Abstract
Background
Obesity increases the risk of asthma and asthma severity and is a well-known risk factor for insulin resistance and the metabolic syndrome (MS) in children and adolescents.
Objective
We aim to examine the association among obesity, insulin sensitivity, metabolic syndrome, and lung function in U.S. adolescents with and without asthma.
Methods
Cross-sectional study 1,429 adolescents aged 12–17 years in the 2007–2010 National Health and Nutrition Examination Survey. Adjusted regression was used to assess the relationships among obesity, insulin sensitivity/resistance, metabolic syndrome, and lung function in children with and without asthma.
Results
Insulin resistance was negatively associated with FEV1 and FVC in adolescents with and without asthma, while MS was associated with lower FEV1/FVC, with a more pronounced decrease among asthmatics; these associations were driven by overweight/obese adolescents. Higher BMI was associated with a decrease in FEV1/FVC among adolescents with insulin resistance. Compared to healthy participants, adolescents with MS had a ~2% decrease in FEV1/FVC; adolescents with asthma had a ~6% decrease; and those with MS and asthma had ~10% decreased FEV1/FVC (p<0.05).
Conclusion
Insulin resistance and MS are associated with worsened lung function in overweight/obese adolescents. Asthma and MS synergistically decrease lung function, as do obesity and insulin resistance. These factors may contribute to the pathogenesis of asthma severity in obese patients and warrant further investigation.
Keywords: asthma, lung function, insulin resistance, adiposity, obesity, metabolic syndrome, NHANES
INTRODUCTION
Asthma and obesity are major public health issues in industrialized countries such as the United States (U.S.), with parallel rises in the prevalence of both diseases over the last few decades(1–4). Epidemiologic studies have showed that childhood obesity is associated with increased risk of incident asthma, increased asthma severity and morbidity, and decreased response to long-term asthma medications(5–8).
Childhood obesity is a known risk factor for insulin resistance, diabetes, and the metabolic syndrome(9, 10). There is growing evidence that metabolic derangements such as hyperglycemia and hyperinsulinemia may lead to airway dysfunction and increased airway responsiveness via several pathways, including epithelial damage and airway smooth muscle proliferation(11). A recent population-based study reported higher rates of acanthosis nigricans (a marker of insulin resistance) in children with asthma than in those without asthma, regardless of body mass index (BMI)(12). Conversely, morbidly obese children and adolescents with asthma have a higher incidence of insulin resistance than morbidly obese children and adolescents without asthma(13, 14). The metabolic syndrome has also been significantly associated with lung function impairment and asthma-like symptoms, with abdominal obesity being the key determinant of this association(15, 16).
We hypothesized that measures of insulin sensitivity (fasting glucose/insulin ratio [G/I], quantitative insulin sensitivity check index [QUICKI]) and insulin resistance (homeostasis model assessment-estimated insulin resistance [HOMA-IR]) are associated with lung function in adolescents, particularly among those with obesity or increased adiposity. We further hypothesized that the metabolic syndrome is associated with worse lung function, and that detrimental effects of insulin resistance and metabolic syndrome on lung function are more pronounced in adolescents with asthma. We examined these hypotheses in a cross-sectional study of adolescents living in the U.S.
METHODS
Subject recruitment
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional nationwide survey designed to assess the health and nutritional status of the non-institutionalized population of the U.S.(17). NHANES combines interviews and physical examinations of participants by highly trained personnel. Participants for the study are selected using a stratified, multistage probability design, and are thus a representative sample of the U.S. population. By design, ethnic minorities (African Americans and Mexican Americans) are over-sampled to increase the statistical power for data analysis in these groups. Adolescents 12 to 17 years of age who participated in the 2007–2008 and 2009–2010 NHANESs were included in this analysis. Current asthma was defined as both having had asthma diagnosed by a doctor or other health care professional and at least one asthma attack in the past year. Participants who had neither diagnosed asthma nor an asthma attack in past year were selected as control subjects. Participants who reported a lifetime diagnosis of asthma but no asthma attacks in the past year were excluded from this analysis. The NHANES was approved by the Institutional Review Board of the National Center for Health Statistics of the Center for Disease Control and Prevention (CDC). Informed consent was obtained from all participants.
Study procedures
Measures of obesity and adiposity were collected by trained health technicians, following recommendations from the Anthropometric Standardization Reference Manual(18). BMI was calculated as weight (in Kg) divided by height (in meters) squared. Percent body fat (PBF) was calculated from tricipital and subscapular skinfolds. For data analysis, all measures were transformed to z-scores, in order to obtain standardized and comparable coefficients: BMI z-scores were calculated using equations based on the 2000 CDC growth charts(19); PBF z-scores were calculated by using reference equations for U.S. children(20); and waist circumference (WC) and waist-to-height ratio (WHtR) were standardized using the distribution of these measures in our study population. Overweight/obese were defined as a z-score > 1.0364 (85th percentile) for each adiposity indicator.
Fasting plasma glucose, serum insulin, high-density lipoprotein (HDL) cholesterol, triglycerides (TG), and C-reactive protein (CRP) were measured at a morning examination session in all NHANES participants aged 12 years and older.. Participants fasting for less than 9 hours, taking insulin or oral medications for diabetes, or refusing phlebotomy were excluded. Insulin sensitivity was measured using two indicators: fasting glucose (μU/mL) to insulin (mg/dL) ratio (G/I) and QUICKI. QUICKI was defined as 1/[log(fasting insulin) + log(fasting glucose)] (21). Conversely, HOMA-IR was used as a measure of insulin resistance and was defined as [fasting insulin x fasting glucose (mmol/L)]/22.5 (22). Systolic blood pressure (SBP) was measured in all NHANES participants following study protocols(23), and was standardized for this analysis by using its distribution in our study population. Metabolic syndrome was defined as meeting at least three of the following five criteria: fasting glucose ≥ 110 mg/dL; WC ≥ 75th percentile; fasting TG ≥ 100 mg/dl; HDL ≤ 50 mg/dl; and SBP ≥ 90th percentile(14, 24).
