Abstract
Dendritic Cells (DCs) can induce peripheral immune tolerance that prevents autoimmune responses. Antigen presentation by peripheral DCs under steady state conditions leads to a conversion of some peripheral CD4+ T cells into Treg cells that require Homeodomain Only Protein (Hopx) to mediate T cell unresponsiveness. However, the roles of these peripheral (p)Treg cells in averting autoimmune responses as well as immunological mechanisms of Hopx remain unknown. Here we report that Hopx+ pTreg cells converted by DCs from Hopxneg T cells are indispensible to sustain tolerance that prevents autoimmune responses directed at self-antigens during experimental acute encephalomyelitis (EAE). Our studies further reveal that Hopx inhibits intrinsic IL-2 expression in pTreg cells after antigenic re-challenge. In the absence of Hopx, increased levels of IL-2 lead to death and decreased numbers of pTreg cells. Therefore formation of Hopx+ pTreg cells represents a crucial pathway of sustained tolerance induced by peripheral DCs and the maintenance of such pTreg cells and tolerance requires functions of Hopx to block intrinsic IL-2 production in pTreg cells.
Introduction
The task of silencing autoimmune responses mediated by autoreactive T cells is a complex process referred to as immune tolerance that begins in the thymus and continues in the peripheral lymphoid system (1–8). Mechanisms of peripheral tolerance can inactivate antigen-specific T cell responses after an exposure to non-inflammatory forms of antigens introduced as soluble peptides/proteins or as cell-bound material. Therefore peripheral tolerance induced by defined tolerizing neural antigens, including myelin oligodendrocyte glycoprotein (MOG), can prevent a specific autoimmune process such as experimental acute encephalomyelitis (EAE), a model of multiple sclerosis (MS) (9–17). DCs play a central role in peripheral tolerance to prevent autoimmune EAE because DCs present specific antigens and induce tolerogenic responses in T cells. Mechanisms of T cell tolerance mediated by DCs include T cell anergy, deletion, skewing of effector T cell responses, expansion of thymically-derived tTreg cells and also de novo induction of pTreg cells (18–27). However it remains unclear what are the relative roles of these mechanisms as well as their specific molecular pathways in tolerance (20–23, 28). We recently discovered that the transcription cofactor Homeodomain Only Protein (Hopx) is required for Treg cell-mediated immune unresponsiveness induced by DCs but the specific roles of Hopx in the regulation of autoimmune responses remain unknown (29). Here we report that maintenance of antigen-specific peripheral tolerance requires de novo induced Hopx+ pTreg cells that develop from HopxnegFoxp3negCD25neg precursors in response to tolerizing antigens presented by DCs. Our findings show indispensible functions of pTreg cells in antigen-specific peripheral tolerance induced by DCs and they also reveal that by inhibiting intrinsic IL-2 expression in induced pTreg cells Hopx promotes maintenance of pTreg cells and peripheral tolerance.
Materials and Methods
Mice
Hopx−/− mice (30) bred on the C57BL/6 background and also crossed with Foxp3RFP mice (31) were previously described (29). They were also bred with MOG-specific TCR transgenic (2D2 TCR tg) mice (32) to produce both 2D2 TCR tg and non-TCR tg / Hopx−/−Foxp3RFP and Hopx+/+Foxp3RFP mice. IL-2−/− mice (33) were bred with Hopx−/− mice and IL-2+/− heterozygotes were used to produce Hopx−/−IL-2−/− and Hopx−/−IL-2+/+ / 2D2 TCR tg Foxp3RFP and non-TCR tg Foxp3RFP mice. HopxFlag-viral2A-GFP reporter mice (34) that faithfully track Hopx expression were first bred on the C57BL/6 background and then crossed with Foxp3RFP reporter mice (31) and also with 2D2 TCR tg mice (32). Sex and age-matched littermates were used for experiments. Mice were used at 6–8 weeks of age except for IL-2−/− and IL-2+/+ littermate control mice that were used at 4–5 weeks of age as donors for adoptive transfers. All mice were maintained in our facility under specific pathogen free conditions and used in accordance with the guidelines of Saint Louis University’s Institutional Animal Care and Use Committee.
Production of chimeric antibodies
Chimeric antibodies were produced as previously described (28, 35). Briefly, antibodies were expressed in A293 cells by transient transfection using calcium/phosphate method. Cells were grown in serum free DMEM supplemented with Nutridoma SP (Roche) and antibodies were purified on protein-G columns. Chimeric antibodies were injected in PBS intraperitoneally.
Flow cytometry and antibodies used for staining
Anti-CD4 (GK1.5), anti-CD25 (PC-61), anti-Vα3.2 (RR3-16), anti-CD45.2 (104), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-ICOS (C398.4A), anti-PD-1 (29F.1A12) and anti-CD73 (TY/11.8) were from BioLegend. Anti-Foxp3 (FJK-16a) was from eBioscience. Cell sorting and analyses were performed on ARIA III, FACS CALIBUR, LSRII, and CANTO (BD). For detection of apoptosis, FITC Annexin V staining kit and Zombie Violet viability dye were used from BioLegend. For intracellular staining cells were fixed and permeabilized using Fixation/Permeabilization buffers from eBioscience and BD according to manufacturers’ manual.
Adoptive transfers
Lymph nodes and spleen cells from multiple mice were pooled and CD4+ T cells were enriched by depletion of CD8+, B220+, CD11c+, CD11b+ and NK1.1+ cells with magnetic microbeads (Miltenyi) and then Foxp3(RFP)neg/CD25neg or Foxp3(RFP)+ cells were purified by subsequent automated cell sorting performed on ARIA III (BD). Cells were washed 3x with PBS and 1×106 Foxp3+ or 10×106 RFPneg/CD25neg cells or 5×106 2D2 TCR tg RFPneg/CD25neg cells were transferred into mice by intravenous injection into a tail vein. In some experiments cells were labeled with 3 μM CFSE (Sigma) in 5% FCS RPMI at 37 °C for 20 min and washed 3x with PBS and 5×106 cells were injected intravenously per mouse.
