Abstract
Recall responses by memory CD8 T cells are impaired in the absence of CD4 T cells. Although several mechanisms have been proposed, the molecular basis is still largely unknown. Using a local influenza virus infection in the respiratory tract and the lung of CD4−/− mice, we show that memory CD8 T cell impairment is limited to the lungs and the lung draining lymph nodes, where viral antigens are unusually persistent and abundant in these mice. Persistent antigen exposure results in prolonged activation of the AKT-mTORC1 pathway in antigen-specific CD8 T cells, favoring their development into effector memory T cells (TEM) at the expense of central memory T cells (TCM), and inhibition of mTORC1 by rapamycin largely corrects the impairment by promoting TCM development. The findings suggest that the prolonged AKT-mTORC1 activation driven by persistent antigen is a critical mechanism underlying the impaired memory CD8 T cell development and responses in the absence of CD4 T cells.
Keywords: Memory CD8 T cell, CD4−/− mice, antigen load, AKT-mTORC1
Introduction
Long-lived memory CD8 T cells provide immune defenses against pathogens such as viruses and intracellular bacteria, and likely some cancers. The mechanisms underlying the development and function of these T cells, following infection or vaccination, has thus been extensively studied. Among the factors shown to have a significant impact on memory CD8 T development are: i) CD4 T cell help (1–4); ii) duration of stimulation by antigen (peptide-MHC-class I complexes) (5, 6); iii) mutation in the T cell receptor (TCR) β chain transmembrane domain (7, 8); iv) activation of AKT, a serine/threonine protein kinase (9); and v) the mechanistic target of rapamycin complex 1 (mTORC1), as indicated by effect of rapamycin on CD8 memory development (10–12). Despite these progresses, an integrated molecular and cellular mechanism that can account the diverse contributing factors to memory CD8 T cell development and recall response remains incomplete.
Based on cell surface marker expression, anatomic localization, persistence, and function, memory CD8 T cells are often classified into two major types, central (TCM) and effector (TEM) memory cells (13). TCM exhibit a phenotype of CD62Lhi CCR7hi and reside primarily in lymphoid tissues, whereas TEM are CD62Llo CCR7lo and widely dispersed in non-lymphoid tissues. The numbers of TCM are stably maintained over long periods by slow homeostatic proliferation. TEM, however, are not maintained by homeostatic proliferation and gradually decline over time. Compared to TEM, which rapidly respond to antigen re-stimulation to express effector functions, but proliferate poorly, TCM respond to antigen re-stimulation by proliferating before exhibiting effector functions (13, 14). Experimentally, recall responses of memory CD8 T cells usually measure the proliferative capacity of TCM by quantifying the number of antigen-specific T cells (14).
CD4 T cells are believed to play a critical role in the recall responses of memory CD8 T cells. Compared to wild type (WT) mice, CD4−/− mice produce relatively normal primary CD8 T cell responses and similar numbers of memory CD8 T cells following a viral or bacterial infection (1–3), but they are deficient in recall responses. Although several mechanisms have been proposed to explain the observed defect, including the contribution of CD4 T helper cells to CD8 T cell activation, expansion, contraction, memory CD8 T cell maintenance, and their response to re-stimulation (15, 16), the question remains unresolved. The fact that CD4−/− mice generate normal numbers of memory CD8 T cells during a primary response but do not produce significant recall responses following re-stimulation (17) suggests that the memory CD8 T cells in CD4−/− mice are of TEM type. However, the molecular mechanism underlying the TCM vs. TEM development in the absence of CD4 T cells has not been examined.
Antigen has also been found to play a critical role in the outcome of TCM vs. TEM development. Following influenza A virus infection in the respiratory tract and the lung in mice, viral antigen is abundant and persists in the lung and lung-draining lymph nodes (DLN) but is scant and transient in non-draining lymph nodes (NDLN). Correspondingly, most persisting CD8 T cells in the lung and DLN exhibit the TEM phenotype, whereas those persisting in NDLN exhibit the TCM phenotype. The importance of antigen is further demonstrated by manipulating antigen persistence: prolonged exposure to antigen leads to TEM development whereas shortened exposure enhances TCM development (5). However, how antigen regulates TCM vs. TEM development at the molecular level has not been elucidated.
Furthermore, studies have shown that alterations in TCR and its signaling pathway also dramatically affect TCM vs. TEM development. Replacement of the most carboxyl-terminal tyrosine with leucine in the TCR β transmembrane domain has only minimal effect on TCR-CD3 interaction but impairs TCR polarization and NF-κB signal in the immunological synapse. Transgenic mice expressing the mutant TCR β chain produce normal primary CD8 T cell responses but have an impaired development and function of memory CD8 T cells (8). Following binding to cognate peptide-MHC complexes, TCR signals through many proteins (18) including AKT kinase (9) and mTORC1 (12). Persistent activation of AKT promotes development of short-lived effector cells, but inhibits development of memory precursor cells (9). Inhibition of mTORC1 by rapamycin greatly improves the quality of memory CD8 T cells and their recall responses by increasing the number of memory precursors during the expansion phase and accelerating memory T cell development during the contraction phase (10, 11). However, whether the effect of antigen and CD4 T cells on memory CD8 T cell development is through TCR, AKT and mTOR at the molecular level has not been determined.
We have studied memory CD8 T cell development in a localized influenza virus infection in the lung and respiratory tracts in mice and identified a critical role of antigen in regulating TEM vs. TCM development (5). In this report, we show that following influenza virus infection of CD4−/− mice, the defective recall response of memory CD8 T cells is limited to the lung and DLN, where antigen is abundant and favors TEM development. In NDLN and spleen, where the antigen load is low and favors TCM development, the recall response is normal or even better. We further show that persistent antigen exposure leads to prolonged activation of the AKT pathway in CD8 T cells. Inhibition of mTORC1 by rapamycin restores TCM development and corrects the limited recall response of memory CD8 T cells in CD4−/− mice. These findings suggest that persistence of antigen in the lung and DLN of CD4−/− mice leads to a prolonged TCR engagement and persistent activation of AKT-mTORC1 pathway, resulting in TEM development and therefore deficient recall responses of memory CD8 T cells in these tissues. Elucidation of antigen and CD4 T cells in regulating TEM vs. TCM development through AKT-mTORC1 pathway helps to develop an integrated mechanism of memory CD8 T cell development and identify possible approaches to improve memory CD8 T cell development in response to vaccination, especially in individuals with impaired or deficient CD4 T cells. In addition, our findings suggest that CD4 T cells do not play a direct role, as several hypotheses postulated, in memory CD8 T cell development.
