Abstract
Symptoms of diabetic gastrointestinal dysmotility indicate neuropathy of the enteric nervous system. Long-standing diabetic enteric neuropathy has not been fully characterized, however. We used prolonged high fat diet ingestion (20 weeks) in a mouse model to mimic human obese and type 2 diabetic conditions, and analyzed changes seen in neurons of the duodenal myenteric plexus. Ganglionic and neuronal size, number of neurons per ganglionic area, density indices of neuronal phenotypes (immunoreactive nerve cell bodies and varicosities per ganglion or tissue area) and nerve injury were measured. Findings were compared with results previously seen in mice fed the same diet for 8 weeks. Compared to mice fed standard chow, those on a prolonged high fat diet had smaller ganglionic and cell soma areas. Myenteric VIP- and ChAT-immunoreactive density indices were also reduced. Myenteric nerve fibers were markedly swollen and cytoskeletal protein networks were disrupted. The number of nNOS nerve cell bodies per ganglia was increased, contrary to the reduction previously seen after 8 weeks, but the density index of nNOS varicosities was reduced. Mice fed high fat and standard chow diets experienced an age-related reduction in total neurons, biasing towards neurons of sensory phenotype. Meanwhile ageing was associated with an increase in excitatory neuronal markers. Collectively, these results support a notion that nerve damage underlies diabetic symptoms of dysmotility, and reveals adaptive ENS responses to the prolonged ingestion of a high fat diet. This highlights a need to mechanistically study long-term diet-induced nerve damage and age-related impacts on the ENS.
Keywords: Intestine, diabetes, neuropathy, neurodegeneration, ageing
Introduction
The prevalence of type 2 diabetes (T2D) and obesity is increasing at an excessive rate worldwide (Smyth and Heron 2006). As frequencies continue to rise, so do the populations of individuals with chronic states of disease (Amos et al. 1997). Those with persistent T2D experience a host of secondary complications, including autonomic neuropathy, which affects many organs in the body (Wang et al. 2008; Brock et al. 2013). Injuries to the enteric nervous system (ENS) manifest through symptoms of gastropathy (dyspepsia, gastroparesis) and gastrointestinal (GI) dysmotility, and these commonly plague T2D patients (Camilleri and Malagelada 1984; Byrtzer et al. 2001; Bagyánszki and Bódi 2012; Yarandi and Srinivasan 2014). Diabetic neuropathy can be caused by hyperglycemia, dyslipidemia, microangiopathy, oxidative stress, abnormal signaling from advanced glycation end products and growth factor deficiencies (Jack and Wright 2012; Yarandi and Srinivasan 2014). The neurons of the ENS are anatomically exposed and susceptible to the influence of these factors (Bagyánszki and Bódi 2012; Yarandi and Srinivasan 2014).
The myenteric plexus controls motility reflexes, including those initiated in the duodenum and stomach, that cause stomach emptying and small bowel transit (Kunze and Furness 1999; Furness 2008). The sensory neurons, interneurons, excitatory and inhibitory motor neurons that compose the myenteric plexus can be defined based on a ‘chemical code’ of neural proteins and transmitters (Sang and Young 1996; Sang et al. 1997; Sang and Young 1998; Qu et al. 2008; Furness 2010; Tan et al. 2010). Morphological, ultrastructural and phenotypic changes of duodenal myenteric plexus neurons during prolonged T2D are not well characterized. This knowledge is fundamental to designing mechanistic studies that elucidate underlying causes and pathophysiology of GI symptoms during diabetes.
Previously, studies of the duodenum ENS in leptin receptor knockout T2D mice have shown a reduction in vasoactive intestinal peptide (VIP) and nitric oxide synthase (nNOS) neurons and expression levels (Spangeus and El-Salhy 2001; Surendran and Kondapaka 2005). Studies by our group have also shown a reduction in total duodenal myenteric neurons, including nNOS/VIP containing neurons, in mice fed a 72% high-fat (HF) diet for 8 weeks (Stenkamp-Strahm et al. 2013a). It is well understood that the ENS undergoes a loss of cells in the duodenum and other segments during normal aging (El-Salhy et al. 1999; Wade 2002; Stenkamp-Strahm et al. 2013b). An analysis of ENS changes in long-standing T2D and parallel age-related changes in animals have yet to be done, however, and will be especially useful to understand GI symptoms in an aging population of human diabetics.
The goal of the present study was to analyze prolonged HF diet ingestion and age-related effects on the packing density (neurons/ganglionic area), neuronal phenotype and nerve injury of cells in the duodenum myenteric plexus of obese T2D mice. The investigation of duodenal neuropathy is critical, as the duodenum is likely affected during the common symptom of gastroparesis. Results show that mice ingesting a HF diet for 20 weeks have a remodeling of this plexus, including ganglionic shrinkage and a reduction in mean neuronal soma sizes. Additional changes include a reduction in VIP immunoreactive (-IR) nerve cell bodies and varicosities, axonal swelling, and cytoskeletal damage. The number of nNOS neurons, originally found to be reduced in mice fed a HF diet for 8 weeks, was found to be increased in mice fed a HF diet for 20 weeks. Interestingly, density indices of nNOS-IR cell bodies plus IR varicosities were reduced during prolonged HF diet ingestion. Age-related changes revealed a reduction in sensory and an increase in excitatory neuron markers. Collectively, our results revealed the nature of duodenal myenteric plexus remodeling in response to HF dietary influence and normal aging. It is possible that alterations and damage revealed herein underlie the GI symptoms experienced by human T2D patients.
Materials and Methods
Mice
C57BL/6J mice used in this study were purchased from Jackson Laboratories (Bar Harbor, ME) at 7 weeks of age and housed in metal cages. Mice were kept on a 12:12 hour light cycle and acclimatized for one week before being fed ad libitum either a standard chow (SC) diet containing 6.2% fat (n = 8; Teklad Global 2018, Teklad Diets, Madison WI) or a high fat (HF) diet with 72% fat (n = 8; modified DIO 70% kcal fat diet with 2% additional corn oil, TestDiet, Richmond, IN). Mice were anesthetized with isofluorane before exsanguination according to procedures approved by the University of Idaho Animal Care and Use Committee.
Assessment of obesity and T2D
HF diet-ingesting mice in the current study were previously shown to have high weight gain, epididymal adipose tissue mass, glucose intolerance and insulin resistance compared to mice ingesting a SC diet (Stenkamp-Strahm et al. 2013a; Stenkamp-Strahm et al. 2013b). Obesity and T2D characteristics were first identified in these mice after 4 weeks of HF diet ingestion, became more progressed at 8 weeks, and markedly progressed after 20 weeks. To correlate nerve cell injuries with symptoms of advanced stage obesity and T2D, mice analyzed in the current study were those having ingested a HF diet for 20 weeks.
Duodenal tissue sampling for scanning transmission electron microscopy (EM), longitudinal muscle-myenteric plexus (LMMP), and cryostat section preparations
Collection of duodenal segments, fixation, dissection, preservation, EM, LMMP, and cryostat tissue sample (full thickness; FT) preparations were processed as previously described (Stenkamp-Strahm et al. 2013a).
Immunohistochemistry of LMMP and FT preparations
The ‘chemical coding’ hypothesis postulates that each class of neuron that can be differentiated functionally contains a unique combination of chemical markers (Sang and Young 1996; Sang and Young 1998; Furness 2010), and this paradigm has been employed to identify and quantify neurons of different phenotype in the mouse ENS (Sang and Young 1996; Sang et al. 1997; Sang and Young 1998; Qu et al. 2008; Stenkamp-Strahm et al. 2013a). The aim of the current study was to determine the effect of prolonged HF diet consumption on total, sensory, excitatory motor and inhibitory motor neurons in the mouse duodenum. This was achieved using chemical coding with neuronal marker antibodies described in Table 1. An antibody against HuC/HuD was used for total neuron assessment. Two antibodies were used for each of the three sub-classes of neurons, respectively: inhibitory neurons (nitric oxide synthase [nNOS] and vasoactive intestinal peptide [VIP]), excitatory neurons (choline acetyltransferase [ChAT] and substance P), and sensory neurons (calbindin and calcitonin gene-related peptide [CGRP]). HuC/HuD, nNOS, ChAT, substance P and calbindin were used to stain and analyze the myenteric plexus in LMMP preparations while VIP, CGRP and calbindin were used to stain and analyze the myenteric plexus and the muscularis externa of FT preparations. Reasoning for the use of FT cryostat sections and/or LMMP preparations for quantification of a given marker has been described previously, as well as the immunohistochemistry staining procedures (Stenkamp-Strahm et al. 2013a). All dilution information for primary antibodies, secondary antibodies and dyes can be found in Table 1.
Table 1.
