Abstract
Voice and swallowing dysfunction as a result of recurrent laryngeal nerve paralysis can be improved with vocal fold injections or laryngeal framework surgery. However, denervation atrophy can cause late-term clinical failure. A major determinant of skeletal muscle physiology is myosin heavy chain (MyHC) expression, and previous protein analyses have shown changes in laryngeal muscle fiber MyHC isoform with denervation. RNA analyses in this setting have not been performed, and understanding RNA levels will allow interventions better designed to reverse processes such as denervation in the future. Total RNA was extracted from bilateral rat thyroarytenoid (TA), posterior cricoarytenoid (PCA), and cricothyroid (CT) muscles in rats. Primers were designed using published MyHC isoform sequences. SYBR Green real time reverse transcription-polymerase chain reaction (SYBR-RT-PCR) was used for quantification. The electropherogram showed a clear separation of total RNA to 28S and 18S subunits. Melting curves illustrated single peaks for all type MyHC primers. All MyHC isoforms were identified in all muscles with various degrees of expression. Quantitative PCR is a sensitive method to detect MyHC isoforms in laryngeal muscle. Isoform expression using mRNA analysis was similar to previous analyses but showed some important differences. This technique can be used to quantitatively assess response to interventions targeted to maintain muscle bulk after denervation.
Keywords: Quantitative PCR, Myosin heavy chain, Rat, Larynx
INTRODUCTION
Effective communication relies on intact voice and speech, and as such, laryngeal muscle activity has a dramatic impact on voice quality. Paralysis of one or more laryngeal muscles can lead to poor voice quality, breathing difficulties, or dysphagia. The most common causes for vocal fold paralysis include surgery in the head and neck divided into the following categories neck surgery not otherwise specified (18.8%), thoracic disease/surgery (15.5%), thyroid surgery (13.3%), and skull base surgery (12.1%). Systemic or peripheral neurologic (2.6%) or inflammatory disease (2.2%) makes up a minority of causes, but in many patients, a definitive cause is not determined (21.4%).(Netterville, 2003) Current treatment for laryngeal paralysis consists of surgical manipulation of the paralyzed vocal fold to place it into a better position to coapt the mobile vocal fold for phonation via injection laryngoplasty or laryngeal framework surgery. Such procedures provide adequate voice but additional treatments can be required over time to address the atrophy that occurs with lack of neural input. (Andrews et al., 2008; Umeno, Chitose, Sato, & Nakashima, 2008)
In addition to paralysis, laryngeal incompetence related to aging is increasingly being recognized as a contributor to poor voice.(Belafsky, Postma, Reulbach, Holland, & Koufman, 2002; Reulbach, Belafsky, Blalock, Koufman, & Postma, 2001) Treatment options for aging is similarly limited to static vocal fold augmentation with injection(Kwon, An, Ahn, Kim, & Sung, 2010) or framework surgery (Omori et al., 1997). Selected patients can have dramatic improvement in voicing after intervention, but there are no clear treatment guidelines. (Behrman, 2004) More importantly just as with intervention for paralysis, the intervention is symptomatic only. It does not address the underlying cause of the insufficiency. In animal models, age related changes have been shown to be a result of both muscle alterations and soft tissue degradation.(Abdelkafy et al., 2007; McMullen & Andrade, 2005; McMullen & Andrade, 2009)
To expand clinical progress in this area, better understanding of the muscle physiology of the larynx is needed. There are traditionally, two different groups of muscles in the larynx. The adductor group which closes and tenses the glottis includes the intrinsic muscles of the larynx including the thyroarytenoid (TA), lateral cricoarytenoid (LCA) and interarytenoid (IA) as well as the intrinsic cricothyroid (CT) muscle. The sole abductor muscle that opens the glottis to allow for full respiration is the posterior cricoarytenoid (PCA). The predominant myosin heavy chain (MyHC) fiber type has a great deal of influence on the contraction speed, endurance, and strength of a given muscle fiber and therefore the muscle bundle. At least five types of MyHC (type I, IIA, IIB, IIX, and IIL—Table 1) can be identified in the rat larynx by immunohistochemistry and SDS-PAGE (DelGaudio & Sciote, 1997; Li, Lehar, Nakagawa, Hoh, & Flint, 2004; Rhee, Lucas, & Hoh, 2004; Shiotani, W., Coleman, Alila, & Flint, 1998; Shiotani, Nakagawa, & Flint, 2001; Wu, Baker, Crumley, & Caiozzo, 2000; Wu, Baker, Marie, Crumley, & Caiozzo, 2004).