Materials and methods
Mice and virus
2C TCR transgenic mice on the RAG1−/− and C57BL/6 background (2C+ rag1−/− Thy1.2+) were used as donors and maintained in the animal facility at the Massachusetts Institute of Technology (MIT). These mice express the 2C TCR on CD8+ T cells specific for SIYRYYGL peptide in association with MHC class I Kb molecule (19). B6, B6 Thy1.1+, CD4−/−, OTI+ rag1−/− Thy1.1+ mice were from the Jackson Laboratories and bred in the animal facility at MIT. Mice were used at 8–16 weeks of age. All animal studies and procedures were approved by MIT’s Committee for Animal Care. Recombinant WSN-SIY virus encoding the SIY epitope in the neuraminidase stalk was constructed by plasmid-based reverse genetics and grown in Madin–Darby canine kidney cells (MDCK)(20).
Antibodies, staining, and flow cytometry
The antibodies for CD62L (MEL-14), CD27 (LG. 3A10), KLRG1 (2F1/KLRG1), Thy1.1 (OX-7), Thy1.2 (53-2.1), and CD69 (H1.2F3) were from Biolegend. The antibodies for pAKT308 (C31E5E) and pAKT473 (D9E) were from Cell Signaling. The antibodies for CD8 (53-6.7), IFN-γ (XMG1.2), and TNF-α (MP6-XT22) were from BD-Biosciences. Single-cell suspensions were prepared from lung, mediastinal (draining) lymph nodes (DLN), spleens, and inguinal and mesenteric lymph nodes (NDLN). The procedures for preparing single-cell suspensions and for the cell surface staining were as described (21).
For SIY/Kb-specific CD8 T cell staining, we added SIY to DimerX-H2Kb-PE per manufacturer’s protocol (BD Bioscience). For the 2C TCR staining, clonotypic antibody 1B2 was used (5). For intracellular IFN-γ and TNF-α staining, cells were stimulated with SIY peptide for 4 h in the presence of GolgiPlug (BD Biosciences). The cells were then fixed and stained with labeled antibodies using an intracellular staining kit (Cytofix/Cytoperm kit; BD Biosciences) according to the manufacturer’s instructions. For the intracellular pAKT308 and pAKT473 staining, the cells were fixed and stained following the description for FOXP3 Fix/Perm Buffer Set (Biolegend). Stained cells were analyzed on FACS LSR-II HTS (BD Biosciences) flow cytometer. Data were analyzed with FlowJo software (Tree Star Inc.)
Infection, adoptive transfer, and preparation of peptide-loaded BMDCs
For influenza virus infection, adoptive transfer, and peptide-loaded BMDC preparation, we followed the procedures described previously (5, 20). For infection, mice were anesthetized and intranasally (i.n.) infected with a sublethal dose (100 pfu) of WSN-SIY virus. For T cell transfer, naïve 2C cells were isolated from 2C rag1−/− mice and adoptively transferred into CD4−/− and WT mice by intravenous injection (2×105 2C cell per recipient). For adoptive transfer of memory 2C cells or endogenous memory CD8 T cells, CD8 T cells were enriched following the protocol of EasySep™ Mouse CD8+ T Cell Isolation Kit (Stem Cell Technologies). Then the enriched CD8 T cells containing 1×104 memory 2C cells or endogenous memory CD8 T cells were intravenously injected into B6 or B6 Thy1.1 mice, respectively. For preparation of peptide-loaded BMDCs, bone marrow derived dendritic cells were prepared and loaded with SIY peptide as earlier described (5) and intravenously injected into CD4−/− and WT mice (5×105 SIY peptide-loaded BMDCs per recipient).
Cells were isolated from lymph nodes of OTI rag1−/− Thy1.1+ mice and 2C rag1−/− Thy1.2+ mice. The lymphocytes were counted and mixed at a ratio of 1 to 1. The cells were incubated with peptides SIYRYYGL (SIY) or SIINEFKL (2 µg/ml) for 2 days and stained and analyzed as above by flow cytometer.
Rapamycin treatment
Rapamycin (Sirolimus, ChemieTek) was dissolved in DMSO to 4 mg/ml as stock and then diluted in PBS to 80 µg/ml for intraperitoneal injection at 600 µg/kg/day (10). The control (without rapamycin) mice were injected with PBS with the same concentration of DMSO.
Statistical analysis
Unpaired two-tailed t-tests were performed for statistical analyses of p values. Error bars represent the standard errors of the means (SEM).
Results
Recall responses of memory CD8 T cells in various tissues in CD4−/− mice
Naïve CD8 T cells expressing the 2C T cell receptor (TCR) were adoptively transferred into WT and CD4−/− mice (2×105 2C cells per recipient) followed by intranasal infection with a sublethal dose [100 plaque-forming unit (pfu)] of influenza A virus, WSN-SIY, which expresses the SIYRYYGL (SIY) epitope recognized by the 2C TCR when presented by H-2Kb (20) (Fig. 1A). At the height of the primary response, 7 days post infection (dpi), the number of 2C cells in the lung, DLN, spleen, and NDLN was quantified by flow cytometry staining for CD8 and the 2C TCR with a clonotypic antibody 1B2. The number of 2C cells and their frequency in the lung, DLN, spleen, and NDLN were similar in WT and CD4−/− mice (Fig. 1B, Supplemental Fig. 1A–B). Similarly, the proportion and the numbers of 2C cells that expressed IFN-γ and TNF-α in DLN and spleen were similar in WT and CD4−/− mice (Supplemental Fig. 1B–D). These results suggest that CD8 T cells do not require CD4 T cells to mount a normal primary response in a local respiratory tract infection, consistent with earlier findings with systemic infections (1–3).