Antibody manufacturer and dilutions used for immunohistochemistry
| Primary Antibody, Dilution | Secondary Antibody, Dilution | Dye, Dilution |
|---|---|---|
| All Neurons | ||
| Ms monoclonal HuC/HuD, 1:200 Molecular Probes, Eugene, OR A21271 |
Dk anti Ms IgG Dylight 488, 1:300 Jackson mmunoResearch, West Grove, PA 715-485-150 |
N/A |
| Inhibitory Motor Neurons | ||
| Rb polyclonal VIP, 1:1000 Abcam, Cambridge, MA Ab43841 |
Biotinylated Gt anti Rb IgG, 1:300 Vector Labs, Burlingame, CA BA1000 |
Streptavidin Alexa Fluor 488 conjugate, 1:600; Jackson ImmunoResearch, West Grove, PA 016-540-084 |
| Rb polyclonal nNOS, 1:500 Invitrogen, Camarillo, CA #61-7000 |
Biotinylated Gt anti Rb IgG, 1:300 Vector Labs, Burlingame, CA BA1000 |
Streptavidin Cy3 conjugate, 1:600 Life Technologies, Grand Island, NY SA1010 |
| Sheep polyclonal nNOS, 1:500 Abcam, Cambridge, MA Ab6175 |
Dk anti Sheep IgG Dylight 594, 1:300 Jackson ImmunoResearch, West Grove, PA 715-485-150 |
N/A |
| Excitatory Motor Neurons | ||
| Gt polyclonal ChAT, 1:200 Millipore, Billerica, MA AB144P |
Biotinylated Rb anti Gt IgG, 1:300 Vector Labs, Burlingame, CA BA5000 |
N/A |
| Rt monoclonal Substance P, 1:150 Millipore, Billerica, MA MAB356 |
Biotinylated Gt anti Rt, 1:300 Invitrogen, Camarillo, CA #62–9540 |
Streptavidin Cy3 conjugate, 1:600 Life Technologies, Grand Island, NY SA1010 |
| Sensory Neurons | ||
| Rabbit polyclonal anti-Calbindin D-28k, 1:1250 CH-1723, Marly 1 (Switzerland) CB 38 |
Biotinylated Gt anti Rb IgG, 1:300 Vector Labs, Burlingame, CA BA1000 |
Streptavidin Cy3 conjugate, 1:600 Life Technologies, Grand Island, NY SA1010 |
| Rb polyclonal CGRP, 1:200 Sigma Aldrich, St. Louis, MO # c8198 |
Biotinylated Gt anti Rb IgG, 1:300 Vector Labs, Burlingame, CA BA1000 |
Streptavidin Cy3 conjugate, 1:600 Life Technologies, Grand Island, NY SA1010 |
| Apoptosis | ||
| Rb polyclonal anti-cleaved Caspase-3, Cell Signaling Technology (ASP 175) |
Dk anti rabbit IgG Dylight 488, 1:300 | N/A |
Ms: Mouse, Dk: Donkey, Rb: Rabbit, Gt: Goat, Rt: Rat
To ensure the specificity of staining, co-localization of nucleated neuronal cells was confirmed through DAPI counterstaining, and primary and secondary antibody negative control staining was performed for all antibodies used.
Quantification of number of neurons and density indices in LMMP
The quantification procedures of immuno-stained neurons in the myenteric ganglia of mouse duodenum has been described previously (Stenkamp-Strahm et al. 2013a). In brief, Metamorph 2 software (Molecular Devices, 163 Sunnyvale, CA) was used to analyze images of myenteric ganglia photographed using a Nikon Eclipse Fluorescent microscope with a 20x objective lens (Nikon, Melville, NY). For each mouse duodenum, one LMMP preparation was used to measure ganglionic sizes and quantify specific neuronal phenotype numbers or density indices. In each LMMP, about 25–35 fields of view were systematically randomly selected and photographed. Areas of all ganglia in photographed fields were measured by tracing boundaries. Delineation of these boundaries was achieved by tracing around stained cell somas (HuC/HuD or stained nerve cells and nerve strands (nNOS, VIP, ChAT, substance P, and calbindin). In nerve strands, boundaries of ganglia were determined as locations where the ganglionic width was less than the width of two stained neurons. Neurons were then counted within ganglia and data were recorded as a ‘packing density’ (HuC/HuD, calbindin, nNOS neurons per ganglionic area). To measure density indices in cases where cells were not countable, areas of ganglia in each photographed field of view were again measured by tracing boundaries. The image was manually thresholded in a blinded fashion after defining a cutoff between specific and nonspecific background staining. This allowed the software to detect only stained nerve cells and fibers. The areas of immunoreactive nerve cell bodies and varicosities in individual ganglia were gathered. ‘Density indices’ (IR area/ganglionic area) were then computed. These density indices are hereafter indicated as ‘density indices of nerve cell bodies plus varicosities’, to distinguish between results taken from LMMP and full thickness preparations.
Large numbers (n > 50 per mouse) of randomly imaged HuC/HuD stained ganglia were used to determine average ganglionic size of SC and HF diet mice. Areas of all ganglia within an image were measured, and mean ganglionic sizes were gathered. Systematic uniform random sampling was used to estimate the sizes of neuron cell bodies. Briefly, individual images were overlaid with a numbered sampling frame, and random numbers were generated to select 2 separate frames for unbiased cell soma size measurement. A tracing tool was used to measure the area of cell somas and mean cell sizes were computed. The number of neuron cell bodies measured was roughly 100–150 for each mouse.
For qualitative demonstration of staining in LMMP preparations, an Olympus Fluoview confocal multiphoton microscope with 60x oil objective and FluoView ASW acquisition software was used to acquire images of HuC/HuD, nNOS, VIP, ChAT, substance P, and calbindin stained myenteric ganglia.
Quantitative analysis of nerve fibers and cells in FT sections
Methods of cryostat (FT) section staining and analysis of muscularis externa have been described previously (Stenkamp-Strahm et al. 2013a). A 20x objective was used to obtain four FT images per animal. Images were taken from different sections stained at different time points by two different individuals to avoid bias and variations that could arise from immunostaining. To obtain the area of muscularis externa, a tracing tool was used to draw around the muscularis externa, excluding mucosa and submucosa areas. The image was then manually thresholded in a blinded fashion to gather areas of stained neurons and nerve fibers residing in muscularis externa. ‘Density indices’ (area of immunoreactive varicosities and nerve cell bodies/area of muscularis externa) were computed. Density indices measured from FT preparations are indicated simply as ‘density indices’ in results, and noted as coming from ‘muscularis externa’ and/or FT preparations.
Analysis of nerve health in muscularis externa with EM
Enteric nerve health has been previously assessed using an EM analysis of muscularis externa tissue in mice (Stenkamp-Strahm et al. 2013a). Using this method, a blinded analysis of at least ten nerve fibers, per mouse, per group was accomplished (n = 4). Nerves within an image were considered healthy due to presence of a normal plasma membrane, normal membranous organelles, neurofilaments, microtubules, secretory vesicles, and lack of axonal swelling. Images that met these criteria were given a score of ‘1’, while images where only half of the nerves met criteria were given a ‘0.5.’ If less than half of the nerves within an image were healthy they received a score of ‘0’. Mean nerve health values of SC and HF diet mice were generated.
Assessment of age-dependent changes between 8- and 20-week SC and HF diet mice
Recently, we performed a similar analysis of neuronal phenotypes in the duodenal myenteric plexus of mice fed SC and HF diets for 8 weeks (Stenkamp-Strahm et al. 2013a). Neuronal packing density and all density indices from mice fed both diets were compiled and compared between 8- and 20-week time points. Data were normalized to values from mice fed a SC diet for 8 weeks, which were given a value of 1 each. Standard errors are recorded as a percentage of the non-normalized density indices or packing density values for each marker.
Statistical analysis
GraphPad Prism 5 software was used to perform statistical analyses (GraphPad Software Inc, LaJolla, CA). An unpaired Student’s t-test was used to compare means of SC and HF diet mice. In cases of multiple comparisons, a repeated measure ANOVA with Bonferroni post-hoc test was used. Significance was determined as P < 0.05, and values within figures are expressed as mean +/− standard error of the mean.
