Abstract
As studies increasingly use transcranial direct-current stimulation (tDCS) to manipulate brain activity, surprising results are emerging. Specifically, research combining tDCS with electrophysiology is showing that the long-lasting effects of tDCS can counterintuitively influence specific neural mechanisms active for as little as 100 milliseconds during the flow of human information processing.
Keywords: transcranial direct current stimulation, neuromodulation, event-related potentials, electrophysiology
Recent research using a novel combination of neuroscience techniques has been yielding surprising results. The combination of techniques is transcranial direct-current stimulation (tDCS) and human electrophysiology, specifically recordings of event-related potentials (ERPs). Here, we discuss some of this recent evidence showing that conventional tDCS, despite its relatively poor spatial resolution compared to intracranial microstimulation, can modulate specific information-processing mechanisms with surprisingly high temporal resolution.
Why is it surprising that tDCS should provide temporally precise effects on specific functions performed by the human brain? Conventional tDCS would seem to be neither temporally nor spatially precise. Unlike transcranial magnetic stimulation (TMS) pulses that are discrete, punctate events that causally manipulate neural activity, tDCS relies on a build up of ionic gradients that take many minutes to realize [1], and then appear to exert effects that can last for many hours [2]. That is, the temporal specificity of the application of tDCS is slow, with it resulting is something like a tonic change of state in the brain. Spatially, tDCS can also be properly criticized for its diffuse spatial resolution. The number, location, and size of anatomical targets for any given tDCS protocol are largely determined by user-defined properties. The electrode sizes, the electrode locations, and stimulation intensity all converge to determine which parts of the brain are influenced. For example, conventional tDCS electrodes are typically connected to a pair of large conductive sponge pads (e.g., 19 cm2 and 52 cm2), and computational models of current flow show that such configurations result in the diffuse spread of current through large swaths of cortex [3, 4] (Fig. 1A). More advanced stimulation technologies, such as high definition tDCS, can help resolve the practical issue of spatial targeting by delivering more focused current flow [3]. However, these technologies do not resolve the inherent conceptual limitations of using anatomical specificity to study neural processes and representations that may be distributed across large-scale neural networks.
Figure 1.
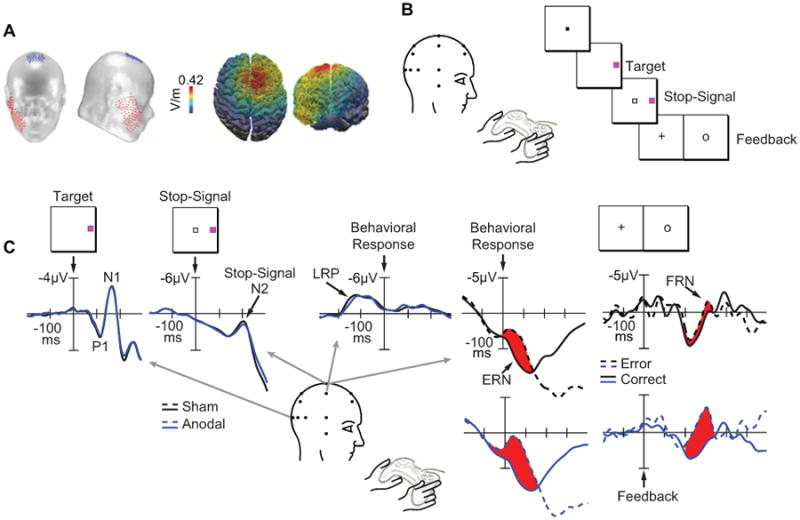
A, Schematic of a typical transcranial direct-current stimulation (tDCS) montage using one anodal electrode (19.25 cm2, red) and one cathodal electrode (52 cm2, blue). A computational model showing the distribution of current flow during anodal tDCS over the medial-frontal cortex (i.e., site FCz of the International 10-20 System) paired with a cathodal electrode over the right cheek projected on top and front views of a 3D reconstruction of the cortical surface. B, The target discrimination task with stop signals requiring subjects to report the color of the target by pressing one of two buttons on a handheld gamepad unless a stop signal appeared. C, Target locked ERPs from correct, no-stop trials shown at lateral occipitotemporal electrodes (OL/OR) contralateral (dotted) to target location across sham (black) or anodal (blue) conditions. Labels show the P1 and N1 components. Stop signal locked ERPs from correct, stop trials shown at the central midline electrode (Cz) across sham (black) and anodal (blue) conditions. The arrow shows the stop signal N2 component. Response locked ERP difference waves (contralateral minus ipsilateral with respect to response hand) from correct, no-stop trials shown at centrolateral electrodes (C3/C4) across sham (black) and anodal (blue) conditions. The arrow shows the lateralized readiness potential (LRP). Response and feedback locked ERPs from correct (solid) and error (dashed) trials shown at Cz across sham (black) and anodal (blue) conditions. Arrows show the error-related negativity (ERN) and feedback-related negativity (FRN). Fig. 1A adapted from Reinhart and Woodman (2015) with permission from the Proceedings of the National Academy of Sciences, USA. Fig. 1B-C adapted from Reinhart and Woodman (2014) with permission from the Society for Neuroscience.
