Abstract
Prevention of seasonal influenza epidemics and pandemics relies on widespread vaccination coverage to induce protective immunity. In addition to a good antigenic match with the circulating viruses, the effectiveness of individual strains represented in the trivalent vaccines depends on their immunogenicity. In this study we evaluated the immunogenicity of H1N1, H3N2 and B seasonal influenza virus vaccine strains delivered individually with a novel dissolving microneedle patch and the stability of this formulation during storage at 25°C. Our data demonstrate that all strains retained their antigenic activity after incorporation in the dissolving patches as measured by SRID assay and immune responses to vaccination in BALB/c mice. After a single immunization all three antigens delivered with microneedle patches induced superior neutralizing antibody titers compared to intramuscular immunization. Cutaneous antigen delivery was especially beneficial for the less immunogenic B strain. Mice immunized with dissolving microneedle patches encapsulating influenza A/Brisbane/59/07 (H1N1) vaccine were fully protected against lethal challenge by homologous mouse-adapted influenza virus. All vaccine components retained activity during storage at room temperature for at least three months as measured in vitro by SRID assay and in vivo by mouse immunization studies. Our data demonstrate that dissolving microneedle patches are a promising advance for influenza cutaneous vaccination due to improved immune responses using less immunogenic influenza antigens and enhanced stability.
Keywords: Dissolving microneedle patches, Metal microneedle arrays, Skin immunization, Influenza vaccines, Mouse model
INTRODUCTION
The standard influenza vaccination is an intramuscular injection of antigens matching the predicted circulating influenza A (H1N1 and H3N2) and influenza B virus strains. The drawbacks of this delivery approach are mainly requirement for cold chain and poor patient compliance. An improved alternative is skin immunization with microneedles which present several immunologicaland logistical advantages when compared to the conventional vaccination route.
Application of microneedle patches to the skin takes advantage of skin resident innate immune cell populations. Although microneedles alone are involved in mechanically-induced inflammation resulting from skin penetration, delivery of an antigen recruits resident antigen presenting cells (APCs) including keratinocytes, Langerhans cells and dermal dendritic cells to the insertion site. Localized inflammatory events are initiated due to chemokine and cytokine secretion by APCs proximal to the site of penetration hence increasing the magnitude of the immune response [1–3].
In the past 10 years microneedles have been extensively investigated for delivery of drugs and vaccines by several groups [4–6] and more recently they are being explored as a diagnostic tool [7–9]. In the field of skin immunization microneedles have shown to present several immunological advantages when compared to conventional routes of delivery for vaccines against influenza [10, 6], Hepatitis B [11], malaria [12], polio [13], West Nile virus and Chikungunya [14] and a growing list of bacterial pathogens such as anthrax, diphtheria, tetanus, tuberculosis, botulism, plague, and staphylococcal toxin [15, 16].
In addition to its immunological advantages microneedles present several logistical advantages that can improve vaccination coverage such as safety and simplified disposal due to elimination of biohazard sharps; ease of distribution particularly during outbreaks and epidemics; requirement of minimally trained personel; minimal discomfort that can increase acceptability of vaccination. Experiments with human volunteers demonstrated very low pain scores from microneedle application as compared to hypodermic needles, and fast skin resealing after removal of microneedles [17–20].
Influenza skin vaccination eliciting robust immune responses has been demonstrated with whole inactivated virus, virus-like particles, subunit vaccines, recombinant HA proteins as well as DNA vaccines coated onto metal microneedles [21–25]. Vaccine delivery using microneedle patches has been shown to result in antigen dose sparing [26, 27] and increased longevity of immunity when compared to systemic immunization [22, 24].
An enhanced immune response would be especially desirable in the case of low immunogenicity strains, such as theB/Brisbane/60/2008 component in 2009–2010 vaccine [28]. In addition, enhanced longevity of immune responses would compensate for the waning protection during the influenza season [29]. It has also been proposed that high risk groups such as young children, older adults and immunocompromized populations will benefit the most from influenza skin vaccination [22, 30] as shown by the improved immune responses to intradermal vaccination in some of these groups [31–33].