EAE Model
To induce EAE mice were injected with 100ug of synthetic Myelin Oligodendrocyte Glycoprotein peptide (MOG35-55, Yale Keck Protein Synthesis Facility) in Complete Freund’s Adjuvant (Difco) subcutaneously in each flank. CFA was enriched with Mycobacterium tuberculosis (10 ml CFA + 40 mg M. Tuberculosis from Difco). Pertussis Toxin (List Biological Laboratories Inc.) was injected 200ng per mouse in PBS intraperitoneally on days 0 and 2 after MOG35-55 injections. Clinical score of EAE was graded on a scale of 1-4 - 0, no clinical signs; 1, flaccid tail; 2, hind limb weakness, abnormal gait; 3, complete hind limb paralysis; 4, complete hind limb paralysis and forelimb weakness or paralysis. Mice were scored daily. Each experimental group was scored in a blinded fashion. Spinal cords were extracted from the spinal columns of experimental mice. The spinal cords were then mashed through 70 μm filters using a 5 ml syringe plunger and prepared for FACS analysis.
Chimeric Antibody and αCD25 Antibody injections
αDEC-MOG or IC-MOG chimeric antibodies in PBS were injected intraperitoneally 15ug per mouse. 250ug per mouse of αCD25 (PC-61.5.3) or rat IgG1 antibodies (BioX cell) were injected in PBS intraperitoneally.
Cell cultures
CD4+ cells were enriched using magnetic microbeads (Miltenyi) and then Foxp3(RFP)neg/CD25neg cells were purified by subsequent automated cell sorting performed on ARIA III (BD). Treg cells were differentiated for 5 days in 96-well plates (Thermo-Fisher) coated with αCD3 (145-2C11) (1 μg/ml), in Click’s media containing 10% FBS, Penicilin-Streptomycin, L-Glutamine, β-Mercaptoethanol (Gibco) and in the presence of soluble αCD28 (37.51) (1.5 μg/ml), recombinant IL-2 (200 units /ml), and TGF-β (4 ng/ml) (all from BioLegend). Foxp3(RFP)+ were then sorted and re-stimulated with PMA (100 ng/ml) for 90 min.
Real-time RT- PCR analysis
RNA was isolated from in vitro cultured iTreg cells using TRIZOL Reagent (Invitrogen) and Qiagen mRNAEasy kit (Qiagen). Total RNA was reverse transcribed and the cDNA was subsequently used for real-time PCR on an ABI Prism instrument using commercial primer-probe sets (Applied Biosystems) for Hypoxanthineguanine phosphoribosyltransferase (HPRT) and IL-2. The results of Q-PCR were standardized to the HPRT expression levels and analyzed by the dd CT method.
Statistical analysis
Mice of particular genotypes were randomly assigned to individual experimental groups. The numbers of groups and mice in each group were determined to achieve statistical significance based on commonly used statistical techniques, two-way and one-way ANOVA and the Student’s t test. All experimental groups and individual mice were included in statistical analysis. Individual P values were calculated using Student’s t-test with Welch’s correction, one-way ANOVA or two-way ANOVA.
Results
Peripherally induced tolerance requires Hopx
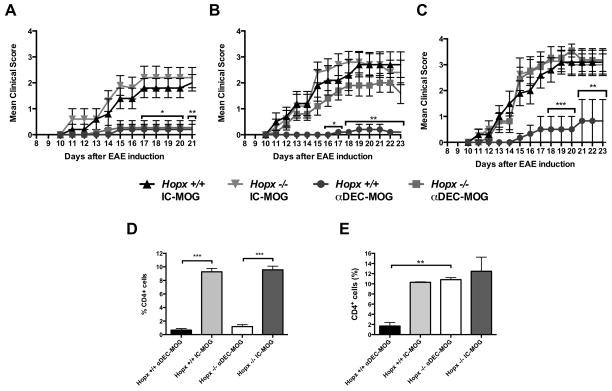
To study peripherally induced tolerance, we used a well-established method to target DCs in vivo with a recombinant chimeric antibody specific for DEC205 (28, 35). MOG is delivered as a fusion protein linked to the C terminus of the heavy chain of a chimeric αDEC-MOG antibody that targets DCs in a process resembling in vivo uptake of self-antigens (21, 28, 35–37). A single treatment of C57Bl/6 mice with αDEC-MOG induces T cell tolerance that protects from autoimmune EAE induced by a subsequent immunization with MOG35-55 peptide (MOG) in Complete Freund’s Adjuvant (CFA) and Pertussis toxin (PT) (21, 28). DCs mediate several distinct pathways of T cell tolerance including a de novo induction of pTreg cells (18–27). Hopx is expressed in Treg cells induced by DCs but the roles of these peripheral (p)Treg cells in averting autoimmune responses as well as immunological mechanisms of Hopx remain unknown (29). To directly examine the role of Hopx in tolerance, we used Hopx−/− mice (30) bred on C57Bl/6 background (29). The induction of T cell tolerance by DCs requires initial T cell proliferation and such T cell activation is independent of Hopx expression in T cells (29, 35, 36). To exclude that the absence of Hopx might affect presentation of tolerogenic antigens by DCs, we transferred CFSE-labeled congenic CD4+ T cells from MOG-specific TCR transgenic (tg) (2D2) mice into Hopx+/+ mice and Hopx−/− mice and measured their proliferation in response to αDEC-MOG-targeted DCs (Supplemental Fig. 1A). We found that a proliferation of the transferred 2D2 T cells in response to MOG-targeted DCs was similar in Hopx+/+ and Hopx−/− mice (Supplemental Fig. 1A). To directly test a requirement for Hopx in tolerance, we induced EAE in Hopx+/+ and Hopx−/− mice either 1 week, 3 weeks or 6 weeks after the initial treatment of these mice with either αDEC-MOG or isotype chimeric antibody control (IC-MOG) (Figure 1A–C). We found that a treatment with αDEC-MOG protected both Hopx+/+ and Hopx−/− mice from EAE that was induced 1 week later (Figure 1A). Further, we found that such a pre-treatment with αDEC-MOG limited a skewing toward Th1 and Th17 effector phenotype similarly in both Hopx+/+ and Hopx−/− T cells (Supplemental Fig. 1B and 1C). We also examined the spinal cords of αDEC-MOG-treated and control mice by flow cytometry to examine CD4+ T cells within the “lymphoid gate” that consists of about 90% live CD45+ cells (Figure 1D) and (Supplemental Fig. 2A and 2B). In contrast to the similar increased numbers of CD4+ T cells in spinal cords of Hopx+/+ or Hopx−/− mice that were not treated with αDEC-MOG and developed EAE, we observed that a treatment with αDEC-MOG prevented such T cell spinal cord infiltration, consistent with the absence of effector T cells (Figure 1D). However, when we delayed an induction of EAE after the initial treatment with αDEC-MOG, we observed in the spinal cords of Hopx−/− mice an infiltration with T cells that was comparable to the T cell infiltration observed in the spinal cords of Hopx+/+ or Hopx−/− mice that were not treated with αDEC-MOG (Figure 1E). Consistent with the presence of encephalitogenic T cells in their spinal cords, such Hopx−/− mice developed symptoms of EAE comparable to either Hopx+/+ or Hopx−/− mice that were not treated with αDEC-MOG (Figure 1B and 1C). In contrast, Hopx+/+ mice remained protected form EAE either 3 weeks (Figure 1B) or 6 weeks (Figure 1C) after the initial treatment of mice with αDEC-MOG. Therefore DCs induced sustained tolerance that requires Hopx to prevent a subsequently triggered autoimmune attack. To confirm that such tolerance induced by DCs is antigen-specific, we used αDEC-OVA that targets unrelated OVA antigen to DCs and we found that treatment with αDEC-OVA did not protect from EAE that was induced by immunization with MOG (Supplemental Fig. 2C).
