Abstract
Background
Information technology is transforming healthcare communication. Using smartphones to remotely monitor incisional wounds via digital photos as well as collect post-operative symptom information has the potential to improve patient outcomes and transitional care. We surveyed a vulnerable patient population to evaluate smartphone capability and willingness to adopt this technology.
Methods
We surveyed 53 patients over a 9-month period on the vascular surgery service at a tertiary-care institution. Descriptive statistics were calculated to describe survey item response.
Results
94% (50 out of 53) of recruited patients participated. The cohort was 50% female, and the mean age was 70 years old (range: 41–87). The majority of patients owned cellphones (80%) and 23% of these cellphones were smartphones. 90% of patients had a friend or family-member that could help take and send photos with a smartphone. 92% of patients reported they would be willing to take a digital photo of their wound via smartphone (68% daily, 22% every-other day, 2% less than every-other day, 8% not at all). All patients reported they would be willing to answer questions related to their health via smartphone. Patient’s identified several potential difficulties with regard to adopting a smartphone wound-monitoring protocol including logistics related to taking photos, health-related questions, and coordination with caretakers.
Conclusions
Our survey demonstrates that an older patient cohort with significant comorbidity is able and willing to adopt a smartphone-based post-operative monitoring program. Patient training and caregiver participation will be essential to the success of this intervention.
Keywords: Telehealth, Telemedicine, Remote technology, mHealth, Transitions in care, Wound care
1. Introduction
The use of telemedicine applications for remote diagnosis and treatment of various health problems is expanding, but implementation of these techniques for transitional care remains relatively limited owing to 1) system and organizational barriers as well as 2) human factors, including patient and doctor engagement.[1] Notwithstanding, interest in telemedicine is growing, primarily because of its potential to address longstanding issues within the health care system including facilitation of patient-provider communication, patient self-management, and the coordination of care across settings.[1]
Post-discharge surveillance of surgical site infection (SSI) and other wound complications is an area of surgical care that stands to benefit from the potential of telemedicine. SSI is the most common nosocomial infection in surgical patients and accounts for 38% of post-operative complications.[2,3] SSI can lead to reoperation, limb loss, or death,[4,5] dramatically increasing health care costs.[6,7] Furthermore, these complications are the leading cause of unplanned, potentially preventable hospital readmissions for surgical patients.[2,4,8,9]
A large proportion of severe wound complications develop after hospital discharge.[2] This is in-part due to shorter lengths of hospitalization, but also because patients rarely recognize the early-stages of a wound infection.[10,11] This absence in clinician monitoring after hospital discharge reveals the potential for a transitional care photo-based telemedicine application. The information contained within a digital wound image may allow diagnose at an early stage, when a SSI can be treated in the outpatient setting with oral antibiotics and wound care, potentially precluding the need for readmission, intravenous antibiotics, and reintervention.
Technologies for wound analysis in the inpatient setting have proven feasible and reliable; interpretation of digital images taken by a photographer of post-operative wounds have been shown to be equivalent to physical examination for determining wound complications.[12–15] However, in contrast to clinician-based inpatient protocols, patient- and caregiver-based outpatient digital wound monitoring protocols for SSI is still an unexplored area and thus patients’ understanding and willingness to use relevant technologies in the post-operative period is poorly understood. This is particularly true for the older surgical patient with multiple comorbidities.
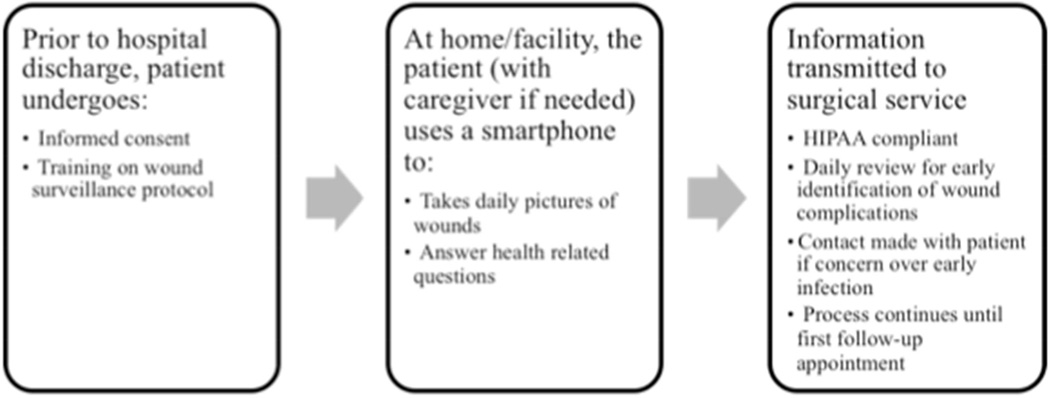
In this study we explore human factors in the context of system and organizational barriers to determine the feasibility of a mobile health (mHealth[16]) smartphone-based intervention for wound monitoring to promote early recognition of wound complications following discharge (fig 1). We surveyed vascular surgery inpatients at a tertiary care center and evaluated smartphone ownership, technological capability, and willingness to adopt new technology in a high-risk, elderly vascular population, for which barriers to adoption might be substantial and the role of caregivers is variable. We focus on vascular surgery because this population has the highest readmission rate among surgical specialties (i.e. approximately 24% in 30-days),[17] and the majority of these readmissions are for treatment of SSI.[5,8]
Figure 1.