Table 1.
MyHC isoform phenotypes
| Isoform | Contraction Rate | Primary Cellular Metabolism |
|---|---|---|
| I (aka βeta) | Slow | Oxidative (Red) |
| IIA | Fast | Mixed |
| IIB | Faster | Glycolytic (White) |
| IIX | Fastest | Glycolytic (White) |
| IIL (aka EO) | Fastest | Glycolytic (White) |
In mammals including both the rat and human, the TA muscle has very short contraction times thus has the fastest MyHC profile (in the range of extraocular muscles for same species) with no slow phenotype fibers identified. (Hoh, 2005; Horton, Rosen, Close, & Sciote, 2008; Shiotani et al., 1998) In the rat, the CT muscle contraction times are two to four times longer than the TA similar to limb muscles. CT muscle therefore has smaller proportions of the fastest MyHC fibers. (Hoh, 2005) In the rat, PCA muscle contraction times are intermediate between the TA and CT. (Hoh, 2005; Shiotani et al., 1998) One study from the human has demonstrated that the three compartments of the PCA muscle (horizontal, oblique, vertical) may have different functions and speeds in that the fast MyHC higher in the vertical fibers of the PCA vs. horizontal fibers of the PCA.(Horton et al., 2008)
Multiple immunochemistry, SDS-PAGE, and proteomic studies have shown denervation leads to detrimental changes in MyHC isoform expression.(Caiozzo, Wu, Baker, & Crumley, 2004; DelGaudio & Sciote, 1997; Li, Lehar, Samlan, & Flint, 2005; Rhee et al., 2004; Shiotani & Flint, 1998) Immunochemistry and SDS-PAGE are at best semiquantitative techniques, however, that may over-represent predominant proteins and under-represent small amounts of protein. Quantification of RNA expression may be a more valuable technique to identify changes in MyHC expression (Jankala, Harjola, Petersen, & Harkonen, 1997). SYBR Green real time RT-PCR RNA analysis demonstrates a high degree of sensitivity when applied to the expression of selected genes from small amounts and limited tissue samples such as rat intrinsic laryngeal muscle and human muscle biopsy samples (Kubota et al., 2003; Overbergh et al., 2003; Shariat et al., 2003). Although muscle fiber type is regulated by multiple mechanisms(Goldspink et al., 1992; Gunning & Hardeman, 1991), studies have suggested MyHC expression is predominantly regulated transcriptionally. In particular recently in young humans, MyHC protein levels were shown to be correlated with mRNA transcript levels (Short et al., 2005) which underscores the importance of better understanding the transcriptional regulation of fiber types.
The purpose of this study is to establish the use of quantitative PCR in laryngeal muscles. This study serves as a basis for additional studies investigating changes resulting in the larynx after denervation, radiation treatment, etc.
MATERIALS AND METHODS
This protocol was approved by the University of Iowa Institutional Animal Care and Use Committee (IACUC). Twelve Sprague–Dawley rats, approximately 280 g, experimental rats were used for this study. After euthanasia, the thyroarytenoid (TA), posterior cricoarytenoid (PCA), cricothyroid (CT), and soleus muscles were removed using a microscopic technique. Total RNA was extracted using a TRIzol® (Invitrogen) extraction protocol. Muscle fibers were loaded into 1.5 ml eppendrof tubes and 1 ml TRIzol® reagent added then the fibers homogenized. Cell lysate was added to the pre-spun phase lock gel-heavy tubes and incubated 5 minutes at room temperature. Chloroform at 0.2 ml per 1 ml TRIzol® reagent initially used was added. Tubes were capped and shaken vigorously for 15 seconds. Samples were centrifuged at 12,000 × g for 10 minutes at 4°C. The clear, aqueous phase containing RNA was transferred to a fresh eppendorf tube. For precipitating RNA, 0.5 ml Isopropyl alcohol was added. Samples were incubated at room temperature for 10 minutes and the centrifuged for 10 minutes at 12,000 × g, 4°C. The supernatant was decanted from the RNA pellet on bottom of tubes. The RNA pellet was washed with ml 75% ethanol and centrifuged at 7,500 × g for 5 minutes at 4°C after which the carefully decanted supernatant was briefly air-dried for 5 minutes. DNase-RNase free water (60 μl) was added to dissolve RNA pallet then incubated at 55–60°C for 10 minutes to facilitate dissolution. RNA concentration was determined using a spectrophotometer.