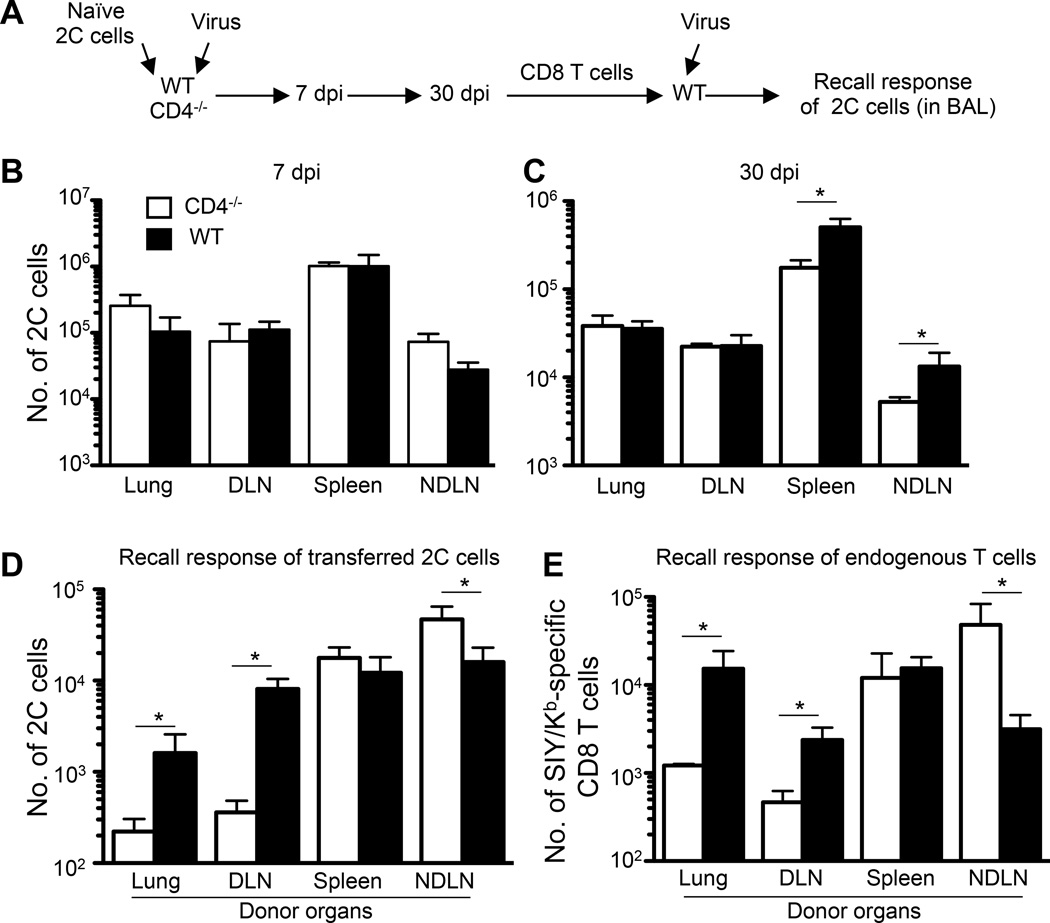
FIGURE 1.
CD8 T cell recall defects differ in various organs of CD4−/− mice. (A) Scheme of experimental procedures. (B–C) Numbers of 2C cells quantified with a clonotypic antibody (1B2) in the indicated sites in CD4−/− and WT mice at 7 dpi and 30 dpi, respectively. (D–E) Number in BAL fluid in recall responses of adoptively transferred 2C cells (D) and endogenous Thy1.2+ SIY/Kb-specific CD8 T cells (SIY/Kb-dimer+ cells) (E). In D and E, the donor tissues are labeled. Error bars: SEM from 3–4 mice per group from one of two independent experiments. * P<0.05.
At 30 dpi, the numbers of memory 2C cells persisting in the lung and DLN were similar in WT and CD4−/− mice, but the numbers of these cells were, respectively, about 2.9 and 2.5 times higher in the spleen and NDLN of WT mice (Fig. 1C and Supplemental Fig. 1E). To determine the recall potential of memory 2C cells, we isolated CD8 T cells at 30 dpi from the lung, DLN, spleen, and NDLN of WT and CD4−/− mice by negative depletion and adoptively transferred 1×104 memory 2C cells into naive WT mice. The recipient mice were then infected intranasally with 100 pfu WSN-SIY influenza virus and 7 days later the numbers of 2C cells in the bronchial alveolar lavage (BAL), the site of virus infection, were counted as a measure of recall potential of donor memory CD8 T cells. As shown in Fig. 1D, the recall response was similar when the transferred memory 2C cells were from spleen of WT and CD4−/− mice, but when they were from the lung and DLN, those from the WT mice gave a much stronger recall response as indicated by a 7–8-fold more 2C cells in the BAL. Unexpectedly, however, when transferred memory 2C cells were from NDLN, those from the CD4−/− mice made 2-fold greater response. These data suggest that the recall response of memory CD8 T cells in different tissues of CD4−/− mice can differ in respiratory tract infection than in systemic infections.
To exclude any possible artifact due to the use of adoptively transferred transgenic T cells, we examined recall responses of the endogenous memory CD8 T cells that arise in the course of influenza virus infection. In this experiment, WT and CD4−/− mice on the Thy1.2 background were infected intranasally with 100 pfu WSN-SIY virus, and 30 dpi total CD8 T cells were isolated from the lung, DLN, spleen, and NDLN and transferred into C57BL/6 recipients on the Thy1.1 background (1×104 SIY/Kb-specific endogenous memory CD8 T cells per recipient). The recipient mice were then infected intranasally with 100 pfu of WSN-SIY virus, and 7 days later the numbers of Thy1.2+ SIY/Kb-dimer+ CD8 T cells were quantified in the BAL. Consistent with the transgenic 2C cell results, similar numbers of Thy1.2+ SIY/Kb-dimer+ CD8 T cells were found in the BAL when the transferred endogenous memory CD8 T cells were from spleen of WT or CD4−/− mice (Fig. 1E). When, however, the memory CD8 T cells came from the lung and DLN those from the WT mice gave a 4–12-greater response; and when transferred memory CD8 T cells were from NDLN, those from the CD4−/− mice gave ~6-fold greater response. Together, these results suggest that the effect of CD4 T cells on recall responses of memory CD8 T cells following a localized respiratory tract infection differ in different tissues. The memory CD8 cell recall response is deficient in the lung and DLN, unaltered in spleen, but surprisingly better in NDLN of CD4−/− mice.
Memory CD8 T cell development at various sites
To determine whether memory CD8 T cell development varies in different organs, we analyzed CD27hi CD62Llo KLRG1lo effector memory precursors (TEMP) and CD27hi CD62Lhi KLRG1lo central memory precursors (TCMP) (22–24) at 7 and 9 dpi. At 7 dpi, the percentages of 2C TEMP and TCMP cells in lung, DLN, spleen, and NDLN were similar in WT and CD4−/− mice (Fig. 2A and 2B). At 9 dpi, the percentages and absolute numbers of 2C TEMP cells in lung, DLN, spleen, and NDLN were not significantly different between WT and CD4−/− mice (Fig. 2C and 2E), probably because the high percentages of 2C TEMP masked the difference in WT and CD4−/− mice at this stage. But the percentages and absolute numbers of 2C TCMP cells in the lung and DLN were significantly higher in WT mice than in CD4−/− mice, respectively (Fig. 2D and 2F). At 30 dpi, the percentages of 2C cells exhibiting the TCM phenotype (CD62Lhi) were significantly lower in DLN of CD4−/− than WT mice, but the percentages of 2C TCM cells were higher in NDLN of CD4−/− than WT mice (Fig. 2G and Supplemental Fig. 1F), consistent with greater recall responses in NDLN of CD4−/− than WT mice (Fig. 1D). The numbers of 2C TCM in DLN and spleen were significantly lower in CD4−/− mice than in B6 mice (Fig. 2H). These data are consistent with the observations that better development of TCMP and TCM is associated with better recall response (13, 14) and point to an effect of CD4 T cell-deficiency on development of memory CD8 T cells.