Offsetting the sluggish and diffuse nature of this causal manipulation of neural activity, tDCS is extremely safe, cost effective, portable, and easy to use, resulting in an increase in popularity [5]. For the cognitive neuroscientist, tDCS also affords the unique opportunity to induce bidirectional changes in the human brain. That is, neural activity in the vicinity of the anodal electrodes is increased, whereas neural activity in the vicinity of the cathodal electrodes is decreased.
Given the apparent lack of temporal and spatial specificity of tDCS, it is surprising that tDCS appears to be able to selectively modulate specific information-processing mechanisms. In other words, the tonic change in the brain that follows the prolonged application of tDCS can have consequences that are highly specific, changing the operation of a single information-processing mechanism, that can operate across a brief 100-millisecond interval. To date, the effective targeting of specific information-processing mechanisms using tDCS has been demonstrated across a wide variety of domains, such as numerical processing [6], visual attention [4], action monitoring [2], perceptual learning [7], and motor skill acquisition [8]. However, the second surprise from the tDCS literature is even more striking. That is, a growing number of studies that have combined tDCS with electrophysiological measurements of brain activity demonstrate that the tonic effects of tDCS can selectively modulate processing during the temporal flow of information processing with high temporal precision.
Recent studies combining tDCS with measurements of electrical brain activity have provided a unique window into the temporal resolution of tDCS manipulations on cognitive functions. For example, tDCS over medial-frontal cortex had selective effects on the electrophysiological responses of the brain to errors (error-related negativity, ERN) and feedback (feedback-related negativity, FRN) during a demanding target discrimination task. However, this stimulation did not change a host of other ERPs indexing mechanisms of perception (P1, N1, N2) and response selection (lateralized-readiness potential or LRP) [2] (Fig. 1B-C). Related work stimulating medial-frontal cortex has shown that during a memory-guided attention task, tDCS modulated two ERP components related to memory storage and covert attention, during two separate 100 ms time windows [4]. However, no other ERP components measured during the 5-second long trials showed any influence of the stimulation. When the stimulation was performed over visual cortex, an early sensory component was affected (i.e., the visual N1 component), but without changing the amplitude of a variety of other sensory, cognitive, and motor-related potentials during this task. That is, by recording electrophysiological activity of the brain researchers have been able to pinpoint the specific neural mechanism modulated by tDCS, and chart its time course and dynamics, separate from mechanisms underlying a variety of other cognitive operations.
This type of highly precise temporal specificity as information processing unfolds is not restricted to studies of humans performing visual tasks following tDCS. Targeting the right cerebellar hemisphere with tDCS, Chen and colleagues [9] found selective and bidirectional changes to a specific ERP known as the mismatch negativity (MMN) that indexes a sensory change-detection mechanism operating between 150-250 ms after the onset of the stimulus. Anodal tDCS increased the amplitude of the somatosensory MMN (Fig. 2A), whereas cathodal stimulation decreased MMN peak amplitude following vibrotactile stimulation of the hand. The selectivity of tDCS to influence the somatosensory MMN was demonstrated by the observation that numerous other ERP components indexing different sensory, perceptual, and cognitive processes were completely unaffected by stimulation (i.e., the N60, P150, N1, P2, and auditory MMN) (Fig. 2A-B). In contrast, anodal tDCS to left prefrontal cortex has been shown to preferentially enhance N1 amplitude in an auditory go/no go discrimination task, without changing responses related to sensory (MMN) or cognitive functions (P3a, P3b) [10].
Figure 2.
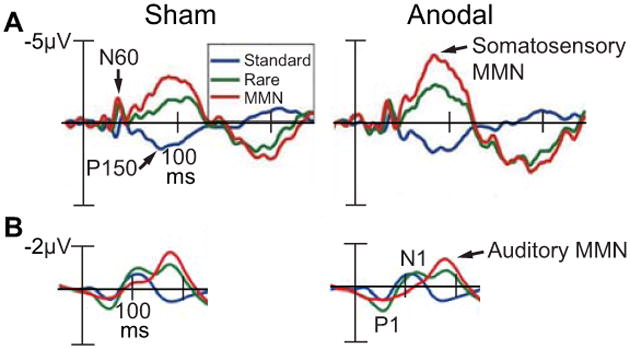
A, Event-related potentials (ERPs) recorded during a vibratory somatosensory discrimination task following 25 min of tDCS over the right cerebellar hemisphere. ERPs elicited from vibratory standard stimuli (blue), rare stimuli (green), and the difference between standard and rare stimuli (i.e., the somatosensory mismatch negativity or MMN, red) shown at the left centrolateral electrode (C3) across sham and anodal conditions. Arrows show the N60, P150, and somatosensory MMN. B, ERPs recorded during an auditory discrimination task following 25 min of tDCS over the right cerebellar hemisphere. ERPs elicited from auditory standard stimuli (blue), rare stimuli (green), and the difference between standard and rare stimuli (i.e., the auditory mismatch negativity or MMN, red) shown at the left centrolateral electrode (C3) across sham and anodal conditions. Arrows show the P1 and N1 elicited from the standard auditory stimulus (blue), and the auditory MMN. Adapted from Chen et al (2014) with permission from The Physiological Society.