Figure 1. Hopx is required to sustain peripherally induced tolerance.
(A–C) Multiple groups of Hopx+/+ and Hopx−/− mice were treated with either αDEC-MOG or IC-MOG 1 week (A), 3 weeks (B) or 6 weeks (C) before immunization with MOG35-55 in CFA + PT to induce EAE. Graphs show mean disease scores (n=10–15 per group from 2 experiments). (D) Multiple groups of Hopx+/+ and Hopx−/− mice were treated as in (A). Results show mean percentages of CD4+ T cells in the “lymphoid” gate of spinal cord cell suspensions 21 days after EAE induction (n=3–4 per group from 2 experiments). (E) Multiple groups of Hopx+/+ and Hopx−/− mice were treated as in (C). Results show mean percentages of CD4+ T cells in the “lymphoid” gate of spinal cord cell suspensions 17 days after EAE induction (n=3–4 per group from 2 experiments). Results in (A–E) show mean +/− SEM, * P<0.05, ** P< 0.01 and *** P< 0.001 determined by two-way ANOVA.
Dendritic cells induce Hopx+ pTreg cells and Treg cell-dependent tolerance
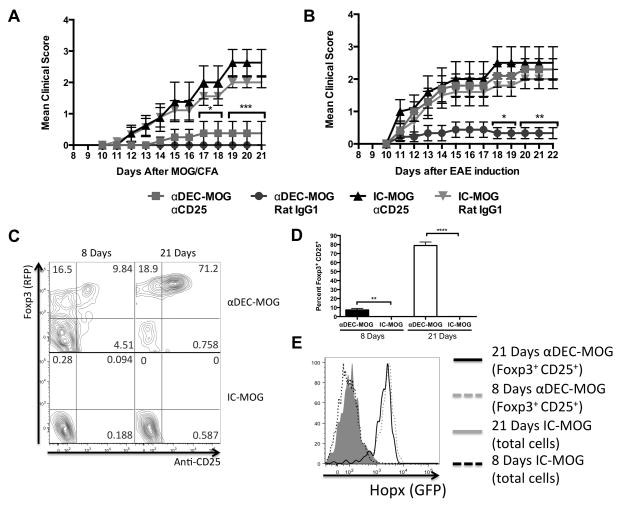
A sustained long-lasting immunological tolerance may require presence and functions of Treg cells (6, 7). Therefore to test the general requirement for Treg cells in such DCs induced tolerance, we used a well-established treatment with αCD25 antibody (PC-61.5.3) to remove Foxp3+CD25+ Treg cells in vivo. A treatment with αCD25 antibody resulted in an about 60% reduction in the numbers of Foxp3+ Treg cells and this reduction lasted for at least 3 weeks (Supplemental Fig. 3A). We treated C57Bl/6 mice with αDEC-MOG or IC-MOG and 5 days later injected αCD25 antibody or an IgG1 isotype control antibody. After another 2 days (or 1 week after chimeric antibody treatment) EAE was induced in these mice (Figure 2A). Alternatively, we treated C57Bl/6 mice with αDEC-MOG or IC-MOG and 7 days later injected αCD25 antibody or an IgG1 isotype control antibody. After another 2 weeks (3 weeks after chimeric antibody treatment) EAE was induced in these mice (Figure 2B). Whereas Treg cell depletion did not affect tolerance to EAE induced 1 week after treatment with αDEC-MOG (Figure 2A), a depletion of Treg cells completely abolished tolerance when EAE was induced 3 weeks after the αDEC-MOG treatment (Figure 2B). To further substantiate these results, we depleted Treg cells 3 weeks after treatment with αDEC-MOG and also found an abolished protection from EAE induced after another 3 weeks (6 weeks after the initial tolerance induction by treatment with αDEC-MOG) (Supplemental Fig. 3B). We conclude that after the initial exposure to MOG, tolerance induced within 1 week by DCs does not rely on Hopx or Treg cells. However, Treg cells are required for tolerance lasting longer than 3 weeks after exposure of DCs to MOG. Therefore DCs induce a sustained, long-lasting tolerance that requires both Treg cells and Hopx.
Figure 2. Dendritic cells induce Hopx+ pTreg cells and Treg cell-dependent long-lasting tolerance.
(A and B) Multiple groups of C57BL/6 mice were treated with αDEC-MOG or IC-MOG. Either (A) 5 days or (B) 1 week after treatment with chimeric antibodies individual groups of mice were injected with either αCD25 or the same dose of rat IgG1. After another (A) 2 days or (B) 2 weeks EAE was induced. Graph shows mean disease scores (n=10 per group from 2 experiments). Results show mean +/− SEM, * P<0.05, ** P< 0.01 and *** P< 0.001 determined by two-way ANOVA. (C–E) GFPneg/RFPneg/CD25neg CD4+ T cells were purified by sorting from 2D2 TCR tg HopxFlag-viral2A-GFP/Foxp3RFP double-reporter mice and then adoptively transferred into CD45.1+ recipient mice. (C) Plots show Foxp3 (RFP) expression (Y-axis) and staining intensity with anti-CD25 (X-axis) of gated populations of adoptively transferred cells at multiple days after treatment with αDEC-MOG as indicated. (D) Graphs show mean percentages of Foxp3+CD25+ pTreg cells among transferred cells as in (C) (n=3–5 per group), ** P<0.01 and **** P< 0.0001 determined by one-way ANOVA with Turkey’s multiple comparisons test. (E) Overlaid histograms show induction of Hopx (GFP) expression from (C) as indicated. The results in C and E represent one of two similar experiments. Results shown in (C–E) are from lymph nodes.