Spirometry was performed following American Thoracic Society (ATS) recommendations(25). The best forced expiratory volume in the 1st second (FEV1) and forced vital capacity (FVC) values were selected for data analysis. Participants were not eligible for spirometry if they were on supplemental oxygen or had painful ear infections, current chest pain or a physical problem with forceful expiration, recent surgery (of the eye, chest or the abdomen), heart disease or tuberculosis. Our main analyses were performed using absolute values (in ml) adjusted for age, gender, height, and height2; confirmatory analyses were performed using NHANES III predictive equations for lung function measures, and are included in the Online Supplement.
Statistical analysis
Primary sampling units and strata for the complex design of NHANES were taken into account for data analysis. Sampling weights, stratification, and clusters provided in the NHANES dataset were incorporated into the analysis to obtain proper estimates and standard errors; fasting sample weights were also used when analyzing fasting glucose and insulin. All multivariate analyses were performed using the linear regression within the SURVEY procedure in SAS (SAS Institute Inc., Cary, NC). All models were adjusted for age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), family history of asthma, health insurance coverage, environmental tobacco smoke (ETS) exposure, CRP, and number of fasting hours; models for FEV1 and FVC were additionally adjusted for height and height2. In a secondary multivariate analysis, we examined the relation between FEV1/FVC and overweight/obesity (BMI ≥85th percentile for age and sex) after stratification by asthma and metabolic syndrome. All statistical analyses were conducted using SAS 9.3 software.
RESULTS
The main characteristics of the 1,429 participating adolescents with and without current asthma are shown in Table 1 (see Supplemental Figure 1 for derivation of study sample from NHANES). Compared to adolescents without asthma, those with asthma were more likely to be black, and to have a low annual household income, health insurance, a family history of asthma, and lower FEV1 and FEV1/FVC (P <0.05 in all instances). Adolescents with asthma also had a non-significant trend towards higher adiposity z-scores than their non-asthmatic counterparts (P<0.10). There were no statistically significant differences in age, gender, ETS exposure, CRP, or indicators of metabolic syndrome or insulin resistance between adolescents with and without asthma. Similar results were found when comparing adolescents with “ever asthma” to healthy controls (Supplemental Table 1).
Table 1.
Characteristics of study participants by asthma status.
| Characteristics | No asthma N=1,334 |
Current asthma N=95 |
P-value |
|---|---|---|---|
| Age (year) | 14.54 ± 0.05 | 14.53 ± 0.24 | 0.97 |
| Male gender | 688 (50.03) | 57 (56.77) | 0.34 |
| Race/ethnicity | 0.007 | ||
| Non-Hispanic White | 439 (60.51) | 25 (47.42) | |
| Non-Hispanic Black | 294 (13.34) | 40 (29.64) | |
| Hispanic | 530 (19.55) | 24 (13.90) | |
| Other | 71 (6.61) | 6 (9.05) | |
| Household income < $20,000/year | 247 (13.81) | 30 (24.95) | <0.05 |
| Health insurance coverage | 1125 (88.45) | 90 (97.18) | 0.002 |
| Family history of asthma | 314 (22.42) | 64 (58.34) | <0.001 |
| ETS exposure | 203 (15.04) | 19 (20.47) | 0.37 |
| Hay fever | 115 (10.02) | 32 (35.32) | 0.006 |
| FEV1 (L)1 | 3.40 ± 0.03 | 3.27 ± 0.13 | 0.02 |
| FVC (L)1 | 3.93 ± 0.03 | 3.94 ± 0.15 | 0.86 |
| FEV1/FVC (%) | 86.82 ± 0.22 | 83.08 ± 1.26 | 0.008 |
| C-reactive Protein | 0.14 ± 0.02 | 0.16 ± 0.03 | 0.67 |
| Body mass index (BMI) z-score | 0.65 ± 0.04 | 0.92 ± 0.17 | 0.09 |
| Percent body fat (PBF) z-score | 0.24 ± 0.04 | 0.45 ± 0.11 | 0.06 |
| Waist circumference (WC) z-score | −0.07 ± 0.04 | 0.16 ± 0.12 | 0.09 |
| Waist-to-Height ratio (WHtR) z-score | −0.11 ± 0.04 | 0.13 ± 0.13 | 0.09 |
| Glucose-to-insulin ratio2 | 9.51 ± 0.38 | 8.31 ± 0.88 | 0.21 |
| QUICKI2 | 0.33 ± 0.002 | 0.32 ± 0.005 | 0.09 |
| HOMA-IR2 | 3.24 ± 0.11 | 3.90 ± 0.55 | 0.24 |
| Metabolic syndrome2 | 58 (9.02) | 7 (15.77) | 0.29 |
Results shown as mean (SE) for continuous variables, and as N (%) for binary variables. Numbers may vary due to missingness. ETS = Environmental tobacco smoking. QUICKI = quantitative insulin-sensitivity check index. HOMA-IR=homeostasis model assessment - insulin resistance.
Adjusted for age, gender, height, and height2.
Data analyzed only for children who were examined after fasting, in morning sessions (n=701).
Table 2 shows the results of the multivariate analysis of the relation between indicators of insulin sensitivity or resistance and lung function measures, among all subjects and after stratification by asthma status. In this analysis, greater insulin sensitivity (defined by higher G/I or QUICKI) was significantly associated with higher FEV1 and FVC in all subjects. Among subjects without asthma, each unit increment in G/I was significantly associated with ~11 mL to 13 mL increments in FEV1 and FVC, respectively; and each 0.01 increment in QUICKI was significantly associated with ~24 mL to 30 mL increments in FEV1 and FVC, respectively. Among adolescents with asthma, these findings were more pronounced: significant increments of ~20 mL in FEV1 and ~45mL in FVC per each unit increment in G/I, and significant increments of ~42 mL in FEV1 and ~59 mL in FVC per each unit increment in QUICKI. Conversely, insulin resistance (defined by higher HOMA-IR) was associated with lower FEV1 and FVC: each unit increase in HOMA-IR was significantly associated with lower FEV1 and FVC among all subjects, and in subjects with and without asthma. Insulin sensitivity or resistance was not significantly associated with FEV1/FVC in all subjects or in subjects without asthma. Among subjects with asthma, both the G/I ratio and the QUICKI were significantly and inversely associated with FEV1/FVC (range for the estimated effect= −0.63% to −0.86% per each unit increment in G/I or QUICKI). In these subjects, the HOMA-IR was significantly and positively associated with FEV1/FVC (Table 2).