Results
Progression of obesity, glucose intolerance and insulin resistance
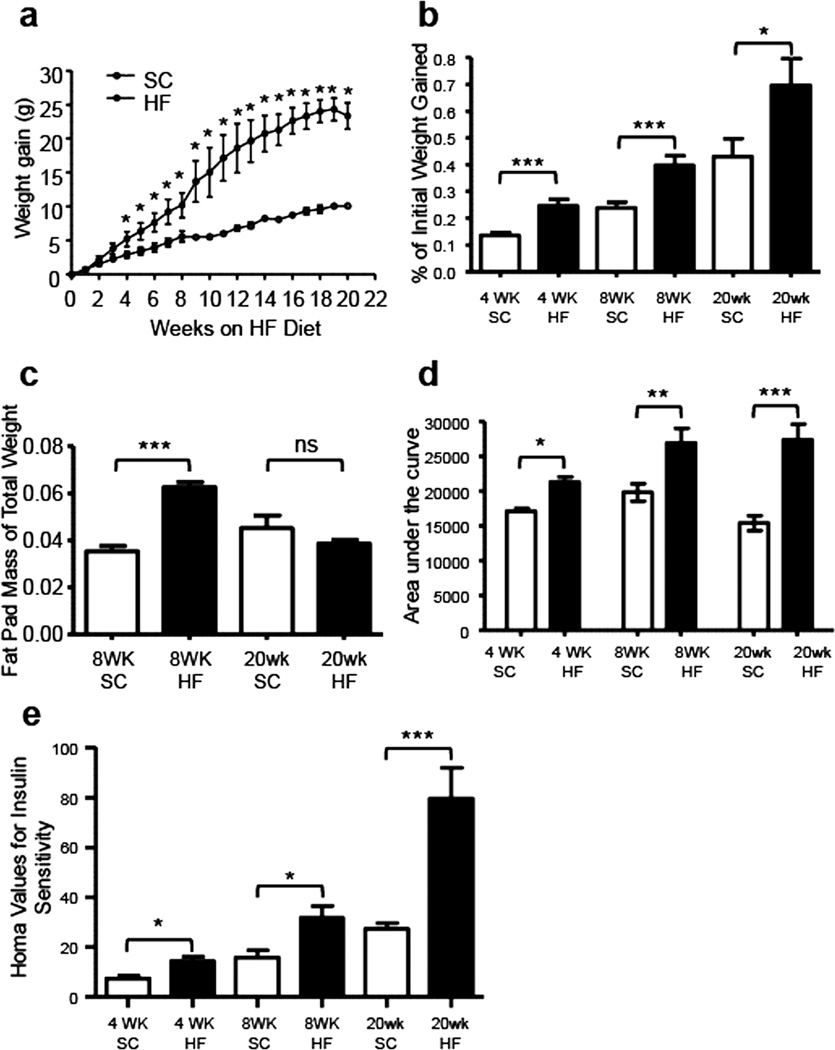
The 72% HF diet-induced T2D disease state was monitored as described previously, through measurement of weight gain, glucose tolerance and insulin resistance at 4, 8 and 20 weeks. Further, epididymal fat pads were excised and measured after euthanasia at 8 and 20 weeks. HF diet mice gained weight much faster than SC diet mice. Compared to mice fed a SC diet, the values of weight gained each week were statistically higher in mice fed a HF diet after 4 weeks (Fig. 1a, P < 0.05, n = 8). Likewise, the amount of weight gained as a proportion of initial body weight (weight gained (g)/initial total weight (g)) was greater in HF diet mice compared to SC diet mice at 4-, 8-, and 20-week time points (1b; 4 weeks, ***P = 0.0002; 8 weeks, ***P = 0.0007; 20 weeks, *P = 0.044; @ n=8). At 8 weeks, the fat pads of HF diet mice comprised a larger percentage of overall body mass when compared to SC diet mice, but these values were not different after 20 weeks (Fig. 1c, 8 weeks, ***P < 0.001, n = 8). Upon ingesting the HF diet for 4 weeks, mice became glucose intolerant compared to SC diet mice. This was indicated by a larger area under the curve (AUC) in curves created using blood glucose values taken after fasting and post-intraperitoneal glucose injection (Fig. 1d, 4 weeks, *P = 0.029, n = 8). The AUC of HF diet mice were significantly greater than that of SC diet mice after 8 and 20 weeks also (Fig. 1d, 8 weeks, **P= 0.0048, n = 8; 20 weeks, ***P = 0.0003, n = 8). Compared to the SC diet mice, higher HOMA values of insulin sensitivity were observed in HF diet mice after 4, 8 and 20 weeks of diet ingestion (Fig. 1e, 4 weeks, *P < 0.019, n = 8; 8 weeks, *P < 0.011, n = 8; 20 weeks, ***P < 0.0003, n = 8), indicating insulin resistance. To summarize, mice ingesting a HF diet were obese (overweight), glucose intolerant and insulin resistant after 4 weeks. The severity of these symptoms continued to increase to the 20-week time point.
Fig. 1.
The severity of HF diet-induced obesity and type 2 diabetes in C57BL6/J mice increases from 4 to 20 weeks of HF diet ingestion. After 4 weeks of HF diet ingestion, mice exhibited greater weight gain (a; n = 8, *P < 0.05, unpaired t-test) and greater proportion of initial body weight gain (b; n = 8, ***P = 0.0002 unpaired t-test) than their SC counterparts. Much greater weight gain and proportion of initial body weight gain were observed after 8 weeks (b; n = 8, ***P = 0.0007 unpaired t-test) and 20 weeks (b; n = 8, *P = 0.044 unpaired t-test) of HF diet ingestion. Mice fed a HF diet had greater epididymal fat pad mass, expressed as a percentage of total body weight (c) at 8 (n = 8, ***P < 0.0001 unpaired t-test) but not 20 weeks of HF diet ingestion (n = 8). The areas under the curves (AUC) of blood glucose levels measured before and at 30 min intervals after 1 g/kg intraperitoneal glucose injection were greater in 4-, 8- and 20-week HF diet mice, indicating that mice were glucose intolerant after 4 weeks (d, n = 8, *P < 0.019; unpaired t-test). Glucose intolerance was greatest after 8 and 20 weeks (d; each n = 8, **P = 0.0048; ***P=0.0003; all unpaired t -test). Analysis of insulin resistance by HOMA showed that HF diet mice had higher HOMA values at 4, 8 and 20 weeks, with insulin resistance becoming greater over time (e; each n = 8, **P = 0.019; *P = 0.011; *P = 0.0003; all unpaired t - test).
Mice fed a HF diet for 20 weeks have reduced myenteric ganglionic areas, neuronal soma sizes, and compromised nerve health
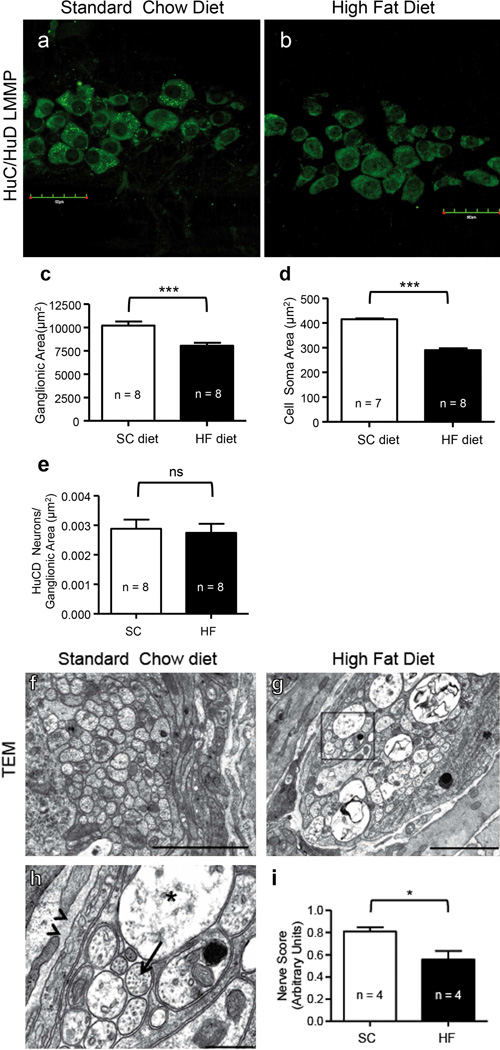
Ganglionic sizes merit assessment, as HF diet ingestion may alter ENS plexuses anatomically (Stenkamp-Strahm et al. 2013a). Compared to SC ingesting mice, an analysis of mean myenteric ganglionic areas of HuC/HuD stained LMMP preparations (Fig. 2a–b) showed a significant reduction by 20 weeks in HF diet mice (Fig. 2c; P < 0.0001, n = 8). Mean HuC/HuD cell soma size was significantly reduced in HF diet mice compared to SC diet mice (Fig. 2d; P < 0.0001, n = 8). Importantly, it has been previously observed that tissue stretch does not significantly impact the size of myenteric ganglia (Karaosmanoglu et al. 1996), indicating that packing density is not affected by tissue stretching during preparation of LMMP. The packing density of HuC/HuD neurons in these 20- week LMMP preparations was not significantly different between SC and HF diet mice (Fig. 2e; P = 0.758, n = 8).
Fig. 2.
The packing density of HuC/HuD neurons in duodenum LMMP of 20-week SC and HF diet mice was similar, but ganglionic and soma shrinkage and nerve injury in the myenteric plexus were seen in HF diet mice only. 20-week SC (a) and HF (b) diet mouse duodenum LMMP preparations, showing HuC/HuD-IR neurons that were used to measure ganglionic sizes, neuronal packing density, and cell soma size. Summary data of these analyses show that HF diet ingestion correlates with a reduction in mean ganglionic area (c; P < 0.001, n = 820 ganglia, unpaired t-test) and average size of HuC/HuD-IR cell bodies (d; P < 0.0001, n = 800, unpaired t-test). Meanwhile, the packing density of HuC/HuD-IR neurons was unaltered (e; P = 0.758, n = 8). EM images from SC (f) and HF diet (g) mouse duodenum preparations were used to assess the overall nerve health of muscularis externa. Duodenal myenteric plexus nerve fibers of HF diet mice (g and inset, h) suffered axonal swelling (h; star, compared to healthy axon indicated by arrow) compared to SC diet mice (f). In some cases, a disruption of neurofilaments and microtubules was also seen (g, h). Nerve health scores from TEM image analysis revealed statistically lower mean health values in HF diet compared to SC diet mice (i; P = 0.046, n = 4, unpaired t-test).
Compared to SC diet, EM showed nerve injuries in mice fed a HF diet (Fig. 2f–h). Changes in duodenal nerves of HF diet mice included axonal swelling (Fig. 2h, star; compared to healthy axon, arrow), and a disrupted appearance of neurofilaments and microtubules. A blinded analysis of nerve health showed that after 20 weeks nerves of HF diet mice became compromised, as health scores were statistically lower compared to SC diet mice (Fig. 2i; P < 0.046, n = 4).