Currently, the only available FDA approved intradermal influenza vaccine is the BD Soluvia system. However, this system is associated with the same disadvantages as an intramuscular flu shot and mainly the requirement for cold chain. In contrast, a dry formulation in microneedle patches could offer practical benefits such as thermostability and independence of cold chain which is required for liquid formulations [34–38].
While dissolving microneedle patch formulations have been used to deliver commercial trivalent influenza vaccine to mice and guinea pigs demonstrating robustness of immune response and hence the validity of the technology, the effects of formulation on each vaccine component were not reported [39–42]. In addition, some dissolving formulations were found to be non- compatible with the single radial diffusion (SRID) vaccine potency assay [43] which is required in the field.
Here we introduce a novel dissolving microneedle patch formulated with the natural polymer gelatin, and assess the immunogenicity of each of the trivalent influenza vaccine components (H1N1, H3N2 and B) in mice in comparison to vaccination by intramuscular injection (IM) which is the recommended vaccination approach in humans. The efficacy of dissolving microneedle patch encapsulating the vaccine components is also compared to mMN coated with each one of these components that have been previously shown to induce potent and long-lived protective immune responses.
MATERIALS AND METHODS
Cells and virus stocks
Madin-Darby canine kidney (MDCK) cells (ATCC, Manassas, VA) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Thermo, Rockford, IL). Influenza virus stocks A/Brisbane/59/2007 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008, generously provided by CDC, were propagated in MDCK cells. The hemagglutination (HA) activity was determined using the WHO protocol [44]. A/Brisbane/59/07 virus was mouse-adapted by serially passaging in lungs of BALB/c mice [45]. The LD50 was determined using the Reed-Munch formula [46] and the viral titer was determined by plaque assay [47].
Vaccine processing
Egg-grown subunit monovalent influenza vaccines were kindly provided by Novartis Vaccines and Diagnostics (Cambridge, MA). The monovalent A/Brisbane/59/2007 strain was concentrated and further lyophilized with 5% w/v sucrose at Novartis Vaccines [48]. Lyophilized vials were reconstituted with 100 mM dibasic potassium phosphate buffer (pH 7.4) prior to use. Liquid monobulk A/Victoria/210/2009 (A/Perth/16/2010-like virus) and B/Brisbane/60/2008 lots were concentrated and buffer exchanged with 100 mM dibasic potassium phosphate using spin filters (Amicon, Billerica, MA and Vivaspin, Sartorius Stedium, Germany). Protein concentration was measured using bicinchoninic acid assay (BCA) with bovine serum albumin as the standard (Thermo, Massachusetts, USA). Hemagglutinin content was measured by single radial immunodiffusion (SRID).
Metal microneedle arrays coated with vaccine
Stainless steel microneedles were fabricated as previously described [49, 50]. Briefly, microneedles were fabricated from stainless steel sheets (Trinity Brand Industries, SS 304, 75 μm thick; McMaster-Carr, Atlanta, GA) by laser cutting. Microneedles were electropolished (model no. E399-100, ESMA, IL) for deburring, cleaning and sharpness in a solution containing glycerin, ortho-phosphoric acid (85%) and deionized water in a ratio of 6:3:1 by volume (Fisher Scientific, Fair Lawn, NJ). The final microneedle geometry was a linear array of five needles with a needle-to-needle spacing of 1575 μm. The concentrated vaccines were combined with an equal volume of coating solution composed of 2% w/v carboxymethylcellulose (CMC) and 30% w/v trehalose dihydrate (Sigma Aldrich, St. Louis, MO). Microneedles were coated by repeated dip-coating into the antigen coating solution using a custom-built coating instrument [49]. The vaccine load per array was estimated with SRID assay and the amount of required vaccine dose per mouse was adjusted by cutting extra needles from the array.