Table 2.
Insulin sensitivity/resistance, metabolic syndrome, and lung function by asthma status.
| Outcome | Glucose:insulin ratio (range: 0.98–37.86) | QUICKI1 (range: 0.24–0.44) | HOMA-IR (range: 0.49–32.83) | Metabolic syndrome |
|---|---|---|---|---|
| All Participants2 (n=545) | ||||
| FEV1 (ml) | 11.36 (2.50, 20.21)* | 24.97 (7.72, 42.22)** | −34.32 (−50.99, −17.66)** | −37.42 (−221.57, 146.729) |
| FVC (ml) | 13.65 (4.26, 23.04)** | 32.74 (14.10, 51.39)** | −42.28 (−63.22, −21.35)** | 68.65 (−144.50, 281.81) |
| FEV1/FVC (%) | −0.01 (−0.17, 0.14) | −0.09 (−0.37, 0.19) | 0.04 (−0.22, 0.30) | −2.34 (−4.03, −0.64)** |
| No asthma (n=519) | ||||
| FEV1 (ml) | 11.09 (2.15, 20.04)* | 24.01 (6.24, 41.79)** | −32.47 (−50.33, −14.61)** | −37.55 (−226.67, 151.57) |
| FVC (ml) | 12.84 (3.53, 22.16)** | 30.73 (11.87, 49.59)** | −39.16 (−61.39, −16.92)** | 58.74 (−158.55, 276.02) |
| FEV1/FVC (%) | −0.01 (−0.16, 0.15) | −0.08 (−0.35, 0.19) | 0.03 (−0.23, 0.29) | −2.04 (−3.92, −0.15)* |
| Current asthma (n=28) | ||||
| FEV1 (ml) | 20.14 (7.82, 32.47)** | 41.84 (15.18, 68.51)** | −37.04 (−59.52, −14.57)** | −530.80 (−721.49, −340.10)** |
| FVC (ml) | 44.58 (29.18, 59.99)** | 59.17 (46.53, 71.81)** | −39.13 (−54.44, −23.81)** | 58.18 (−99.95, 216.31) |
| FEV1/FVC (%) | −0.63 (−0.83, −0.42)** | −0.86 (−1.01, −0.72)** | 0.54 (0.50, 0.59)** | −12.56 (−17.61, −7.50)** |
Data presented as beta coefficient (95% confidence interval). All models adjusted for age, gender, race/ethnicity, health insurance coverage, family history of asthma, ETS exposure, fasting hours, C-reactive protein, and BMI z-score. FEV1 and FVC additionally adjusted for height and height2.
p<0.05,
p<0.01.
Results shown per 0.01 unit of QUICKI increment in each lung function measure.
Additionally adjusted for asthma status.
Table 3 shows the results of the analysis of the relation between indicators of insulin sensitivity or resistance and lung function measures, after stratification by obesity (defined using BMI). In a multivariate analysis among adolescents of normal weight, there was no significant association between any indicator of insulin sensitivity or resistance and lung function. Among overweight or obese adolescents, insulin sensitivity (higher G/I or QUICKI) was significantly associated with higher FEV1 (range of estimated effect= 28.6 mL to 46.1 mL per each unit increment) and FVC, and insulin resistance (HOMA-IR) was associated with lower FEV1 (estimated effect= −36.3 mL per each unit increment) and FVC. Similar results were obtained when defining obesity/adiposity on the basis of PBF, WC, or WHtR (see Supplemental Table 2) or when analyzing overweight and obese subjects separately (data not shown).
Table 3.
Insulin sensitivity/resistance, metabolic syndrome, and lung function by obesity status.
| Outcome | Glucose:insulin ratio (range: 0.98–37.86) | QUICKI1 (range: 0.24–0.44) | HOMA-IR (range: 0.49–32.83) | Metabolic syndrome |
|---|---|---|---|---|
| BMI < 85th percentile (n=316) | ||||
| FEV1 (ml) | 2.11 (−8.73, 12.95) | −0.68 (−23.63, 22.28) | −0.87 (−37.98, 36.23) | −16.30 (−261.99, 229.38) |
| FVC (ml) | 1.14 (−8.13, 10.42) | 0.71 (−18.83, 20.25) | −2.48 (−39.75, 34.80) | 80.95 (−129.61, 291.50) |
| FEV1/FVC (%) | 0.03 (−0.14, 0.19) | −0.04 (−0.40, 0.32) | 0.01 (−0.65, 0.66) | −3.07 (−6.50, 0.35) |
| BMI ≥ 85th percentile (n=229) | ||||
| FEV1 (ml) | 28.55 (10.41, 46.68)** | 46.13 (21.87, 70.40)** | −36.34 (−56.75, −15.93)** | 32.88 (−187.15, 525.91) |
| FVC (ml) | 33.75 (15.31, 52.19)** | 52.61 (30.09, 75.13)** | −40.09 (−59.85, −20.32)** | 160.77 (−88.10, 409.64) |
| FEV1/FVC (%) | 0.01 (−0.21, 0.23) | 0.06 (−0.26, 0.37) | −0.08 (−0.32, 0.16) | −2.33 (−3.97, −0.68)** |
Data presented as beta (95% confidence interval). All models adjusted for age, gender, race/ethnicity, health insurance coverage, family history of asthma, ETS exposure, fasting hours, C-reactive protein, and asthma status. FEV1 and FVC additionally adjusted for height and height2.
p<0.05,
p<0.01.
Results shown per 0.01 unit of QUICKI increment in each lung function measure.