It remains unknown if Hopx-expressing pTreg cells induced by DCs can develop from Hopxneg precursor T cells. To study the expression of Hopx and Foxp3 in T cells upon induction of tolerance by DCs, we produced a HopxFlag-viral2A-GFP/Foxp3IRES-RFP double-reporter mouse (here referred to as HopxGFPFoxp3RFP reporter mice). We sorted GFPneg(Hopxneg)RFPneg(Foxp3neg)CD25neg CD4 cells from 2D2 TCR tg HopxGFPFoxp3RFP mice and transferred such isolated T cells into new recipient mice (Figure 2C–E and Supplemental Fig. 3C). We found that within 21 days after treatment of the recipients with αDEC-MOG, over 70% of T cells responding to MOG in lymph nodes and spleens became Foxp3+CD25+ double positive pTreg cells and such pTreg cells also induced expression of Hopx. In contrast no induction of CD25, Foxp3 or Hopx expression was observed in MOG-specific T cells either 8 or 21 days after treatment with IC-MOG (Figure 2C–E and Supplemental Fig. 3C) Therefore presentation of MOG by DCs to T cells leads to an induction of Hopx+ pTreg cells from precursor T cells.
Hopx+ pTreg cells are indispensible for tolerance
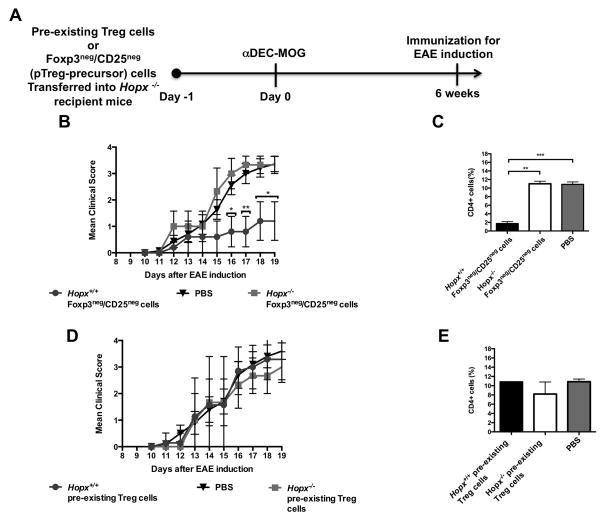
In addition to converting pTreg cells, tolerogenic stimulation by DCs can increase numbers and enhance functions of pre-existing Foxp3+CD25+ tTreg cells and therefore it remains unclear whether the newly induced pTreg cells or the expanded tTreg cells are crucially required to ameliorate symptoms of EAE and MS (17, 19–21, 23, 38, 39). To distinguish between the roles of these different types of Treg cells in tolerance, we attempted to restore DCs-induced and Hopx-dependent tolerance by transferring either pre-existing Foxp3+ Treg cells that include mostly tTreg cells or the Foxp3negCD25neg precursors of pTreg cells (6, 7) into Hopx−/− mice that were treated with αDEC-MOG and then 6 weeks later immunized to induce EAE (Figure 3). In separate experiments, we determined the numbers of such transferred pre-existing tTreg cells and the pTreg cells that were induced from the transferred Foxp3negCD25neg cells 6 weeks after the initial presentation by dendritic cells of MOG delivered by αDEC-MOG. We found about 6 times more remaining pre-existing tTreg cells than the induced pTreg cells (Supplemental Fig. 3D–F). Further, by using either Hopx+/+ or Hopx−/− transferred cells, we also directly tested the role of Hopx in Treg cells to confer tolerance. As expected, in the absence of any cells transferred (PBS only) treatment with αDEC-MOG failed to induce tolerance in Hopx−/− mice. However, we found that mice transferred with Hopx+/+ Foxp3negCD25neg precursors of pTreg cells were protected from EAE. In contrast, mice transferred with Hopx−/− Foxp3negCD25neg cells developed similar symptoms of EAE and similar infiltrations of spinal cords as observed in mice that did not receive precursors of pTreg cell (Figure 3B and 3C). However, a transfer of neither Hopx+/+ nor Hopx−/− tTreg cells prevented symptoms of EAE or blocked T cell infiltration of spinal cords in recipient mice (Figure 3D and 3E). Thus we conclude that tolerance induced by DCs depends on Hopx expression in de novo-induced pTreg cells.
Figure 3. Hopx+ pTreg cells are indispensible for peripherally-induced tolerance.
(A) Schematic outline of an experimental design to restore induced tolerance in Hopx−/− mice. (B) Multiple groups of Hopx−/− mice were transferred with PBS or Foxp3negCD25neg CD4+ cells purified by sorting from either Hopx+/+ or Hopx−/− Foxp3RFP mice as indicated. All mice were treated with αDEC-MOG 6 weeks before EAE induction by immunization with MOG35-55 in CFA + PT. Graph shows mean disease scores (n=6–8 per group from 2 experiments). (C) Multiple groups of Hopx−/− mice each were treated as in (B). Results show mean percentages of CD4+ T cells in the “lymphoid” gate of spinal cord cell suspensions 19 days after EAE induction (n=3–4 per group from two experiments). (D) Multiple groups of Hopx−/− mice were transferred with PBS or Foxp3+ CD4+ cells purified by sorting from either Hopx+/+ or Hopx−/− Foxp3RFP mice as indicated. All mice were treated with αDEC-MOG 6 weeks before EAE induction. Graph shows mean disease scores (n=6–7 per group from 2 experiments). (E) Multiple groups of Hopx−/− mice were treated as in (D). Results show mean percentages of CD4+ T cells in the “lymphoid” gate of spinal cord cell suspensions 19 days after EAE induction (n=2–3 per group from two experiments). Results in (B–E) show mean +/− SEM, * P< 0.05, ** P< 0.01 and *** P< 0.001 determined by one-way or two-way ANOVA.