Polymer microneedle patches containing influenza vaccine
Polymer microneedle patches (pMN) were prepared by a two-step micro-molding process. The vaccine formulation consisting of concentrated monovalent vaccine, sucrose, fish gelatin and sulforhodamine B dye (Sigma Aldrich) in 100 mM dibasic potassium phosphate buffer pH 7.4 was cast onto a PDMS mold (100 microneedles per array; each microneedle measuring 700 μm in length and 200 μm in width at the base. Vacuum was applied to ensure that the formulation filled the entire microneedle cavity and the formulation was allowed to air dry at room temperature overnight.
In the second step, the backing formulation consisting of fish gelatin and sucrose in 100 mM dibasic potassium phosphate buffer pH 7.4 was cast onto the mold under vacuum and subsequently dried at room temperature overnight before demolding the microneedle patch. This drying protocol for fish gelatin microneedles has consistently given sharp and robust structures. The patches had a moisture content less than 2% as tested with the Karl Fischer titration method [51] and were stored with a dessicant in individuals pouches. The strength of needles was checked before and after short-term insertion in pig skin explant for bending and brittleness under the microscope. The patches were adjusted to obtain a final dose of 3 μg per patch and further mounted onto a 1 cm2 paper backing for insertion.
For vaccine stability and delivery studies the patches were prepared by the same protocol except that the dye was not added. The patches were stored at + 25°C in sealed aluminum pouches with desiccant until use. Three patches of each strain were analyzed by SRID at five time points within a three month storage period (days 1, 5, 31, 57 and 81).
Single Radial Immunodiffusion (SRID) assay
Antigen content in vaccine monobulks, pMN and mMN was quantified by SRID assay based on published protocols [52, 53] with modifications for smaller RID plates (Binding Site Group, Birmingham, UK). Reference reagents were obtained from the Center for Biologics Evaluation and Research (Kensington, MD). Antigen from pMN and coated mMN was extracted in Dulbeccos’s phosphate-buffered saline without calcium and magnesium (dPBS) (Mediatech, Manassas, VA). Gelatin was added to the standards to match its concentration in patch extracts to avoid misinterpretation of data from gelatin interference. SRID rings were measured with a digital RID plate reader model AD400 (Binding Site, Birmingham, UK). Hemagglutinin content in both liquid formulations and in patch extracts was calculated from the calibration curve constructed with six duplicate dilutions of standards. Three pMN of each strain were analyzed each in triplicate and averaged; antigen dissolved from five coated mMNof each strain was pooled for analysis.
Animals
Six- to eight weeks old female BALB/c mice (Charles River Laboratory, Wilmington, MA) were used for this study. Infections were performed in a Biosafety Level 2 facility at Emory University (Atlanta, GA). All animal studies were approved by the IACUC at Emory University.
Immunization, challenge and sample collection
Groups of five mice where immunized with a single dose containing on average 3 μg of HA of monovalent vaccine (H1N1, H3N2 or B) either formulated in the pMN or coated onto mMN, or intramuscularly. For cutaneous immunization, an area on the dorsal side of the mice was prepared by hair trimming and treatment with depilatory cream (Nair, Church and Dwight, Ewing, NJ). Metal microneedle arrays or pMN were manually inserted into skin and held in place for 5 minutes or for 10 minutes, respectively, to allow dissolution of the coating or dissolving microneedles in skin. For intramuscular immunizations, groups of mice received either untreated vaccine or concentrated vaccine with the excipients used for microneedle fabrication (sucrose, gelatin and dye) at similar amounts as in dissolving microneedles. Vaccines were diluted in dPBS and a final dose of 3 μg of HA was injected into the upper quadrant of the hind leg. Blood was collected at weeks 2, 4, and 10 weeks post-immunization and serum was stored at −20°C until analysis. For the vaccine stability study, mice were vaccinated once with A/Brisbane/59/2007 vaccine formulated in dissolving microneedle patches stored for 3 months at room temperature or intramuscularly with the patch vaccine extract, and bled on days 0, 14 and 28. At week 11 post immunization, groups of mice were challenged intranasally with 5xLD50 of mouse-adapted influenza A/Brisbane/59/07 virus under isoflurane anesthesia. In challenge studies, animals were monitored daily for morbidity (body weight loss, hunched posture, ruffled hair and decreased mobility) and mortality for 2 weeks. Mice reaching 75% of their expected weight were euthanized according to IACUC guidelines.