Taken together, these electrophysiological studies demonstrate that the causal manipulations of neural activity by conventional tDCS, although spatially diffuse in its application, can nonetheless lead to remarkably precise changes in population-level dynamics measured by whole-brain scalp electrophysiology [see also 11]. More broadly these findings highlight the advantage of using noninvasive stimulation methods in conjunction with electrophysiological measurements to understand the mechanisms of human information processing.
The temporal precision of tDCS effects also informs our understanding of tDCS itself. Specifically, the non-specific tDCS signal applied to the head may serve to nudge the complex system underlying a specific information-processing mechanism into an alternate state or mode of functioning. If this is true, then switching the state of a large-scale system with tDCS might enhance or inhibit the processing of information, resulting in an amplitude or latency shift of an electrophysiological signal indexing the cortical population-level activity of that cognitive subsystem. This view for how tDCS might influence large-scale networks is supported by human research showing that tDCS can enhance a mental process at the expense of another [12], and animal work showing that direct-current stimulation differentially modulates incoming afferent inputs, enhancing some, while inhibiting others [13]. This perspective generates testable predictions for future work. For example, if this model is correct, then bi-stable neuronal networks and so-called gating mechanisms should be especially vulnerable to tDCS.
In summary, accumulating evidence shows that conventional tDCS, despite its poor anatomical specificity, can modulate specific information-processing mechanisms with high temporal resolution. Electroencephalographic (EEG) or magnetoencephalographic (MEG) recordings are revealing the fine-grained functional changes induced by conventional tDCS, allowing researchers to establish causal links between brain activity and behavior, and ultimately explain how neural activity gives rise to perception, cognition, and action.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-EY019882, R01- EY025275, P30-EY08126, F31-MH102042, and T32-EY007135).
Footnotes
Author Information: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annual Review of Biomedial Engineering. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 2.Reinhart RMG, Woodman GF. Causal control of medial-frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. Journal of Neuroscience. 2014;34(12):4214–4227. doi: 10.1523/JNEUROSCI.5421-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Datta A, et al. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation. 2009;2(4):201–7. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinhart RMG, Woodman GF. Enhancing long-term memory with stimulation tunes visual attention in one trial. Proceedings of the National Academy of Sciences of the USA. 2015;112(2):625–630. doi: 10.1073/pnas.1417259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaghi S, et al. Noninvasive brain stimulation with low-intensity electrical currents: putative mechanisms of action for direct and alternating current stimulation. Neuroscientist. 2010;16(3):285–307. doi: 10.1177/1073858409336227. [DOI] [PubMed] [Google Scholar]
- 6.Kadosh RC, et al. Modulating neuronal activity produces specific and long-lasing changes in numerical competence. Current Biology. 2010;20:2016–2020. doi: 10.1016/j.cub.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sehm B, et al. Facilitation of inferior frontal cortex by transcranial direct current stimulation induces perceptual learning of severely degraded speech. Journal of Neuroscience. 2013;33(40):15868–15878. doi: 10.1523/JNEUROSCI.5466-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reis J, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the USA. 2009;106(5):1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JC, et al. Bi-directional modulation of somatosensory mismatch negativity with transcranial direct current stimulation: an event related potential study. Journal of Physiology. 2014;592(Pt 4):745–757. doi: 10.1113/jphysiol.2013.260331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knechtel L, et al. Transcranial direct current stimulation of prefrontal cortex: An auditory event-related potential and proton magnetic resonance spectroscopy study. Neurology, Psychiatry and Brain Research. 2014;20(4):96–101. [Google Scholar]
- 11.Griskova I, et al. Does electroconvulsive therapy (ECT) affect cognitive components of auditory evoked P300? Acta Neurobiologiae Experimentalis. 2005;65:73–77. doi: 10.55782/ane-2005-1541. [DOI] [PubMed] [Google Scholar]
- 12.Iuculano T, Kados RC. The mental cost of cognitive enhancement. Journal of Neuroscience. 2013;33(10):4482–4486. doi: 10.1523/JNEUROSCI.4927-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radman A, et al. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. Journal of Physiology. 2013;591(Pt 10):2563–2578. doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]