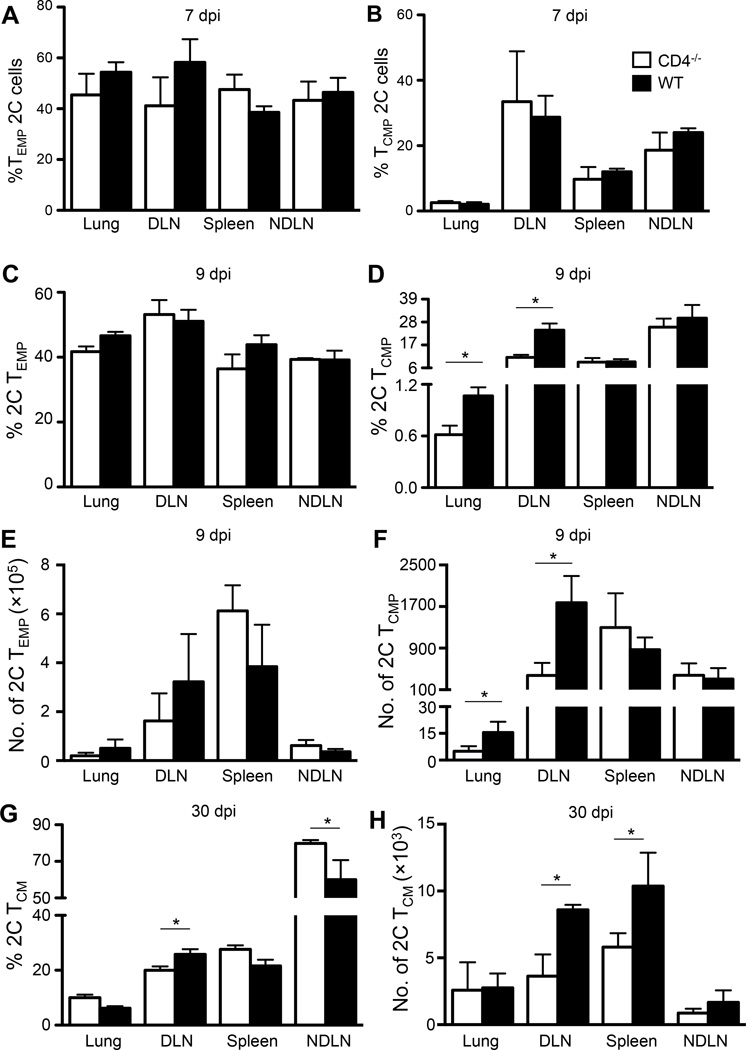
FIGURE 2.
Memory CD8 T cell development differs in WT and CD4−/− mice. The experimental procedures were the same as in Fig. 1A. (A–B) Percentage of TEMP (CD27hi CD62Llo KLRG1lo) 2C cells (A) and TCMP (CD27hi CD62Lhi KLRG1lo) 2C cells (B) in the indicated organs in CD4−/− and WT mice at 7 dpi. (C–D) Percentage of 2C TEMP (CD27hi CD62Llo KLRG1lo) cells (C) and 2C TCMP (CD27hi CD62Lhi KLRG1lo) cells (D) in the indicated organs in CD4−/− and WT mice at 9 dpi. (E–F) Absolute numbers of 2C TEMP cells (E) and 2C TCMP cells (F) in the indicated organs in CD4−/− and WT mice at 9 dpi. (G-H) Percentage (G) and number (H) of 2C TCM (CD62Lhi) cells in the indicated organs in CD4−/− and WT mice at 30 dpi. Error bars: SEM from 4 mice per group from one of two experiments. * P<0.05.
Persistence of influenza virus and sustained AKT activation
As antigen abundance has been shown to affect TCM vs. TEM development (5), it seemed possible that the different antigen levels in different tissues may be involved in the tissue-variable effect of CD4 T cell-deficiency on memory CD8 T cell development and recall responses. To test this possibility, we quantified the duration of infectious influenza virus in BAL of WT and CD4−/− mice by plaque assay as virus titer in BAL accurately reflects that in the lung parenchyma (25). As shown in Fig. 3A, at 5 dpi the virus level was similar in CD4−/− and WT mice, but in WT mice the virus level dropped precipitously by 7 dpi and was below detection by 8 dpi. In contrast, significant levels of virus persisted in CD4−/− mice, and were detectable 10 dpi (Fig. 3A). These results show that infectious virus persists in the lungs of CD4−/− mice much longer than in WT mice, where viral antigen has been found to persist long after virus is cleared (25–27).
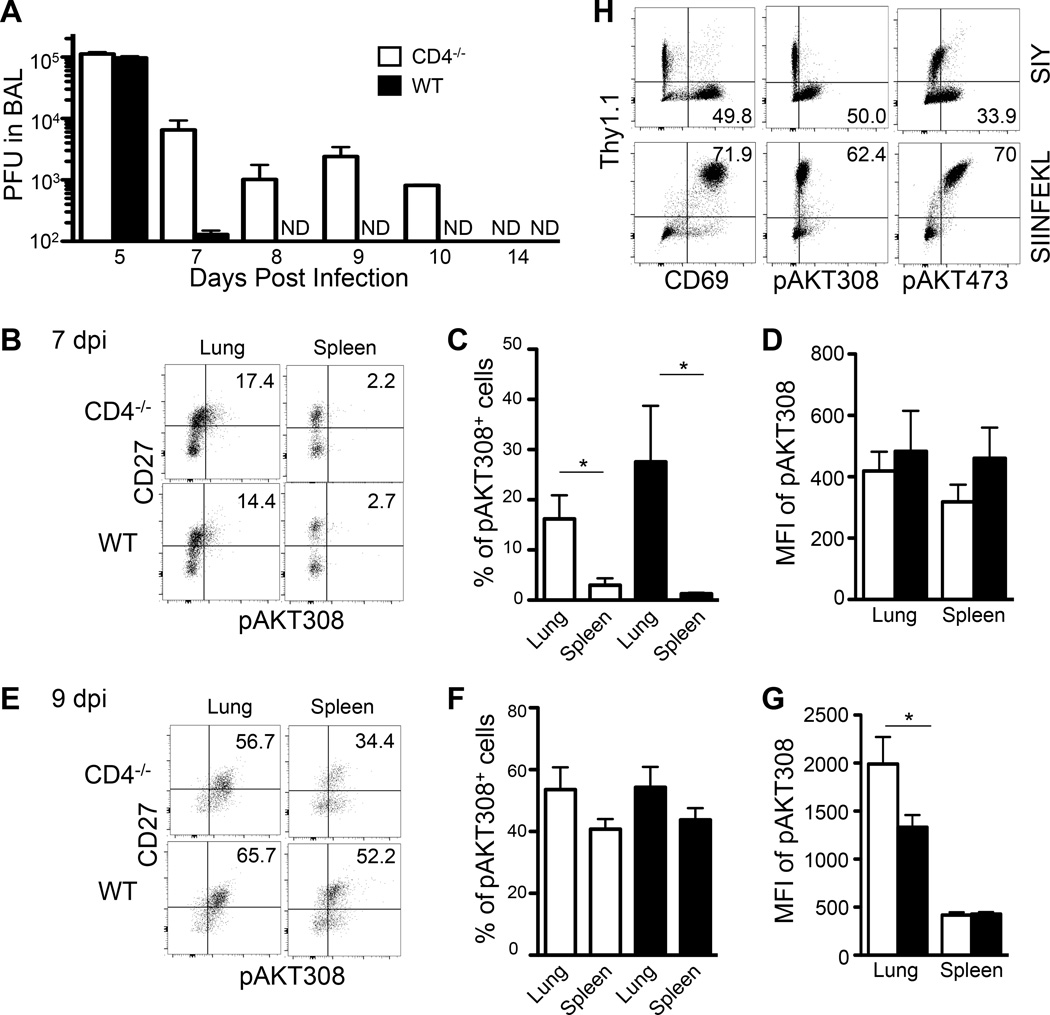
FIGURE 3.