Delivery efficiency of polymer microneedle patches
For the vaccine delivery study freshly prepared dissolving pMN (n=6) encapsulating A/Brisbane/59/2007 monovalent vaccine were cut in halves. One half was manually applied to previously depilated mouse skin, firmly held in place for 1 minute and left on skin for total 10 minutes while the other half was stored until use. The patches were applied on either dry or pre-wetted with distilled water skin; both unused and used halves were extracted in the same volume of PBS and analyzed by SRID for HA content as shown in Supplementary Fig. 1S.
Humoral immune responses
Virus-specific antibody levels in blood were determined by ELISA as previously described [45] using Nunc 96-well Maxisorb plates (Rochester, NY) coated with 100 ng total protein of untreated vaccine per well. Hemagglutination inhibition (HAI) titers were assessed based on the WHO protocol [44] and neutralizing antibody titers were determined using heat-inactivated mouse sera by microneutralization for A/Brisbane/59/2007 and A/Victoria/210/2009 as described previously [24] or by plaque reduction for B/Brisbane/60/2008 [47].
Statistics
The statistical significance was calculated for selected groups by two-tailed unpaired Students t-test and p ≤ 0.05 was considered significant. HAI and NT titers were converted to log2 titers for statistical analysis. One-way ANOVA with Dunnett post hoc test was applied to the analysis of antigen stability during pMN storage. Unless otherwise stated the antibody assays (ELISA, HAI, microneutralization) were at least duplicated,
RESULTS
Vaccine processing, loading and delivery
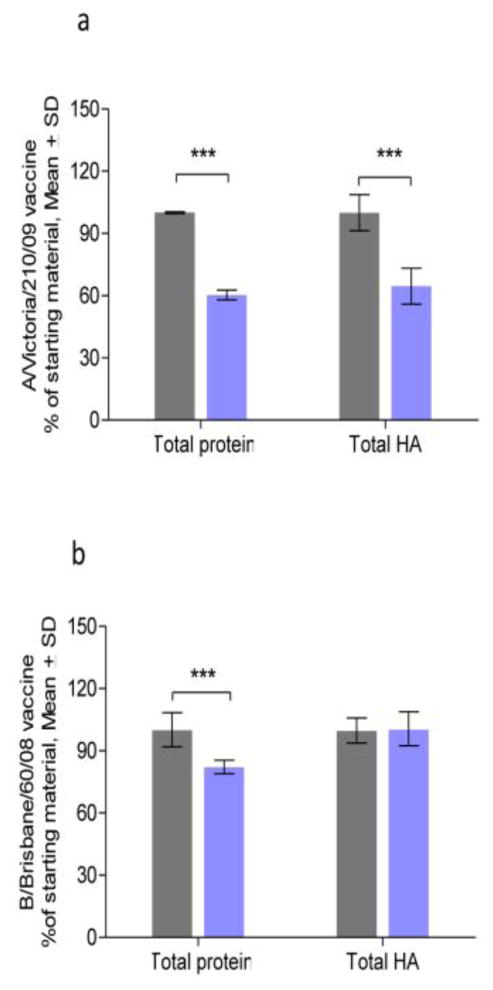
Since the loading capacity of the molds used to make pMN is limited to ≤1 μl in volume per needle, vaccines were concentrated prior to loading. A/Victoria/210/2009 monobulk was concentrated 50-fold with 60% protein retention after processing (Fig. 1a) whereas B/Brisbane/60/2008 was concentrated 123-fold with 82% protein retention (Fig. 1b). We observed that after the concentration step the specific HA activity per unit protein for A/Victoria/210/2009 was increased by 7% and for B/Brisbane/60/2008 by 22% (Table 1, Fig. 1a, b), suggesting removal of non-antigenic, low molecular weight proteins during filtration. The A/Brisbane/59/2007 vaccine was provided as a lyophilized material, which yielded 0.59 μg HA/μg protein after solubilization in potassium phosphate buffer. After concentration and excipient addition the vaccines were incorporated in pMN or coated onto mMN.