Various methods including spectrophotometry and gel electrophoresis have raditionally been used to analyze RNA quality. The bioanalyzer (Agilent Technologies 2100 Bioanalyzer, DNA core, University of Iowa) provides a very sensitive qualitative analysis of total RNA in small quantities compared to traditional methods. The samples were electrophoretically separated into peaks of 18S and 28S ribosomal RNA. Since the intrinsic laryngeal muscle samples were small (0.5 – 1 mg), the peaks in the bioanalyzer signal were difficult to resolve. To verify our method of RNA extraction, total RNA was extracted from the soleus muscle and diluted x10, x100 and x500 to the same concentration of our intrinsic laryngeal muscles samples.
Prior to RT-PCR, DNase treatment (Promega) was performed. Each reaction contained 1–8 μl RNA in water, 1 μl 10X buffer, 1u/ug RNA RNase-free DNase and DNase-RNase free water to a final volume of 10 μl. The samples were incubated at 37°C for 30 minutes after which time1 μl of stop solution was added to terminate the reaction and the samples incubated at 65°C for 10 minutes to inactivate DNase. All samples were normalized to a common concentration.
Oligonucleotide primers were designed using NCBI published nucleotide sequences complementary to selected regions of the rat gene encoding MyHC with NCBI accession numbers detailed in Table 2. Since the original search was performed, regular NCBI automation has collapsed some of the sequences into accession numbers NM_001135158.1 and NM_001135157.1. Commonly used housekeeping genes for real time quantitative PCR normalization include glyceraldehydes-3-phosphate dehydrogenase (GAPDH), 18S and 28S ribosomal RNA, β-actin, β-2-microglobulin. A recent report regarding the viability of using said genes as housekeeping genes recommended GAPDH as the most stable in response to exercise in slow- and fast- twitch human muscle fibers (Jemiolo & Trappe, 2004). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, TaqMan Rodent GAPDH) primer was purchased from Applied Biosystems to be used as a control housekeeping gene.
Table 2.
MyHC isoform Primer oligonucleotide sequence
| Isoform | Forward primer | Reverse primer | NCBI Accession No. |
|---|---|---|---|
| I | GAA TGG CAA GAC GGT GAC TGT | GGA AGC GTA CCT CTC CTT GAG A | x 15939 |
| IIA | ATG ACA ACT CCT CTC GCT TTG G | TTA AGC TGG AAA GTG ACC CGG | xm_340817 |
| IIB | GAA CAC GAA GCG TGT CAT CCA | AGG TTT CGA TAT CTG CGG AGG | xm_340818 |
| IIL | AAG AGA TGA CTT ACC AGG CCG A | GCC TGC CTC TTG TAG GAC TTC A | xm_340820 |
| IIX | CCA ATG AGA CTA AGA CGC CTG G | GCT ATC GAT GAA TTG TCC CTC G | xm_213345 |
Superscript III reverse transcriptase (Invitrogen) was used to generate the first cDNA strand from RNA. A two-step RT was performed in a total reaction volume of 20 μl. The first reaction mix contained: 1 μl Random hexomer, 1 μl Oligo (dT)18, 1 μl 10mM dNTP mix (Biosciences, Clontech), 8 μl extracted total RNA, and 2 μl DNase-RNase free water. The mixture was incubated 65°C for 5 minutes, chilled on ice for at least 1 minute, and added the reaction mixture of the second step which contained: 4 μl 5X First strand buffer, 1 μl 0.1M DDT, 1 μl Superscript III RT, 1 μl RNaseOUT (Invitrogen). The final mixture was incubated at 25°C for 10 minutes and then was incubated at 42°C for 50 minutes, followed by reaction termination at 85°C for 5 minutes. To remove RNA complementary to the cDNA1 μl of 2 Units of E. coli RNase H (New England, BioLabs) was added and incubated at 37°C for 20 minutes then stored at −20°C until use.