Hopx is required for maintenance of DCs-induced pTreg cells after antigenic re-challenge under inflammatory conditions
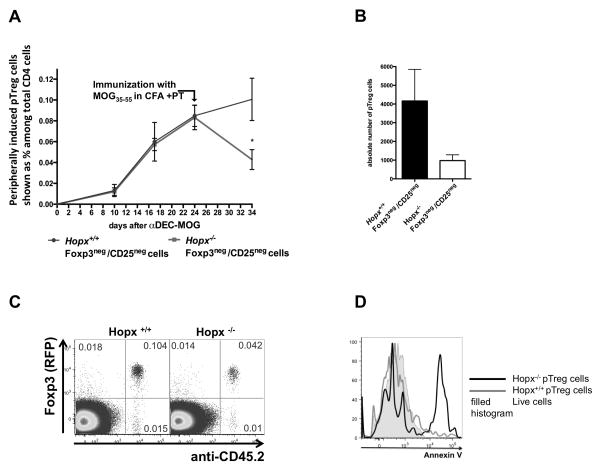
To examine Hopx-dependent responses in pTreg cells, we transferred Foxp3negCD25neg CD4 T cells isolated from either Hopx+/+ or Hopx−/− 2D2 TCR tg Foxp3RFP mice into individual groups of recipient mice that we subsequently treated with αDEC-MOG. We then analyzed expression of Foxp3 and CD25 in the transferred cells (Supplemental Fig. 4A). We found that populations of both Hopx+/+ and Hopx−/− pTreg cells developed similarly after treatment with αDEC-MOG (Supplemental Fig. 4A). Also in agreement with our previous studies (29), we found similar expression of PD-1, ICOS and CD73 in Hopx+/+ and Hopx−/− pTreg cells (Supplemental Fig. 4B). To further examine the fate of such pTreg cell populations, we examined the percentage of the Foxp3+CD25+ cells converted from the transferred Foxp3negCD25neg cells among the total CD4 cells in recipient mice before and after immunization with MOG35-55 in CFA and PT and also determined the absolute numbers of such pTreg cells (Figures 4A and 4B). We found that after a similar initial formation of Hopx+/+ and Hopx−/− pTreg cells, we recovered more than twice as many Hopx+/+ pTreg cells as Hopx−/− pTreg cells after we immunized the recipient mice with MOG (Figure 4A and 4B). To examine a specific down-regulation of Foxp3 expression, we analyzed Foxp3 expression in the remaining Hopx−/− and Hopx+/+ cells (Figure 4C). We observed only negligible numbers of Foxp3neg T cells among Hopx−/− and Hopx+/+ remaining cells, consistent with a lack of down-regulation of Foxp3 and therefore suggesting deletion as a possible mechanism behind the decreased numbers of Hopx−/− pTreg cells (Figure 4C). To confirm an apoptotic death of pTreg cells in the absence of Hopx, we examined binding of Annexin V and found an increased staining with Annexin V of Hopx−/− pTreg cells (Figure 4D). We conclude that maintenance of pTreg cells after antigenic re-challenge depends on Hopx.
Figure 4. Hopx is required for maintenance of pTreg cells after antigenic re-challenge under inflammatory conditions.
(A–D) Foxp3negCD25neg CD4+ T cells were purified by sorting from either Hopx+/+ or Hopx−/− 2D2 TCR tg Foxp3RFP mice and then adoptively transferred into CD45.1+ recipient mice that were then treated with αDEC-MOG. (A) Results show mean percentages of induced Hopx+/+ or Hopx−/− Foxp3+CD25+ pTreg cells among the total CD4+ cells in the recipients at indicated time points before and after immunization with MOG35-55 in CFA + PT (n=5 per group for each time point and type of cells transferred). (B) Absolute numbers of induced Hopx+/+ or Hopx−/− Foxp3+CD25+ pTreg cells after immunization with MOG in CFA + PT as in (A), (n=5 per group). Results in (A and B) show mean +/− SEM, * p<0.05 determined by two-way ANOVA or Student’s T test with Welch’s correction. (C) Plots show Foxp3 (RFP) expression (Y-axis) and staining intensity with anti-CD45.2 (X-axis) among CD4+ cells in mice transferred with either Hopx+/+ or Hopx−/− cells after immunization with MOG in CFA + PT as in (A). The results represent one of three similar experiments. (D) Overlaid histograms show staining intensity with Annexin V in Hopx+/+ or Hopx−/− Foxp3+CD25+ pTreg cells 5 days after immunization with MOG in CFA + PT. The results shown represent one of two similar experiments. Results shown in (A–D) are from lymph nodes and similar results were obtained from spleens.