Fig. 1. Preservation of total protein and active hemagglutinin content during vaccine processing as estimated by protein (BCA) and SRID assays, respectively.
a) A/Victoria/210/09 vaccine; b) B/Brisbane/60/08 vaccine, HA: hemagglutinin. Gray and blue columns represent original and concentrated monobulks, respectively. (*** p < 0.0001.)
Table 1.
Recovery of Hemagglutinin after concentration of vaccine monobulks
| Vaccine strain | Before concentration (Mean ± SD) | After concentration (Mean ± SD) | ||
|---|---|---|---|---|
|
| ||||
| HA, mg/ml | HASP* | HA, mg/ml | HASP* | |
| A/Victoria/210/09(H3N2) | 0.276 ± 0.024 (n=11) | 0.55 | 13.8 ± 1.8 (n=8) | 0.59 |
| B/Brisbane/60/08 | 0.142 ± 0.013 (n=12) | 0.46 | 17.4 ± 1.3 (n=6) | 0.56 |
Hemagglutinin to protein ratio, [mg/mg]
Antigen content was consistent between patches with an average loading of all strains per pMN 12.13 ± 0.094 μg HA, and 2.5 ± 0.12 μg HA per mMN array (Table 2). Independent experiments studying insertion of dissolving microneedles in murine skin showed that less than 30 % of the vaccine remained in the used patches (Supplementary Fig. 1S and Table 1S), with an efficiency of delivery similar to PVP microneedle patches [54]. There was no clear difference observed between application of patches on wet or dry skin (Table 1S).
Table 2.
Antigen content in dissolving and metal coated microneedles
| Strain | Load per pMN, μg HA (Mean ± SD) | Load per mMN, μg HA |
|---|---|---|
| A/Brisbane 57/09/(H1N1) | 12.2 ± 0.8 (n=3) | 2.67 |
| A/Victoria/210/09(H3N2) | 12.0 ± 0.8 (n=3) | 2.47 |
| B/Brisbane/60/08 | 12.2 ± 0.5 (n=3) | 2.38 |
Humoral responses induced by influenza A (H1N1) vaccine
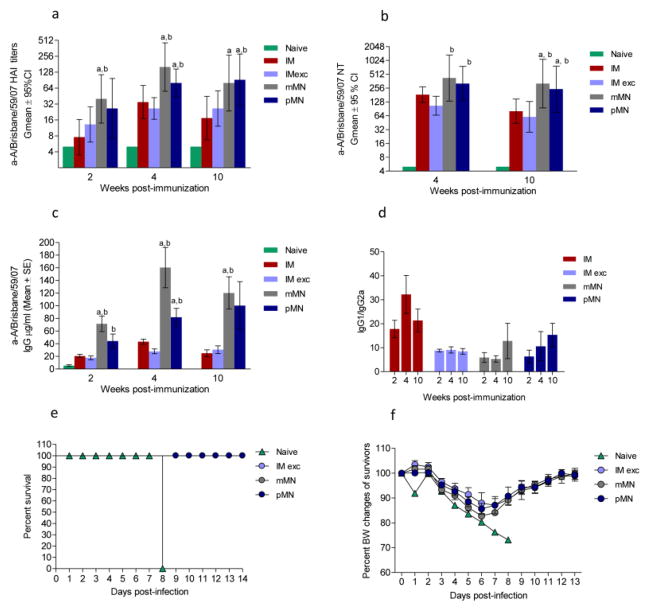
Mice cutaneously vaccinated with H1N1 antigen using pMN or mMN developed about two-fold higher HAI antibody titers than mice immunized intramuscularly with unprocessed vaccine (IM) (p=0.008 mMN vs. IM) or concentrated vaccine mixed with excipients (IM exc.) (p=0.046 mMN vs IM exc.) as early as two weeks post-immunization. The statistically significant differences between these groups were maintained at week 4 (p = 0.040 pMN vs. IM; p=0.004 pMN vs. IM exc; p=0.011 mMN vs. IM; p=0.003 mMN vs. IM exc.) (Fig. 2a). By week 10, the pMN group showed higher antibody titers than the IM (p=0.014) and IM exc. (p=0.034) groups, whereas the mMN group also showed higher titers than the IM group (p=0.025). Similarly, at week 4, the neutralizing titers induced after skin immunization were two to three-fold higher than those observed after intramuscular injection of