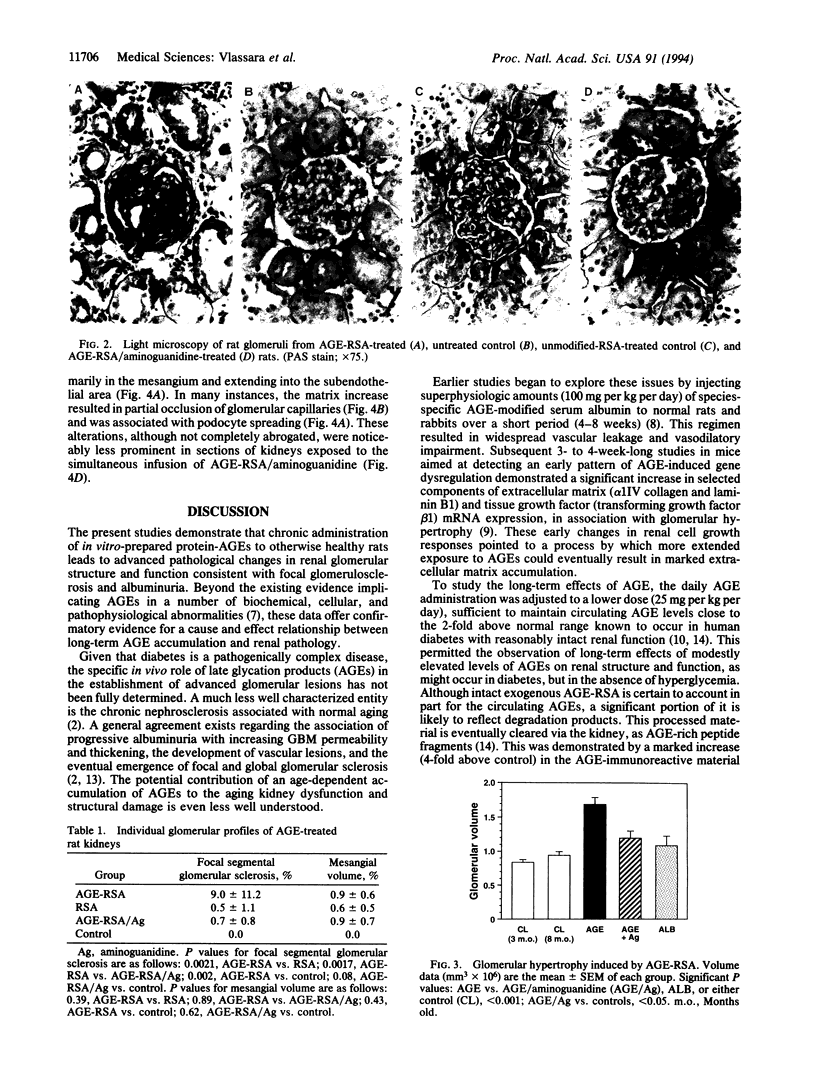
High levels of tissue advanced glycation end products (AGEs) that result from the spontaneous modification of proteins by glucose occur in diabetes and aging. To address the potential pathogenic role of AGEs in the glomerulosclerosis of diabetes or nephrosclerosis of aging, doses of AGE-modified rat albumin (25 mg per kg per day, i.v.) sufficient to elevate circulating AGE levels to the range of diabetic serum were administered daily to healthy rats alone or in combination with the AGE inhibitor aminoguanidine. After 5 months, the AGE content of renal tissues in AGE-treated rats rose to 50% above controls (P < 0.025), whereas serum contained 2.8-fold greater AGE levels (P < 0.025). Light and electron microscopy of kidneys from AGE-treated rats revealed a more than 50% increase in glomerular volume compared to controls (P < 0.001), significant periodic acid/Schiff reagent-positive deposits, basement membrane widening, and mesangial extracellular matrix increase and indicated significant glomerulosclerosis compared to untreated (P < 0.002) or albumin-treated controls (P < 0.002). These changes were associated with significant loss of protein (P < 0.005) and albumin (P < 0.002) in the urine of AGE-treated rats compared to controls. Cotreatment with aminoguanidine markedly limited both the structural and functional defects. These in vivo data demonstrate that AGEs influence glomerular structure and function in a manner leading to glomerulosclerosis. The effects are AGE-specific, as they are ameliorated by a pharmacological AGE inhibitor, aminoguanidine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Brenner B. M. Effects of aging on the renal glomerulus. Am J Med. 1986 Mar;80(3):435–442. doi: 10.1016/0002-9343(86)90718-7. [DOI] [PubMed] [Google Scholar]
- Bilous R. W., Mauer S. M., Sutherland D. E., Steffes M. W. Mean glomerular volume and rate of development of diabetic nephropathy. Diabetes. 1989 Sep;38(9):1142–1147. doi: 10.2337/diab.38.9.1142. [DOI] [PubMed] [Google Scholar]
- Bucala R., Makita Z., Vega G., Grundy S., Koschinsky T., Cerami A., Vlassara H. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Edwards C. A., O'Brien W. D., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980 Jun 10;104(2):161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- Fogo A., Ichikawa I. Evidence for the central role of glomerular growth promoters in the development of sclerosis. Semin Nephrol. 1989 Dec;9(4):329–342. [PubMed] [Google Scholar]