Hopx mediated inhibition of IL-2 expression is required for maintenance of induced pTreg cells
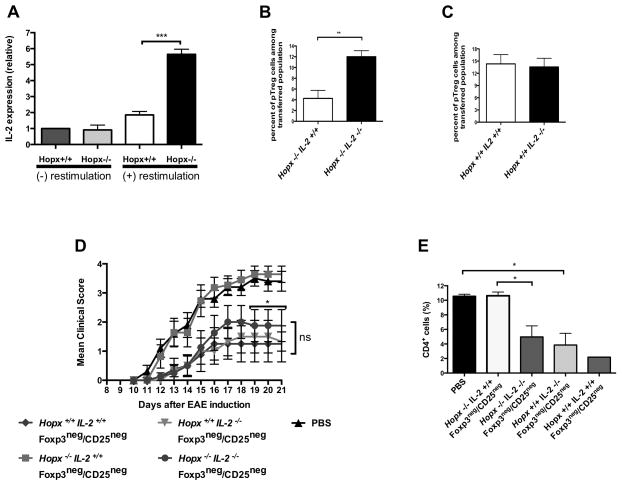
The c-fos/c-jun AP-1 complex induces IL-2 expression in T cells (6, 40, 41). Since Hopx inhibits the expression of c-fos and c-jun in various types of cells including T cells (29, 30, 42), we hypothesized that Hopx could govern IL-2 expression in induced pTreg cells. Since Hopx−/− pTreg cells that express IL-2 are expected to die rapidly in vivo (Figure 4), we examined IL-2 expression by quantitative PCR in Hopx+/+ or Hopx−/− in vitro induced Treg cells after a brief re-stimulation (Figure 5A). We found expression of IL-2 to be about 3 times higher in Hopx−/− than in Hopx+/+ induced Treg cells (Figure 5A). To directly examine the impact of endogenously produced IL-2 on functions of Hopx−/− pTreg cells, we used T cells from IL2−/− mice that we crossed with Hopx+/+ and Hopx−/− mice. IL2−/− Treg cells can develop in the presence of exogenous IL-2 (43). Therefore we first confirmed induction of IL2−/− pTreg cells in a IL-2-sufficient environment by adoptively transferring sorted Hopx−/−IL2+/+ and Hopx−/−IL2−/− Foxp3negCD25neg 2D2 cells into IL2+/+ recipient mice that were then treated with αDEC-MOG. We found that both Hopx−/−IL2+/+ and Hopx−/−IL2−/− Foxp3negCD25neg cells converted to Foxp3+CD25+ pTreg cells at a similar rate (Supplemental Fig. 4C). To examine the impact of antigenic re-challenge on Hopx−/−IL2−/− and Hopx−/−IL2+/+ pTreg cells, we immunized the recipient mice that harbored pre-formed pTreg cells with MOG35-55 in CFA and PT. We recovered about 3 times fewer Hopx−/−IL2+/+ pTreg cells than Hopx−/−IL2−/− pTreg cells (Figure 5B). In contrast, we recovered similar numbers of Hopx+/+IL2+/+ and Hopx+/+IL2−/− pTreg cells under the same conditions in vivo (Figure 5C). We also determined that neither Hopx −/− nor Hopx+/+ IL-2-deficient pTreg cells underwent apoptotic cell death following antigenic re-challenge under pro-inflammatory conditions (Supplemental Fig. 4D). Therefore an increased expression of IL-2 in Hopx−/− pTreg cells adversely affects their maintenance after antigenic re-challenge. To examine directly the impact of such increased expression of IL-2 on tolerance, we followed the experimental design introduced in Figure 3. We transferred multiple groups of Hopx−/− mice with Hopx+/+IL2+/+, Hopx+/+IL2−/−, Hopx−/−IL2+/+ or Hopx−/−IL2−/− Foxp3negCD25neg cells or PBS only. We treated all the recipients with αDEC-MOG and after another 6 weeks immunized them to induce EAE (Figure 5D and 5E). As expected, all Hopx-sufficient Foxp3negCD25neg cells restored tolerance, whereas Hopx−/−IL2+/+ Foxp3negCD25neg cells failed to restore tolerance and prevent symptoms of EAE. In contrast, Hopx−/−IL2−/− Foxp3negCD25neg cells prevented EAE symptoms and T cell infiltration of spinal cords to a similar extent that we observed with the Hopx-sufficient Foxp3negCD25neg cells (Figure 5D and 5E). Thus a genetic deletion of IL-2 restores defective tolerance in the absence of Hopx. We conclude that Hopx blocks intrinsically expressed IL-2 from disrupting pTreg cell-dependent maintenance of long-term tolerance.
Figure 5. Hopx mediated inhibition of IL-2 expression is required for maintenance of pTreg cells and tolerance.
(A) IL-2 transcripts from Hopx+/+ or Hopx−/− Treg cells induced in vitro and then either re-stimulated or not re-stimulated were analyzed by real-time PCR. The results were normalized for expression of Hprt and standardized by the dd CT method to expression of IL-2 in not re-stimulated Hopx+/+ iTreg cells. Error bars represent SD, ***P ≤ 0.001 determined by one-way ANOVA. Results represent one of two similar experiments. (B) Foxp3negCD25neg CD4+ T cells were purified by sorting from either Hopx−/− IL-2+/+ or Hopx−/− IL-2−/− /2D2 TCR tg Foxp3RFP mice and then adoptively transferred into CD45.1+ recipient mice. Graphs show percentages of induced Foxp3+CD25+ pTregs cells among adoptively transferred cells in the indicated groups of mice after treatment with αDEC-MOG and then immunization with MOG35-55 in CFA + PT (n=2–4 per group from two experiments). (C) Foxp3negCD25neg CD4+ T cells were purified by sorting from either Hopx+/+ IL-2+/+ or Hopx+/+ IL-2−/− /2D2 TCR tg Foxp3RFP mice and then adoptively transferred into CD45.1+ recipient mice that were then treated as in (B) (n=3–4 per group from two experiments). Results shown in (B and C) are from lymph nodes and similar results were obtained from spleens. (D) Multiple groups of Hopx−/− mice were transferred with PBS or Foxp3negCD25neg cells purified by sorting from Hopx+/+ IL-2+/+, Hopx−/− IL-2+/+, Hopx−/− IL-2−/− or Hopx+/+ IL-2−/− Foxp3RFP mice as indicated. All mice were treated with αDEC-MOG 6 weeks before induction of EAE by immunization with MOG35-55 in CFA + PT. Graph shows mean disease scores (n=6–11 per group from 2 experiments). (E) Multiple groups of Hopx−/− mice were treated as in (D). Results show mean percentages of CD4+ T cells in the “lymphoid” gate of spinal cord cell suspensions 21 days after EAE induction (n=2–3 per group from 2 experiments). All results show mean +/− SEM, * P< 0.05 and ** P< 0.01 determined by one-way or two-way ANOVA.
Discussion
We propose that under steady state tolerogenic conditions DCs confer long-lasting tolerance that is sustained by peripherally-induced pTreg cells whose maintenance depends on functions of Hopx to inhibit intrinsically produced IL-2. Tolerogenic presentation by peripheral DCs of antigens, such as peptides from neural proteins, either expressed in DCs or targeted to these antigen presenting cells in vivo can inactivate T cell responses by several mechanisms including T cell anergy, skewing of effector T cell responses and induction of Treg cell functions preventing a subsequent induction of EAE (1, 3–8, 20–24, 28, 36). However, it has remained unknown how these DCs-dependent mechanisms of tolerance are orchestrated and maintained.
A division of labor between various types of regulatory T cells results in different contributions of either expanded pre-existing tTreg cells or de novo induced pTreg cells to the prevention of general auto-inflammatory responses, maternal-fetal conflict and mucosal tolerance in the airways and gut (44–47). However, Treg cells that accumulate during pro-inflammatory processes such as EAE, a model of multiple sclerosis (MS), are inefficient in controlling autoimmunity (48, 49). Such Treg cells appear to originate from expanded pre-existing Foxp3+ Treg cells that then may fail to maintain their regulatory phenotype (49, 50). Our present results now establish that sustained antigen-specific tolerance induced by DCs specifically relies on functions of de novo induced, Hopx-expressing pTreg cells.
