Abstract
Insufficient nutrients disrupt physiological homeostasis resulting in diseases and even death. Considering the physiological and pathological consequences of this metabolic stress, the adaptive responses that cells utilize under this condition are of great interest. We show that under low glucose conditions, cells initiate adaptation followed by apoptosis responses using PERK/Akt and MEK1/ERK2 signaling, respectively. For adaptation, cells engage the endoplasmic reticulum stress-induced unfolded protein response, which results in PERK/Akt activation and cell survival. Sustained and extreme energetic stress promotes a switch to isoform-specific MEK1/ERK2 signaling, induction of GCN2/eIF2α phosphorylation and ATF4 expression, which overrides PERK/Akt-mediated adaptation and induces apoptosis through ATF4-dependent expression of pro-apoptotic factors including Bid and Trb3. ERK2 activation during metabolic stress contributes to changes in TCA cycle and amino acid metabolism, and cell death, which is suppressed by glutamate and α-ketoglutarate supplementation. Taken together, our results reveal promising targets to protect cells or tissues from metabolic stress.
Keywords: Energetic stress, Glucose, ERK2, ERK1, apoptosis, eIF2α, ATF4, glutamate
Introduction
Physical and psychological stress disrupts homeostasis and promotes diseases such as diabetes, obesity, cancer, neurological disorders and even death. Preventing this requires the maintenance of a physiologic steady state by sensing, and then responding via positive and negative feedback control mechanisms to maintain biological health even though the external environment is constantly changing. Homeostasis mechanisms maintain pH, temperature, energy, immunity, etc (Grayson et al., 2013). Metabolic homeostasis also requires a balance between food intake (nutrients), hormone production and secretion, and proper maintenance of organ physiology (Grayson et al., 2013). Glucose is a primary component of metabolic homeostasis as it is a major energy source and is used for the synthesis of DNA, RNA, proteins, and lipids (Cantor and Sabatini, 2012). Improper maintenance of glucose levels is of great physiological and pathological importance. Patients with diabetes have elevated glucose levels that can result in blindness, renal failure, and vascular diseases (Szablewski, 2011). On the contrary, mildly or severely reduced glucose causes symptoms ranging from mild discomfort, nausea, dizziness, to severe confusion, fainting, seizures, coma, brain damage, and even death, highlighting the need to maintain the perfect balance of glucose (Szablewski, 2011).
Although our knowledge of the detailed mechanisms of cell fate decisions under mildly or severely reduced glucose conditions is limited, it is known that cells first operate an adaptation/survival system to protect themselves. One of the general mechanisms for this is inhibition of mRNA translation. As energetic sources are depleted, cells suppress translation to save energy for their survival (Inoki et al., 2003). This is achieved by inhibition of ribosome biogenesis (Shaw et al., 2004), prevention of ribosomal RNA (rRNA) transcription through epigenetic modification of ribosomal DNA (rDNA) (Murayama et al., 2008), and inhibition of translational factors (Inoki et al., 2003). Mammalian/mechanistic target of rapamycin (mTOR) and p53 are involved in the regulation of mRNA translation under these conditions (Choo et al., 2010; Roberts et al., 2014). However, when extensive stress overcomes the cells’ capacity to adapt, cells activate cell death mechanisms. Little is known about the changes in cell signaling that promote this transition, but, it is known that low glucose can induce cell death through disruption of mitochondrial integrity and activation of pro-apoptotic molecules (Danial et al., 2003; Lowman et al., 2010).
Therapeutic approaches that take advantage of metabolic stress-induced cell death or ones that attempt to reverse this stress have been actively investigated. For example, 2-deoxyglucose, a compound that induces a glucose deprivation-like state at high concentrations, has proven to be a potentially promising treatment of polycystic kidney disease (PKD) (Rowe et al., 2013). However, in spite of the physiological, pathological, and therapeutic importance of metabolic stress induced by mildly or severely low glucose, the molecular mechanisms by which cells actively respond to this stress remain unclear (Altman and Rathmell, 2012). In the present study, we have investigated the signaling mechanisms utilized during mild to severe glucose deprivation to promote cell survival or cell death. We have found that mTORC1, Akt, and ERK activities fluctuate during glucose deprivation-induced stress and that ERK2 activation is the major signal used to promote the cell death fate through its regulation of GCN2/eIF2α/ATF4-dependent expression of pro-apoptotic molecules. Furthermore, blocking the ERK2/ATF4 pathway protects cells from cell death induced by this stress. Importantly, suppression of ERK2 under glucose starvation conditions results in reprogramming of metabolism such as amino acid metabolism. Among the many amino acids whose levels are altered by ERK2 inhibition, glutamate was sufficient to protect cells from low glucose-mediated cell death.
Results
Metabolic stress caused by low glucose levels induces cell apoptosis
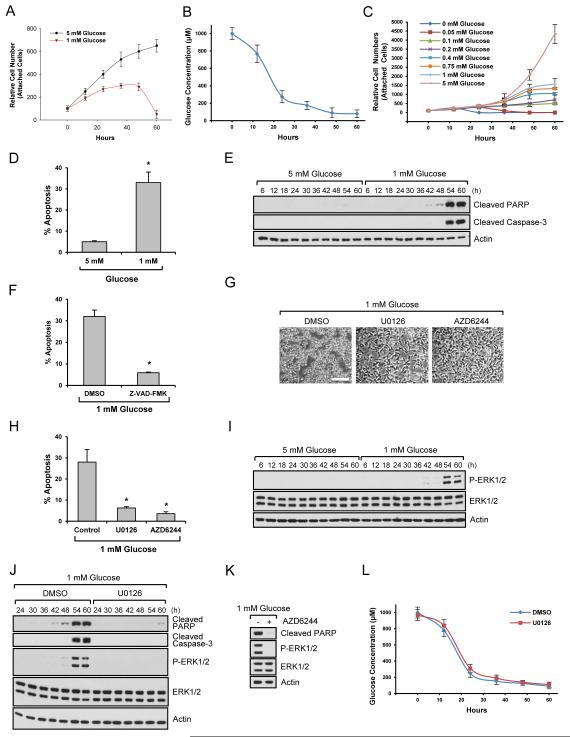
A normal blood glucose level is ~ 4-5 mM, and hypoglycemia is defined by a level less than 2.2 mM (Szablewski, 2011). Many studies on the functions of low glucose on cellular processes use 0 ~ 1 mM glucose as starting levels of glucose (Choo et al., 2010; Inoki et al., 2003; Lee et al., 2014; Murayama et al., 2008; Roberts et al., 2014). We used 1 mM glucose as starting low glucose conditions and 5 mM glucose as a control. Cells growing in the presence of 5 mM glucose continuously proliferated over time course of 60 hrs (Fig. 1A). At 1 mM glucose, cell numbers increased for the first 24 h, but cell proliferation was not sustained (Fig. 1A). Under these conditions, glucose levels decreased dramatically for the first 24 h, after which they changed gradually (Fig. 1B). At around 50 h, cell death was observed morphologically in low glucose conditions (Fig. S1A). By maintaining glucose levels over a 60h time course, we determined that certain levels of glucose are needed for optimal proliferation (Fig. 1C). To determine if cell death starting at around 50 h was via apoptosis, we measured the sub-G1 population, an indicator of DNA fragmentation, a hallmark of cell apoptosis. Growth in 1 mM glucose led to a profound increase in cell death (Fig. 1D). We next examined the expression of a critical executioner of apoptosis, caspase 3, which is activated by cleavage. Cleaved caspase-3 was increased during growth in low glucose conditions and the level of cleaved poly ADP-ribose polymerase (PARP), an apoptosis marker, was also increased (Fig. 1E). To determine if the cell death was caspase dependent or independent apoptosis, we used a pan caspase inhibitor, Z-VAD-FMK, and found that this inhibitor suppressed cell apoptosis induced by low glucose (Fig. 1F). Taken together, these results suggest that upon depletion of extracellular glucose, there is a concomitant increase in cell death that is caspase-dependent.
Fig. 1. Low glucose induces cell apoptosis through ERK.