Conceptual model of mHealth smartphone-based intervention protocol for wound monitoring to promote early recognition of wound complications following discharge
2. Methods
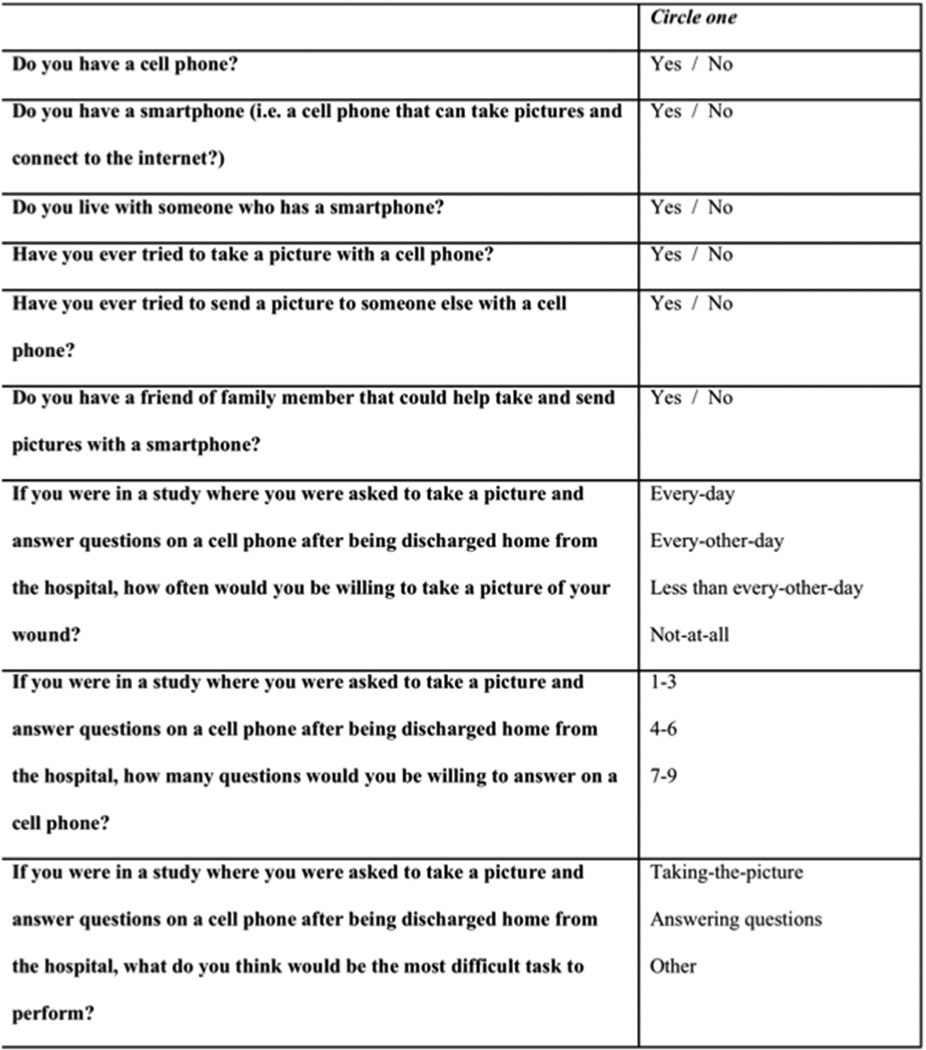
A preliminary draft of the survey was developed and informed by a community-based research advisory focus group: Community Advisors on Research Design and Strategies (CARDS).[18] CARDS are trained focus groups that advise researchers; they reflect the views of racial, ethnic and socioeconomic groups rarely represented in research planning or activities. The CARDS input and feedback provided a “patient’s perspective” that informed survey development. The preliminary set of survey questions were then reviewed by a multidisciplinary team of vascular surgery faculty, surgery residents, and health services researchers who provided a “professional perspective” (fig 2).
Figure 2.
Survey: Post-operative would surveillance
The University of Wisconsin Madison Health Sciences Institutional Review Board approved the finalized survey and associated protocol. The study was conducted from September 2013 to May 2014 at the University of Wisconsin Hospitals and Clinics. The population of interest was vascular surgery inpatients older than 18 years and not in the intensive care unit. Vascular surgery nurse practitioners identified and recruited eligible participants. The survey was administered to each participant in a private hospital room to ensure that the subject's verbal responses and participation were maintained in confidence.
Statistical analysis was performed with SAS software, version 9.3 (SAS Institute Inc., Cary, NC). Descriptive statistics were calculated to describe survey item response. We qualified a cellphone as a smartphone if it had the capability to take digital pictures, carry an operating system capable of running downloaded applications, and connect to the internet. We created a composite variable for patient experience with technology (i.e. an answer of “yes” to having tried taking or sending a picture) and a composite variable for caregiver resourcefulness (i.e. an answer of “yes” to living with someone who owns a smartphone or having someone who could help take and send pictures with a smartphone) and used Fisher’s exact test to examine the relationship between these respective variables.