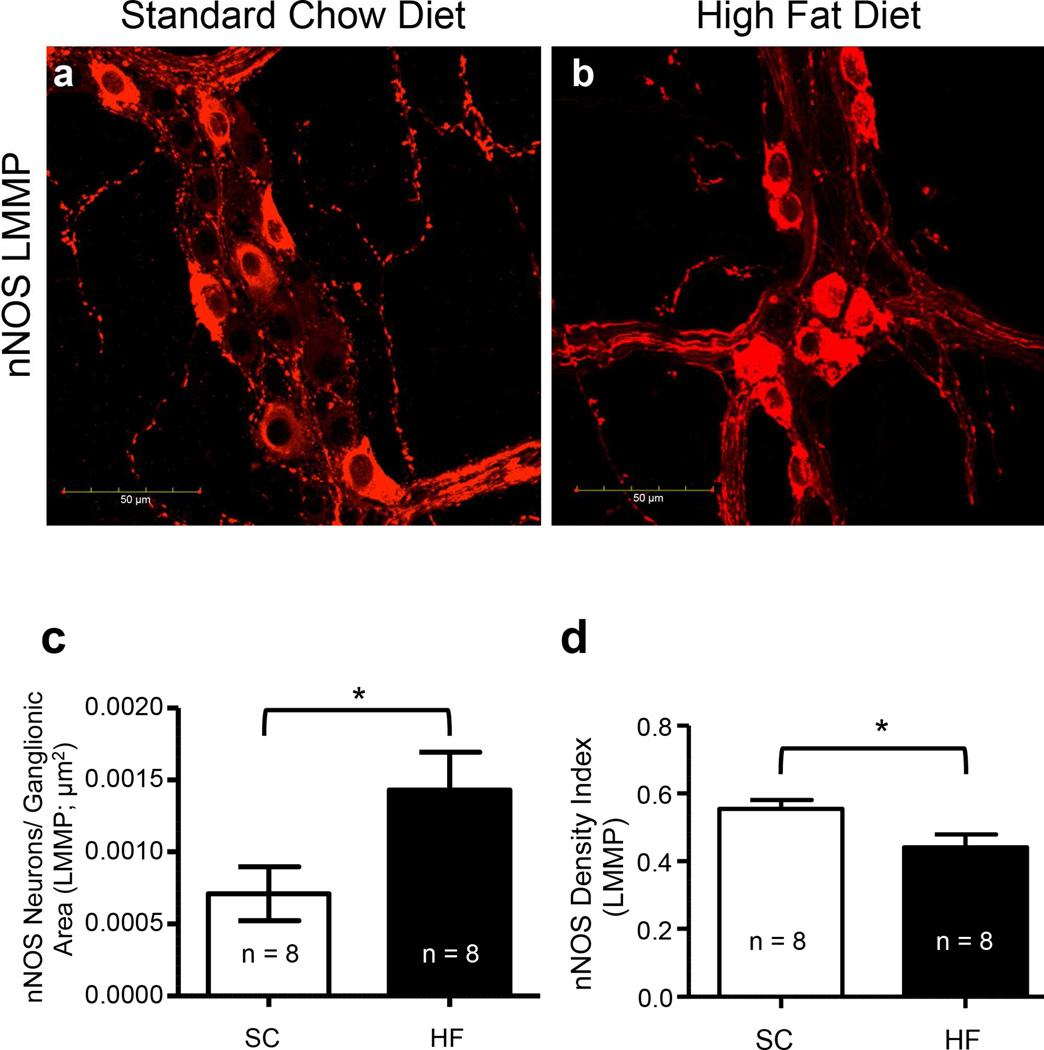
During prolonged HF diet ingestion, packing density of myenteric nNOS-IR nerve cell bodies was increased, while the density index of nNOS-IR nerve cell bodies plus varicosities was reduced
nNOS-IR nerve cell bodies were counted in the myenteric ganglia in LMMP preparations of SC and HF diet mice and the packing density of both groups was computed. The density index of nNOS-IR nerve cell bodies and varicosities in the myenteric ganglia were analyzed and compared in a similar manner (Fig. 3a–b). There was an increase in the number of nNOS-IR nerve cell bodies in the myenteric plexus in duodena of mice fed a HF diet compared to those fed a SC diet (Fig. 3c; P < 0.042, n = 8). In contrast to the packing density, the density index of nNOS-IR nerve cell bodies plus varicosities was reduced in mice fed a HF diet (Fig. 3d; P < 0.026, n = 8).
Fig. 3.
The number of nNOS-IR nerve cell bodies in the myenteric ganglia of 20-week HF diet mice increased, while the density index of nNOS-IR nerve cell bodies plus varicosities was reduced. LMMP preparations from 20-week SC (a) and HF (b) diet mice were stained with an antibody for nNOS to assess the packing density of nNOS IR nerve cell bodies and the density index of nNOS-IR cell bodies plus IR varicosities in the ganglia. Neuronal NOS-IR cell body packing density was increased in HF diet mice compared to SC diet mice (c; P = 0.042, n = 8, unpaired t-test). In contrast, the density index of nNOS-IR cell bodies and IR varicosities was reduced (d; P = 0.026, n = 8, unpaired t-test).
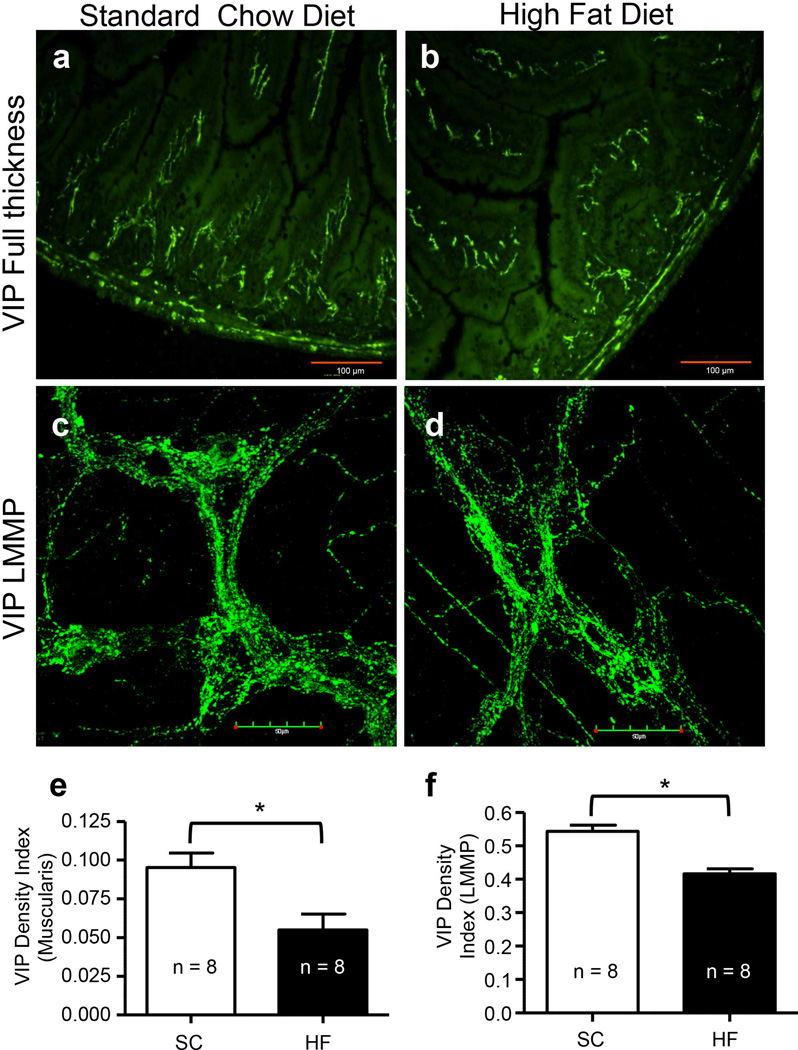
During prolonged HF diet ingestion, VIP density indices were reduced in muscularis externa and myenteric ganglia
To further analyze the effect of a HF diet on inhibitory motor neurons, an antibody against the inhibitory neurotransmitter VIP was used to stain FT and LMMP preparations (Fig. 4a–d), and density indices of 20-week SC and HF diet mice were compared. Mean areas of muscularis externa did not differ between SC and HF diet mice after 20 weeks (data not shown). The density indices of VIP-IR varicosities in muscularis externa (Fig. 4e; P < 0.012, n = 8) and IR nerve cell bodies and varicosities in the myenteric ganglia (Fig. 4f; P < 0.012, n = 8) of HF diet mice were statistically lower than those of SC diet mice.
Fig. 4.
Vasoactive intestinal polypeptide (VIP) density indices were reduced in 20-week HF diet mice, compared to 20-week SC. VIP IR in muscularis externa (a–b) and LMMP (c–d) were used to assess density indices of IR in nerve fibers and ganglia of muscularis externa (“muscularis”), and myenteric ganglia of LMMP in 20-week SC diet (a, c) and HF diet (b, d) mice. Compared to 20-week SC diet mice, VIP density indices of 20-week HF diet mice were reduced in both the muscularis externa (e; P = 0.012, n = 8, unpaired t-test) and in the myenteric ganglia (f; P = 0.012, n = 8, unpaired t-test).