Data are representative of at least three independent experiments. Where applicable, data are the means ± SEM of three separate experiments performed in triplicate. Results were statistically significant (*, p < 0.01) as assessed by using the Student’s t-test. (A) HEK293 cells growing in the presence of 5 mM or 1 mM glucose were counted at the indicated time points. (B) HEK293 cells were incubated in 1 mM glucose media as starting levels of glucose. Media were collected at the indicated time points and glucose levels were measured. (C) HEK293 cells were incubated in the media with constant glucose levels and cell numbers were counted at the indicated time points. (D) HEK293 cells were incubated in the presence of 5 mM or 1 mM glucose for 54 h, and apoptosis was measured as the percentage of subG1 cells. (E) HEK293 cells growing in the presence of 5 mM or 1 mM glucose were lysed and immunoblot analysis was performed. (F) HEK293 cells growing in the presence of 1 mM glucose for 36 h were treated with a caspase inhibitor for an additional 18 h and cell apoptosis was measured. (G-H) HEK293 cells treated with 10 M U0126 or 4 M AZD6244 were grown in the presence of 1 mM glucose for 54 h. Cell images were taken (G) or apoptosis assay was performed (H). Scale bar, 50 m. (I) HEK293 cells were incubated in the presence of 5 mM or 1 mM glucose. Cells were lysed at the indicated time points and immunoblot analysis was performed. (J-K) HEK293 cells treated with 10 M U0126 (J) or 4 M AZD6244 (K) were grown in the presence of 1 mM glucose. Cells were lysed at the indicated time points (J) or at 54 h (K), and immunoblot analysis was performed. (L) HEK293 cells grown in the presence of 1 mM glucose as starting levels of glucose were treated with DMSO or 10 M U0126. Media were collected at the indicated time points and glucose levels were measured. See also Fig. S1.
ERK is a key regulator of low glucose-induced cell apoptosis
To reveal the molecular mechanism by which low glucose induced cell apoptosis, we performed a kinase inhibitor screen. Surprisingly, we found that a MEK/ERK pathway inhibitor, U0126, potently suppressed cell death induced by low glucose (Fig. 1G and 1H). This was interesting because ERKs (ERK1&2) are generally regarded as survival kinases. Furthermore, another highly specific inhibitor of the MEK/ERK pathway, AZD6244, and also found to inhibit cell death as determined by cell morphology (Fig. 1G) and by measuring the sub-G1 population (Fig. 1H). Because inhibition of the ERK pathway blocked cell apoptosis under our conditions, we next determined whether glucose depletion induced ERK activation, and if so, when during the starvation process ERKs were activated. ERKs were not activated when cells were first incubated in 1mM glucose but by ~50 hrs ERKs became robustly activated (Fig. 1I) and this coincided with cleavage of PARP (Fig. 1E). We also measured cleaved PARP and active caspase-3 in cells growing in the presence of low glucose with U0126 or AZD6244. As shown in Fig. 1J and 1K, ERK pathway inhibition suppressed expression of these apoptosis markers. These results suggest that ERKs are important mediators of cell death induced under low glucose conditions. This finding was also confirmed in other cell types including cortical epithelial cells (MCT) (Fig. S1B), ureteric bud (UB) cells (Fig. S1C), and tubular epithelial cells (HK-2) (Fig. S1D). Inhibition of ERK pathway also protected primary endothelial cells (Fig. S1E), primary prostate cells (Fig. S1F), and primary fibroblasts (Fig. S1G) from cell death induced by low glucose. We suspected that inhibition of ERK pathway might enable cells to consume less glucose, thereby maintaining cells in the media with higher levels of glucose than control. However, interestingly, changes of glucose levels in the media were similar irrespective of U0126 treatment (Fig. 1L).
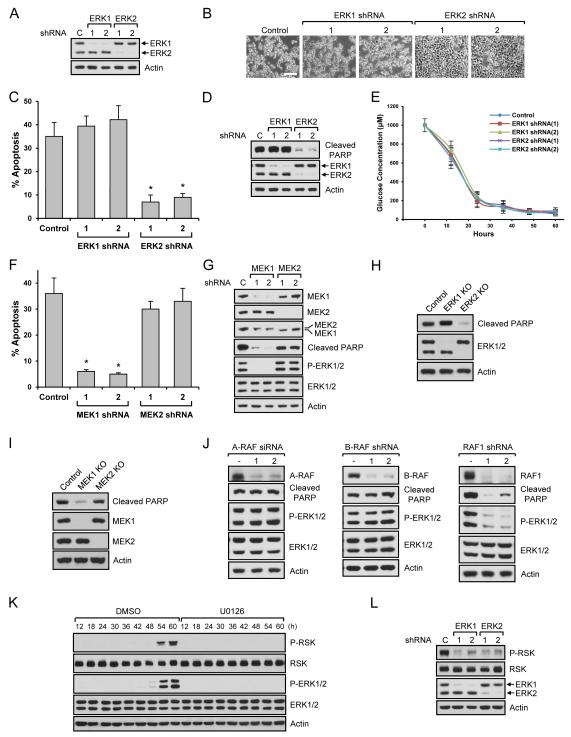
MEK1 and ERK2, but not MEK2 and ERK1, are responsible for cell death induced by low glucose
It is generally believed that ERK1 and ERK2 play identical roles based on their high sequence homology (~85%). However, some reports have identified differences in ERK1 vs. ERK2 signaling (Li et al., 2014; Shin et al., 2010). Because MEK inhibitors, U0126 and AZD6244, block both ERK1 and ERK2, we wondered if either or both were responsible for cell death. For this, we knocked down ERK1 or ERK2 using shRNAs (Fig. 2A) and measured cell apoptosis. As shown in Fig. 2B and 2C, low glucose induced morphological changes and apoptosis of control cells and ERK1 knockdown cells. However, cells with ERK2 knockdown did not induce cell morphological changes (Fig. 2B) and failed to undergo apoptosis (Fig. 2C) when cultured in low glucose. We also measured changes of cleaved PARP. As shown in Fig. 2D, only ERK2 knockdown suppressed PARP cleavage. These results indicate that ERK2 but not ERK1 is responsible for cell death. Using different cell lines such as MCT (Fig. S2A), UB (Fig. S2B), and HK-2 (Fig. S2C) cells, we confirmed that only ERK2 activation correlated with low glucose-induced cell death. To determine if knockdown of ERK1 or ERK2 enabled cells to use glucose differentially, we measured glucose levels in the media. As shown in Fig. 2E, cells consumed similar amounts of glucose irrespective of the ERK isoforms. ERKs are directly regulated by MEKs. The MEK family consists of MEK1 and MEK2, and they also share high sequence homology (~85%). Despite the current dogma, evidence also exists suggesting different roles for MEK1 and MEK2 under certain conditions (Belanger et al., 2003; Scholl et al., 2009). Therefore, we knocked down MEK1 or MEK2 and determined their respective roles in cell death. Interestingly, only MEK1 knockdown cells showed resistance to cell apoptosis (Fig. 2F) and morphology change (Fig. S2D). We also measured ERK1/2 phosphorylation in these cells. Consistent with other results, knockdown of MEK1 but not MEK2, inhibited ERK1/2 phosphorylation and expression of cleaved PARP (Fig. 2G). To confirm isoform-specific functions of ERKs and MEKs, we generated knockout cells using CRISPR systems. Similar to the results from ERK and MEK knockdown, knockout of ERK2 or MEK1 blocked low-glucose induced PARP cleavage (Fig. 2H and 2I). Taken together, these results suggest that MEK1 is responsible for ERK1/2 phosphorylation under glucose-deprivation conditions and that only ERK2 plays an essential role in the observed cell death. Intrigued by these findings, we also investigated Raf regulation under low glucose conditions. Rafs are comprised of A-Raf, B-Raf, C-Raf (Raf-1). Using isoform specific knockdown, we found that C-Raf is responsible for ERK activation and cell death in low glucose conditions (Fig. 2J). Because of the isoform-specific functions of ERKs and their upstream molecules, we wondered if activation of RSK, a downstream target of ERK, was also dependent on ERK isoforms. We found that RSK was activated when ERK was phosphorylated and inhibition of ERK blocked RSK phosphorylation under low glucose conditions (Fig. 2K). However, both ERK1 and ERK2 knockdown decreased RSK phosphorylation, suggesting that RSK phosphorylation is not ERK isoform specific (Fig 2L).