Hopx is an evolutionarily conserved, homedomain-containing, small transcription cofactor expressed in stem cells, tumor cells, myocytes and various lymphocytes (29, 30, 42, 51–60). Expression of Hopx in effector-memory T cells has been linked with their increased survival, consistent with versatile and context-dependent functions of Hopx (60). However, Hopx is not required for the functions of encephalitogenic effector T cells and Hopx−/− mice succumb to EAE at a similar rate and severity as Hopx-sufficient mice. Further, Hopx is dispensable for the initially developing tolerance shortly after an exposure of DCs to MOG. Instead, such Hopx-independent and Treg cell-independent tolerance appears to rely on anergic cell-intrinsic mechanisms. Within 3 weeks such initially-tolerized T cells develop into pTreg cells that sustain tolerance long-term. However, only Hopx+/+ pTreg cells can survive the antigenic re-challenge and then prevent subsequent infiltration of CNS by the newly-activated encephalitogenic T cells. Therefore Hopx−/− mice fail to sustain antigen-induced tolerance long-term. Consistent with the specific Hopx-dependent mechanisms in pTreg cells, only Hopx+/+ pTreg cells can restore the defective tolerance in Hopx−/− mice. Hopx is also expressed in thymically-derived tTreg cells but functions of Hopx in these T cells remain unknown. Since tTreg cells are crucial for the maintenance of general immune homeostasis, future studies would elucidate any role of such tTreg cells in the Hopx-dependent functions of pTreg cells. Overall, our results establish a crucial role for Hopx expressing-pTreg cells in the maintenance of antigen-specific peripheral tolerance induced by DCs.
Although IL-2-mediated signals are essential for Treg cell development, Treg cells block their own expression of IL-2 through multiple mechanisms including Foxp3 and Helios-dependent pathways and such intrinsic inhibition of IL-2 production in Treg cells may be crucial for some suppressor functions of these cells (6, 61, 62). For example, a capture by Treg cells of IL-2 made by effector cells may enhance immune regulation by depriving such effector T cells of IL-2 in addition to other mechanisms including blocking of IL-2 production in effector T cells (41, 63, 64). Since Treg cells rely on extracellular sources of IL-2 for their proliferation and survival, a treatment with recombinant IL-2 promotes proliferation and functions of regulatory T cells (6, 41, 49, 65–71). However, the increased IL-2 concentrations in vivo also lead to a disappearance of tTreg cell populations following their initial expansion (67).
The c-fos/c-jun AP-1 complex induces IL-2 expression in T cells (6, 40, 41). We now established that consistent with its inhibition of the expression of c-fos and c-jun, Hopx governs IL-2 expression in induced pTreg cells. We further established that an increased production of IL-2 caused by the absence of Hopx is deleterious for pTreg cell maintenance and functions and genetic deletion of IL-2 restores numbers and functions in tolerance of Hopx−/− pTreg cells. Therefore we propose that Hopx-dependent inhibition of IL-2 expression in pTreg cells is necessary for their maintenance and tolerance.
In conclusion, our results define how DCs orchestrate sustained tolerance to transiently available neural antigens by inducing antigen-specific pTreg cells. Further, by establishing the role for Hopx as an indispensible regulator of pTreg cell and tolerance maintenance in vivo, our results could provide the foundation for more selective and efficient immune therapies for specific immune disorders.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by grants from the National Multiple Sclerosis Society (RG5019A) and National Institute Of Allergy and Infectious Diseases of the National Institutes of Health (R01AI113903) (both to D. Hawiger). Generation of the original HopxFlag-viral2A-GFP reporter mice was supported by R01 HL071546 (to J.A. Epstein).
The authors would like to thank Dr. Eric Olson for sharing Hopx−/− mice, Joy Eslick and Sherri L. Koehm for expert help with flow cytometry.
Footnotes
This publication is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
References
- 1.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 2.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 4.von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- 5.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 6.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 8.Bollrath J, Powrie FM. Controlling the frontier: Regulatory T-cells and intestinal homeostasis. Semin Immunol. 2013;25:352–357. doi: 10.1016/j.smim.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J Exp Med. 1979;149:758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Critchfield JM, Racke MK, Zuniga-Pflucker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 11.Smilek DE, Gautam AM, Pearson C, Steinman L, McDevitt HO. EAE: a model for immune intervention with synthetic peptides. Int Rev Immunol. 1992;9:223–230. doi: 10.3109/08830189209061792. [DOI] [PubMed] [Google Scholar]
- 12.Whitham RH, Kotzin BL, Buenafe AC, Weinberg AD, Jones RE, Hashim GA, Hoy CM, Vandenbark AA, Offner H. Treatment of relapsing experimental autoimmune encephalomyelitis with T cell receptor peptides. J Neurosci Res. 1993;35:115–128. doi: 10.1002/jnr.490350202. [DOI] [PubMed] [Google Scholar]
- 13.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 14.Wasserman HA, Evavold BD. Induction of anergy by antibody blockade of TCR in myelin oligodendrocyte glycoprotein-specific cells. J Immunol. 2008;180:7259–7264. doi: 10.4049/jimmunol.180.11.7259. [DOI] [PubMed] [Google Scholar]
- 15.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, King NJ, Miller SD. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011;187:2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Podojil JR, Chang J, Luo X, Miller SD. TGF-beta-induced myelin peptide-specific regulatory T cells mediate antigen-specific suppression of induction of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:6629–6636. doi: 10.4049/jimmunol.0904044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJ, Shea LD, Miller SD. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nature Immunology. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 20.Yogev N, Frommer F, Lukas D, Kautz-Neu K, Karram K, Ielo D, von Stebut E, Probst HC, van den Broek M, Riethmacher D, Birnberg T, Blank T, Reizis B, Korn T, Wiendl H, Jung S, Prinz M, Kurschus FC, Waisman A. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity. 2012;37:264–275. doi: 10.1016/j.immuni.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. 2013;123:844–854. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern JN, Keskin DB, Kato Z, Waldner H, Schallenberg S, Anderson A, von Boehmer H, Kretschmer K, Strominger JL. Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17280–17285. doi: 10.1073/pnas.1010263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K. Targeting of autoantigens to DEC205(+) dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol. 2013;191:2938–2947. doi: 10.4049/jimmunol.1202592. [DOI] [PubMed] [Google Scholar]