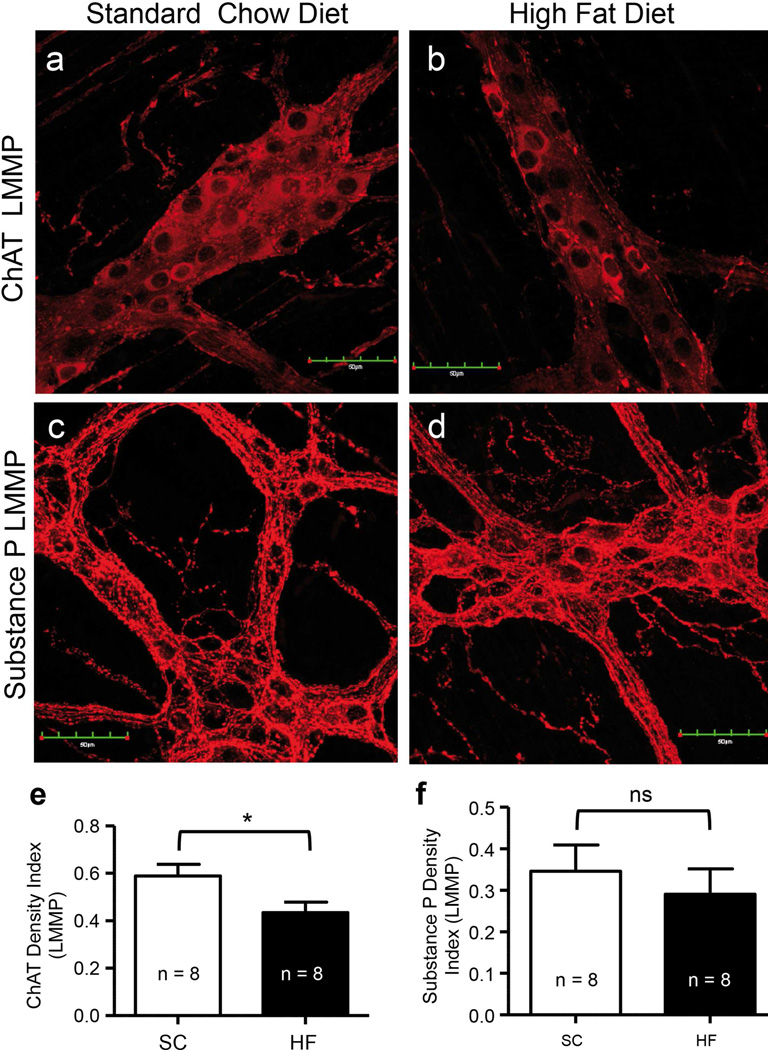
Density index of ChAT-IR nerve bodies and varicosities was reduced, while that of substance P was not altered by prolonged HF diet ingestion
To investigate changes in excitatory motor neurons, antibodies for ChAT and substance P were used to stain duodenal LMMP preparations of 20-week SC and HF diet mice (Fig. 5a–d). Compared to those on a SC diet, mice on a HF diet for 20 weeks had significantly reduced density indices of ChAT-IR nerve cell bodies and varicosities (Fig. 5e; P = 0.039; n = 8). Computation of density indices of substance P-IR nerve cell bodies and varicosities of both mouse groups did not show significant differences (Fig. 5f, P = 0.546, n = 8).
Fig. 5.
Myenteric ganglia excitatory neurons were variably affected by the ingestion of a HF diet for 20 weeks. Duodenal LMMP preparations from 20-week SC and HF diet mice showing ChAT-IR (a, b) and substance P-IR (c, d) in the myenteric plexus of mouse duodenum. ChAT density indices were reduced by a HF diet (e; P = 0.039; n = 8, unpaired T-test), but substance P density indices were not different between 20-week SC and HF diet mice (f; P = P = 0.546; n = 8, unpaired T-test).
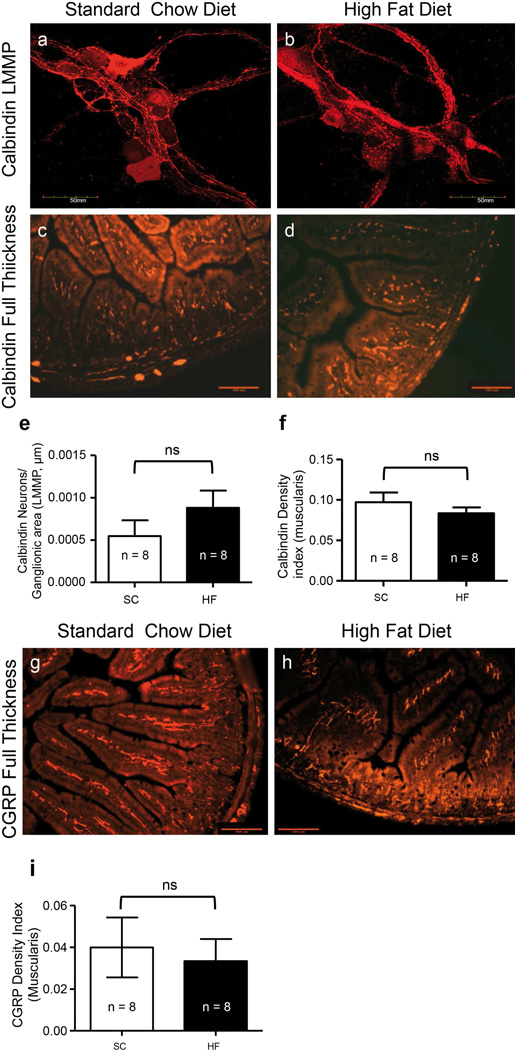
The number of myenteric ganglia calbindin neurons and calbindin and CGRP density indices of muscularis externa were unchanged in prolonged HF diet mice compared to SC diet mice
The packing density of calbindin-IR neurons was measured in myenteric ganglia in LMMP preparations (Fig. 6a–b). The density index of calbindin-IR was measured in the muscularis externa of FT sections also (Fig. 6c–d). The packing density of calbindin neurons in myenteric ganglia was not different between SC and HF diet mice after 20 weeks (Fig. 6e; P = 0.246, n = 8), and the density index of calbindin in muscularis externa was unaltered by consumption of a HF diet as well (Fig. 6f; P = 0.347, n = 8).
Fig. 6.
Analysis of calbindin-IR and CGRP-IR showed that sensory neurons were not altered in the duodenum myenteric plexus of HF diet mice after 20 weeks. An anti-calbindin antibody was used to stain LMMP duodenal preparations from mice fed SC (a) and HF (b) diets. Anti-calbindin was also used to stain muscularis externa of duodenal preparations from the same 20-week SC (c) and HF (d) diet mice. The packing density of calbindin-IR neurons in the myenteric ganglia was no different between diet groups (e; P = 0.246, n = 8, unpaired t-test), and the same was seen when density indices were measured in the muscularis externa (f; P = 0.347, n = 8, unpaired t-test). Immunoreactivity for CGRP in muscularis externa of duodenal preparations from 20-week SC (g) and HF (h) diet mice was also assessed. Similar to calbindin, CGRP density indices in muscularis externa of 20-week SC and HF diet mice were similar (i; P =0.719, n = 8, unpaired t-test).
CGRP immunoreactivity was analyzed in the muscularis externa of FT sections only (Fig. 6g–h). CGRP density indices were unaltered by consumption of a HF diet (Fig. 6i; P = 0.714, n = 8).
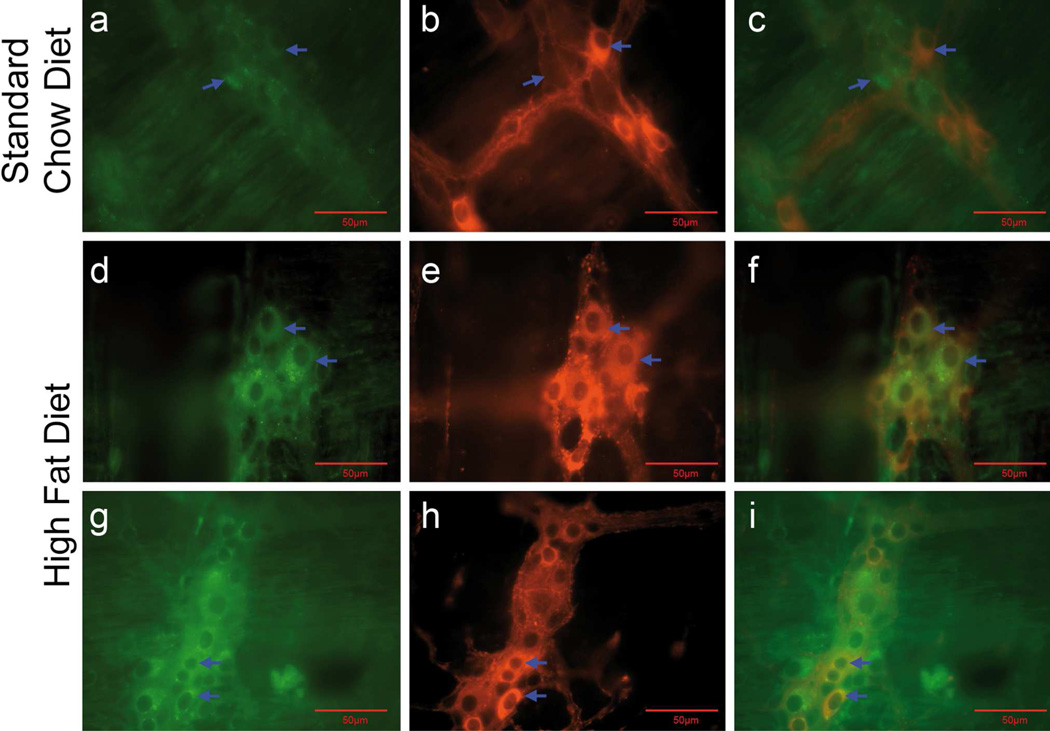
Prolonged HF diet induces the activation of apoptosis in duodenal myenteric nNOS and ChAT neurons
We and other investigators have reported that apoptosis and loss of myenteric neurons, specifically nNOS neurons, occurs in the duodenum and colon of mice fed a HF diet for 6–8 weeks (Stenkamp-Strahm et al. 2013a, Nezami et al., 2014). We hypothesized that activation of apoptosis persisted in nNOS neurons, and was perhaps detectable in ChAT neurons, of the duodenal myenteric plexus of 20-week HF diet mice. Immunohistochemical analysis for apoptotic activation was assessed using an antibody directed against cleaved caspase-3. The immunoreactivity to cleaved caspase-3 was absent or observed in a few nerve cell bodies in the myenteric ganglia of mice fed the SC diet (Fig. 7a–c). In contrast, caspase-3-IR was observed in the myenteric ganglia of mice fed a HF diet for 20 weeks in co-localized nNOS-IR (Fig. 7d–f) and ChAT-IR (Fig. 7g–i) neurons. This suggests an activation of apoptosis in these classes of neurons. Quantification of caspase-3- IR neurons per ganglionic area showed significant differences between mice fed the SC diet and those fed the HF diet (Fig. 7i; P = 0.0017, n =5). The populations of caspase-3-IR neurons that were also immunoreactive for nNOS or CHAT were not quantified due to a lack of adequate LMMP preparations.