Fig. 2. MEK1 and ERK2 regulate low glucose-induced cell apoptosis.
Data are representative of at least three independent experiments. Where applicable, data are the means ± SEM of three separate experiments performed in triplicate. Results were statistically significant (*, p < 0.01) as assessed by using the Student’s t test. (A-D) Stable HEK293 cells were generated using ERK1 or ERK2 shRNAs (A). Cells were grown in the presence of 1 mM glucose for 54 h. Cell images were taken (B), apoptosis assay was performed (C), or immunoblot analysis was performed (D). Scale bar, 50 m. (E) Stably knocked down HEK293 cells with ERK1 or ERK2 shRNAs were grown in the presence of 1 mM glucose as starting levels of glucose. Media were collected at the indicated time points and glucose levels were measured. (F-G) Stable HEK293 cells were generated using MEK1 or MEK2 shRNAs. Cells were grown in the presence of 1 mM glucose for 54 h. Apoptosis assay was performed (F) or immunoblot analysis was performed (G). (H-I) Stable ERK1 or ERK2 (H), MEK1 or MEK2 (I) knockout HEK293 cells were generated using CRISPR systems. Cells were grown in the presence of 1 mM glucose for 54 h and immunoblot analysis was performed. (J) HEK293 cells knocked down with A-Raf siRNAs, B-Raf shRNAs, and Raf1 (C-Raf) shRNAs were incubated in the presence of 1 mM glucose for 54 h and immunoblot analysis was performed. (K) HEK293 cells treated with DMSO or 10 M U0126 were grown in the presence of 1 mM glucose. Cells were lysed at the indicated time points and immunoblot analysis was performed. (L) Stable HEK293 cells were generated using ERK1 or ERK2 shRNAs. Cells were grown in the presence of 1 mM glucose for 54 h and immunoblot analysis was performed. See also Fig. S2.
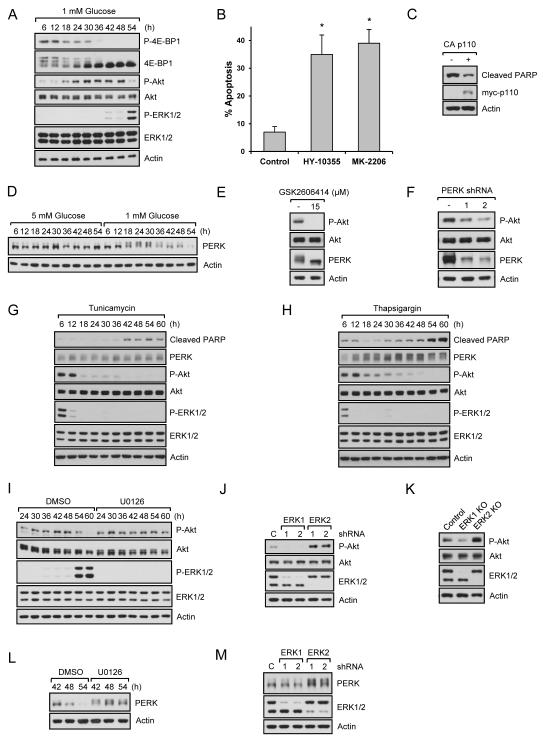
ERK2 inhibits PERK/Akt-mediated adaptation/survival signaling
As glucose levels are reduced, cells initially respond to the increasing metabolic stress by activating adaptation/survival signaling to promote cell survival. However, survival cannot be maintained with continued metabolic stress and eventually cell death signals are activated. One of the main mechanisms for the protection of cells during metabolic stress is inhibition of mRNA translation as this is a major energy consuming process. mTORC1 is a key complex that senses nutrient availability and controls mRNA translation. To monitor mTORC1 activity, we measured phosphorylation of 4E-BP1, a direct substrate of mTORC1. As shown in Fig. 3A, 4E-BP1 phosphorylation started to decrease around 18 h. We next examined Akt, a well-known survival factor and upstream regulator of mTORC1. Surprisingly, Akt phosphorylation started to increase around the time when mTORC1 became inactive, and decreased around the time when ERK2 was highly phosphorylated and cell apoptosis occurred (Fig. 3A). We confirmed Akt and ERK signaling using a flow cytometer (Fig. S3A). To determine if Akt functions as a survival factor, we treated cells with Akt-specific inhibitors, MK-2206 and HY-10355. These inhibitors efficiently blocked Akt phosphorylation (Fig. S3B), accelerated cell apoptosis in low glucose conditions (Figs. 3B and S3C). However, Akt inhibitors did not induce apoptosis of cells growing in 5 mM glucose conditions (Figs. S3D and S3E). We also expressed a constitutively active membrane targeted form of the catalytic domain (p110) of human PI3K, an upstream activator of Akt, and also observed inhibition of PARP cleavage in low glucose conditions (Fig. 3C). We next investigated the mechanism by which Akt was activated by metabolic stress. Glucose is important for protein folding and stability because glucose metabolites are used by the endoplasmic reticulum (ER) for protein N-linked glycosylation, a post-translational modification that attaches carbohydrates to proteins (Altman and Rathmell, 2012). Therefore, reduced glucose evokes ER stress that activates the unfolded protein response (UPR) which suppresses mRNA translation and reduces ER stress (Altman and Rathmell, 2012). Among three key proteins for UPR, PERK is the first to be activated by autophosphorylation and plays an essential role in UPR and cell adaptation/survival (Szegezdi et al., 2006). Therefore, we assessed whether low glucose induced PERK activation as evidenced by a band shift. As shown in Fig. 3D, low glucose increased PERK activation. Interestingly, PERK activity was upregulated (18h) (Fig. 3D) when Akt was activated (18h) (Fig. 3A). Therefore, we asked if PERK was responsible for Akt activation. For this, we used a highly specific PERK inhibitor, GSK2606414 (Axten et al., 2012). As shown in Fig. 3E, this inhibitor decreased PERK activity and Akt phosphorylation. We also knocked down PERK and found that decreased PERK protein inhibited Akt phosphorylation (Fig. 3F). Taken together, these results suggest that low glucose induces the UPR response that activates PERK and Akt to protect cells from death. We then determined if low glucose-induced signaling is similar to the signaling pathways induced by pharmacological inducers of ER stress or apoptosis. We first used tunicamycin and thapsigargin that inhibit the first step of glycosylation and the ER Ca-ATPase, respectively. As shown in Fig. 3G and 3H, ER stress inducers, unlike low glucose, increased PERK activation and inhibited phosphorylation of Akt and ERK at early time points. In addition, inhibition of ERKs did not prevent cell death induced by these ER stress inducers (Fig. S3F). We next used stress and death inducers that are involved in inhibition of transcription (actinomycin D), inhibition of mRNA translation (cycloheximide), inhibition of a broad spectrum of kinases (staurosporine), and induction of DNA damage and reactive oxygen species (camptothecin and etoposide). As shown in Fig. S3G, the stress and death responses induced by these pharmacological drugs were not similar to low glucose-induced signaling. Furthermore, inhibition of ERK pathway did not prevent cell death induced by these drugs (Fig. S3H). These results suggest that low glucose-induced cell protection and death signaling are different from pharmacological inducers of ER stress and cell death.
Fig. 3. PERK/Akt mediates survival signaling in low glucose conditions and Inhibition of ERK2 pathway sustains PERK/Akt pathway.