Comparison of virus persistence and AKT phosphorylation in WT and CD4−/− mice. (A) WT and CD4−/− mice were infected intranasally with 100 pfu of WSN-SIY virus. BAL was harvested at the indicated dpi and virus titer measured by plaque assays. ND, not detected. (B–G) Naive 2C T cells were adoptively transferred into WT and CD4−/− mice followed by intranasal infection with WSN-SIY. At 7 and 9 dpi, cells from the lung and spleen were stained for CD8, 2C TCR, CD27 and pAKT308. Shown are CD27 vs. pAKT308 staining profiles gating on CD8+ 2C TCR+ cells at 7 dpi (B) and 9 dpi (E), percentages of CD27hi pAKT308+ 2C cells at 7 dpi (C) and 9 dpi (F), and MFI of pAKT308 of CD27hi 2C cells at 7 dpi (D) and 9 dpi (G). (H) Naïve OTI and 2C cells were isolated from OTI rag1−/− Thy1.1+ and 2C rag1−/− Thy1.2+ mice, respectively. These cells were mixed at the 1 to 1 ratio and activated by either SIY or SIINFEKL peptides. Two days later, cells were stained for Thy1.1, Thy1.2, CD8 plus CD69 or pAKT308 or pAKT473. Shown are Thy1.1 vs. CD69 or pAKT308 or pAKT473 staining profiles gating on CD8+ cells. Error bars: SEM from 4 mice per group in one of two experiments. * P<0.05.
We hypothesized that persistent viral antigens may lead to persistent activation of the AKT-mTORC1 pathway and thereby affect development of memory precursors TCMP and TEMP. To determine AKT activation status we assayed phosphorylation of AKT at positions 308 (28) and 473 (29) in CD27+ 2C T memory precursors, because phosphorylation of Thr-308 is required for activating AKT kinase activity to phosphorylate the downstream substrates (28, 30) and phosphorylation of Ser-473 regulates AKT kinase activity (29, 30). At 7 dpi, the percentage of CD27+ 2C T cells positive for pAKT308 was higher in the lung than spleen of both CD4−/− and WT mice (Fig. 3B–C), although the mean fluorescence intensity (MFI) of pAKT308 was indistinguishable in the same tissues between 2C T cells from CD4−/− and WT mice (Fig. 3D). At 9 dpi, the percentage of CD27+ 2C T cells positive for pAKT308 increased significantly in the lung and spleen of both CD4−/− and WT mice (Fig. 3E–F). Furthermore, the MFI of pAKT308 was significantly higher in CD27+ 2C T memory precursors in the lung of CD4−/− mice than WT mice (Fig. 3G). Similarly, the percentage of CD27+ 2C T memory precursors positive for pAKT473 was higher in the lung than spleen of both CD4−/− and WT mice at both 7 and 9 dpi (Supplemental Fig. 2B–G). However, the MFI of pAKT473 did not change significantly at both 7 and 9 dpi (Supplemental Fig. 2D and 2G).
To determine if antigen can directly activate AKT phosphorylation in the absence of CD4 T cells, naïve 2C T cells (Thy1.2+) were mixed with naïve OT1 T cells (Thy1.1+) at 1 to 1 ratio and stimulated with either SIY peptide or SIINFEKL (recognized by the 2C or OT1 TCR, respectively). Two days later, the cells were analyzed for T cell activation marker CD69 and AKT phosphorylation. When the cell mixture was stimulated with SIY peptide, 2C T cells, but not OT1 T cells, were activated to express CD69 and phosphorylate AKT at 308 and 473 positions (Fig. 3H and Supplemental Fig. 2H). When the cell mixture was stimulated with SIINFEKL peptide, OT1 T cells, but not 2C T cells, were activated to express CD69 and phosphorylate AKT at 308 and 473 positions. Thus, antigen can specifically activate AKT phosphorylation in CD8 T cells in the absence of CD4 T cells.
To examine if persistent antigen stimulation sustains AKT phosphorylation, naïve OT1 T cells were stimulated with SIINFEKL (2 µg/ml) for 0, 15, 30, 60, 120 minutes, 1 or 2 days. Upregulation of CD69 was rapidly induced between 30 and 120 min following stimulation (Supplemental Fig. 3A–B). The MFI of AKT phosphorylation at positions 308 and 473 on CD69+ OT1 T cells increased quickly within 30 min of stimulation, decreased by 90 and 120 min, then increased by 1 and 2 days of stimulation (Supplemental Fig. 3A, 3C, and 3D). To determine if antigen dose affects AKT phosphorylation, naïve OT1 T cells were stimulated for 1 day with SIINFEKL at concentrations from 0, 10−9, 10−8, 10−7, 10−6, 10−5, 10−4, 10−3, 10−2, 10−1, to 1 µg/ml. Upregulation of CD69 was induced in a dose-dependent manner from the concentration 10−8 to 10−3 µg/ml (Supplemental Fig. 3E–3F). Similarly, higher antigen doses also induced higher MFI of AKT phosphorylation (Supplemental Fig. 3E, 3G, and 3H). These data suggest that persistent and higher dose of antigen stimulation induces a sustained AKT phosphorylation.
Rescue of TCM development and recall responses by rapamycin
Phosphorylation of AKT at position 308 is known to lead to activation of mTORC1 (12). We reasoned that blocking the AKT-mTORC1 pathway with rapamycin should correct the impaired TCM development and CD8 T cell recall defects in CD4−/− mice. WT and CD4−/− mice were infected intranasally with WSN-SIY virus and given rapamycin (600 µg/kg) (10) daily between 8 to 37 dpi (Fig. 4A). SIY/Kb-specific endogenous CD8 T cells were quantified at 7 and 37 dpi. At 7 dpi just before starting rapamycin treatment, the numbers of SIY/Kb-specific CD8 T cells in the lung, DLN, spleen and NDLN were similar in CD4−/− and WT mice (Supplemental Fig. 4A), as well as the percentages of IFN-γ+ and TNF-α+ CD8 T cells (Supplemental Fig. 4B–C).