Fig. 7.
A subpopulation of duodenal myenteric neurons of mice fed a HF diet for 20 weeks exhibited activated apoptotic pathways. LMMP preparations were used to demonstrate co-localization of cleaved caspase-3-IR with nNOS-IR (a–f) and ChAT-IR (g–i) neurons in the myenteric ganglia. Compared to mice fed a SC diet for 20 weeks (ac; caspase-3 + nNOS), those fed a HF diet showed caspase-3-IR in nNOS-IR nerve cell bodies (d-f; caspase-3 + nNOs) and in ChAT-IR nerve cell bodies (g-I; caspase-3 + ChAT) indicating an activation of apoptosis in nNOS and ChAT neurons. Arrows of a-c indicate a lack of co-localization of caspase-3 and nNOS-IR. Arrows in d-f and g-I show co-localization of these markers (d–f), as well as co-localization of caspase-3 and ChAT (g–i). J, Caspase-3-IR nerve cell bodies per ganglion area were computed before staining the preparations to localize caspase-3-IR with nNOS and ChAT. Summary data show an increased number of myenteric caspase-3-IR nerve cell bodies per ganglion area in mice fed a HF diet for 20 weeks (P= 0.0017, n = 5, unpaired t-test) compared to mice fed a SC diet.
Comparison of 8- and 20-week SC and HF diet mice revealed age-dependent plasticities among subpopulations of enteric neurons
It is well understood that the ENS undergoes age-related changes in the mouse and other species (Gomes et al. 1997; El-Salhy et al. 1999; Wade 2002). Mice in the current study began dietary intervention at 8 weeks of age, which continued through 28 weeks of age. This places mice in the category of ‘adult’ throughout the study period (Fox et al. 2006). We observed an age-dependent decline in duodenal packing density of myenteric neurons in both SC and HF diet mice from 8 to 20 weeks of age (Table 2; HuC/HuD neurons/ ganglionic area).
Table 2.
Comparison of packing density and density indices of 8- and 20-week SC and HF diet mice
| Antibody Used Measurement |
8wk SC diet |
8wk HF diet | 20wk SC diet |
20wk HF diet | Age-wise Comparison |
|
|---|---|---|---|---|---|---|
| 8wk vs. 20wk SC diet |
8wk vs. 20wk HF diet |
|||||
| HuC/HuD neurons/ganglia LMMP | 1 +/− 0.015 | 0.910 +/− 0.025 *P = 0.05 |
0.543 +/− 0.058 | 0.510 +/− 0.051 P = 0.67 |
***P <0.0001 | ***P = 0.0001 |
| nNOS neurons/ganglia LMMP | 1 +/− 0.091 | 0.82 +/− 0.168 ***P<0001 |
0.958 +/− 0.250 | 1.956 +/− 0.507 P = 0.103 |
P = 0.870 | P= 0.198 |
| VIP, Density Index Muscularis externa | 1 +/− 0.15 | 0.570 +/− 0.16 *P = 0.05 |
1.233 +/− 0.136 | 0.729 +/− 0.150 *P = 0.029 |
P = 0.112 | P = 0.899 |
| ChAT, Density Index LMMP | 1 +/− 0.15 | 1.24 +/− 0.14 P = 0.09 |
2.598 +/− 0.289 | 1.934 +/− 0.289 P = 0.061 |
*** <0.0013 | **P = 0.0026 |
| Substance P Density Index LMMP | 1 +/− 0.12 | 0.90 +/− 0.1 P = 0.5 |
3.223 +/− 0.664 | 2.600 +/− 0.554 P = 0.4 |
**P= 0.0048 | P = 0.113 |
| Calbindin neurons/ganglia LMMP | 1 +/− 0.06 | 1.07 +/− 0.10 P = 0.5 |
0.676 +/− 0.230 | 1.091 +/− 0.250 *P = 0.022 |
***P= 0.0001 | P = 0.981 |
| CGRP, Density Index Muscularis externa | 1 +/− 0.008 | 0.87+/− 0.013 P = 0.65 |
1.114+/− 0.430 | 0.893 +/− 0.307 P = 0.681 |
P= 0.858 | P = 0.642 |
LMMP: longitudinal muscle myenteric plexus, n = 4 for all 8wk mice and n=8 for all 20wk mice, all values were normalized to 8wk SC diet mice.
(*P) indicate significant differences between compared groups.
Ageing was not associated with a change in the packing density of nNOS IR neurons in duodenal myenteric ganglia of mice fed SC and HF diets for 20 weeks (Table 2; nNOS, neurons/ ganglionic area, LMMP). Age-dependent analysis of the other inhibitory neuron marker, VIP, showed no alteration in density indices of muscularis externa between 8 and 20 weeks, and this was in both mouse groups (Table 2; VIP Density Index).
Evaluation of age-induced changes of excitatory markers revealed an increase in ChAT and substance P density indices of nerve cell bodies and varicosities after 20 weeks, in both diet groups (Table 2; ChAT Density Index LMMP; Substance P Density Index LMMP).
Ageing affected one sensory/interneuron marker in both mouse groups. The number of calbindin neurons/ganglionic area was significantly reduced in mice fed a SC diet for 20 weeks. However, calbindin neurons/ ganglionic area in mice fed a HF diet for 8 and 20 weeks did not show a significant difference (Table 2; Calbindin neurons/ ganglionic area). CGRP density indices in duodenal muscularis externa of SC and HF diet mice did not change due to age (Table 2; CGRP Density Index).
Non-normalized mean values and SEM for all neuronal packing densities and density indices assessed herein can be found in Supplemental Table 1.
Discussion
It is postulated that damage to the ENS plays a key role in diabetic gastroparesis, diarrhea and constipation (Chandrasekharan et al. 2011; Yarandi and Srinivasan 2014). As such, a better understanding of ENS alterations associated with HF diet induced obesity and T2D may eventually lead to more efficient treatment strategies for patients. The goal of this study was to characterize the effect of prolonged HF diet ingestion on the duodenal myenteric plexus. We found that mice fed a HF diet for 20 weeks experienced hallmarks of obesity and T2D beginning at 4 weeks. Weight gain, glucose intolerance and insulin resistance of 20-week HF diet mice was much higher than that of 8-week HF diet mice indicating a progression of metabolic imbalance, and a prolonged disease state that human T2D patients with poor glucose and dietary management often experience. Key findings of this study include a correlation between prolonged HF diet ingestion, reductions in ganglionic and nerve cell body sizes, and a decline in nerve health in the duodenal myenteric plexus. These findings were associated with a decrease in density indices of VIP-IR and nNOS-IR, despite the increased number of nNOS-IR nerve cell bodies per ganglionic areas. Prolonged consumption of a HF diet also caused a decline in the density index of ChAT-IR in the myenteric ganglia. Age-related changes seen in the myenteric ganglia of these non-senescing adult mice included an overall decrease in the packing density of neurons, and an increase in the density index of cholinergic neurons regardless of diet. Of note, the density index of substance P in the myenteric ganglia increased with aging in 20-week SC diet mice, but not in HF diet mice. Ageing was also associated with a decline in the packing density of calbindin-IR neurons in 20-week SC diet mice, but not in HF diet mice.
Changes in the duodenum myenteric plexus after 20 weeks of HF diet ingestion
The packing density of HuC/HuD myenteric neurons in 20-week HF and SC diet mice was similar, suggesting the total population of duodenal myenteric neurons was unchanged due to disease state or HF diet ingestion. Interestingly, we observed significantly increased caspase-3-IR neurons in 20-week HF mice, which suggests activation of apoptosis. Our results coincide with studies showing no change in total duodenal neurons during streptozotocin induced type 1 diabetes (Furlan et al. 1999) and ob/ob transgenic type 2 diabetes (Spangeus and El-Salhy 2001). However, they conflict with our own previous results showing neuronal loss at 8 weeks of the same high fat diet-induced T2D (Stenkamp-Strahm et al. 2013a). It is likely that the difference between studies depends on fatty acid composition in the feed (Voss et al. 2013; Nezami et al. 2014), and presumably the impact of the gut microbiota (Anitha et al. 2006). These new results suggest that after 8 weeks of HF diet ingestion myenteric neurons are potentially protected from T2D–induced apoptosis, which is supported by a study showing 12 week HF diet protection to nerves of the antral ENS (Baudry et al. 2012). Our results support the theory that plasticity in the ENS occurs over time and after injury, and that regeneration of axonal connections, synapses, and even nerve cells may be possible (Giaroni et al. 1999; Azan et al. 2011; Laranjeira et al. 2011).
Prolonged HF diet consumption correlated with a shrinkage in ganglia (~18%) and nerve cell body (~21%) size in the duodenum myenteric plexus. Previously, a similar shrinkage in ganglia was seen in the diabetic guinea pig (LePard 2005). Mean ganglionic sizes were not significantly different between 8-week SC diet, 8- week HF diet, and 20-week SC diet-ingesting mice (8-week data not shown), showing that the shrinkage was independent of age. It is unlikely that this ganglionic shrinkage affected the packing density as nerve cell soma sizes were reduced as well. These findings suggest that ganglia and neuronal cell soma size reductions are remodeling strategies initiated in response to early neuron loss, nerve fiber damage, or perhaps changes in GI function experienced after HF diet ingestion.