Data are representative of at least three independent experiments. Where applicable, data are the means ± SEM of three separate experiments performed in triplicate. Results were statistically significant (*, p < 0.01) as assessed by using the Student’s t test. (A) HEK293 cells growing in the presence of 1 mM glucose were lysed and immunoblot analysis was performed. (B) HEK293 cells growing in the presence of 1 mM glucose for 24 h were treated with 5 μM MK-2206 or 5 M HY-10355 for additional 20 h, and apoptosis assay was performed. (C) Control or constitutively active PI3K subunit (CAAX-p110) overexpressing cells were grown in the presence of 1 mM glucose for 54 h and immunoblot analysis was performed. (D) HEK293 cells growing in the presence of 5 mM or 1 mM glucose were lysed and immunoblot analysis was performed. (E) HEK293 cells growing in the presence of 1 mM glucose for 12 h were treated with 15 M GSK2606414 for an additional 12 h. Cells were lysed and immunoblot analysis was performed. (F) PERK knockdown HEK293 cells grown in 1 mM glucose media for 24 h were lysed and immunoblot analysis was performed. (G-H) HEK293 cells treated with tunicamycin (G) or thapsigargin (H) were grown in the complete media. Cells were lysed at the indicated time points and immunoblot analysis was performed. (I) HEK293 cells growing in the presence of 1 mM glucose with or without U0126 were lysed and immunoblot analysis was performed. (J-K) ERK1 or ERK2 knockdown (J) or knockout (K) HEK293 cells were grown in 1 mM glucose media for 54 h, lysed, and immunoblot analysis was performed. (L) The same method was used as described in (I). (M) The same method was used as described in (J). See also Fig. S3.
Since ERK pathway inhibition led to suppression of low glucose-induced cell apoptosis, we wondered if this was linked to the cellular adaptation/survival mechanism utilizing UPR and Akt phosphorylation and activation. For this, we blocked the ERK pathway and found that ERK inhibition resulted in sustained Akt phosphorylation in low glucose conditions (Fig. 3I). ERK2 knockdown or knockout cells also exhibited higher Akt phosphorylation (Figs. 3J and 3K). Since PERK was responsible for Akt phosphorylation, we examined the relationship between PERK and ERK. As shown in Fig. 3L and Fig. 3M, cells with ERK inhibition or ERK2 knockdown showed increased PERK activation. These results suggest cells activate PERK/Akt survival signaling under the initial metabolic stress conditions, but subsequent activation of ERK2 as energetic stress increases, inhibits PERK and Akt activation and cell survival. Similar results were observed in primary endothelial cells (Fig. S3I), primary prostate cells (Fig. S3J), and primary fibroblasts (Fig. S3K).
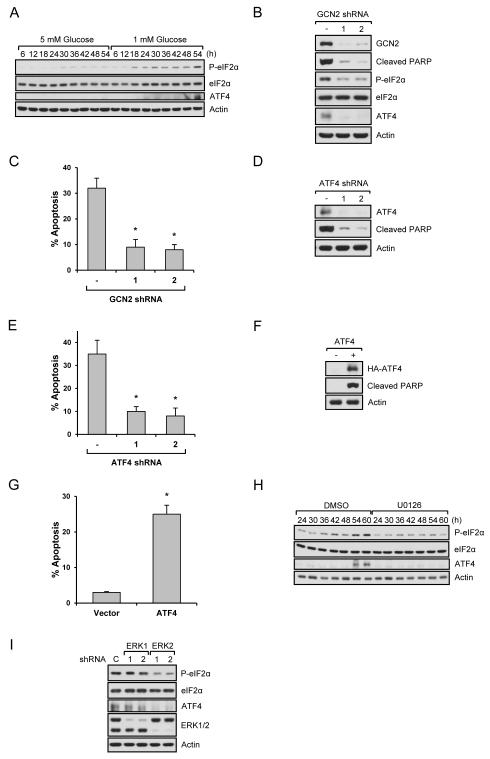
Low glucose-induced ERK2 phosphorylation activates the death machinery
Next we investigated the mechanisms by which ERK2 promotes cell death. Although ER stress-induced UPR protects cells from cell death, long-term ER stress eventually leads to cell death. However, the mechanisms responsible for this transition are unclear. Based on the change of PERK activity (Fig. 3D) and PERK’s role as an eIF-2α kinase, we examined the phosphorylation of eIF2α, an important regulator of mRNA translation. We expected that eIF-2α phosphorylation would be strongly increased upon PERK activation and decreased by PERK inactivation. As shown in Fig. 4A, 5 mM glucose did not induce a significant change in eIF2α phosphorylation, however, under low glucose conditions, an increase of eIF2α phosphorylation was observed around the time when PERK was activated. Interestingly, however, eIF-2α phosphorylation was further increased at the later time point (54 h) (Fig. 4A) when ERK became highly phosphorylated (Fig. 3A). Intrigued by this finding, we examined a downstream target of eIF2α, ATF4. Although eIF2α phosphorylation suppresses general mRNA translation, it also promotes the translation of certain transcripts such as ATF4. We found that the ATF4 protein level was increased at a later time point (54 h) when p-eIF2α was further increased, but not around 18h when PERK phosphorylation increases (Fig. 4A). These findings suggested that other pathways, possibly another eIF2α kinase, were responsible for eIF2α phosphorylation and ATF4 expression. Therefore, we performed additional studies to further define the mechanism. It has been shown that different stresses increase eIF-2α phosphorylation by different kinases. For example, PERK, GCN2, PKR, and HRI directly phosphorylate eIF-2α induced by ER stress, amino acid deprivation, dsRNA, and low levels of heme, respectively (Koromilas and Mounir, 2013). Based on the fact that glucose is used for biosynthesis of amino acids, nucleotides, and lipids (Cantor and Sabatini, 2012), we hypothesized that the shortage of glucose might disrupt amino acid metabolism, which activates GCN2 and then increases eIF-2α phosphorylation and subsequent ATF4 expression. To prove this, we first knocked down GCN2 and analyzed phospho-eIF2α/ATF4 levels and cell apoptosis. As shown in Fig. 4B and Fig. 4C, knockdown of GCN2 blocked the increase of phospho-eIF-2α and ATF4 levels, and inhibited cell death induced by low glucose. ATF4 has dual functions, anti-apoptosis or pro-apoptosis, depending on cellular context (Hetz, 2012). Therefore, we next determined if ATF4 played a role in cell apoptosis under low glucose conditions. For this, ATF4 was knocked down or overexpressed in cells. As shown in Fig. 4D, 4E, and S4A, knockdown of ATF4 inhibited PARP cleavage and apoptosis. Whereas overexpression of ATF4 accelerated cell death at an early time point when most control cells were still alive (Fig. 4F, 4G, and S4B). Taken together, our results suggest that the GCN2/eIF-2α/ATF4 pathway is required for low glucose-induced cell death. We next asked if ERK2 is responsible for the activation of this pathway. As shown in Fig. 4H, inhibiting the ERK pathway decreased phospho-eIF-2α and ATF4 expression as did knockdown of ERK2, but not ERK1 (Fig. 4I).
Fig. 4. ERK2 regulates GCN2/eIF2α/ATF4 pathway.
Data are representative of at least three independent experiments. Where applicable, data are the means ± SEM of three separate experiments performed in triplicate. Results were statistically significant (*, p < 0.01) as assessed by using the Student’s t test. (A) HEK293 cells growing in the presence of 5 mM or 1 mM glucose were lysed and immunoblot analysis was performed. (B-C) GCN2 knockdown HEK293 cells were grown in 1 mM glucose media for 54 h. Immunoblot analysis was performed (B) or cell apoptosis rate was measured (C). (D-E) Control or ATF4 knockdown cells were incubated in 1 mM glucose media for 54 h. Immunoblot analysis (D) or apoptosis assay (E) was performed. (F-G) Control or ATF4 overexpressing cells were incubated in 1 mM glucose media for 30 h. Immunoblot analysis (F) or apoptosis assay (G) was performed. (H) DMSO or U0126 treated HEK293 cells growing in the presence of 1 mM glucose were lysed and immunoblot analysis was performed. (I) ERK1 or ERK2 knockdown cells growing in the presence of 1 mM glucose for 54 h were lysed and immunoblot analysis was performed. See also Fig. S4.