Quantitative PCR was performed using ABI-Prism qPCR machine and Platinum SYBR green qPCR Super Mix UDG (Invitrogen). Each reaction contained: 12.5 μl of Platinum SYBR green qPCR Super Mix-UDG, 0.5 μl ROX reference dye, 0.33 μl 100 μM forward and reverse primer, 5 μl cDNA and 6.34 μl DNase-RNase free water to a final volume of 25 μl. Amplification was placed in 96-well optical reaction plate and caps (ABI Prism). PCR parameters were as follows: 2-step cycling: 50°C for 2 minutes and then initial denatuation at 95°C for 2 minutes followed by 50 cycles of 15 seconds at 95°C, and 30 seconds at 60°C. Following the final cycle, melting curve analysis (dissociation curve) was performed for each run, and data from dissociation curves that showed evidence for primer-dimers or mirrored the negative control were eliminated (Vandesompele, De Paepe, & Speleman, 2002). The cycle number where the amplification curve crossed the threshold of the standard curve was noted as critical threshold (Ct) and used for all further analysis.
RESULTS
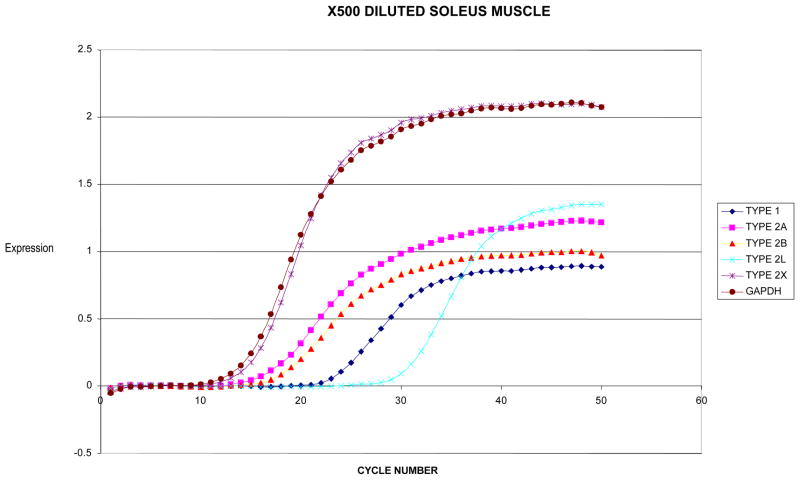
To examine the quality of extracted RNA, a dilution (10X) of soleus muscle (50mg) total extracted RNA using the aforementioned protocol showed 28S and 18S peaks with low noise between peaks as well as minimal low molecular weight contamination (Figure 1A). The soleus total RNA was then further diluted (500X) to a low concentration at approximating the concentration of total RNA extracted from a laryngeal muscle. The soleus muscle showed a small peak of 28S and 18S ribosomal RNA again with minimal contamination (Fig. 1B). The 500X dilution sample then showed good results when run using the SYBR green RT-PCR (Fig. 2).
Figure 1.
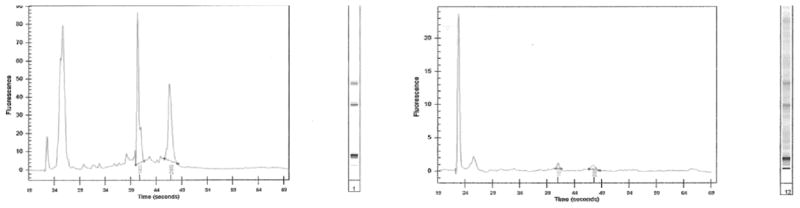
Bioanalyzer output from total RNA extracted from soleus muscle diluted 10X (A) and 500X (B).
Figure 2.
SYBR Green real time PCR output of X500 diluted soleus muscle.