3. Results
Fifty of 53 recruited vascular surgery inpatients participated in the survey (94% response rate). Of the 50 participating subjects, half were female (n=25). The mean age was 70 years old (range: 41–87).
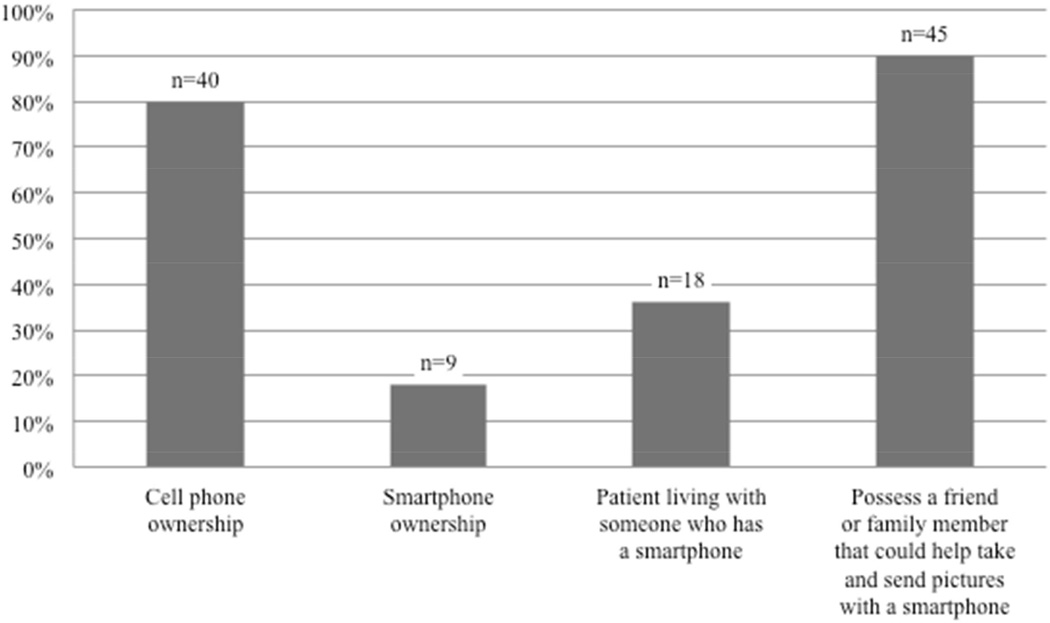
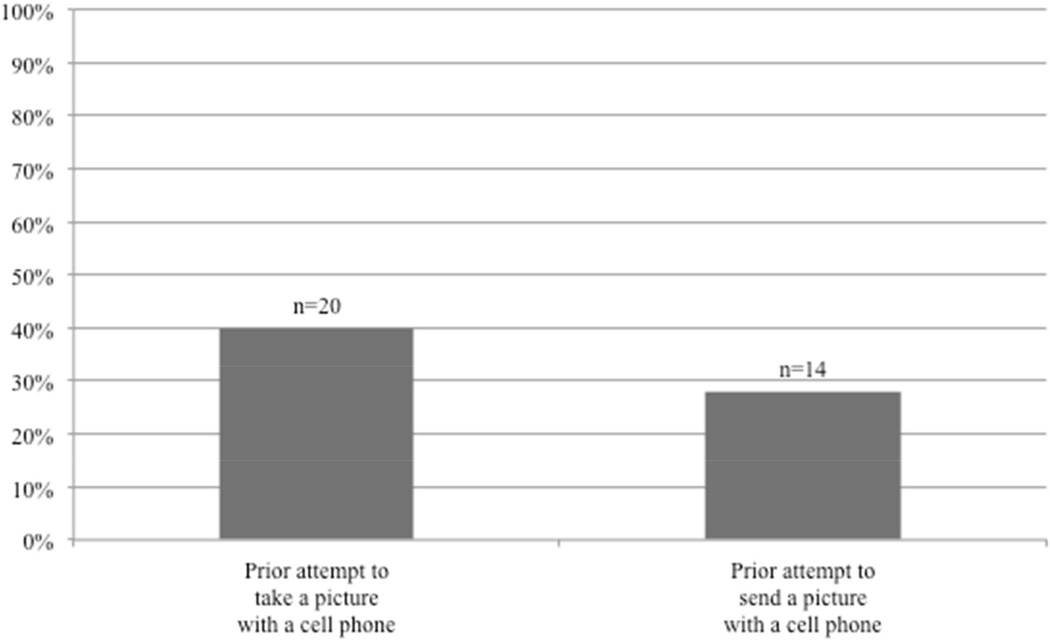
The results for patient ownership, prior experience, and access to mobile technology are presented in figures 3 and 4. The majority of all patients surveyed own cellphones (80%); 23% of these cellphones were smartphones. Younger age was associated with smartphone ownership after controlling for gender (p=0.012). Eighteen patients (36%) were living with someone who owned a smartphone. Twenty patients (40%) had tried to take a picture with a cell phone, and 14 patients (28%) had attempted to send a picture to someone else with a cell phone. Forty-five patients (90%) had a friend or family-member that could help take and send photos with a smartphone.