ERK2 regulates low glucose-induced cell death by modulating ATF4-dependent c-Fos, Bid, and TRB3 expression
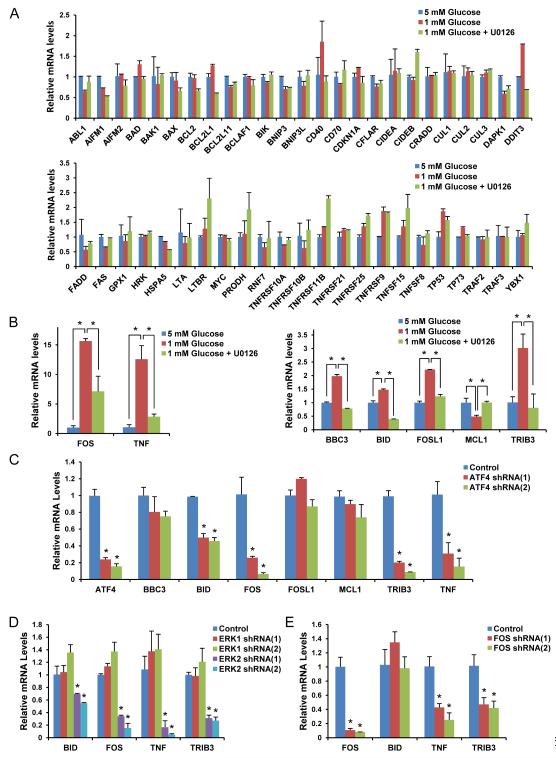
Since ATF4 is a transcription factor, we wondered which genes were regulated by the ERK2/ATF4 pathway. For this, we screened for genes known to be involved in cell apoptosis (Fig. S5) using qPCR analysis after ERK pathway inhibition (Fig. 5A). Among these, BBC3 (PUMA), BID, FOSL1 (FRA1), MCL1, TRIB3 (TRB3), FOS (c-Fos), and TNF (TNFα) mRNA levels were changed significantly by low glucose and these changes were blunted by ERK pathway inhibition (Fig. 5B). To determine if these genes were regulated by ATF4, we performed qPCR in ATF4 knockdown cells. As shown in Fig. 5C, mRNA levels of BID, FOS, TRIB3, and TNF were affected, whereas BBC3, FOSL1, and MCL1 mRNA levels were not significantly changed by ATF4 knockdown. Next, we determined if mRNA levels of the genes affected by ATF4 were regulated by only ERK2. As shown in Fig. 5D, only ERK2 not ERK1 affected their expression. The transcription factor c-Fos drew our attention because it can interact with ATF4 to form a transcriptional heterodimer with increased activity (Hai and Curran, 1991) and therefore could potentially be responsible for the induction of some of the other genes regulated by ERK2/ATF4 pathway (Figs 5B and 5C). Indeed, we found that knockdown of c-Fos significantly suppressed the expression of TNFα and TRB3 (Fig. 5E).
Fig. 5. ERK2/ATF4 regulates mRNA levels of genes involved in apoptosis.
Data are representative of at least three independent experiments. Where applicable, data are the means ± SEM of three separate experiments performed in triplicate. Results were statistically significant (*, p < 0.01) as assessed by using the Student’s t test. (A-B) qPCR was performed using mRNA from the HEK293 cells grown in the presence of 5 mM or 1 mM glucose with/without U0126 for 50 h (A). Significantly changed mRNA levels of certain genes are shown in (B). (C-E) qPCR was performed using the mRNA from ATF4 (C), ERK1/2 (D), or c-Fos (E) knockdown HEK293 cells grown for 50 h in the presence of 1 mM glucose. See also Fig. S5.
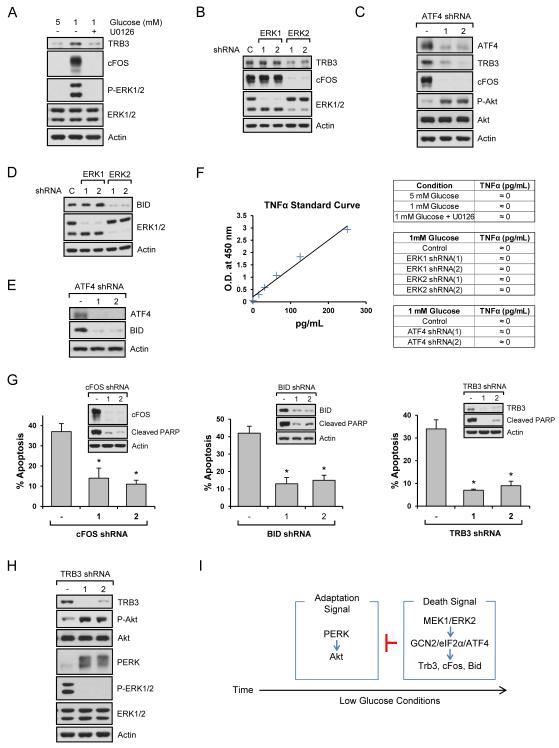
Phosphorylation of eIF2α suppresses general translation, but activates translation of certain mRNAs under stress conditions. Therefore, we wondered if the mRNAs regulated by ERK2/ATF4 corresponded with increased protein levels. As shown in Fig. 6A, TRB3 and c-Fos protein levels were increased by low glucose and decreased by ERK inhibition. Like mRNA levels, TRB3 and c-Fos proteins were regulated by ERK2 not ERK1 (Fig. 6B) and ATF4 (Fig. 6C). Because ERK inhibition dramatically decreased mRNA levels of Bid, a well-known proapoptosis molecule, we also examined its protein level and found that knockdown of ERK2 (Fig. 6D) and ATF4 (Fig. 6E) decreased Bid levels. Although TNFα mRNA levels increased, protein levels were not affected under low glucose conditions (Fig. 6F). We next examined if these proteins are involved in cell death. Using shRNAs against c-Fos, Bid, and Trb3, we found that knockdown of these genes inhibited low glucose-induced cell death that was measured at 54 h (Fig. 6G and Fig. S6A). Whereas Fos and Trb3 knockdown, like ERK inhibition, delayed the onset of cell death even at 66 h, Bid knockdown did not (Fig. S6B), suggesting that Fos/Trb3 pathway may be the main targets of ERK-mediated cell death in low glucose conditions. We also used a TNFα antagonist, but it did not block cell apoptosis (Fig. S6C). Interestingly, TRB3 is known as an Akt inhibitory protein by directly binding to it (Du et al., 2003). Therefore, we knocked down TRB3 and found that reduced TRB3 levels increased Akt phosphorylation (Fig. 6H). TRB3 is also known to positively regulate ERK signaling (Izrailit et al., 2013). Based on our finding that inhibition of ERK sustained PERK activation (Figs. 3L-3M), we were also interested in the link between TRB3 and PERK. As shown in Fig. 6H, TRB3 knockdown decreased ERK phosphorylation while it induced PERK activation. Combining our results and a previous report that TRB3 is a direct inhibitor of Akt (Du et al., 2003), it appears that ERK-induced TRB3 suppresses Akt activity via direct binding and indirectly by further promoting ERK signaling as part of a positive feedback switch that inhibits PERK/Akt (Figs. 3I-3M). Taken all together, our results suggest that ERK2/ATF4 coordinates the upregulation of another transcription factor (c-Fos), a pro-apoptotic Bcl-2 family member (Bid), and negative regulator of Akt (Trb3) to regulate cell death (Fig. 6I).
Fig. 6. ERK2/ATF4 regulates TRB3, c-Fos, and Bid to induce apoptosis.
Data are representative of at least three independent experiments. Where applicable, data are the means ± SEM of three separate experiments performed in triplicate. Results were statistically significant (*, p < 0.01) as assessed by using the Student’s t test. (A-C) Immunoblot analysis was performed using the lysates from (A) HEK293 cell grown in 5 mM or 1 mM glucose with/without U0126 for 54 h, (B) ERK1 or ERK2 knockdown cells, or (C) ATF4 knockdown cells grown in the presence of 1 mM glucose for 54 h. (D-E) Bid protein levels were determined using ERK1 or ERK2 (D), or ATF4 (E) knockdown cells grown in the presence of 1 mM glucose for 54 h. (F) Extracellular TNF-α levels were measured from HEK293 cells grown in 5 mM or 1 mM glucose with/without U0126 for 54 h, ERK1 or ERK2 knockdown cells, or ATF4 knockdown cells grown in the presence of 1 mM glucose for 54 h. (G) Stably knocked down HEK293 cells with c-Fos, Bid, or Trb3 shRNAs were grown in the presence of 1 mM glucose for 54 h. Immunoblot analysis was performed or cell death rate was measured. (H) Immunoblot analysis was performed using the lysates from Trb3 knockdown cells grown in the presence of 1 mM glucose for 54 h. (I) Schematic diagram of cell adaptation and death signalings under low glucose conditions. See also Fig. S6.