vaccine mixed with excipients (p=0.015 and 0.014 for mMN and pMN respectively). By week 10 these titers decreased by 50% in the IM and IM exc. groups whereas they decreased by 25% in cutaneously vaccinated mice (Fig. 2b). These findings agree with previous reports that humoral immune responses induced by skin immunization with microneedle patches show improved duration vs. those elicited by intramuscular vaccination [22].
Fig. 2. Humoral immune responses and protection of selected groups against lethal challenge in mice immunized with A/Brisbane/59/07 (H1N1) vaccine.
(a) Anti-A/Brisbane/59/07 HAI titers; (b) Microneutralization titers; (c) Total IgG titers; (d) IgG1 to IgG2a titer ratio. (E) Survival after challenge with homologous virus; (F) Body weight losses of the surviving mice. Naive: unimmunized mice; IM: vaccine administered intramuscularly; IM exc.: intramuscular delivery of vaccine mixed with excipients of the microneedle formulation; mMN: vaccine coated metal microneedles; pMN: dissolving microneedle patches encapsulating the vaccine. ELISA antibody data for ratios represent the mean ± SEM, HAI titers and NT represent the geometric mean ± 95%CI. Asterisks indicate statistical significance of difference between skin and IM groups: ap ≤ 0.05 mMN or pMN compared to the IM group, bp ≤ 0.05 mMN or pMN compared to the IM exc. group at the same post-immunization time points.
The presence of excipients did not significantly affect the outcome of IM vaccination with the H1N1 vaccine strain. Although the A/Brisbane/59/2007-specific total IgG levels of the pMN group were initially only half of those elicited by the mMN group, they steadily increased and reached similar levels by week 10. The IM and IM exc. groups exhibited 3 to 4-fold lower total influenza-specific IgG titers than the skin-immunized groups without any marked increases from week 2 to 10 (Fig. 2c). The IgG1 to IgG2a ratios were similar (p>0.05) among IM exc., mMN and pMN groups, suggesting that the delivery route does not affect the isotype profile at least at early time points (Fig. 2d).
Protective immunity against H1N1 infection
Despite the differences in kinetics of immune responses observed in the vaccinated cohorts, all groups survived lethal challenge with homologous virus almost 3 months after vaccination (Fig. 2e). The body weight losses did not exceed 17% and no significant differences were observed among groups (Fig. 2e). Thus, skin immunization with pMN encapsulating subunit influenza vaccine was as protective as vaccine-coated mMN or IM vaccination.