- Fukui M., Nakamura T., Ebihara I., Shirato I., Tomino Y., Koide H. ECM gene expression and its modulation by insulin in diabetic rats. Diabetes. 1992 Dec;41(12):1520–1527. doi: 10.2337/diab.41.12.1520. [DOI] [PubMed] [Google Scholar]
- Hayase F., Nagaraj R. H., Miyata S., Njoroge F. G., Monnier V. M. Aging of proteins: immunological detection of a glucose-derived pyrrole formed during maillard reaction in vivo. J Biol Chem. 1989 Mar 5;264(7):3758–3764. [PubMed] [Google Scholar]
- Makita Z., Bucala R., Rayfield E. J., Friedman E. A., Kaufman A. M., Korbet S. M., Barth R. H., Winston J. A., Fuh H., Manogue K. R. Reactive glycosylation endproducts in diabetic uraemia and treatment of renal failure. Lancet. 1994 Jun 18;343(8912):1519–1522. doi: 10.1016/s0140-6736(94)92935-1. [DOI] [PubMed] [Google Scholar]
- Makita Z., Radoff S., Rayfield E. J., Yang Z., Skolnik E., Delaney V., Friedman E. A., Cerami A., Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991 Sep 19;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Cerami A., Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992 Mar 15;267(8):5133–5138. [PubMed] [Google Scholar]
- Miyazaki S., Kawasaki K., Yaoita E., Yamamoto T., Kihara I. Bovine serum albumin (BSA) nephritis in rats. III. Antigen distribution in various organs. Clin Exp Immunol. 1985 Feb;59(2):293–299. [PMC free article] [PubMed] [Google Scholar]
- Skolnik E. Y., Yang Z., Makita Z., Radoff S., Kirstein M., Vlassara H. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodelling and diabetic nephropathy. J Exp Med. 1991 Oct 1;174(4):931–939. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker G. E., Peten E. P., Carome M. A., Pesce C. M., Schmidt K., Yang C. W., Elliot S. J., Striker L. J. The kidney disease of diabetes mellitus (KDDM): a cell and molecular biology approach. Diabetes Metab Rev. 1993 Apr;9(1):37–56. doi: 10.1002/dmr.5610090105. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Bucala R., Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994 Feb;70(2):138–151. [PubMed] [Google Scholar]
- Vlassara H., Fuh H., Makita Z., Krungkrai S., Cerami A., Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. W., Vlassara H., Peten E. P., He C. J., Striker G. E., Striker L. J. Advanced glycation end products up-regulate gene expression found in diabetic glomerular disease. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9436–9440. doi: 10.1073/pnas.91.20.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]