Inhibition of ERK2 protects cells from death by modulating glutamate and glutamine
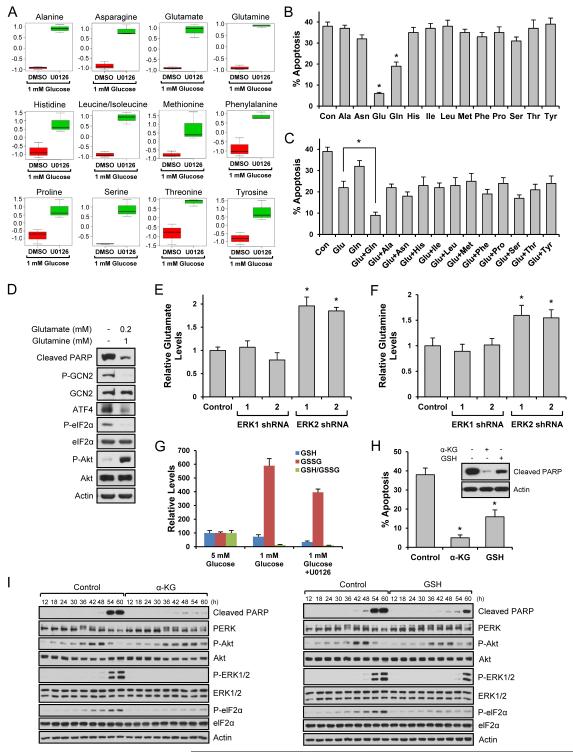
Our results suggest that when energetic stress becomes extreme, cells activate ERK2 to irreversibly induce cell death by inhibiting Akt survival signaling engaged during the adaptation phase and by inducing activators of the apoptotic machinery. To investigate this process further, we characterized the relationship between ERK2 and cellular metabolism in cells growing in low glucose with or without an ERK pathway inhibitor. Metabolomic analysis by targeted mass spectrometry (Yuan et al., 2012) indicated a correlation with ERK2 inhibition and a dramatic increase in the levels of most amino acids with the exception of aspartate, which was decreased (Figs. S7A, S7B, 7A, and S7C). To determine if the change in amino acid levels contributed to cell survival, we supplemented the culture media with specific amino acids. Re-addition of many amino acids such as serine did not show any protective effect against cell death induced by low glucose-induced stress (Fig. 7B). However, re-addition of glutamine partially blocked cell death and interestingly, glutamate dramatically blocked cell death (Fig. 7B). Addition of a stress-induced amino acid, aspartate, did not accelerate cell death (Fig. S7C). Using low concentrations of glutamate, we determined if re-addition of any of the other amino acids had additive or synergistic effects on cell death. As shown in Fig. 7C, only the combination of low concentration of glutamate with glutamine exhibited a very strong anti-apoptotic effect. We next determined if glutamate and/or glutamine affected cell adaptation and death machineries. As shown in Fig. 7D, the combination of these amino acids blocked the increase of phospho-eIF2α and ATF4, and increased Akt phosphorylation. Further studies revealed that only ERK2 knockdown increased glutamate and glutamine (Figs. 7E and 7F). Taken together our results suggest that low glucose induces apoptosis through the ERK2/eIF-2α/ATF4-dependent pathway and blocking the ERK pathway leads to changes of cellular metabolism, which result in sustaining the Akt-dependent adaptation and inhibition of eIF-2α/ATF4-dependent cell death. Intrigued by our findings, we further studied the mechanisms by which glutamate protected cell death induced by low glucose. Through the transamination process, glutamate plays a central role in synthesizing new amino acids (Newsholme et al., 2003). Glutamate is also involved in biosynthesis of α-ketoglutarate and glutathione, a key intermediate in TCA cycle and an important antioxidant, respectively (Newsholme et al., 2003). Because the addition of other amino acids did not show a profound protective effect, we examined glutamate’s possible connection with α-ketoglutarate and glutathione and found that α-ketoglutarate level was increased by ERK pathway inhibition (Fig. S7D). Interestingly, other TCA cycle intermediates exhibited the same pattern (Fig. S7D). Under low glucose conditions, oxidized glutathione (GSSG) dramatically increased and ERK inhibition reduced its level (Fig. 7G). However, reduced glutathione (GSH) level was further decreased by ERK inhibition making the GSSG/GSH ratio, a marker of oxidative stress, similar irrespective of ERK inhibition under low glucose conditions (Fig. 7G). These results suggest that α-ketoglutarate not glutathione may be linked to glutamate’s protective effect under low glucose conditions. We next examined if addition of α-ketoglutarate rescued cell death under low glucose conditions. As shown in Fig. 7H, it potently blocked cell death. Although glutathione exhibited a protective effect, it was not as profound as α-ketoglutarate (Fig. 7H). Since both α-ketoglutarate and glutathione showed protective effects, we wondered if α-ketoglutarate and glutathione could function similarly to ERK inhibition or glutamate in low glucose conditions. As shown in Fig. 7I, α-ketoglutarate decreased ERK phosphorylation, eIF2α phosphorylation and cleaved PARP, while it increased PERK and Akt phosphorylation. Glutathione also showed similar effects but they were not as profound as α-ketoglutarate.
Fig. 7. Glutamate protects cells from cell death induced by low glucose.
Data are representative of at least three independent experiments. Where applicable, data are the means ± SEM of three separate experiments performed in triplicate. Results were statistically significant (*, p < 0.01) as assessed by using the Student’s t test. (A) Metabolites from HEK293 cells grown in the presence of 1 mM glucose for 54 h with or without U0126 were analyzed. Box plots show individual amino acids significantly changed by U0126. (B) HEK293 cells grown in the presence of 1 mM glucose for 36 h were treated with 2 mM amino acids. After 18 h, cell death was measured. (C) HEK293 cells grown in the presence of 1 mM glucose for 36 h were treated with both glutamate (200 M) and other amino acids (1 mM). After 18 h, cell death was measured. (D) HEK293 cells grown in the presence of 1 mM glucose for 36 h were treated with 200 M glutamate and 1 mM glutamine. After 18 h, cells were lysed and immunoblot analysis was performed. (E-F) ERK1 or ERK2 knockdown HEK293 cells were grown in 1 mM glucose media for 54 h, and glutamate (E) and glutamine (F) levels were measured. (G) The levels of GSH and GSSG from HEK293 cells grown in the presence of 1 mM glucose for 54 h with or without U0126 were analyzed. (H) HEK293 cells grown in the presence of 1 mM glucose for 36 h were treated with α-ketoglutarate (1 mM) or cell permeable glutathione (1 mM). After 18 h, cell death was measured or cells were lysed and immunoblot analysis was performed. (I) HEK293 cells treated with α-ketoglutarate (1 mM) or cell permeable glutathione (1 mM) were grown in the presence of 1 mM glucose. Cells were lysed at the indicated time points and immunoblot analysis was performed. See also Fig. S7.