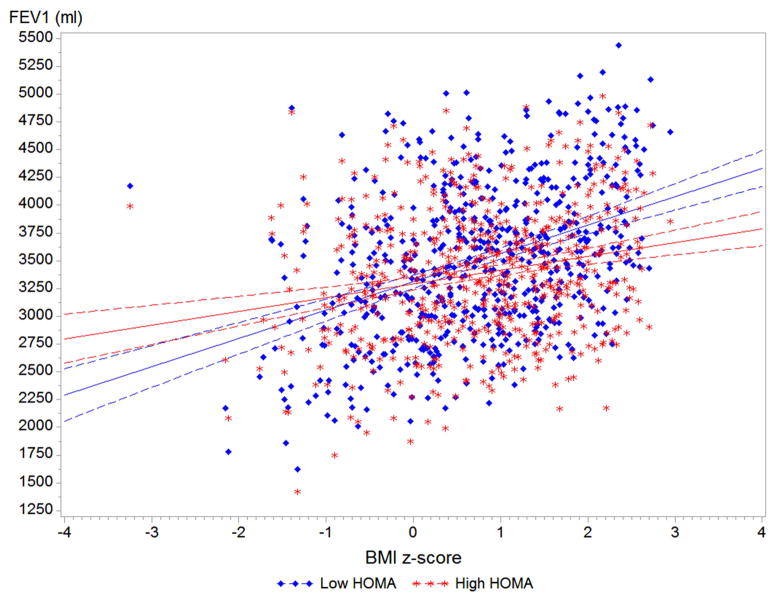
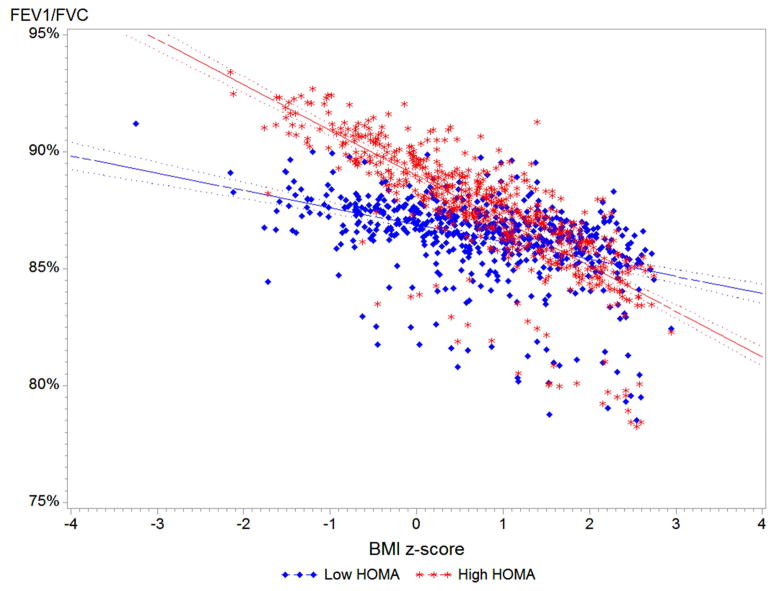
On the basis of our results, we examined the relation between BMI z-score and lung function after stratification by insulin resistance (Figure 1). Among adolescents without insulin resistance (HOMA-IR < 3.0), each 1.0 z-score increment in BMI was associated with ~200 mL higher FEV1. Among adolescents with insulin resistance (HOMA-IR > 3.0), the increment in FEV1 per each 1.0 z-score increment in BMI was lower at ~70mL (P for interaction = 0.0006). On the other hand, each z-score unit increment in BMI was associated with a significant decrement in FEV1/FVC among adolescents with insulin resistance, but with a non-significant decrement in FEV1/FVC among adolescents with normal sensitivity (P for interaction= 0.02).
Figure 1. BMI z-score and lung function by insulin resistance status.
Predicted values for FEV1 and FEV1/FVC by insulin resistance status (HOMA-IR<3.0 vs >3.0). All models adjusted for age, gender, race/ethnicity, asthma status, health insurance coverage, family history of asthma, ETS exposure, fasting hours, and C-reactive protein. Interaction P-value for FEV1 = 0.0006, and for FEV1/FVC = 0.02.
We then focused on the metabolic syndrome (Table 2). Among all subjects or subjects without asthma, the metabolic syndrome was significantly associated with a 2% to 2.3% decrement in FEV1/FVC but not significantly associated with FEV1 or FVC. Among subjects with asthma, the metabolic syndrome was significantly associated with a 12.6% decrement in FEV1/FVC and a 530 mL decrement in FEV1. After stratifying this analysis by obesity status (Table 3), there was no significant association between the metabolic syndrome and lung function measures among subjects of normal weight. Among subjects who were overweight or obese, the metabolic syndrome was significantly associated with (2.3%) lower FEV1/FVC but not with FEV1 or FVC. In an analysis of the relation between each criterion used to define metabolic syndrome and lung function, there was no particular measure driving our results (Supplemental Table 3).
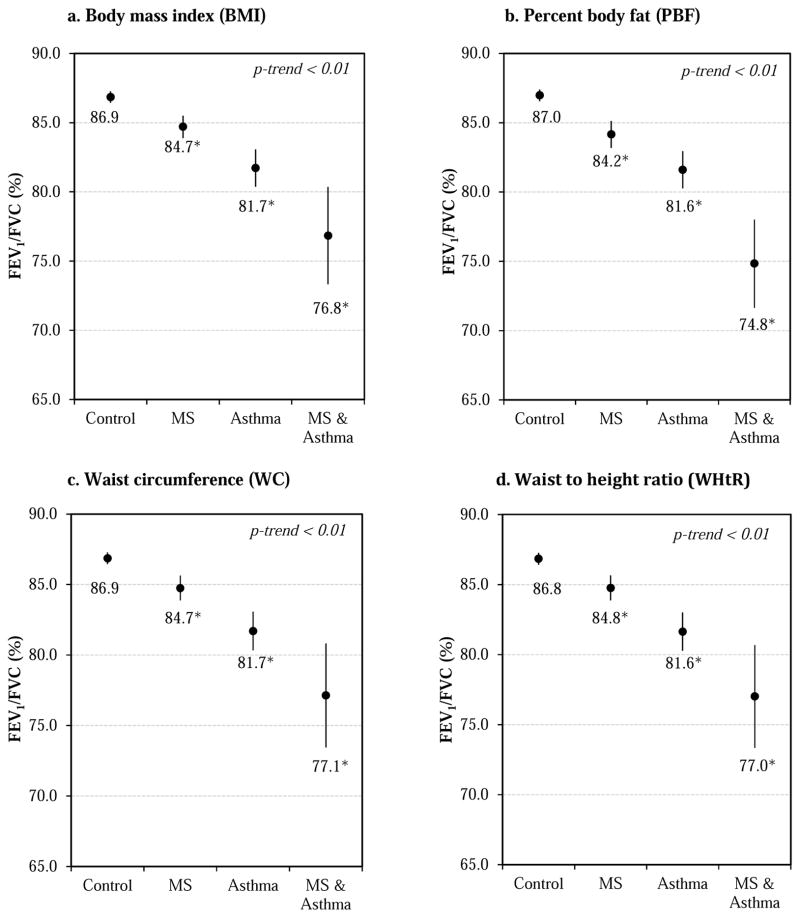
Finally, we repeated the multivariate analysis of FEV1/FVC after stratification by asthma and metabolic syndrome status. Compared to healthy controls (subjects without asthma or metabolic syndrome), those with metabolic syndrome had an ~2% lower FEV1/FVC, those with asthma had an ~5% lower FEV1/FVC, and those with both asthma and metabolic syndrome had an ~10% lower FEV1/FVC (P for linear trend <0.01). These findings remained significant after additional adjustment for any of the adiposity indicators (BMI, PBF, WC, or WHtR, see Figure 2). We obtained similar but non-statistically significant results for FEV1 (Supplemental Figure 2).