Figure 3.
Patient ownership and access to mobile technology
Figure 4.
Prior experience with mobile technology
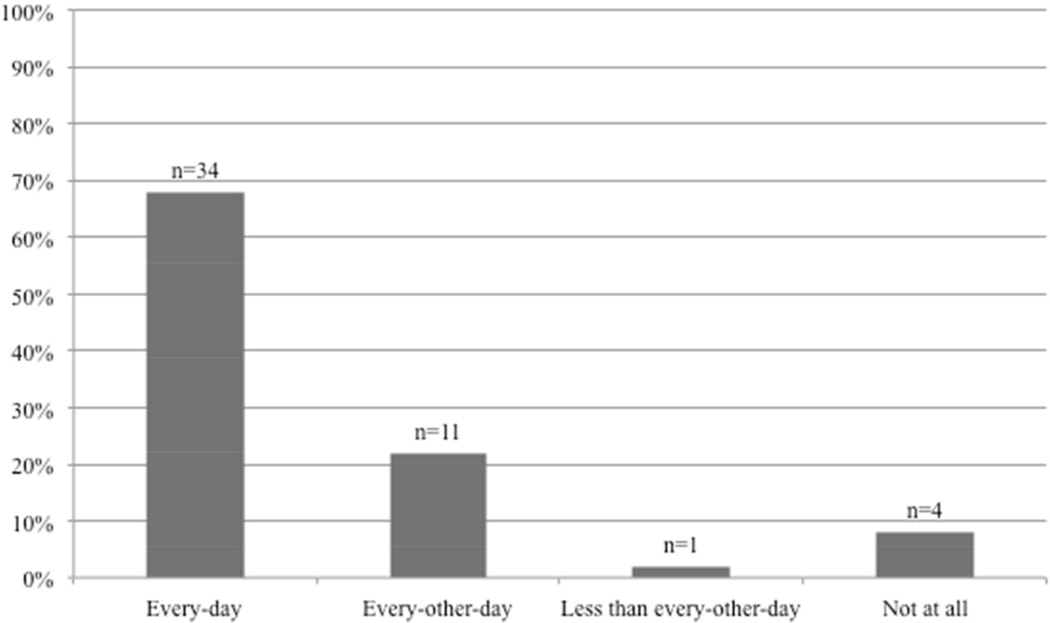
Forty-six patients (92%) reported that they would be willing to take a digital photo of their wound via smartphone (fig 5). Of these 46 patients, 68% reported they would take a picture daily, 22% every-other day, and 2% less than every-other day.
Figure 5.
Patients’ willingness to take a digital photograph of his or her wound using a smartphone
Patient experience with technology was independent of caregiver resourcefulness (p=.07). Out of 30 patients who did not have experience with technology, 25 (83%) had a resourceful caregiver. Only 5 patients (10%) did not have experience with technology and also did not have a resourceful caregiver.
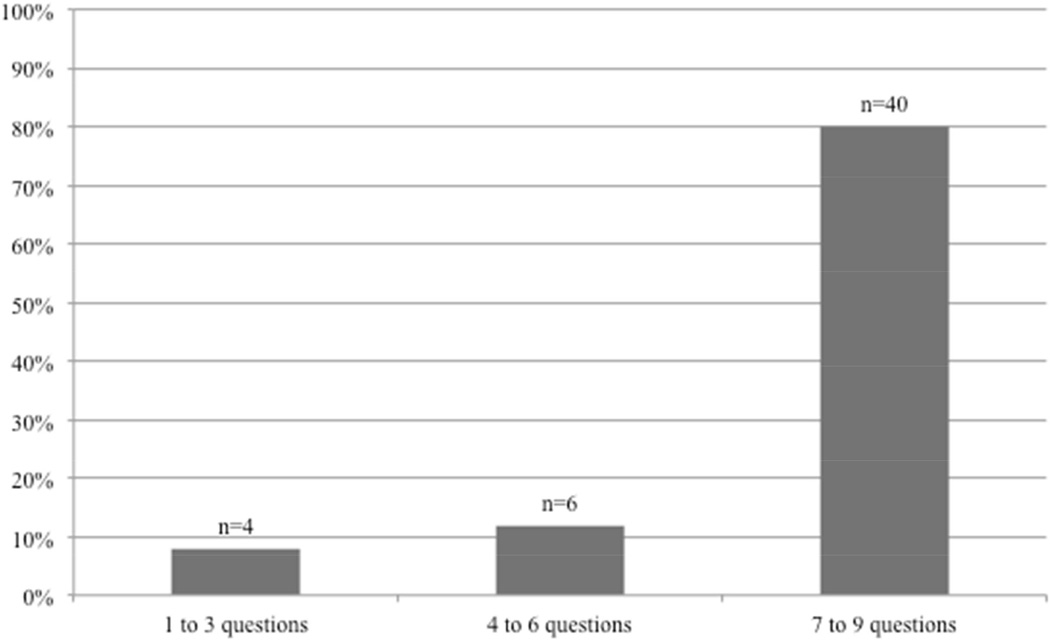
All participants reported a willingness to answer health-related questions via smartphone with the following distribution: 80% were willing to answer between 7 and 9 questions, 12% were willing to answer 4 to 6 questions and 8% were willing to answer a maximum of 3 questions (fig 6).