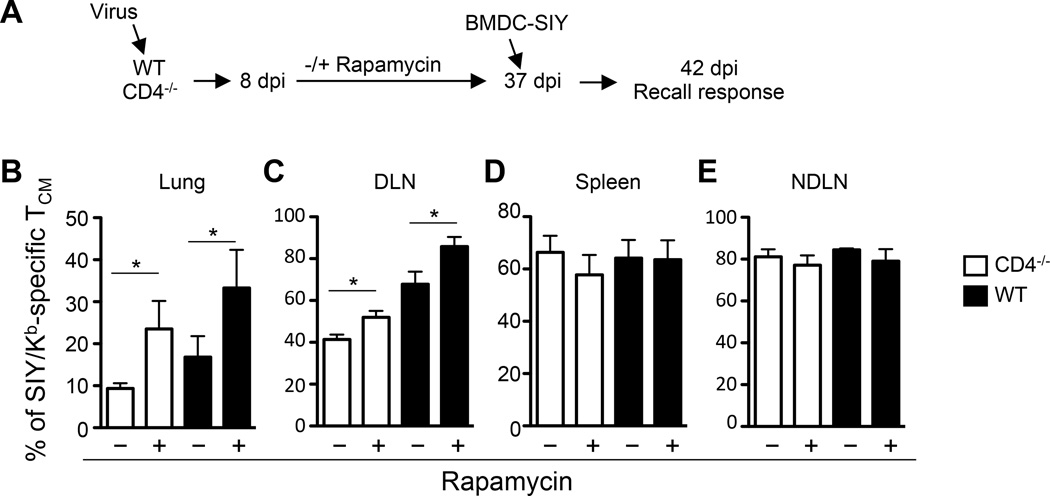
FIGURE 4.
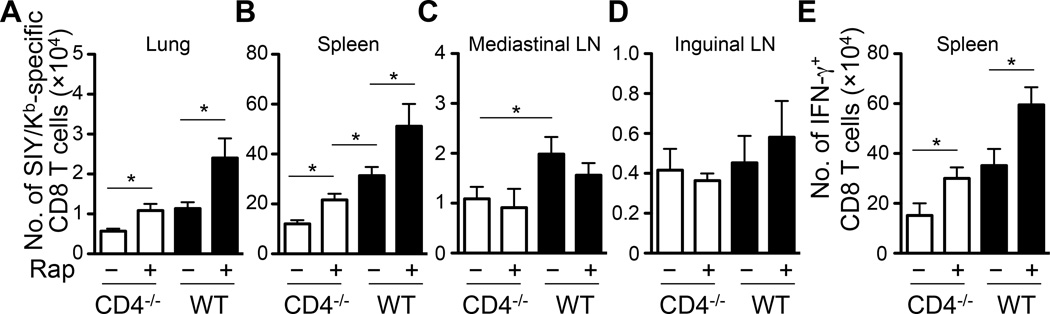
Rapamycin treatment enhances TCM development. (A) Scheme of experimental procedures. CD4−/− and WT mice were infected i.n. with 100 pfu of WSN-SIY virus. The SIY/Kb-specific endogenous memory CD8 T cells in the indicated tissues were analyzed 37 dpi by staining for CD8, CD62L and SIY/Kb-dimer. (B–E) The percentage of CD8+ CD62Lhi SIY/Kb-specific TCM cells among total CD8+ cells at 37 dpi in the lung (B), DLN (C), spleen (D), NDLN (E) in CD4−/− and WT mice with and without rapamycin treatment. Error bars: SEM from 5–6 mice per group from one of three representative experiments. * P<0.05.
At 37 dpi, the numbers of SIY/Kb-specific CD8 T cells in various tissues were largely the same in CD4−/− and WT mice either with or without rapamycin treatment (Supplemental Fig. 4D–E). However, following rapamycin treatment the percentage of SIY/Kb-Dimer+ TCM (CD62Lhi) in the lung and DLN of both CD4−/− and WT mice increased significantly (Fig. 4B–C and Supplemental Fig. 4D). But there was no significant difference in the percentage of TCM in spleen and NDLN (Fig. 4D–E) of rapamycin-treated CD4−/− and WT mice.
To determine whether rapamycin inhibition of mTORC1 corrects the CD8 T cell recall defects in CD4−/− mice, we injected the rapamycin-treated mice intravenously (i.v.) with 5×105 bone marrow-derived dendritic cells pulsed with SIY peptide (BMDC-SIY). The SIY/Kb-specific CD8 T cell recall responses were quantified 5 days later (Fig. 4A). Except in the lymph nodes (Fig. 5A–E), significantly more SIY/Kb-specific CD8 T cells were recovered in the lung and spleen of rapamycin-treated CD4−/− and WT mice (Fig. 5A–B). More CD8 T cells were also induced to express IFN-γ in the spleen of both CD4−/− and WT mice following rapamycin treatment (Fig. 5E). Moreover, following rapamycin treatment the number of SIY/Kb-specific CD8 T cells in the lung of CD4−/− mice reached the same level as that in the lung of WT mice without rapamycin treatment (Fig. 5A). The numbers of SIY/Kb-specific CD8 T cells from the lung and spleen of rapamycin-treated WT mice were, however, still significantly higher than those of rapamycin-treated CD4−/− mice. Together, these results suggest that inhibition of the AKT-mTORC1 pathway by rapamycin restores TCM development and recall responses in CD4−/− mice to the levels found in WT mice not treated with rapamycin (Fig. 6) .
FIGURE 5.
Rapamycin treatment partially corrects CD8 T cell recall defects in CD4−/− mice. The mice were injected i.v. with bone marrow derived dendritic cell pulsed with SIY peptides (BMDC-SIY) 37 dpi (Fig. 4A). The numbers of T cells specific for SIY/Kb and expressing IFN-γ were analyzed 5 days later. (A–E) The number of SIY/Kb-specific CD8 T cells in the lung (A), spleen (B), mediastinal LN (C) and inguinal LN (D) of CD4−/− and WT mice with or without rapamycin treatment. (E) The number of IFN-γ+ CD8 T cells in the spleen of CD4−/− and WT mice with or without rapamycin treatment. Error bars: SEM from 5–6 mice per group from one of three representative experiments. * P<0.05.