Figure 2. Predicted FEV1/FVC by asthma and metabolic syndrome status.
All models adjusted for age, gender, race/ethnicity, health insurance coverage, family history of asthma, ETS exposure, fasting hours, C-reactive protein, and z-score for each respective adiposity indicator (BMI, PBF, WC, or WHtR). MS=metabolic syndrome. No asthma & no MS n=496; MS only n=58; asthma only n=23; and MS & asthma n=7. *P<0.05 compared to control group (no asthma & no MS).
As a confirmatory analysis, we reran the models for Tables 2, 3, and 4 with FEV1, FVC, and FEV1/FVC as percent-of-predicted using NHANES III predictive equations for lung function measures (see Supplemental Tables 4, 5, and 6). Although there was a ~16–19% decrement in sample size (as non Mexican-American Hispanic participants were excluded, given lack of appropriate predictive equations) and coefficients are expressed in different units (“% of predicted” instead of ml), the results of this analysis are very similar to those of our main analyses. The direction and significance of all results for the whole sample and for non-asthmatics remained the same, while among asthmatics (n=25) only the association between MS and FEV1/FVC remained significant (Table 2 and Supplemental Table 4). As in the main analysis, all results continued to be driven by overweight/obese adolescents (Table 3 and Supplemental Table 5).
Table 4.
Metabolic syndrome criteria and FEV1/FVC by asthma and obesity status.
| SBP ≥ 90th percentile | HDL < 50 mg/dL | Triglyceride ≥ 100 mg/dL | WC ≥ 75th percentile | Fasting glucose ≥ 110 mg/dL | |
|---|---|---|---|---|---|
| All participants1 | −0.38 (−2.15, 1.40) | −0.65 (−2.08, 0.78) | −0.40 (−2.46, 1.66) | −1.65 (−3.37, 0.08) | −1.68 (−6.26, 2.90) |
| Non-asthmatics | 0.14 (−1.72, 2.01) | −0.63 (−2.14, 0.88) | −0.34 (−2.48, 1.80) | −1.94 (−3.79, −0.10)* | −1.70 (−6.33, 2.93) |
| Asthmatics | −12.73 (−16.97, −8.50)** | 1.61 (−1.25, 4.47) | 0.46 (−0.87, 1.80) | 2.82 (1.06, 4.57)** | n/a2 |
| Normal weight1 | −0.53 (−3.08, 2.03) | 0.33 (−1.73, 2.39) | −0.51 (−3.45, 2.44) | n/a2 | −2.85 (−8.79, 3.08) |
| Overweight/obese1 | −0.12 (−2.64, 2.39) | −1.93 (−3.66, −0.20)* | −0.20 (−2.10, 1.69) | −1.54 (−3.21, 0.23) | 2.07 (−1.32, 5.45) |
Data presented as β (95% CI). All models adjusted for age, gender, race/ethnicity, health insurance coverage, family history of asthma, ETS exposure, fasting hours, and CRP.
Defined on basis of BMI z-score; additionally adjusted for current asthma.
All asthmatics had fasting glucose < 110mg/dL, and all children of normal weight had WC < 75th pct.
p<0.05,
p<0.01.
DISCUSSION
To the best of our knowledge, this is the first study of insulin resistance or the metabolic syndrome and lung function in a representative sample of U.S. adolescents with and without asthma. Among adolescents with and without asthma, we found that insulin resistance is associated with decreased FEV1 and FVC, and that the metabolic syndrome is associated with lower FEV1/FVC. Both findings were significant only in overweight or obese adolescents, and were more pronounced for FEV1 and FEV1/FVC among adolescents with asthma.
Insulin resistance, an immediate precursor of type-2 diabetes, has been associated with other illnesses such as hepatosteatosis and cardiovascular disease. We found that insulin resistance (or decreased insulin sensitivity) is associated with lower lung function in adolescents, particularly those who are overweight or obese. For example, an increment in HOMA-IR from 1.98 (the 25th percentile in our sample) to 4.47 (75th percentile) would be associated with ~90 mL lower FEV1 and ~100 mL lower FVC, while a similar decrease in QUICKI would lead to ~171 mL lower FEV1 and ~195mL lower FVC. These reductions were rather symmetrical, consistent with studies in adults that report a much higher prevalence of restrictive deficit among subjects with insulin resistance(26, 27).
We also show significant modification of the estimated effect of BMI on lung function by insulin resistance, whereby a higher BMI in adolescents with insulin resistance is associated with a smaller increment in FEV1 (~70 mL vs ~200 ml per 1.0 BMI z-score increase) but a greater decrement in FEV1FVC than in their insulin-sensitive counterparts. Although prior studies have reported an association between a higher BMI and either higher FEV1 or lower FEV1/FVC in children(28–30); this is the first study reporting that insulin resistance may modify or influence this association.