- 24.Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, Kuchroo VK, Robson SC, Quintana FJ. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14:1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Hawiger D, Wan YY, Eynon EE, Flavell RA. The transcription cofactor Hopx is required for regulatory T cell function in dendritic cell-mediated peripheral T cell unresponsiveness. Nat Immunol. 2010;11:962–968. doi: 10.1038/ni.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin CH, Liu ZP, Passier R, Zhang CL, Wang DZ, Harris TM, Yamagishi H, Richardson JA, Childs G, Olson EN. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell. 2002;110:725–735. doi: 10.1016/s0092-8674(02)00933-9. [DOI] [PubMed] [Google Scholar]
- 31.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 34.Takeda N, Jain R, Leboeuf MR, Padmanabhan A, Wang Q, Li L, Lu MM, Millar SE, Epstein JA. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development. 2013;140:1655–1664. doi: 10.1242/dev.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 37.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient Targeting of Protein Antigen to the Dendritic Cell Receptor DEC-205 in the Steady State Leads to Antigen Presentation on Major Histocompatibility Complex Class I Products and Peripheral CD8(+) T Cell Tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 41.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen F, Kook H, Milewski R, Gitler AD, Lu MM, Li J, Nazarian R, Schnepp R, Jen K, Biben C, Runke G, Mackay JP, Novotny J, Schwartz RJ, Harvey RP, Mullins MC, Epstein JA. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002;110:713–723. doi: 10.1016/s0092-8674(02)00932-7. [DOI] [PubMed] [Google Scholar]
- 43.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 44.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation.[see comment] Nature Medicine. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA. Self-antigen-Driven Activation Induces Instability of Regulatory T Cells during an Inflammatory Autoimmune Response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kee HJ, Kim JR, Nam KI, Park HY, Shin S, Kim JC, Shimono Y, Takahashi M, Jeong MH, Kim N, Kim KK, Kook H. Enhancer of polycomb1, a novel homeodomain only protein-binding partner, induces skeletal muscle differentiation. Journal of Biological Chemistry. 2007;282:7700–7709. doi: 10.1074/jbc.M611198200. [DOI] [PubMed] [Google Scholar]
- 52.Hamamori Y, Schneider MD. HATs off to Hop: recruitment of a class I histone deacetylase incriminates a novel transcriptional pathway that opposes cardiac hypertrophy.[comment] Journal of Clinical Investigation. 2003;112:824–826. doi: 10.1172/JCI19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kook H, Lepore JJ, Gitler AD, Lu MM, Wing-Man Yung W, Mackay J, Zhou R, Ferrari V, Gruber P, Epstein JA. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop.[see comment] Journal of Clinical Investigation. 2003;112:863–871. doi: 10.1172/JCI19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kook H, Yung WW, Simpson RJ, Kee HJ, Shin S, Lowry JA, Loughlin FE, Yin Z, Epstein JA, Mackay JP. Analysis of the structure and function of the transcriptional coregulator HOP. Biochemistry. 2006;45:10584–10590. doi: 10.1021/bi060641s. [DOI] [PubMed] [Google Scholar]
- 55.Yin Z, Gonzales L, Kolla V, Rath N, Zhang Y, Lu MM, Kimura S, Ballard PL, Beers MF, Epstein JA, Morrisey EE. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2006;291:L191–199. doi: 10.1152/ajplung.00385.2005. [DOI] [PubMed] [Google Scholar]
- 56.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katoh H, Yamashita K, Waraya M, Margalit O, Ooki A, Tamaki H, Sakagami H, Kokubo K, Sidransky D, Watanabe M. Epigenetic silencing of HOPX promotes cancer progression in colorectal cancer. Neoplasia. 2012;14:559–571. doi: 10.1593/neo.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheung WK, Zhao M, Liu Z, Stevens LE, Cao PD, Fang JE, Westbrook TF, Nguyen DX. Control of Alveolar Differentiation by the Lineage Transcription Factors GATA6 and HOPX Inhibits Lung Adenocarcinoma Metastasis. Cancer Cell. 2013;23:725–738. doi: 10.1016/j.ccr.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe H, Meyerson M. Hopping between differentiation states in lung adenocarcinoma. Cancer Cell. 2013;23:707–709. doi: 10.1016/j.ccr.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albrecht I, Niesner U, Janke M, Menning A, Loddenkemper C, Kuhl AA, Lepenies I, Lexberg MH, Westendorf K, Hradilkova K, Grun J, Hamann A, Epstein JA, Chang HD, Tokoyoda K, Radbruch A. Persistence of effector memory Th1 cells is regulated by Hopx. Eur J Immunol. 2010;40:2993–3006. doi: 10.1002/eji.201040936. [DOI] [PubMed] [Google Scholar]
- 61.Yamaguchi T, Kishi A, Osaki M, Morikawa H, Prieto-Martin P, Wing K, Saito T, Sakaguchi S. Construction of self-recognizing regulatory T cells from conventional T cells by controlling CTLA-4 and IL-2 expression. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2116–2125. doi: 10.1073/pnas.1307185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baine I, Basu S, Ames R, Sellers RS, Macian F. Helios induces epigenetic silencing of IL2 gene expression in regulatory T cells. J Immunol. 2013;190:1008–1016. doi: 10.4049/jimmunol.1200792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 66.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grigorian A, Mkhikian H, Demetriou M. Interleukin-2, Interleukin-7, T cell-mediated autoimmunity, and N-glycosylation. Ann N Y Acad Sci. 2012;1253:49–57. doi: 10.1111/j.1749-6632.2011.06391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim MG, Koo TY, Yan JJ, Lee E, Han KH, Jeong JC, Ro H, Kim BS, Jo SK, Oh KH, Surh CD, Ahn C, Yang J. IL-2/Anti-IL-2 Complex Attenuates Renal Ischemia-Reperfusion Injury through Expansion of Regulatory T Cells. J Am Soc Nephrol. 2013;24:1529–1536. doi: 10.1681/ASN.2012080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, Humblet-Baron S, Schonefeldt S, Herold MJ, Hildeman D, Strasser A, Bouillet P, Lu LF, Matthys P, Freitas AA, Luther RJ, Weaver CT, Dooley J, Gray DH, Liston A. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, Procaccini C, La Cava A, Matarese G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014;20:69–74. doi: 10.1038/nm.3411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.