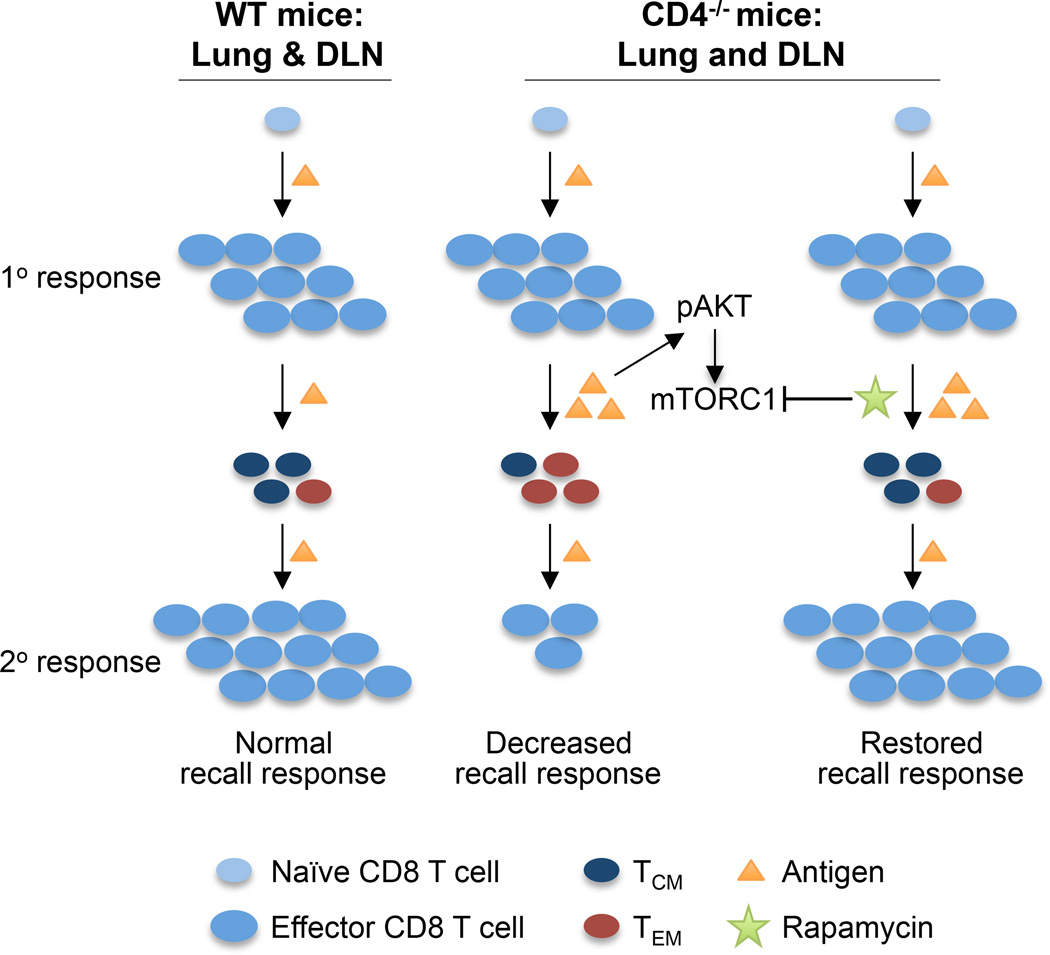
FIGURE 6.
Schematic comparison of memory CD8 T cell development and responses in WT and CD4−/− mice and how rapamycin restores memory CD8 T cell development and responses in CD4−/− mice.
Discussion
Various mechanisms have been proposed to explain CD4 T cell help in the development and recall response of memory CD8 T cells. Using influenza virus infection in the respiratory tract and examining memory CD8 T cells at various tissues, we show that the recall responses of memory CD8 T cells vary in different tissues. The recall responses of memory CD8 T cells from the lungs and DLN, but not spleen, are deficient in CD4−/− mice, as compared to those of WT mice. Surprisingly, memory CD8 T cells from NDLN of CD4−/− mice actually produce better recall responses than those from WT mice. Thus the impact of CD4 T cells on recall responses of memory CD8 T cells differ from that reported previously in CD4−/− mice following systemic infections (1–3), where recall responses of memory CD8 T cells were examined only in the spleen.
In our study, in order to compare the recall responses of memory CD8 T cells from different tissues, we isolated memory CD8 T cells from spleen, DLN, NDLN and lung, adoptively transferred them into naïve recipient mice, and then quantified the numbers of donor-derived influenza-specific CD8 T cells at the site of infection (BAL) at the peak of responses (7 dpi). One potential caveat is that the observed defect might be due to differential trafficking of the transferred memory CD8 T cells from the lung tissue into the airways. A recent study has shown that CXCR3 regulates memory CD8 T cell migration into the lung and BAL (31). Influenza-specific memory CD8 T cells in the lung uniformly express a high level of CXCL3 whereas those in the spleen exhibit a more heterogeneous CXCL3 expression. Transfer of memory CD8 T cells from the lung tissue would have favored their trafficking into the lung. Furthermore, following influenza virus challenge, antigen-specific CD8 T cells proliferate in the DLN, acquire effector function, and then migrate to the site of infection (lung). Any difference in trafficking of memory CD8 T cells from different tissues would be irrelevant following their re-activation, proliferation and acquisition of effector function. Thus, trafficking is unlikely a significant factor contributing to the observed defect of memory CD8 T cells from the lung tissue.
The recall defects of memory CD8 T cells in CD4−/− mice have been attributed to various mechanisms, including i) obligatory CD4 T cell involvement in memory CD8 T cell and antigen presenting cell interactions (32), ii) critical role of interleukin 2 (IL-2), which is predominantly secreted by CD4 T cells (15), and iii) requirement of CD4 T cells in the maintenance of memory CD8 T cells (17). Our findings suggest an alternative mechanism (Fig. 6). In the absence of CD4 T cells, clearance of influenza virus from the site of infection is delayed. The prolonged persistence of antigen in the respiratory tract and DLN (5) leads to persistent activation of the AKT-mTORC1 pathway, shifting local balance to favor TEM development at the expenses of TCM. Because TEM do not proliferate significantly upon re-stimulation (14), the recall responses of memory CD8 T cells, as measured by the numbers of antigen-specific T cells, are therefore limited.
Supporting this view, we show that influenza virus was detected for at least 3 more days in BAL of virus-infected CD4−/− mice than in similarly infected WT mice (Fig. 3A), consistent with previous observations showing a critical role of CD4 T cells in immune responses against various infections including influenza viruses (16, 26). It is worth noting that CD4−/− mice have significant numbers of CD8 T cells that are restricted by MHC class II (33). However, these CD8 T cells do not substitute for CD4 T cell function in response to influenza virus infection. Consistently, MHC II−/− mice also exhibit a defect in recall response of memory CD8 T cells as the CD4−/− mice (2). We have shown previously that prolonged antigen exposure drives activated CD8 T cells to undergo further proliferation and differentiation into TEM (5). Consistently, in the lungs and DLN of CD4−/− mice, where viral antigen is abundant and persistent, the percentages of TCMP and TCM decreased significantly (Fig. 2), correlating with poor recall responses of memory CD8 T cells in these tissues (Fig. 1D–E). In contrast, in spleen and NDLN in CD4−/− mice, where viral antigen is scant, the percentages of TCMP and TCM are normal or elevated, correlating with normal or enhanced recall responses, respectively. These results suggest that the effect of CD4 T cells on memory CD8 T cell development and recall responses is likely indirect. It is the persistence of antigen, as a result of CD4 T cell deficiency, that alters memory CD8 T cell development and recall responses.