Prolonged HF diet ingestion was associated with an increase in nerve injury, including axonal swelling and loss of cytoskeletal structures. These results correspond with observations made in stomach biopsies from diabetic humans that experienced gastroparesis (Faussone-Pellegrini et al. 2012) as well as dysmotility and enteropathy of the large bowel (Schmidt et al. 1984). They support a notion that nerve tissues in HF diet mice were not unscathed, even though neuronal packing density showed no decline. Indeed, loss of fibers during diabetes with little or no loss of nerve cells has been seen previously in stomach tissues (Pasricha et al. 2008; Wang et al. 2008). Although functional analyses were not the goal of this work, nerve injuries shown here suggest that HF diet mice likely have symptoms of dysmotility, since damages of this nature have been seen in type 1 diabetic mice that had symptoms of delayed gastric emptying and intestinal transit (Anitha et al. 2006).
VIP content of myenteric ganglia and muscularis externa in the duodenum of 20-week HF diet mice was reduced compared to SC diet, coinciding with our previous results of loss of VIP neurons at 8 weeks. These observations are also similar to findings in 20-week ob/ob transgenic T2D mice (Spangeus and El-Salhy 2001). As VIP and nNOS are expressed by the same myenteric neuronal phenotype and nNOS-IR varicosities were reduced, the change suggests that disrupted microfilament damage and swelling of axons occurred in VIP/nNOS fibers, resulting in altered vesicular transport and VIP/nNOS depletion in nerves fibers of the muscularis externa. This remains to be determined, however.
One of the novel findings of this study is that the number of nNOS-IR cell bodies in the myenteric plexus of HF diet mice increased compared to SC diet mice after 20 weeks of ingestion. An increase in nNOS nerve cells has been seen in the duodenum of type 1 diabetic rats after 1-week (Furlan et al. 1999). However, a reduction in nNOS expression or nNOS-IR neurons is more commonly reported both in type 1 (Spangeus et al. 2000; Shotton and Lincoln 2006; Bagyánszki and Bódi 2012) and T2D (Spangeus and El-Salhy 2001; Surendran and Kondapaka 2005; Pasricha et al. 2008; Chandrasekharan et al. 2011; Bagyánszki and Bódi 2012). Importantly, this includes our own findings in mice fed the same HF diet for 8 weeks (Stenkamp-Strahm et al. 2013a) and other findings using HF diets to model obesity, T2D and non-alcoholic fatty liver disease (Voss et al. 2013; Nezami et al. 2014; Voss et al. 2014; Rivera 460 et al. 2014). This observation raises the question of why nNOS neurons are susceptible to early stage HF diet-induced T2D loss, while being spared at later stages. A larger presence of nNOS has been seen in axons of diabetics early in disease, with degeneration of fibers causing nNOS accumulation in cell bodies at later stages (Cellek et al. 2004). Similar observations were made in the present study, as the increased packing density of nNOS-IR nerve cell bodies was concurrently seen with a decrease in the overall density index of nNOS-IR varicosities. When taken together with EM results of disrupted cytoskeletal elements in nerve fibers, our findings suggest impaired nerve fiber transport/translocation of enzymes from the cell soma during later stages of T2D. Neuronal NOS is constitutively expressed in the GI tract and several splice variants exist, but processes regulating specific transcription are poorly understood. It is possible that changes in the expression of nNOS splice variants (Savidge 2011) and shifts in neuronal phenotype occurred between 8 and 20 weeks of diet ingestion, to compensate for early neuropathies (Giaroni et al. 1999). To fully understand the plasticity of nNOS neurons during T2D, these ideas will need to be addressed in future studies.
HF diet ingestion for 20 weeks decreased the density index of ChAT neurons in the myenteric ganglia, suggesting that ChAT neurons are vulnerable to HF diet-induced damage. The observation of activated caspase-3-IR in ChAT-IR neurons supports this assumption. Contrary to this, HF diet ingestion did not alter myenteric substance P neurons and sensory neurons (calbindin, CGRP), which parallels results reported by our group at 8 weeks (Stenkamp-Strahm et al. 2013a). It remains possible that damage to nerve fibers seen through EM analysis occurred in these neuronal phenotypes, as this may happen without marked changes in protein expression.
Age-related changes
An age-associated loss in total neurons was seen in both diet groups, which parallels a commonly seen age-related loss of ENS neurons (El-Salhy et al. 1999; Phillips et al. 2004; Phillips and Powley 2007). Aging did not affect inhibitory motor neurons because it did not affect the packing density of nNOS neurons in SC diet mice, or VIP indices of both mouse groups. This is consistent with previous observations that ENS inhibitory neurons survive more readily compared to those of alternate phenotypes during the ageing process (Wade 2002; Phillips and Powley 2007).
Reactive oxygen species (ROS) accumulation in neurons causes age related neurodegeneration (Thrasivoulou et al. 2006). It has been suggested that the nNOS neuron loss commonly seen during diabetes is also due to oxidative stress (Chandrasekharan et al. 2011; Voukali et al. 2011; Yarandi and Srinivasan 2014). In addition, the synergistic actions of advanced glycation end products and endogenous nitric oxide are thought to underlie diabetic selective apoptotic cell death of nNOS neurons (Cellek et al. 2004). Therefore, the combination of age-related and diet-induced oxidative stress may be cumulative (Anitha et al. 2006; Papanas and Ziegler 2012; Bagyánszki and Bódi 2012). New research has shown that palmitate can cause apoptosis in nNOS neurons by triggering mitochondrial dysfunction and endoplasmic reticulum stress (Voss et al. 2013; Nezami et al. 2014; Voss et al. 2014). Importantly, palmitate constitutes about 30% of lard and the HF diet used in this study contained 35% lard. Considering all factors, one would expect extensive degeneration of nNOS neurons after 20 weeks of HF diet ingestion, contrary to the findings of this study. This highlights a need to further identify the exact causes of T2D nerve cell death, and adaptive responses of myenteric neurons to a HF diet.
20-week SC and HF diet mice experienced an increase in ChAT content in the myenteric plexus, relative to 8-week mice. The content of substance P in the myenteric plexus of both 8-week and 20-week SC and HF diet mice did not differ. Previous studies in aged animals have commonly shown declines in cholinergic neuron populations and activity (Lopes et al. 2007; Phillips and Powley 2007). This is the first study to look at excitatory motor neuron markers in the duodenum of adult and middle-aged adult mice. Whether the patterns seen are regular temporal plasticities, and whether age-related degeneration of these neurons occurs at a later age stage, remains to be determined.
In the myenteric ganglia, packing density of calbindin-IR neurons, which are considered to be intrinsic sensory and interneurons (Tan et al. 2010), was reduced in 20- compared to 8- week SC diet mice. This parallels a trend in aged animals showing that sensory nerves may be the most prone to age-induced cell death (Wade 2002; Thrasivoulou et al. 2006; Phillips and Powley 2007). The packing density of calbindin-IR neurons in HF diet mice and calbindin and CGRP density indices of muscularis externa did not show this age-related alteration, however. The absence of age-related change in the packing density of calbindin-IR neurons among HF diet mice correlated with a trend toward an increase in calbindin-IR neurons (37%) in mice fed the HF diet for 20 weeks. Causes of the difference between these dietary effects are not known.
Guidelines for histological techniques and quantitative methods for measuring enteric neuron populations recommend a normalization of data to intestinal length (Knowles et al. 2009). For the present study, this would require an estimation of individual sample length and width, and measurement of stretching effects. For our results, measurements of neurons were referenced to ganglionic area. This allowed immediate specimen collection for EM experiments (Karaosmanoglu et al. 1996).
Conclusions
The present study suggests that prolonged HF diet ingestion causes nerve cell soma and ganglionic shrinkage in the duodenal myenteric plexus. Prolonged high fat diet ingestion also exacerbates nerve damage and injury to inhibitory motor neurons, regardless of an increase in the actual number of nNOS neurons. In addition, ChAT neurons become vulnerable to apoptotic cell death. The evidence of ganglionic remodeling, nerve cell damage and age-related changes seen in mice fed HF and SC diets for 20 weeks indicates a need to investigate mechanisms behind age and diet interactions that underlie enteric neuron plasticities. Specifically, the mechanisms underlying changes in nNOS/VIP, ChAT and calbindin neurons should be sought, as these are likely to cause alterations in GI function and gut brain signaling in T2D patients. It would also be useful to analyze compounding effects of senescence and T2D on the ENS during long-term dietary intervention in a mouse model.
Supplementary Material
Acknowledgements
This study was funded by the University of Idaho, IBEST and Idaho INBRE (NIH Grant Nos. P20 RR016454 and P20 GM103408). Work done by Gericke, M was supported by Deutsche Forschungsgemeinschaft grant number DFG-SFB 1052/1. The authors would like to acknowledge Joe T. Schmaltz, Rachel Siemens, Sky Hembree and Spencer Dean for their assistance with staining and quantification procedures.