Insulin resistance –and the resulting metabolic imbalances– may affect lung function via several pathways. Insulin growth factor 1 (IGF-1) is known to alter airway smooth muscle contractility (31, 32) and stimulate its proliferation(33). Several studies have reported links between adiponectin or leptin and asthma, asthma severity, or lung function(34–36). Selective leptin resistance may lead to loss of the anorexigenic and metabolic effects of leptin, while rising levels of this hormone continue to affect other organs (e.g. chronically activating the sympathetic system)(37, 38). Resistin, an adipokine implicated in insulin resistance, has pro-inflammatory properties in the lung via the recruitment and activation of neutrophils(39). Likewise, cytokines from the resistin-link molecule (RELM) family have been shown to induce airway inflammation and fibroblast proliferation in murine models(40). Prior studies in healthy adults have also found an association between insulin resistance and airway hyper-responsiveness (AHR)(41). On the other hand, murine models have shown that allergic airway inflammation may lead to reduced levels of insulin-degrading enzyme and decreased insulin receptor phosphorylation in the brain, producing changes that may induce insulin resistance and a pro-inflammatory response(42).
The metabolic syndrome has been associated with asthma in large cross-sectional(43) and longitudinal(44) studies in adults (mean ages 55 and 43 years, respectively). Similarly, a large study of almost 122,00 adults (mean age= 46 years) reported an association between the metabolic syndrome and lower FEV1 and FVC after adjusting for potential confounders (including smoking status, alcohol consumption, BMI, and activity level)(15). However, this and other studies in adults have shown that decrements in lung function associated with the metabolic syndrome result in a restrictive ventilatory deficit (low FEV1 and FVC, but normal FEV1/FVC) (45, 46). In contrast, we report a decreased FEV1/FVC ratio in adolescents with metabolic syndrome, consistent with an obstructive deficit. This estimated effect of metabolic syndrome on FEV1/FVC was separate from and additive with the effect of asthma; thus, adolescents with both asthma and metabolic syndrome had ~530 mL lower FEV1 and 10% lower FEV1/FVC than their healthy counterparts (both reductions being clinically significant in adolescents). Based on these results, one may speculate that the mechanisms or effects of MS on lung function in children and adolescents may differ from those in adults.
One previous study has reported low FEV1/FVC in morbidly obese adults with metabolic syndrome. However, that study did not include subjects with asthma, and the observed association was likely mediated by peripheral blood eosinophilia(47). We did not find that one particular component of the metabolic syndrome explained our results; however, WC was most consistently associated with the outcomes of interest. Of note, a low HDL was an additional, independent risk factor for low FEV1/FVC in adolescents who are overweight or obese.
Several mechanisms may explain the decrements in lung function associated with the metabolic syndrome. In addition to insulin resistance, in the metabolic syndrome increased protein turnover may lead to altered arginine metabolism and elevated methyl-arginine molecules such as asymmetric dimethylarginine (ADMA); this in turn limits arginine availability for nitric oxide generation, which may result in epithelial damage and dysfunction(48). In mouse models of allergy with diet-induced metabolic syndrome, electron microscopy has shown stressed and dysfunctional mitochondria in the bronchial epithelium, leading to AHR even in the absence of allergen exposure(49). Chemokine (C-X-C motif) receptor 3 (CXCR3), involved in immune cell regulation and allergic airway inflammation(50), has also been reported to modulate diet-induced insulin resistance and visceral adipose tissue macrophage infiltration and inflammation in mice(51). As with insulin resistance, some studies have shown that the decline in lung function in some cases precedes the development of metabolic syndrome(52); future studies should try to elucidate whether this is a cause-effect relationship, or whether both are consequences of the same process (e.g. obesity and inflammation), with the decline in lung function merely being an earlier manifestation of disease.
Our study has considerable strengths, including the use of a representative sample of U.S. adolescents, performance of standardized procedures by uniformly trained personnel, and adjustment of data analysis for several potential confounders, including obesity/adiposity and CRP. We also acknowledge several limitations of our findings. Firstly, NHANES is a cross-sectional study, and therefore a temporal relationship between insulin resistance, metabolic syndrome, and lung function cannot be determined. Secondly, we had limited statistical power for subgroup analyses, due to the small number of adolescents with current asthma. Thirdly, the metabolic syndrome may be defined following several sets of guidelines. The definition we used had less stringent criteria and thus allowed us to classify more subjects as having metabolic syndrome; using stricter criteria (International Diabetes Federation(53, 54)) yielded much fewer children classified as such (n=19), but results for FEV1/FVC remained the same (data not shown). Fourthly, our definition of current asthma could have excluded a portion of participants with asthma who had low exacerbation risk but higher impairment in the year prior to the study. Finally, we could not control for all factors related to obesity, insulin resistance, or metabolic syndrome, such as diet or family history of diabetes.
In summary, insulin resistance and MS are associated with significantly worsened lung function in overweight/obese adolescents. The effect of MS is worse among adolescents with asthma. Insulin resistance and MS may thus contribute to the pathogenesis of asthma severity in obese patients and warrant further investigation.
Supplementary Material
Clinical Implications.
Insulin resistance and the metabolic syndrome may impact lung function in adolescents. Management of these conditions should be part of the treatment of obese asthmatic children and adolescents.
Acknowledgments
Sources of Funding: Dr. Forno’s contribution was supported by NIH grant HD052892. Dr. Celedón’s contribution was supported by NIH grants HL079966 and HL117191, and by an endowment from the Heinz Foundation. Dr. Forno had full access to all of the data and takes responsibility for the integrity and accuracy of the analysis. None of the funding sponsors had any role in study design, data analysis, or manuscript preparation or approval. Dr. Celedón served as a single-time consultant for Genentech in 2011 on a topic unrelated to this manuscript.