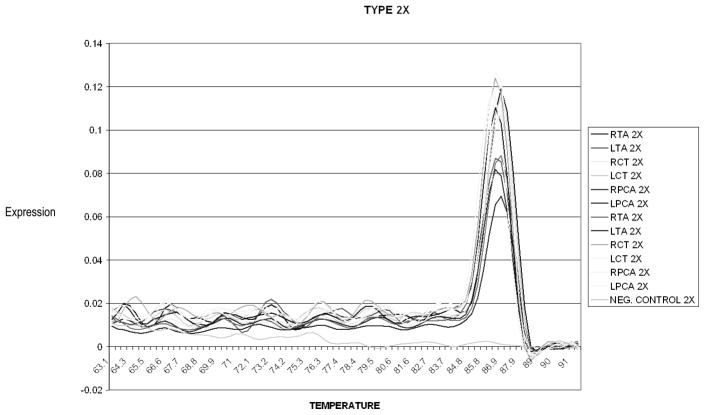
To confirm primer quality, the primers were subjected a dissociation analysis following the final PCR cycle. Figure 3 illustrates a typical melting curve for MyHC type IIX PCR products with only a single dissociation peak at 86.5 °C. Dissociation of PCR constantly produced single peaks for MyHC type I at 86.2 C, type IIA at 83.1 C, type IIB at 85.5C, type IIL at 85.1, and the housekeeping gene (GAPDH) at 87.6C suggesting the presence of only one product of each PCR primer. Results were verified in triplicate on the same plates, and in duplicate on different plates for all isoforms of the PCA muscle.
Figure 3.
Typical melting (heat dissociation) curve of PCR product for MyHC type 2X in the sample. PCR yielding single peak at at 86.5 °C.
Type I had a higher Ct value than other MyHC isoform in the TA and PCA muscles and type IIL had a higher Ct value than other MyHC isoform in CT muscle. The TA muscle showed expression of all MyHC isoforms with type I form showed relatively low levels of expression compared to isoform IIL due to the higher Ct values for type I compared to type IIL(Table 3). The PCA muscle showed expression of all MyHC isoform expression with all type II isoforms showing similar levels of expression to that of GAPDH, but type I was expressed at low levels compared to the type II isoforms as a whole(Table 3). The CT muscle showed expression of all MyHC isoform in a pattern opposite of TA (Table 3). The type I form showed relatively high levels of expression compared to isoform IIL due to the higher Ct values for type IIL compared to type I.
Table 3.
Average MyHC and GAPDH Ct as well as average and standard deviation (SD) of the difference between GAPDH and MyHC by muscle and isoform
| MyHC | GAPDH | Difference | SD | |
|---|---|---|---|---|
| Thyroarytenoid | ||||
| I | 36.46 | 23.57 | 12.71 | 7.98 |
| IIA | 26.80 | 23.87 | 2.94 | 6.66 |
| IIB | 24.27 | 23.87 | 0.41 | 4.16 |
| IIL | 19.16 | 23.52 | −2.80 | 6.10 |
| IIX | 24.44 | 23.87 | 0.93 | 4.88 |
|
| ||||
| Posterior Cricoarytenoid | ||||
| I | 31.31 | 21.28 | 9.60 | 3.11 |
| IIA | 22.16 | 21.33 | 0.45 | 1.74 |
| IIB | 23.92 | 21.38 | 2.16 | 3.40 |
| IIL | 23.09 | 21.43 | 1.76 | 4.02 |
| IIX | 22.53 | 20.46 | 0.47 | 1.89 |
|
| ||||
| Cricothyroid | ||||
| I | 26.29 | 22.12 | 6.03 | 2.99 |
| IIA | 22.61 | 22.12 | 0.49 | 4.14 |
| IIB | 27.33 | 22.12 | 5.20 | 4.30 |
| IIL | 31.26 | 20.95 | 1.56 | 4.02 |
| IIX | 21.57 | 22.12 | 0.47 | 1.89 |
DISCUSSION
While previous analyses have focused on SDS-PAGE and immunochemical analyses, this study establishes the ability to reliably extract high-quality myosin heavy chain (MyHC) mRNA from laryngeal muscles, the thyroarytenoid (TA), posterior cricoarytenoid (PCA), and cricothyroid (CT) in control animals. Similar to previous studies, isoform IIL expression was relatively higher in TA and PCA muscles compared to CT muscle. However unlike other studies, CT muscle did demonstrate low levels of IIL expression. Type I expression has previously only been reported in CT muscles without any in TA or PCA muscle. This ability to detect low levels of expression based upon reliable standard primers points to the improved sensitivity of the quantitative PCR technique. This finding that all MyHC isoforms are expressed in all laryngeal muscles has significance. It indicates the basic framework is present in all muscle and that transcriptionally based modulation is possible. If no isoform is being transcribed even at low levels, it may be more difficult to drive the cells to express that particular isoform with exogenous stimuli (either chemical, mechanical, or electrical).