References
- Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;5(14 Suppl):S1–S85. [PubMed] [Google Scholar]
- Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azan G, Low WC, Wendelschafer-Crabb G, et al. Evidence for neural progenitor cells in the human adult enteric nervous system. Cell Tissue Res. 2011;344:217–225. doi: 10.1007/s00441-011-1130-9. [DOI] [PubMed] [Google Scholar]
- Bagyánszki M, Bódi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes. 2012;3:80–93. doi: 10.4239/wjd.v3.i5.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry C, Reichardt F, Marchix J, et al. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and glial cell line-derived neurotrophic factor. J Physiol. 2012;590:533–544. doi: 10.1113/jphysiol.2011.219717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C, Søfteland E, Gunterberg V, et al. Diabetic autonomic neuropathy affects symptom generation and brain-gut axis. Diabetes Care. 2013;36:3698–3705. doi: 10.2337/dc13-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrtzer P, Talley J, Leemon M, et al. Prevalence of Gastrointestinal Symptoms Associated With Diabetes Mellitus. Arch Intern Med. 2001;161:189–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Malagelada JR. Abnormal intestinal motility in diabetics with the gastroparesis syndrome. Eur J Clin Invest. 1984;14:420–427. doi: 10.1111/j.1365-2362.1984.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Cellek S, Qu W, Schmidt AM, Moncada S. Synergistic action of advanced glycation and products and endogenous nitric oxide leads to neuronal apoptosis in vitro: A new insight into selective nitrergic neuropathy in diabetes. Diabetologia. 2004;47:331–339. doi: 10.1007/s00125-003-1298-y. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan B, Anitha M, Blatt R, et al. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131–138. doi: 10.1111/j.1365-2982.2010.01611.x. e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Salhy M, Sandström O, Holmlund F. Age-induced changes in the enteric nervous system in the mouse. Mech Ageing Dev. 1999;107:93–103. doi: 10.1016/s0047-6374(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini M, Grover M, Pasricha P, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med. 2012;16:1573–1581. doi: 10.1111/j.1582-4934.2011.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Barthold S, Davisson M. The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models (American College of Laboratory Animal Medicine) 2006;3 [Google Scholar]
- Furlan M, de Miranda Neto M, Sant’ana Dde M, Molinari S. Number and size of myenteric neurons of the duodenum of adult rats with acute diabetes. Arq Neuropsiquiatr. 1999;57:740–745. doi: 10.1590/s0004-282x1999000500003. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system: Normal functions and enteric neuropathies. Neurogastroenterol Motil. 2008;20:32–38. doi: 10.1111/j.1365-2982.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- Furness JB. Enteric Nervous System: Neural Circuits and Chemical Coding. Encycl. Neurosci. Elsevier Ltd. 2010:1089–1095. [Google Scholar]
- Giaroni C, De Ponti F, Cosentino M, et al. Plasticity in the enteric nervous system. Gastroenterology. 1999;117:1438–1458. doi: 10.1016/s0016-5085(99)70295-7. [DOI] [PubMed] [Google Scholar]
- Gomes OA, de Souza RR, Liberti EA. A preliminary investigation of the effects of aging on the nerve cell number in the myenteric ganglia of the human colon. Gerontology. 1997;43:210–217. doi: 10.1159/000213852. [DOI] [PubMed] [Google Scholar]
- Jack M, Wright D. Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl Res. 2012;159:355–365. doi: 10.1016/j.trsl.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaosmanoglu T, Aygun B, Wade P, Gershon M. Regional differences in the number of neurons in the myenteric plexus of the guinea pig small intestine and colon: an evaluation of markers used to count neurons. Anat Rec. 1996;244:470–480. doi: 10.1002/(SICI)1097-0185(199604)244:4<470::AID-AR5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Knowles CH, De Giorgio R, Kapur RP, et al. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009;118:271–301. doi: 10.1007/s00401-009-0527-y. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- Laranjeira C, Sandgren K, Kessaris N, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121:3412–3424. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePard KJ. Choline acetyltransferase and inducible nitric oxide synthase are increased in myenteric plexus of diabetic guinea pig. Auton Neurosci. 2005;118:12–24. doi: 10.1016/j.autneu.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lopes GS, Smaili SS, Neto AC, et al. Aging-induced decrease of cholinergic response and calcium sensitivity on rat jejunum contractions. J Gerontol A Biol Sci Med Sci. 2007;62:264–270. doi: 10.1093/gerona/62.3.264. [DOI] [PubMed] [Google Scholar]
- Nezami B, Mwangi S, Lee J, et al. MicroRNA 375 mediates palmitate657 induced enteric neuronal damage and high-fat diet-induced delayed intestinal transit in mice. Gastroenterology. 2014;146:473–83.e3. doi: 10.1053/j.gastro.2013.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanas N, Ziegler D. Prediabetic Neuropathy: Does It Exist? Curr Diab Rep. 2012;12:376–383. doi: 10.1007/s11892-012-0278-3. [DOI] [PubMed] [Google Scholar]
- Pasricha PJ, Pehlivanov ND, Gomez G, et al. Changes in the gastric enteric nervous system and muscle: A case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. doi: 10.1186/1471-230X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Kieffer EJ, Powley TL. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol (Berl) 2004;209:19–30. doi: 10.1007/s00429-004-0426-x. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: Patterns of aging. Auton Neurosci Basic Clin. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu ZD, Thacker M, Castelucci P, et al. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- Reeves N, Najafi B, Crews R, Bowling F. Aging and type 2 diabetes: consequences for motor control, musculoskeletal function, and whole-body movement. J Aging Res. 2013;2013:50875. doi: 10.1155/2013/508756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera LR, Leung C, Pustovit RV, et al. Damage to enteric neurons occurs in mice that develop fatty liver disease but not diabetes in response to a high-fat diet. Neurogastroenterol Motil. 2014;26:1188–1199. doi: 10.1111/nmo.12385. [DOI] [PubMed] [Google Scholar]
- Sang Q, Williamson S, Young HM. Projections of chemically identified myenteric neurons of the small and large intestine of the mouse. J Anat. 1997;190(Pt 2):209–222. doi: 10.1046/j.1469-7580.1997.19020209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Q, Young HM. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- Sang Q, Young HM. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat Rec. 1998;251:185–199. doi: 10.1002/(SICI)1097-0185(199806)251:2<185::AID-AR6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Savidge TC. S-nitrosothiol signals in the enteric nervous system: lessons learnt from big brother. Front Neurosci. 2011;5:31. doi: 10.3389/fnins.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Riemann JF, Schmid A, Sailer D. Ultrastructure of diabetic autonomic neuropathy of the gastrointestinal tract. Klin Wochenschr. 1984;62:399–405. doi: 10.1007/BF01742296. [DOI] [PubMed] [Google Scholar]
- Shotton HR, Lincoln J. Diabetes only affects nitric oxide synthase containing myenteric neurons that do not contain heme oxygenase 2. Brain Res. 2006;1068:248–256. doi: 10.1016/j.brainres.2005.11.057. [DOI] [PubMed] [Google Scholar]
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- Spangeus A, El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes) Histol Histopathol. 2001;16:159–165. doi: 10.14670/HH-16.159. [DOI] [PubMed] [Google Scholar]
- Spangeus A, Suhr O, El-Salhy M. Diabetic state affects the innnervation of gut in an animal model of human type 1 diabetes. Histol Histopathol. 2000;15:739–744. doi: 10.14670/HH-15.739. [DOI] [PubMed] [Google Scholar]
- Stenkamp-Strahm CM, Kappmeyer AJ, Schmalz JT, et al. High-fat diet ingestion correlates with neuropathy in the duodenum myenteric plexus of obese mice with symptoms of type 2 diabetes. Cell Tissue Res. 2013a;354:381–394. doi: 10.1007/s00441-013-1681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp-Strahm C, Patterson S, Boren J, et al. High-fat diet and age dependent effects on enteric glial cell populations of mouse small intestine. Auton Neurosci basic Clin. 2013b:1–12. doi: 10.1016/j.autneu.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran S, Kondapaka SB. Altered expression of neuronal nitric oxide synthase in the duodenum longitudinal muscle-myenteric plexus of obesity induced diabetes mouse: implications on enteric neurodegeneration. Biochem Biophys Res Commun. 2005;338:919–922. doi: 10.1016/j.bbrc.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Tan LL, Bornstein JC, Anderson CR. The neurochemistry and innervation patterns of extrinsic sensory and sympathetic nerves in the myenteric plexus of the C57Bl6 mouse jejunum. Neuroscience. 2010;166:564–579. doi: 10.1016/j.neuroscience.2009.12.034. [DOI] [PubMed] [Google Scholar]
- Thrasivoulou C, Soubeyre V, Ridha H, et al. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–257. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- Voss U, Sand E, Olde B, Ekblad E. Enteric neuropathy can be induced by high fat diet in vivo and palmitic acid exposure in vitro. PLoS One. 2013;8:e81413. doi: 10.1371/journal.pone.0081413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss U, Turesson MF, Robaye B, et al. The enteric nervous system of P2Y13 receptor null mice is resistant against high-fat-diet- and palmitic-acid induced neuronal loss. Purinergic Signal. 2014 doi: 10.1007/s11302-014-9408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voukali E, Shotton HR, Lincoln J. Selective responses of myenteric neurons to oxidative stress and diabetic stimuli. Neurogastroenterol Motil. 2011;23:964–e411. doi: 10.1111/j.1365-2982.2011.01778.x. [DOI] [PubMed] [Google Scholar]
- Wade PR. Aging and neural control of the GI tract. I. Age-related changes in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2002;283:G489–G495. doi: 10.1152/ajpgi.00091.2002. [DOI] [PubMed] [Google Scholar]
- Wang X, Pitchumoni CS, Chandrarana K, Shah N. Increased prevalence of symptoms of gastroesophageal reflux diseases in type 2 diabetics with neuropathy. World J Gastroenterol. 2008;14:709–712. doi: 10.3748/wjg.14.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil. 2014;26:611–624. doi: 10.1111/nmo.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.