Abbreviations
- BMI
Body mass index
- CRP
C-reactive protein
- FEV1
Forced expiratory volume in the first second
- FVC
Forced vital capacity
- G/I
Glucose to insulin ratio
- HOMA-IR
Homeostasis model assessment-estimated insulin resistance
- HDL
High-density lipoprotein
- MS
Metabolic syndrome
- NHANES
National Health and Nutrition Examination Survey
- PBF
Percent body fat
- QUICKI
Quantitative insulin sensitivity check index
- WC
Waist circumference
- WHtR
Waist-to-height ratio
Footnotes
Author contributions: Conception and study design: E.F., Y-Y.H. and J.C.C.; Data analysis and interpretation: E.F. and Y-Y.H; drafting of the manuscript for intellectual content: E.F., Y-Y.H., R.M. and J.C.C. All authors approved the final version of the manuscript prior to submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the united states, 2001–2010. NCHS data brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the united states, 1980–2007. Pediatrics. 2009;123 (Suppl 3):S131–145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United states, 2011–2012. NCHS data brief. 2013:1–8. [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the united states, 1999–2004. JAMA: the journal of the American Medical Association. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedon JC, Shore SA, Lung D American Thoracic Society Ad Hoc Subcommittee on O. An official american thoracic society workshop report: Obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 6.Forno E, Acosta-Pérez E, Brehm J, Han YY, Alvarez M, Colón-Semidey A, Canino G, Celedón JC. Obesity and adiposity indicators, asthma and atopy in puerto rican children. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedon JC Childhood Asthma Management Program Research G. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrell LN, Nguyen EA, Roth LA, Oh SS, Tcheurekdjian H, Sen S, Davis A, Farber HJ, Avila PC, Brigino-Buenaventura E, Lenoir MA, Lurmann F, Meade K, Serebrisky D, Rodriguez-Cintron W, Kumar R, Rodriguez-Santana JR, Thyne SM, Burchard EG. Childhood obesity and asthma control in the gala ii and sage ii studies. Am J Respir Crit Care Med. 2013;187:697–702. doi: 10.1164/rccm.201211-2116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among u.S. Adolescents: A population-based study. Diabetes care. 2006;29:2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 10.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. The New England journal of medicine. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal A, Mabalirajan U, Ahmad T, Ghosh B. Emerging interface between metabolic syndrome and asthma. American journal of respiratory cell and molecular biology. 2011;44:270–275. doi: 10.1165/rcmb.2010-0141TR. [DOI] [PubMed] [Google Scholar]
- 12.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. American journal of respiratory and critical care medicine. 2011;183:441–448. doi: 10.1164/rccm.201004-0603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Shawwa BA, Al-Huniti NH, DeMattia L, Gershan W. Asthma and insulin resistance in morbidly obese children and adolescents. J Asthma. 2007;44:469–473. doi: 10.1080/02770900701423597. [DOI] [PubMed] [Google Scholar]
- 14.Del-Rio-Navarro BE, Castro-Rodriguez JA, Garibay Nieto N, Berber A, Toussaint G, Sienra-Monge JJ, Romieu I. Higher metabolic syndrome in obese asthmatic compared to obese nonasthmatic adolescent males. The Journal of asthma: official journal of the Association for the Care of Asthma. 2010;47:501–506. doi: 10.3109/02770901003702808. [DOI] [PubMed] [Google Scholar]
- 15.Leone N, Courbon D, Thomas F, Bean K, Jego B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: The critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 16.Lee EJ, In KH, Ha ES, Lee KJ, Hur GY, Kang EH, Jung KH, Lee SY, Kim JH, Shin C, Shim JJ, Kang KH, Yoo SH. Asthma-like symptoms are increased in the metabolic syndrome. The Journal of asthma: official journal of the Association for the Care of Asthma. 2009;46:339–342. doi: 10.1080/02770900802660931. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 2007–2008 national health and nutrition examination survey (nhanes) survey operations manuals, brochures, consent documents. 2012 [cited 2014 February 18]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/current_nhanes_07_08.htm.
- 18.Lohman TG, Roche AF. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 19.Centers for Disease Control and Prevention. A sas program for the cdc growth charts. 2011 [cited 2014 February 18]. Available from: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 20.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA. Skinfold equations for estimation of body fatness in children and youth. Human biology. 1988;60:709–723. [PubMed] [Google Scholar]
- 21.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. The Journal of clinical endocrinology and metabolism. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 22.Wallace TM, Levy JC, Matthews DR. Use and abuse of homa modeling. Diabetes care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 23.Ostchega Y, Prineas RJ, Paulose-Ram R, Grim CM, Willard G, Collins D. National health and nutrition examination survey 1999–2000: Effect of observer training and protocol standardization on reducing blood pressure measurement error. Journal of clinical epidemiology. 2003;56:768–774. doi: 10.1016/s0895-4356(03)00085-4. [DOI] [PubMed] [Google Scholar]
- 24.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in american adolescents: Findings from the third national health and nutrition examination survey. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 25.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. The European respiratory journal. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 26.Lecube A, Sampol G, Munoz X, Lloberes P, Hernandez C, Simo R. Insulin resistance is related to impaired lung function in morbidly obese women: A case-control study. Diabetes/metabolism research and reviews. 2010;26:639–645. doi: 10.1002/dmrr.1131. [DOI] [PubMed] [Google Scholar]
- 27.Lim SY, Rhee EJ, Sung KC. Metabolic syndrome, insulin resistance and systemic inflammation as risk factors for reduced lung function in korean nonsmoking males. Journal of Korean medical science. 2010;25:1480–1486. doi: 10.3346/jkms.2010.25.10.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL Childhood Asthma Management Program Research G. Association of body mass with pulmonary function in the childhood asthma management program (camp) Thorax. 2003;58:1036–1041. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vo P, Makker K, Matta-Arroyo E, Hall CB, Arens R, Rastogi D. The association of overweight and obesity with spirometric values in minority children referred for asthma evaluation. J Asthma. 2013;50:56–63. doi: 10.3109/02770903.2012.744035. [DOI] [PubMed] [Google Scholar]