Figure 6.
Patients’ willingness to answer health-related questions using a smartphone
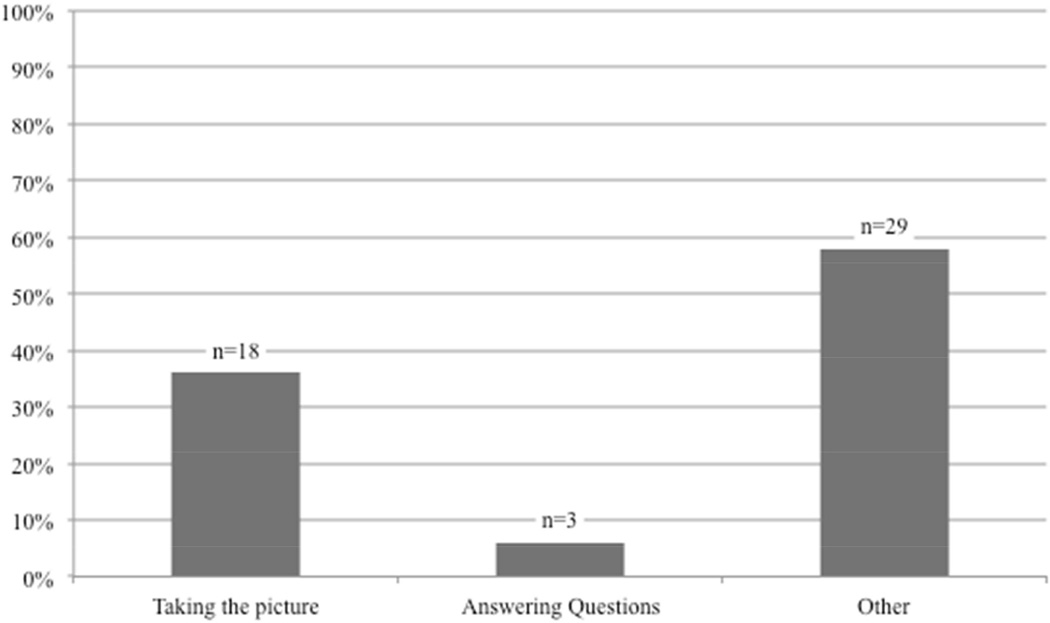
Patient’s identified several potential difficulties with regard to a mHealth approach to post-operative wound management including logistics related to taking photos, interpretation and content of health-related questions, coordination with caretakers, and finding the necessary help to complete the smartphone module (fig 7). Patients voiced concerns about lack of knowledge or experience taking photos with a cellphone and potentially dysfunctional factors (his or her ability to properly visualize the wound-area or uncertainty about their “state-of-mind” after surgery). “Other” concerns about participating in an outpatient wound surveillance program included coordinating a mutual time with a caregiver’s schedule; many patients anticipated that they would need assistance performing the protocol as described in figure 1. Additional areas of potential concern included understanding how to incorporate the protocol into a daily schedule. Only one patient expressed concern about privacy protection during transmission of data from a smartphone to the hospital server.
Figure 7.
Categorization of patient identified potential difficulties with regard to a mHealth approach to post-operative wound management
4. Discussion
The high prevalence of wound complications in surgical patients and the fact that SSI largely develops or progresses in the outpatient setting makes transitional care coordination an important focus of post-operative care; and thus, organized development and implementation of a wound surveillance program has the potential to improve the outcomes of patients suffering from early post-operative wound complications.[2] In the setting of over 5 billion people worldwide owning a cell phone,[16] along with the computing power and paralleled widespread use of smartphones,[19] a mHealth-based approach to outpatient monitoring is inherently attractive. However there is limited data on current system and organizational barriers and a paucity of knowledge around essential human factors specific to implementation of a mHealth-based transitional care wound surveillance protocol. Therefore, we administered a survey to explore patients’ and caregivers’ willingness to utilize the associated technology.
The success of a smartphone photo-based initiative is dependent upon patients’ and caregivers’ willingness to utilize smartphone technology. Our data suggest that surgical patients are motivated to participate in such a program. The majority (92%) of sampled patients were willing to take pictures of their post-operative wounds, and 68% of these patients were willing to perform this task on a daily basis. Furthermore, all that we surveyed were willing to answer basic questions about their health via a smartphone. Our findings are consistent with existing research demonstrating a 90% participation and compliance rate by dermatological patients (mean age: 30 years) for submission of digital images via email of skin lesions for physician review.[20] With the mean age of our surveyed cohort being 70 years old, our results suggest that high levels of participation in image capture and transmission may be transferable after appropriate training in a more elderly population.