Discussion
Little is known about the detailed connections between ER stress, UPR, and cell death. Moreover, most studies on ER stress-induced UPR and cell death use high doses of various pharmacological inducers (e.g. tunicamycin, thapsigargin, and DTT) of ER stress (Hetz, 2012). As a result, UPR-mediated adaptation signaling and death signaling occur with virtually identical kinetics, making it difficult to dissect the mechanisms underlying these different signaling processes (Hetz, 2012). Considering this, it is not unexpected that there are similarities and discrepancies between our low glucose-induced ER stress results and other reports. For example, some studies using pharmacological ER stress inducers show Akt inactivation by ER stress, sustained high PERK activity during ER stress and apoptosis, or rapid cell death by PERK-mediated eIF2α phosphorylation (Hetz, 2012; Merksamer and Papa, 2010). Our studies suggest that there are three distinct but connected signaling phases regulated under mild to severe metabolic stress conditions. In the first phase, cells grow and proliferate with well-characterized and interconnected homeostatic pathways. In the second phase, cells respond to mild metabolic stress by inhibiting mTORC1 signaling and activating PERK/Akt-mediated adaptation/survival signaling. Finally, during the last phase, when cells cannot overcome more severe stress, they respond by activating ERK2/ATF4-mediated signaling, which blocks adaptation/survival signaling and induces cell death.
Here we show that during the last phase, the ERK2/ATF4 pathway determines cell fate by inducing the c-Fos transcription factor, the Akt inhibitor and anti-survival protein Trb3, and the pro-apoptosis protein Bid. Interestingly, ATF4 has been shown to homodimerize or heterodimerize with other transcription factors such as c-Fos (Hai and Curran, 1991), and ERK stabilizes the c-Fos protein by direct phosphorylation (Murphy et al., 2002). Based on these and our findings, it appears that in the context of ER and energetic stress, ERK2 induces ATF4, ATF4 increases c-Fos mRNA/protein, and ERK2 stabilizes c-Fos protein to further activate ATF4, thus positively regulating each other to synergistically induce cell death. Additionally, the common target of ATF4 and c-Fos was Trb3. ERK2 activation increased Trb3, a direct binding inhibitor of Akt (Du et al., 2003). Akt promotes cell survival under low glucose conditions (Chopra et al., 2012; Lee et al., 2014) and ER stress conditions (Bobrovnikova-Marjon et al., 2012), and it prevents GCN2 activation by an unknown mechanism (Koromilas and Mounir, 2013). However, Trb3 also promotes ERK activation (Izrailit et al., 2013). Therefore, it is possible that ERK2/GCN2/eIF2α/ATF4 dependent Trb3 expression inhibits Akt survival signaling while also further promoting ERK and GCN2 activation and induction of ATF4-dependent apoptosis. Lastly, we show that ERK2 regulates Bid (BH3-interacting domain death agonist). Bid regulates mitochondrial apoptotic pathway and amplifies caspase signaling (Czabotar et al., 2014). Therefore, it seems that ERK2/ATF4 also regulates Bid expression to collaborate with Trb3 and Fos to execute cell apoptosis under metabolic stress. Taken together, our results suggest that ERK2-dependent cell death under metabolic stress requires cellular signaling that mediates communication between cell survival molecules and apoptotic molecules.
Here, we demonstrate that under conditions of energetic and ER stress, only MEK1 activated ERKs, and ERK2 not ERK1 induced cell death through an ATF4-dependent regulation of c-Fos, Trb3, and Bid expression. Interestingly, MEK1 or ERK2 knockouts are embryonic lethal whereas MEK2 and ERK1 are not (Belanger et al., 2003). It is still not fully understood why MEK and ERK isoforms are functionally similar in many cellular processes but different under certain conditions. Some of the reasons for this functional difference may be due to different interacting molecules or differential cellular localization, which are dependent on cellular context. Our results and growing evidence from others (Li et al., 2014; Shin et al., 2010) suggest that although protein isoforms are physically and structurally similar, they are not functionally alike in certain cellular processes such as metabolic stress-induced cell death process.
ERKs are also generally regarded as positive regulators of mRNA translation (Hay and Sonenberg, 2004). However, our study shows that ERK2 may attenuate mRNA translation under metabolic stress conditions by increasing phospho-eIF2α although translation of certain transcripts such as ATF4 is not inhibited. Taken together, it seems that ERKs can function as pro-apoptotic molecules under certain highly stressful conditions. Since ERKs are regarded as strong therapeutic targets in many diseases, it will be important to determine which functions of ERKs, survival or cell death, predominate in certain diseases and tissues.
Since a variety of cellular stresses lead to disruption of metabolic homeostasis and subsequent cell damage and death, finding a way to reorganize metabolism for cell survival is of physiological and pathological importance. From metabolite profiling, we found that ERK2 inhibition changed amino acid levels significantly, and glutamate, alone or with glutamine, protected cell death induced by metabolic stress. Recent studies showed that amino acid-induced signaling is essential for cell survival and proliferation. For example, leucine, arginine, and glutamine activate the mTOR/S6K pathway for cell proliferation and survival (Gallinetti et al., 2013). Serine has also received much attention recently because its metabolism plays a central role in the synthesis of nucleotides, proteins, and lipids required for cell proliferation (Amelio et al., 2014). Although inhibition of ERK led to changes in the levels of many amino acids, glutamate was the major survival factor as cell death could be rescued upon its re-addition to the low glucose media. It is not clear why glutamate is more effective than glutamine considering glutamine can be converted to glutamate. It is possible that the efficiency of the metabolism of glutamine to glutamate and/or glutamine uptake is low under low glucose conditions. The function of glutamate is not understood in many cell types and under various cellular conditions. Glutamate is not only an important source of nitrogen metabolism that is used for the synthesis of new amino acids and TCA cycle intermediates, but also a key factor for balancing redox potential (Newsholme et al., 2003). Our studies suggest that ERK2 is involved in reprogramming glutamate metabolism and the TCA cycle under low glucose conditions. Controlled cell growth, proliferation, and survival require maintenance of at least three major factors: energy (ATP), macromolecules (carbohydrates, proteins, lipids, and nucleic acids), and redox balance (Cairns et al., 2011). The TCA cycle functions as a central hub to provide ATP and building blocks of many macromolecules such as nucleotides and amino acids (Cairns et al., 2011). In addition, the TCA cycle is one of the sources of NADPH, which provides reducing power that is important for macromolecule biosynthesis and ROS scavenging (Cairns et al., 2011). Considering the key roles of this cycle in the maintenance of cell growth and survival, reprogramming of the TCA cycle under low glucose conditions may provide stronger anti-apoptotic effects than modulating any single factor such as redox balance. In this regard, ERK2 seems to be an important mediator between cell metabolism and death under low glucose-mediated stress.
Metabolic homeostasis is adjusted and maintained through both external and internal factors. Despite the intricate mechanisms evolved to maintain levels of nutrients in cells and tissues, mildly or severely decreased glucose levels can be caused by inadequate intake/absorption of food (e.g. due to fasting, improper/unbalanced diet, excess alcohol consumption, effects from medication), or unbalanced internal factors such as in endocrine disorders (e.g. adrenal insufficiency, pituitary gland malfunction), organ failure (e.g. liver failure, kidney failure), and cancer (e.g. pancreatic cancer, liver cancer). A common example in which maintaining metabolic homeostasis is a struggle can occur in type I diabetics following insulin injection, which can result in plasma glucose concentrations of less than 1 mM (Cryer, 2007). The symptoms of hypoglycemia range from mild discomfort to severe confusion, seizures, coma, and brain damage (Szablewski, 2011). Furthermore, some diseases such as diabetes, chronic kidney disease, and acute coronary syndromes are associated with higher death rate due to hypoglycemia (Cryer, 2012; Kosiborod et al., 2008; Moen et al., 2009). Although the mechanisms for this are not well understood, recent clinical evidence suggests that even acute hypoglycemia induces endothelial dysfunction in humans (Ceriello et al., 2013; Wang et al., 2012), which is one of the reasons of increased cardiovascular risk and vascular complication in diabetes (Wright and Frier, 2008). Based on our results that connect cellular signaling, metabolism, and cell fate under metabolic stress conditions, our studies suggest that certain metabolites such as glutamate, or manipulation of the signaling enzymes such as ERK2 that regulate metabolism, can provide beneficial effects for these pathophysiological conditions and disorders.