- 30.Forno E, Acosta-Perez E, Brehm J, Han YY, Alvarez M, Colon-Semidey A, Canino G, Celedon JC. Obesity and adiposity indicators, asthma, and atopy in puerto rican children. J All Clin Immun. 2013 doi: 10.1016/j.jaci.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noveral JP, Bhala A, Hintz RL, Grunstein MM, Cohen P. Insulin-like growth factor axis in airway smooth muscle cells. The American journal of physiology. 1994;267:L761–765. doi: 10.1152/ajplung.1994.267.6.L761. [DOI] [PubMed] [Google Scholar]
- 32.Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol. 2009;41:494–504. doi: 10.1165/rcmb.2008-0251OC. [DOI] [PubMed] [Google Scholar]
- 33.Cohen P, Noveral JP, Bhala A, Nunn SE, Herrick DJ, Grunstein MM. Leukotriene d4 facilitates airway smooth muscle cell proliferation via modulation of the igf axis. The American journal of physiology. 1995;269:L151–157. doi: 10.1152/ajplung.1995.269.2.L151. [DOI] [PubMed] [Google Scholar]
- 34.Sood A, Shore SA. Adiponectin, leptin, and resistin in asthma: Basic mechanisms through population studies. Journal of allergy. 2013;2013:785835. doi: 10.1155/2013/785835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, Szefler SJ, Sorkness CA, Morgan WJ, Teach SJ, Gan VN. Asthma control, adiposity, and adipokines among inner-city adolescents. The Journal of allergy and clinical immunology. 2010;125:584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in german schoolchildren. Pediatr Allergy Immunol. 2008 Mar 12;2008 doi: 10.1111/j.1399-3038.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 37.Mark AL. Selective leptin resistance revisited. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305:R566–581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasrallah MP, Ziyadeh FN. Overview of the physiology and pathophysiology of leptin with special emphasis on its role in the kidney. Seminars in nephrology. 2013;33:54–65. doi: 10.1016/j.semnephrol.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Jiang S, Park DW, Tadie JM, Gregoire M, Deshane J, Pittet JF, Abraham E, Zmijewski JW. Human resistin promotes neutrophil proinflammatory activation and neutrophil extracellular trap formation and increases severity of acute lung injury. J Immunol. 2014;192:4795–4803. doi: 10.4049/jimmunol.1302764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra A, Wang M, Schlotman J, Nikolaidis NM, DeBrosse CW, Karow ML, Rothenberg ME. Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. American journal of physiology Lung cellular and molecular physiology. 2007;293:L305–313. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 41.Kim KM, Kim SS, Lee SH, Song WJ, Chang YS, Min KU, Cho SH. Association of insulin resistance with bronchial hyperreactivity. Asia Pacific allergy. 2014;4:99–105. doi: 10.5415/apallergy.2014.4.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarlus H, Wang X, Cedazo-Minguez A, Schultzberg M, Oprica M. Chronic airway-induced allergy in mice modifies gene expression in the brain toward insulin resistance and inflammatory responses. Journal of neuroinflammation. 2013;10:99. doi: 10.1186/1742-2094-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee EJ, In KH, Ha ES, Lee KJ, Hur GY, Kang EH, Jung KH, Lee SY, Kim JH, Lee SY, Shin C, Shim JJ, Kang KH, Yoo SH. Asthma-like symptoms are increased in the metabolic syndrome. J Asthma. 2009;46:339–342. doi: 10.1080/02770900802660931. [DOI] [PubMed] [Google Scholar]
- 44.Brumpton BM, Camargo CA, Jr, Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic syndrome and incidence of asthma in adults: The hunt study. Eur Respir J. 2013;42:1495–1502. doi: 10.1183/09031936.00046013. [DOI] [PubMed] [Google Scholar]
- 45.Fimognari FL, Pasqualetti P, Moro L, Franco A, Piccirillo G, Pastorelli R, Rossini PM, Incalzi RA. The association between metabolic syndrome and restrictive ventilatory dysfunction in older persons. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62:760–765. doi: 10.1093/gerona/62.7.760. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima K, Kubouchi Y, Muneyuki T, Ebata M, Eguchi S, Munakata H. A possible association between suspected restrictive pattern as assessed by ordinary pulmonary function test and the metabolic syndrome. Chest. 2008;134:712–718. doi: 10.1378/chest.07-3003. [DOI] [PubMed] [Google Scholar]
- 47.van Huisstede A, Cabezas MC, Birnie E, van de Geijn GJ, Rudolphus A, Mannaerts G, Njo TL, Hiemstra PS, Braunstahl GJ. Systemic inflammation and lung function impairment in morbidly obese subjects with the metabolic syndrome. Journal of obesity. 2013;2013:131349. doi: 10.1155/2013/131349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agrawal A, Mabalirajan U, Ahmad T, Ghosh B. Emerging interface between metabolic syndrome and asthma. American journal of respiratory cell and molecular biology. 2011;44:270–275. doi: 10.1165/rcmb.2010-0141TR. [DOI] [PubMed] [Google Scholar]
- 49.Leishangthem GD, Mabalirajan U, Singh VP, Agrawal A, Ghosh B, Dinda AK. Ultrastructural changes of airway in murine models of allergy and diet-induced metabolic syndrome. ISRN allergy. 2013;2013:261297. doi: 10.1155/2013/261297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rojas-Dotor S, Segura-Mendez NH, Miyagui-Namikawa K, Mondragon-Gonzalez R. Expression of resistin, cxcr3, ip-10, ccr5 and mip-1alpha in obese patients with different severity of asthma. Biological research. 2013;46:13–20. doi: 10.4067/S0716-97602013000100002. [DOI] [PubMed] [Google Scholar]
- 51.Deiuliis JA, Oghumu S, Duggineni D, Zhong J, Rutsky J, Banerjee A, Needleman B, Mikami D, Narula V, Hazey J, Satoskar AR, Rajagopalan S. Cxcr3 modulates obesity-induced visceral adipose inflammation and systemic insulin resistance. Obesity. 2014;22:1264–1274. doi: 10.1002/oby.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsiao FC, Wu CZ, Su SC, Sun MT, Hsieh CH, Hung YJ, He CT, Pei D. Baseline forced expiratory volume in the first second as an independent predictor of development of the metabolic syndrome. Metabolism. 2010;59:848–853. doi: 10.1016/j.metabol.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Prevalence of the metabolic syndrome among u.S. Adolescents using the definition from the international diabetes federation. Diabetes Care. 2008;31:587–589. doi: 10.2337/dc07-1030. [DOI] [PubMed] [Google Scholar]
- 54.Agudelo GM, Bedoya G, Estrada A, Patino FA, Munoz AM, Velasquez CM. Variations in the prevalence of metabolic syndrome in adolescents according to different criteria used for diagnosis: Which definition should be chosen for this age group? Metabolic syndrome and related disorders. 2014;12:202–209. doi: 10.1089/met.2013.0127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.