Our study has now directly addressed how antigen regulates TCM vs. TEM development at the molecular level. We show that antigen stimulation of CD8 T cells in vitro leads to phosphorylation of AKT in the absence of CD4 T cells (Fig. 3H). More importantly, the persistence of viral antigen in CD4−/− mice also leads to a prolonged activation of AKT as indicated by the elevated levels of AKT phosphorylation in the lungs of CD4−/− mice (Fig. 3E–3G). Prolonged activation of AKT is known to promote development of short-lived effector cells, but inhibit development of memory precursors (9). Our results showing the impaired TCMP and TCM development and therefore the impaired memory responses in the lung and DLN of CD4−/− mice are consistent with this previous report. Furthermore, AKT exerts its effect partly through the mTORC1 pathway, inhibition of which has been shown to improve the quality of memory CD8 T cells and their recall responses (10, 11). Similarly, inhibition of mTORC1 by rapamycin restores the CD8 T cell recall responses in the lung of CD4−/− mice to the same level as in the lung of the WT mice without rapamycin treatment (Fig. 5). Rapamycin has been shown to enhance central memory T cell differentiation through several mechanisms independent of AKT. Although we could not exclude these mechanisms, our results are most consistent with the interpretation that antigen promotes TEM development at the expense of TCM through the prolonged activation of the AKT-mTORC1 pathway.
Modulation of TCM vs. TEM development in different tissues by antigen may help to minimize the adverse effect associated with the great expansion of antigen-specific effector T cells during a recall response. For example, following respiratory influenza virus infection TEM development is favored in the lung and DLN whereas TCM development predominates in the spleen and NDLN. With a subsequent influenza infection the expansion of memory CD8 T cells is limited in the lung and DLN and therefore avoids the potential massive destruction of the lung parenchyma. On the other hand, TCM in the spleen and NDLN can proliferate and help to eradicate the acute infection in the respiratory tract and the lung.
Our study also sheds light on the effect of rapamycin in reducing secondary cancers. Chronic immunosuppression by rapamycin in recipients of organ transplants, such as kidney allografts, is associated with a reduced frequency of secondary cancers, especially squamous cell and skin cancers (34). Chronic exposure to tumor antigen is known to favor effector CD8 T cell development and eventual exhaustion (35). The benefit of rapamycin treatment may be through promoting the development of tumor-specific TCM, which inhibit tumor development by mounting robust recall responses to newly emerged tumor cells. Similarly, rapamycin has been shown to restrict HIV and HCV replication in AIDS patients (36). Again, rapamycin may promote TCM development following chronic exposure to HIV and HCV antigens and therefore help to suppress viral replication.
Supplementary Material
Acknowledgements
We thank Ling Wang, Glenn Paradis and the Flow Cytometry Core facility of the Koch Institute Swanson Biotechnology Center at MIT for technical support.
Grant support
This work was supported in part by National Institutes of Health Grant AI69208, the National Research Foundation Singapore through the Singapore–MIT Alliance for Research and Technology’s Interdisciplinary Research Group in Infectious Disease research program, Ivan R. Cottrell Professorship and Research Fund, and the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute.
Footnotes
Disclosure
The authors have no financial conflict of interest.
References
- 1.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 2.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 4.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen CH, Talay O, Mahajan VS, Leskov IB, Eisen HN, Chen J. Antigen-bearing dendritic cells regulate the diverse pattern of memory CD8 T-cell development in different tissues. Proc Natl Acad Sci U S A. 2010;107:22587–22592. doi: 10.1073/pnas.1016350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballesteros-Tato A, León B, Lee BO, Lund FE, Randall TD. Epitope-specific regulation of memory programming by differential duration of antigen presentation to influenza-specific CD8(+) T cells. Immunity. 2014;41:127–140. doi: 10.1016/j.immuni.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate 'decisions' and effector function. Nat Immunol. 2014;15:815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, Palmer E. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 2009;323:502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- 9.Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, Suresh M. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188:4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 14.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaech SM, Ahmed R. Immunology. CD8 T cells remember with a little help. Science. 2003;300:263–265. doi: 10.1126/science.1084511. [DOI] [PubMed] [Google Scholar]
- 16.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 17.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro MN, Cantrell DA. Serine-threonine kinases in TCR signaling. Nat Immunol. 2014;15:808–814. doi: 10.1038/ni.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Eisen HN, Kranz DM. A model T-cell receptor system for studying memory T-cell development. Microbes Infect. 2003;5:233–240. doi: 10.1016/s1286-4579(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 20.Shen CH, Ge Q, Talay O, Eisen HN, García-Sastre A, Chen J. Loss of IL-7R and IL-15R expression is associated with disappearance of memory T cells in respiratory tract following influenza infection. J Immunol. 2008;180:171–178. doi: 10.4049/jimmunol.180.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu G, Chen J. A genome-wide regulatory network identifies key transcription factors for memory CD8+ T-cell development. Nat Commun. 2013;4:2830. doi: 10.1038/ncomms3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerlach C, Rohr JC, Perié L, van Rooij N, van Heijst JW, Velds A, Urbanus J, Naik SH, Jacobs H, Beltman JB, de Boer RJ, Schumacher TN. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 2013;340:635–639. doi: 10.1126/science.1235487. [DOI] [PubMed] [Google Scholar]
- 23.Buchholz VR, Flossdorf M, Hensel I, Kretschmer L, Weissbrich B, Gräf P, Verschoor A, Schiemann M, Höfer T, Busch DH. Disparate individual fates compose robust CD8+ T cell immunity. Science. 2013;340:630–635. doi: 10.1126/science.1235454. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207:1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zammit DJ, Turner DL, Klonowski KD, Lefrançois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 30.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slütter B, Pewe LL, Kaech SM, Harty JT. Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus. Immunity. 2013;39:939–948. doi: 10.1016/j.immuni.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 33.Tyznik AJ, Sun JC, Bevan MJ. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J Exp Med. 2004;199:559–565. doi: 10.1084/jem.20031961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, Molinolo AA, Gutkind JS. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65:9953–9961. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 35.Bak SP, Barnkob MS, Bai A, Higham EM, Wittrup KD, Chen J. Differential requirement for CD70 and CD80/CD86 in dendritic cell-mediated activation of tumor-tolerized CD8 T cells. J Immunol. 2012;189:1708–1716. doi: 10.4049/jimmunol.1201271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Benedetto F, Di Sandro S, De Ruvo N, Montalti R, Ballarin R, Guerrini GP, Spaggiari M, Guaraldi G, Gerunda G. First report on a series of HIV patients undergoing rapamycin monotherapy after liver transplantation. Transplantation. 2010;89:733–738. doi: 10.1097/TP.0b013e3181c7dcc0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.