The success of a smartphone photo-based initiative is also dependent upon patients’ and caregivers’ reliable access and proper use of smartphone technology. Rate of smartphone ownership is one potential barrier with approximately 1 out of 5 patients in our cohort owning a smartphone. Our results are concordant with national survey data from the Pew Research Center, whose researchers found that only 18% of individuals 65 and older and 39% of individuals between 55 and 64 owned a smartphone.[21] Although adults over age 65 have the lowest rates of smartphone ownership, the number of older adults with smartphones is rising.[22,23] Instead of waiting for smartphone ownership levels to increase to an adequate level, a potential solution to the low rate of smartphone ownership in older patients is to allow a patient to borrow a smartphone from a family-member or a friend, or to provide patients with a loaned smartphone, providing access to the tools needed to participate in the protocol. There is the risk that without these strategies, mHealth might exacerbate existing healthcare disparities in patients at lower socioeconomic levels whom may not able to participate without such support. Furthermore, these same patients who lack smartphone ownership may also lack familiarity with newer technology and thus need considerable training to learn how to use the device. The usability of the smartphone application and associated protocol must incorporate evidence-based approaches in systems design and human factors to ensure the protocol is simple, intuitive, and patient-centered. Furthermore, development of the smartphone application should allow patients and/or caregivers to transmit digital photos seamlessly with care to avoid the complexity of opening multiple smartphone applications or navigating the process of uploading digital content. This will lower the barrier to implementation and avoid the pitfalls of believing technology alone will improve quality without spawning disparities in care.
Patient engagement will be critical to the success of a wound monitoring protocol. The current discharge process consists of a clinician describing the “warning signs” of a wound infection to a patient followed by instruction to seek attention if these signs should appear. This approach is problematic because even after routine instruction, many patients voice concerns of not knowing how to take care of their wound.[24] Moreover, research has shown that many patients do not recognize the early signs of a wound infection.[10,11] Promoting patient engagement may counteract these shortcomings as prior research has shown that engaged patients are more likely than others to participate in preventative practices, self-manage their conditions, and attain superior outcomes.[25,26] Consequently, utilization of a smartphone based protocol as a centering point for which patient engagement can originate may provide a foundation for effective discharge teaching and also by default encourage ongoing communication between providers and patients.
Patient caregiver engagement will also be critical to the success of a wound monitoring protocol, particularly when caregivers are caring for older adult patients. We report that 90% of patients interviewed had a family member or friend that was willing to help him or her take daily pictures of their wound. Involving these caregivers in a monitoring protocol is vital because even after rigorous patient-centered instruction a subset of patients will be unable to take self-wound pictures owing to physical limitations. Examples include a patient with a below-the-knee amputation who is unable to view his stump or an elderly patient suffering from dementia, a condition affecting 11% of individuals age 65 and older.[27,28] Fortunately the prevalence of established caregiving relationships are common; in the United States up to 90% of dependent community-dwelling individuals with acute and chronic illness are provided daily care by family caregivers.[29–31] An additional concern is caregiver proximity; the number of caregivers who are geographically distant is rising, and maintaining daily image capture for individuals requiring caregiver help may not be feasible.[32,33] Thus, creating a protocol that is both patient-centered and caregiver-centered may help address these unique challenges.
Practical concerns of implementing a wound monitoring protocol include provider-level barriers (e.g. provider buy-in, increased clinical workloads) and system-level barriers (e.g. disruption of the existing clinical workflow, integration of mHealth information in medical records). To overcome these barriers, an mHealth wound monitoring protocol design must employ user-centered strategies based on provider-feedback at each clinical level; they also must allow seamless integration of new data into established electronic health records and existing care processes that will facilitate care coordination across settings and promote efficiency. If these barriers are properly addressed, implementation of a mHealth wound monitoring protocol has the ability to decrease surgical morbidity while also reducing costs by means of decreasing patient visits, readmissions and reinterventions. The potential for a telemedicine-based approach is particularly appealing in an accountable care organization environment where hospitals are responsible for post-discharge outcomes.[34]
Another practical concern for both patients and providers is data protection.[35] Protocol design must ensure the upmost privacy and security within daily workflow and adhere to Health Information Portability and Accountability Act (HIPAA).[36] Four stages at which security is critical (and methods for addressing these issues) are as follows: (1) patient consent and training procedures (standardized protocol teaching and patient education on security risks), (2) smartphone security (password protection; instant transmission of the digital photos/data without storage on smartphone device), (3) security of transmitted photos and information (encryption of images/data), and (4) security once the transmission reaches hospital servers (digital data protection and continuous monitoring of medical record for tampering). Addressing these fundamental security and privacy issues will entrust patients and practitioners, ensuring safety of electronic health information.
As we continue researching proper development of a mHealth smartphone-based intervention for wound monitoring after hospital discharge, it is essential to incorporate observations from our analysis with those in the field who have previously shown effectiveness in implementing telemedicine and technology on a large scale. For example, many lessons can be learned from the Veterans Affairs hospital system, which has been a pioneer in telemedicine and has successfully implemented numerous patient portals for over 1.8 million individuals accessing virtual care.[37,38] The Veterans Affairs hospital system has also incorporated data such as serial record of vital signs and secure messaging in their protocols for improving diagnostic ability.[38] As future iterations of our mHealth smartphone-based intervention are developed and trialed, incorporating these lessons and technologies will enrich the care delivered to an expanding number of patients.