This study is limited in that we obtained all muscle fibers contained in an individual muscle and analyzed them in bulk. Previous animal studies have shown three dimensional differences in the dog thyroarytenoid muscle.(Bergrin, Bicer, Lucas, & Reiser, 2006) The current study did not account for intra-muscular differences. However in the future, laser capture microdissection and single fiber analysis can overcome this limitation to more clearly define the molecular characteristics of this hybrid muscle. (Wu, Crumley, & Caiozzo, 2000) In particular, the sensitivity of this analysis will allow for more comprehensive evaluation of the natural state of these muscles as well as changes.
This study was peformed in rats which limits its application to mouse models of musclular or systemic disorders. Rats were selected primarily because of the size of the larynx muscle fibers and extensive previous literature regarding muscle fiber type profiles. The underlying methods of this study can be easily applied to a mouse model by simply substituting mouse primers for rat. In addition to use in mouse models, it has become clear that microRNA regulation is important in muscle physiology and may possibly have a role in reinnervation. (Williams et al., 2009) This study provides a crucial foundation to be able to study microRNA models in a way we have not been able to in the past.
The findings of this study have limited direct clinical utility. As stated in the introduction, the most direct application of this data is for future research in denervation associated with laryngeal paralysis or atrophy with aging. However, it is likely that the fibrosis and atrophy and resultant poor voice, swallowing, etc associated with radiation treatment for head and neck cancers also has a molecular basis. Very little is known about the details of those changes(Lidegran, Forsgren, Dahlqvist, Franzen, & Domeij, 1999; Nagler, Baum, Miller, & Fox, 1998), but the techniques in this study are sensitive enough to first quantify those changes as well as measure the effect of interventions designed to reduce the effect. For instance if inflammation plays a significant role, might treatment with anti-inflammatories limit long-term complications?
In summary, this study has demonstrated the ability to sensitively extract RNA from rat laryngeal muscle in a reproducible fashion. Application of this technique to future studies will allow better characterization of changes associated with disease.
LEARNING OUTCOMES.
Readers will be able to describe the relationship between myosin heavy chain expression and muscle contractile properties.
Readers will be able to separate myosin heavy chain isoforms into slow and fast twitch phenotypes.
Readers will be able to describe differential muscle isoform expression between different laryngeal muscles.
Readers will be able to compare this study to other modalities of determining muscle fiber type.
References
- Abdelkafy WM, Smith JQ, Henriquez OA, Golub JS, Xu J, Rojas M, et al. Age-related changes in the murine larynx: Initial validation of a mouse model. The Annals of Otology, Rhinology, and Laryngology. 2007;116(8):618–622. doi: 10.1177/000348940711600810. [DOI] [PubMed] [Google Scholar]
- Andrews BT, Van Daele DJ, Karnell MP, McCulloch TM, Graham SM, Hoffman HT. Evaluation of open approach and injection laryngoplasty in revision thyroplasty procedures. Otolaryngology--Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery. 2008;138(2):226–232. doi: 10.1016/j.otohns.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Behrman A. Evidence-based treatment of paralytic dysphonia: Making sense of outcomes and efficacy data. Otolaryngologic Clinics of North America. 2004;37(1):75–104. vi. doi: 10.1016/S0030-6665(03)00169-5. [DOI] [PubMed] [Google Scholar]
- Belafsky PC, Postma GN, Reulbach TR, Holland BW, Koufman JA. Muscle tension dysphonia as a sign of underlying glottal insufficiency. Otolaryngology--Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery. 2002;127(5):448–451. doi: 10.1067/mhn.2002.128894. [DOI] [PubMed] [Google Scholar]