Experimental Procedures
TNFα assay
Secreted TNFα level was determined using TNFα ELISA kit (Life Technologies) according to the manufacturer’s instruction. Briefly, cell culture media with different conditions were collected and used with or without concentration using Amicon centrifugal filter units (Millipore). TNFα standards and cell culture samples were incubated in human TNFα antibody coated wells for various time (2 h ~ overnight). Manufacture’s instruction was used with these various conditions.
Quantitative RT-PCR analysis
Total RNA was isolated using RNeasy mini kit (Qiagen) and first strand cDNA was prepared using superscript III first-strand synthesis supermix kit (Life Technologies) according to the manufacturers’ protocol. Quantitative PCR was performed using QuantiTect SYBR green qPCR kit on Roche LightCycler 480. Pre-designed KiCqStart SYBR Green Primers were purchased from Sigma and melting curve analysis was performed at the end of PCR. All experiments were performed in triplicate and repeated at least three times.
Metabolite profiling and analysis
Cells were harvested in triplicate, and intracellular metabolites were extracted using 80% (v/v) aqueous methanol. Targeted liquid chromatography - tandem mass spectrometry (LC-MS/MS) was performed using a 5500 QTRAP triple quadrupole mass spectrometer (AB/SCIEX) coupled to a Prominence UFLC HPLC system (Shimadzu) with Amide HILIC chromatography (Waters). Data were acquired in selected reaction monitoring (SRM) mode using positive/negative ion polarity switching for steady-state polar profiling of more than 260 molecules. Peak areas from the total ion current for each metabolite SRM transition were integrated using MultiQuant v2.0 software (AB/SCIEX). Informatics analysis was carried out using MetaboAnyst.ca free online software.
Glutamate and glutamine analysis
Intracellular glutamate and glutamine levels were measured using the kits for glutamate assay (Sigma) and glutamine assay (Abnova) according to the manufacturers’ instructions.
Flow cytometry
To measure cell apoptosis, the sub-G1 cell population was measured using flow cytometry as described previously (Chen et al., 2010). Cellular phospho-Akt and phospho-ERK levels were measured using Cell Signaling Technology antibodies according to the manufacture’s instruction.
Supplementary Material
Acknowledgement
We thank Dr. Andrew L. Kung and Dr. David Baltimore for generously providing reagents. This work was supported by a start-up fund from the University of Cincinnati College of Medicine and the Marlene Harris-Ride Cincinnati Pilot Grant Program (S.O. Yoon) and NIH grants GM51405, HL121266 and CA46595 (J. Blenis). We thank Min Yuan for help with mass spectrometry experiments. The work was partially funded by NIH grants P01CA120964 (J.M.A.) and P30CA006516 (J.M.A.).
Footnotes
Author contributions
S.S., J.B., and S.O.Y. designed experiments. S.S., G.R.B., and L.W. performed experiments. S.S., D.R.P., J.M.A., J.B., and S.O.Y. analyzed data. S.S., G.R.B., J.B., and S.O.Y wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman BJ, Rathmell JC. Metabolic stress in autophagy and cell death pathways. Cold Spring Harb Perspect Biol. 2012;4:a008763. doi: 10.1101/cshperspect.a008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci. 2014;39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J Med Chem. 2012;55:7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- Belanger LF, Roy S, Tremblay M, Brott B, Steff AM, Mourad W, Hugo P, Erikson R, Charron J. Mek2 is dispensable for mouse growth and development. Mol Cell Biol. 2003;23:4778–4787. doi: 10.1128/MCB.23.14.4778-4787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovnikova-Marjon E, Pytel D, Riese MJ, Vaites LP, Singh N, Koretzky GA, Witze ES, Diehl JA. PERK utilizes intrinsic lipid kinase activity to generate phosphatidic acid, mediate Akt activation, and promote adipocyte differentiation. Mol Cell Biol. 2012;32:2268–2278. doi: 10.1128/MCB.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, Bucciarelli L, Rondinelli M, Genovese S. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care. 2013;36:4104–4108. doi: 10.2337/dc13-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Azad MB, Gibson SB. Methods for detecting autophagy and determining autophagy-induced cell death. Can J Physiol Pharmacol. 2010;88:285–295. doi: 10.1139/Y10-010. [DOI] [PubMed] [Google Scholar]
- Choo AY, Kim SG, Vander Heiden MG, Mahoney SJ, Vu H, Yoon SO, Cantley LC, Blenis J. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38:487–499. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I, Li HF, Wang H, Webster KA. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia. 2012;55:783–794. doi: 10.1007/s00125-011-2407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest. 2007;117:868–870. doi: 10.1172/JCI31669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE. Severe hypoglycemia predicts mortality in diabetes. Diabetes Care. 2012;35:1814–1816. doi: 10.2337/dc12-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- Gallinetti J, Harputlugil E, Mitchell JR. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 2013;449:1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson BE, Seeley RJ, Sandoval DA. Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci. 2013;14:24–37. doi: 10.1038/nrn3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Izrailit J, Berman HK, Datti A, Wrana JL, Reedijk M. High throughput kinase inhibitor screens reveal TRB3 and MAPK-ERK/TGFbeta pathways as fundamental Notch regulators in breast cancer. Proc Natl Acad Sci U S A. 2013;110:1714–1719. doi: 10.1073/pnas.1214014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koromilas AE, Mounir Z. Control of oncogenesis by eIF2alpha phosphorylation: implications in PTEN and PI3K-Akt signaling and tumor treatment. Future Oncol. 2013;9:1005–1015. doi: 10.2217/fon.13.49. [DOI] [PubMed] [Google Scholar]
- Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, Masoudi FA, Marso SP, Spertus JA. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117:1018–1027. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- Lee JV, Carrer A, Shah S, Snyder NW, Wei S, Venneti S, Worth AJ, Yuan ZF, Lim HW, Liu S, et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014;20:306–319. doi: 10.1016/j.cmet.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ma H, Wang Y, Cao Z, Graves-Deal R, Powell AE, Starchenko A, Ayers GD, Washington MK, Kamath V, et al. Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon cancer. J Clin Invest. 2014;124:2172–2187. doi: 10.1172/JCI71103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman XH, McDonnell MA, Kosloske A, Odumade OA, Jenness C, Karim CB, Jemmerson R, Kelekar A. The proapoptotic function of Noxa in human leukemia cells is regulated by the kinase Cdk5 and by glucose. Mol Cell. 2010;40:823–833. doi: 10.1016/j.molcel.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Merksamer PI, Papa FR. The UPR and cell fate at a glance. J Cell Sci. 2010;123:1003–1006. doi: 10.1242/jcs.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen MF, Zhan M, Hsu VD, Walker LD, Einhorn LM, Seliger SL, Fink JC. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1121–1127. doi: 10.2215/CJN.00800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama A, Ohmori K, Fujimura A, Minami H, Yasuzawa-Tanaka K, Kuroda T, Oie S, Daitoku H, Okuwaki M, Nagata K, et al. Epigenetic control of rDNA loci in response to intracellular energy status. Cell. 2008;133:627–639. doi: 10.1016/j.cell.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate--their central role in cell metabolism and function. Cell Biochem Funct. 2003;21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Tan-Sah VP, Ding EY, Smith JM, Miyamoto S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol Cell. 2014;53:521–533. doi: 10.1016/j.molcel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med. 2013;19:488–493. doi: 10.1038/nm.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl FA, Dumesic PA, Barragan DI, Harada K, Charron J, Khavari PA. Selective role for Mek1 but not Mek2 in the induction of epidermal neoplasia. Cancer Res. 2009;69:3772–3778. doi: 10.1158/0008-5472.CAN-08-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Dimitri CA, Yoon SO, Dowdle W, Blenis J. ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol Cell. 2010;38:114–127. doi: 10.1016/j.molcel.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szablewski L. Glucose Homeostasis - Mechanism and Defects. In: Rigobelo E, editor. Diabetes - Damages and Treatments. 2011. pp. 227–256. (InTech) [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J, Gutterman DD, Widlansky ME. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol. 2012;32:712–720. doi: 10.1161/ATVBAHA.111.227389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev. 2008;24:353–363. doi: 10.1002/dmrr.865. [DOI] [PubMed] [Google Scholar]
- Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.