There are several limitations to our study. First, our survey included a small number of questions. The questions were created following consultation with community-dwelling adults as well as with health care professionals whom were mindful to minimize the response burden on subjects who would be taking the survey (i.e. post-operative vascular surgery inpatients). Nevertheless we believe these questions capture the relevant content needed to inform the design of a mHealth intervention to monitor wounds. Second, we did not explore specific barriers to implementation or potential solutions. For example, we did not specifically inquire about a patients’ ability to follow a standard protocol (same setting, same lighting, etc). Third, our sampled population may limit generalizability. The patients interviewed were vascular surgery patients and may not be representative of the overall surgical population. Our survey was administered in a tertiary care center that acts as a referral center for neighboring counties, and therefore may not be representative of hospitals absent these characteristics. Furthermore, the majority of patients, although representative of varying levels of socioeconomic position, were English-speaking and primarily of white race. Most importantly, the survey was conducted among patients who were of pre-discharge status and may not accurately anticipate their post-discharge capabilities; although many of the patients interviewed had surgery previously, their responses represented a hypothetical situation. Lastly, only patients (rather than caregivers) were interviewed, and the survey results may not fully represent the capability of the “team” who will ultimately participate in a smartphone intervention.
5. Conclusions
In summary, a mHealth-based wound surveillance program using smartphone digital photos has the potential to improve the outcomes of patients who develop early post-operative wound complications following discharge. Our survey demonstrates that an older patient cohort with significant comorbidity is able and willing to adopt a smartphone-based post-operative monitoring program. These preliminary results are encouraging regarding the potential mHealth has to decrease the burden associated with wound infections and complications, even among patient populations with significant disabilities and low rates of familiarity with technology.
Acknowledgments
We would like to thank Mary Randel NP and Lauren Dallman NP for their assistance in identification of patient subjects.
Funding Sources
Dr Wiseman is supported by the National Institutes of Health T32 University of Wisconsin Research Training in Vascular Surgery grant HL110853. Dr Kent and Dr Fernandes-Taylor are supported by an AHRQ R21 grant HS 023395.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Jason T. Wiseman contributed to study conception and design, data collection, statistical analysis, analysis and interpretation, writing the article, critical revision of the article, and final approval of the article. Sara Fernandes-Taylor contributed to study conception and design, statistical analysis, analysis and interpretation, writing the article, critical revision of the article, and final approval of the article. Maggie L. Barnes contributed to study conception and design, data collection, critical revision of the article, and final approval of the article. Adela Tomsejova contributed to writing the article, critical revision of the article, and final approval of the article. R. Scott Saunders contributed to study conception and design, data collection, analysis and interpretation, and final approval of the article. K. Craig Kent contributed to study conception and design, data collection, analysis and interpretation, writing the article, critical revision of the article, and final approval of the article.
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
References
- 1.Bashshur R, Shannon G, Krupinski E, Grigsby J. Sustaining and Realizing the Promise of Telemedicine. Telemed E-Health. 2013;19:339–345. doi: 10.1089/tmj.2012.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Am J Infect Control. 1999;27:97–134. [PubMed] [Google Scholar]
- 3.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 4.Kirkland K, Briggs J, Trivette S. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 5.Engelbert TL, Fernandes-Taylor S, Gupta PK, Kent KC, Matsumura J. Clinical characteristics associated with readmission among patients undergoing vascular surgery. J Vasc Surg. 2014;59:1349–1355. doi: 10.1016/j.jvs.2013.10.103. [DOI] [PubMed] [Google Scholar]
- 6.Gibson A, Tevis S, Kennedy G. Readmission after delayed diagnosis of surgical site infection: a focus on prevention using the American College of Surgeons National Surgical Quality Improvement Program. Am J Surg. 2014;207:832–839. doi: 10.1016/j.amjsurg.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173:2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 8.Wiseman J, Guzman A, Fernandes-Taylor S, Engelbert T, Saunders R, Kent K. General and vascular surgery readmissions: A systematic review. J Am Coll Surg. 2014;219:552–569. doi: 10.1016/j.jamcollsurg.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitby M, McLaws M-L, Collopy B, Looke DFL, Doidge S, Henderson B, et al. Post-discharge surveillance: can patients reliably diagnose surgical wound infections? J Hosp Infect. 2002;52:155–160. doi: 10.1053/jhin.2002.1275. [DOI] [PubMed] [Google Scholar]