- Bergrin M, Bicer S, Lucas CA, Reiser PJ. Three-dimensional compartmentalization of myosin heavy chain and myosin light chain isoforms in dog thyroarytenoid muscle. American Journal of Physiology Cell Physiology. 2006;290(5):C1446–58. doi: 10.1152/ajpcell.00323.2005. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Wu YZ, Baker MJ, Crumley R. Effects of denervation on cell cycle control in laryngeal muscle. Arch Otolaryngol Head Neck Surg. 2004;130(9):1056–68. doi: 10.1001/archotol.130.9.1056. [DOI] [PubMed] [Google Scholar]
- DelGaudio JM, Sciote JJ. Changes in myosin expression in denervated laryngeal muscle. Ann Otol Rhinol Laryngol. 1997;106(12):1076–81. doi: 10.1177/000348949710601212. [DOI] [PubMed] [Google Scholar]
- Goldspink G, Scutt A, Loughna PT, Wells DJ, Jaenicke T, Gerlach GF. Gene expression in skeletal muscle in response to stretch and force generation. The American Journal of Physiology. 1992;262(3 Pt 2):R356–63. doi: 10.1152/ajpregu.1992.262.3.R356. [DOI] [PubMed] [Google Scholar]
- Gunning P, Hardeman E. Multiple mechanisms regulate muscle fiber diversity. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 1991;5(15):3064–3070. doi: 10.1096/fasebj.5.15.1835946. [DOI] [PubMed] [Google Scholar]
- Hoh JF. Laryngeal muscle fibre types. Acta Physiol Scand. 2005;183(2):133–49. doi: 10.1111/j.1365-201X.2004.01402.x. [DOI] [PubMed] [Google Scholar]
- Horton MJ, Rosen C, Close JM, Sciote JJ. Quantification of myosin heavy chain RNA in human laryngeal muscles: Differential expression in the vertical and horizontal posterior cricoarytenoid and thyroarytenoid. The Laryngoscope. 2008;118(3):472–477. doi: 10.1097/MLG.0b013e31815c1a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankala H, Harjola VP, Petersen NE, Harkonen M. Myosin heavy chain mRNA transform to faster isoforms in immobilized skeletal muscle: A quantitative PCR study. J Appl Physiol. 1997;82(3):977–82. doi: 10.1152/jappl.1997.82.3.977. [DOI] [PubMed] [Google Scholar]
- Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: Validation of internal control with exercise. Biochem Biophys Res Commun. 2004;320(3):1043–50. doi: 10.1016/j.bbrc.2004.05.223. [DOI] [PubMed] [Google Scholar]
- Kubota K, Nakanishi H, Hiki N, Shimizu N, Tsuji E, Yamaguchi H, et al. Quantitative detection of micrometastases in the lymph nodes of gastric cancer patients with real-time RT-PCR: A comparative study with immunohistochemistry. Int J Cancer. 2003;105(1):136–43. doi: 10.1002/ijc.11031. [DOI] [PubMed] [Google Scholar]
- Kwon TK, An SY, Ahn JC, Kim KH, Sung MW. Calcium hydroxylapatite injection laryngoplasty for the treatment of presbylaryngis: Long-term results. The Laryngoscope. 2010;120(2):326–329. doi: 10.1002/lary.20749. [DOI] [PubMed] [Google Scholar]
- Li ZB, Lehar M, Nakagawa H, Hoh JF, Flint PW. Differential expression of myosin heavy chain isoforms between abductor and adductor muscles in the human larynx. Otolaryngol Head Neck Surg. 2004;130(2):217–22. doi: 10.1016/j.otohns.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Li ZB, Lehar M, Samlan R, Flint PW. Proteomic analysis of rat laryngeal muscle following denervation. Proteomics. 2005;5(18):4764–4776. doi: 10.1002/pmic.200401329. [DOI] [PubMed] [Google Scholar]
- Lidegran M, Forsgren S, Dahlqvist A, Franzen L, Domeij S. Short- and long-term effects of irradiation on laryngeal mucosa of the rat. Acta Oncologica (Stockholm, Sweden) 1999;38(8):1081–1091. doi: 10.1080/028418699432400. [DOI] [PubMed] [Google Scholar]
- McMullen CA, Andrade FH. Contractile dysfunction and altered metabolic profile of the aging rat thyroarytenoid muscle. J Appl Physiol. 2005 doi: 10.1152/japplphysiol.01066.2005. [DOI] [PubMed] [Google Scholar]
- McMullen CA, Andrade FH. Functional and morphological evidence of age-related denervation in rat laryngeal muscles. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2009;64(4):435–442. doi: 10.1093/gerona/gln074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler RM, Baum BJ, Miller G, Fox PC. Long-term salivary effects of single-dose head and neck irradiation in the rat. Archives of Oral Biology. 1998;43(4):297–303. doi: 10.1016/s0003-9969(97)00120-9. [DOI] [PubMed] [Google Scholar]
- Netterville James, Billante Cheryl. The immobile vocal fold. In: Ossoff RA, editor. The Larynx. Philadelphia, NY: Lippincott Williams & Wilkins; 2003. p. 270. [Google Scholar]
- Omori K, Slavit DH, Matos C, Kojima H, Kacker A, Blaugrund SM. Vocal fold atrophy: Quantitative glottic measurement and vocal function. The Annals of Otology, Rhinology, and Laryngology. 1997;106(7 Pt 1):544–551. doi: 10.1177/000348949710600702. [DOI] [PubMed] [Google Scholar]
- Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech. 2003;14(1):33–43. [PMC free article] [PubMed] [Google Scholar]
- Reulbach TR, Belafsky PC, Blalock PD, Koufman JA, Postma GN. Occult laryngeal pathology in a community-based cohort. Otolaryngology--Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery. 2001;124(4):448–450. doi: 10.1067/mhn.2001.114256. [DOI] [PubMed] [Google Scholar]
- Rhee HS, Lucas CA, Hoh JF. Fiber types in rat laryngeal muscles and their transformations after denervation and reinnervation. J Histochem Cytochem. 2004;52(5):581–90. doi: 10.1177/002215540405200503. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Roudier MP, Wilcox GE, Kattan MW, Scardino PT, Vessella RL, et al. Comparison of immunohistochemistry with reverse transcription-PCR for the detection of micrometastatic prostate cancer in lymph nodes. Cancer Res. 2003;63(15):4662–70. [PubMed] [Google Scholar]
- Shiotani A, Flint PW. Myosin heavy chain composition in rat laryngeal muscles after denervation. Laryngoscope. 1998;108(8 Pt 1):1225–9. doi: 10.1097/00005537-199808000-00023. [DOI] [PubMed] [Google Scholar]
- Shiotani A, Nakagawa H, Flint PW. Modulation of myosin heavy chains in rat laryngeal muscle. Laryngoscope. 2001;111(3):472–7. doi: 10.1097/00005537-200103000-00017. [DOI] [PubMed] [Google Scholar]
- Shiotani A, WOB, Coleman ME, Alila HW, Flint PW. Reinnervation of motor endplates and increased muscle fiber size after human insulin-like growth factor I gene transfer into the paralyzed larynx. Hum Gene Ther. 1998;9(14):2039–47. doi: 10.1089/hum.1998.9.14-2039. [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Coenen-Schimke JM, Rys P, et al. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol. 2005;99(1):95–102. doi: 10.1152/japplphysiol.00129.2005. [DOI] [PubMed] [Google Scholar]
- Umeno H, Chitose S, Sato K, Nakashima T. Efficacy of additional injection laryngoplasty after framework surgery. The Annals of Otology, Rhinology, and Laryngology. 2008;117(1):5–10. doi: 10.1177/000348940811700102. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Paepe A, Speleman F. Elimination of primer-dimer artifacts and genomic coamplification using a two-step SYBR green I real-time RT-PCR. Anal Biochem. 2002;303(1):95–8. doi: 10.1006/abio.2001.5564. [DOI] [PubMed] [Google Scholar]
- Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, et al. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science (New York, NY) 2009;326(5959):1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YZ, Baker MJ, Crumley RL, Caiozzo VJ. Single-fiber myosin heavy-chain isoform composition of rodent laryngeal muscle: Modulation by thyroid hormone. Arch Otolaryngol Head Neck Surg. 2000;126(7):874–80. doi: 10.1001/archotol.126.7.874. [DOI] [PubMed] [Google Scholar]
- Wu YZ, Baker MJ, Marie JP, Crumley R, Caiozzo VJ. The plasticity of denervated and reinnervated laryngeal muscle: Focus on single-fiber myosin heavy-chain isoform expression. Arch Otolaryngol Head Neck Surg. 2004;130(9):1070–82. doi: 10.1001/archotol.130.9.1070. [DOI] [PubMed] [Google Scholar]
- Wu YZ, Crumley RL, Caiozzo VJ. Are hybrid fibers a common motif of canine laryngeal muscles? single-fiber analyses of myosin heavy-chain isoform composition. Arch Otolaryngol Head Neck Surg. 2000;126(7):865–73. doi: 10.1001/archotol.126.7.865. [DOI] [PubMed] [Google Scholar]