- 11.Seaman M, Lammers R. Inability of patients to self-diagnose wound infections. J Emerg Med. 1991;9:215–219. doi: 10.1016/0736-4679(91)90416-d. [DOI] [PubMed] [Google Scholar]
- 12.Murphy RX, Bain Ma, Wasser TE, Wilson E, Okunski WJ. The reliability of digital imaging in the remote assessment of wounds: defining a standard. Ann Plast Surg. 2006;56:431–436. doi: 10.1097/01.sap.0000202146.92893.6a. [DOI] [PubMed] [Google Scholar]
- 13.Wirthlin DJ, Buradagunta S, Edwards Ra, Brewster DC, Cambria RP, Gertler JP, et al. Telemedicine in vascular surgery: feasibility of digital imaging for remote management of wounds. J Vasc Surg. 1998;27:1089–1099. doi: 10.1016/s0741-5214(98)70011-4. discussion 1099–100. [DOI] [PubMed] [Google Scholar]
- 14.Engel H, Huang J, Tsao C, Lin C, Chou P, Brey E, et al. Remote real-time monitoring of free flaps via smartphone photography and 3G wireless internet: A prospective study evidencing diagnostic accuracy. Microsurgery. 2011;31:589–595. doi: 10.1002/micr.20921. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Pedersen PC, Strong D, Tulu B, Agu E. Wound image analysis system for diabetics. Med Imaging. 2013;8669:1–14. [Google Scholar]
- 16.Hampton T. Recent Advances in Mobile Technology Benefit Global Health, Research, and Care. J Am Med Assoc. 2012;307:2013–2014. doi: 10.1001/jama.2012.4465. [DOI] [PubMed] [Google Scholar]
- 17.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 18.Wisconsin Network for Research Support | Innovative Community Engagement Services for Researchers and Educators. [accessed October 13, 2014]; n.d. http://winrs.son.wisc.edu/ [Google Scholar]
- 19.Boulos MNK, Wheeler S, Tavares C, Jones R. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. Biomed Eng Online. 2011;10:24. doi: 10.1186/1475-925X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimner T, Blozik E, Fischer Casagrande B, Von Overbeck J. Digital skin images submitted by patients: an evaluation of feasibility in store-and-forward teledermatology. Eur J Dermatol. 2010;20:606–610. doi: 10.1684/ejd.2010.1019. [DOI] [PubMed] [Google Scholar]
- 21.Smith A. Smartphone Ownership – 2013 Update. Washington, DC: 2013. [Google Scholar]
- 22.Mobile Health Market Trends and Figures 2013–2017. [accessed December 1, 2014];Res Mark. 2013 http://www.researchandmarkets.com/reports/2603873/mobile_health_trends_and_figures_20132017. [Google Scholar]
- 23.Blodget H. Actually, The US Smartphone Revolution Has Entered The Late Innings. Bus Insid. [accessed December 1, 2014]; n.d. http://www.businessinsider.com/blodget-the-us-smartphone-revolution-is-already-entering-the-late-innings-2012-9. [Google Scholar]
- 24.Pieper B, Sieggreen M. Discharge knowledge and concerns of patients going home with a wound. J Wound Ostomy Cont Nurs. 2007;34:245–253. doi: 10.1097/01.WON.0000270817.06942.00. [DOI] [PubMed] [Google Scholar]
- 25.Ricciardi L, Mostashari F, Murphy J, Daniel JG, Siminerio EP. A national action plan to support consumer engagement via e-health. Health Aff (Millwood) 2013;32:376–384. doi: 10.1377/hlthaff.2012.1216. [DOI] [PubMed] [Google Scholar]
- 26.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood) 2013;32:207–214. doi: 10.1377/hlthaff.2012.1061. [DOI] [PubMed] [Google Scholar]
- 27.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Chicago: 2013. [DOI] [PubMed] [Google Scholar]
- 29.Mitnick S, Leffler C, Hood VL. Family caregivers, patients and physicians: ethical guidance to optimize relationships. J Gen Intern Med. 2010;25:255–260. doi: 10.1007/s11606-009-1206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feinberg L. Options for supporting family caregivers. A policy paper of the Family Caregiver Alliance. 1997 [Google Scholar]
- 31.Levine C. Introduction to family caregiving: current challenges for a time honored practice. J Am Soc Aging. 2003;27:5–8. [Google Scholar]
- 32.National Alliance for Caregiving and AARP. Caregiving in the U.S. [accessed October 2, 2014];:1–85. n.d. http://www.caregiving.org/data/04finalreport.pdf. [Google Scholar]
- 33.Benefield LE, Beck C. Reducing the distance in distance-caregiving by technology innovation. Clin Interv Aging. 2007;2:267–272. [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher ES, Shortell SM. Accountable care organizations: Accountable for What, to Whom, and How. J Am Med Assoc. 2010;304:1715–1716. doi: 10.1001/jama.2010.1513. [DOI] [PubMed] [Google Scholar]
- 35.Hassol A, Walker J, Kidder D. Patient experiences and attitudes about access to a patient electronic health care record and linked web messaging. J Am Med Informatics Assoc. 2004;11:505–513. doi: 10.1197/jamia.M1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Department of Health & Human Services. Health Information Privacy. [accessed September 28, 2014]; n.d. http://www.hhs.gov/ocr/privacy/
- 37.United States Department of Veterans Affairs. VA Telehealth Services. [accessed April 2, 2015];2014 http://www.telehealth.va.gov.
- 38.United States Department of Veterans Affairs. [accessed April 7, 2015];Executive summary. 2014 :1–57. http://www.va.gov/budget/docs/report/2014-VAparPartI.pdf.