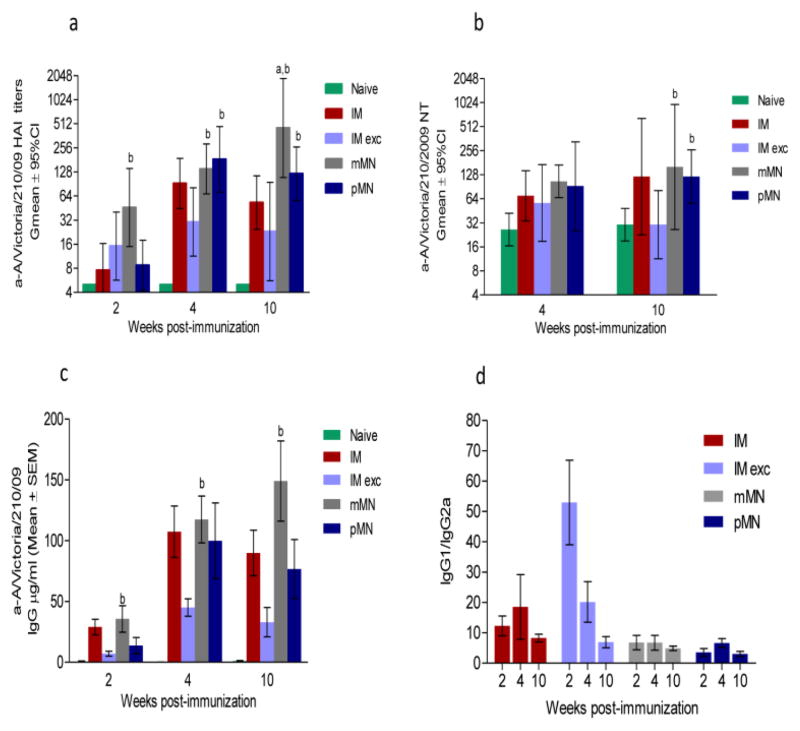
Humoral responses to influenza A H3N2 vaccine
Influenza A (H3N2) viruses generally cause more severe disease than influenza A (H1N1) [55, 56]. Therefore, we examined whether skin immunization can improve immunogenicity of the A/Victoria/210/2009 H3N2 antigen. While at week 2 only the mMN group showed HAI titers above 40 which are generally considered protective, at later time points (weeks 4 and 10) the pMN group displayed consistently 6-fold higher titers (p=0.006 and 0.02) than the IM exc. group (Fig. 3a) whereas the mMN group had up to 20-fold higher titers than the IM cohort (p==0.004) by week 10. On the other hand, the neutralizing antibody titers were similar between groups at weeks 4 and 10, with the exception of the IM exc. immunized mice in which titers dropped to the unimmunized control level by week 10 (Fig. 3b). In contrast to H1N1 vaccine, the neutralizing titers induced by H3N2 vaccine were lower in all groups indicating that this subtype was less immunogenic. Total influenza-specific IgG titers (Fig. 3c) demonstrated the same trend as HAI titers. Interestingly, the lowest isotype ratio among all H3N2-immunized groups was demonstrated in the pMN immunized cohort (IgG1/IgG2a=3). In contrast, the IMexc. group had the highest ratio indicative of clear Th2 bias (Fig. 3d), suggesting that for this strain the presence of excipients in the liquid formulation may have negatively affected vaccine antigenicity which was otherwise preserved in dry formulation as seen in mMN Therefore, A/Victoria/210/2009 vaccineTh1-induced responses were improved with skin microneedle delivery. The group that displayed the lowest antibody titers was the one immunized intramuscularly with concentrated vaccine mixed with excipients. (Fig. 3a–c).
Fig. 3. Humoral immune responses in mice immunized with A/Victoria/210/09 (H3N2) vaccine.
(a) Anti-A/Victoria/210/09 HAI titers; (b) Neutralizing antibody titers; (c) Total IgG titers; (d) IgG1 to IgG2a titer ratios; Abbreviations are as listed in the legend for Fig. 3. ELISA antibody data for ratios represent the mean ± SEM, HAI titers and NT represent the geometric mean ± 95%CI. ap ≤ 0.05 mMN or pMN compared to the IM group. bp ≤ 0.05 mMN or pMN compared to the IM exc. group at the same post-immunization time points.
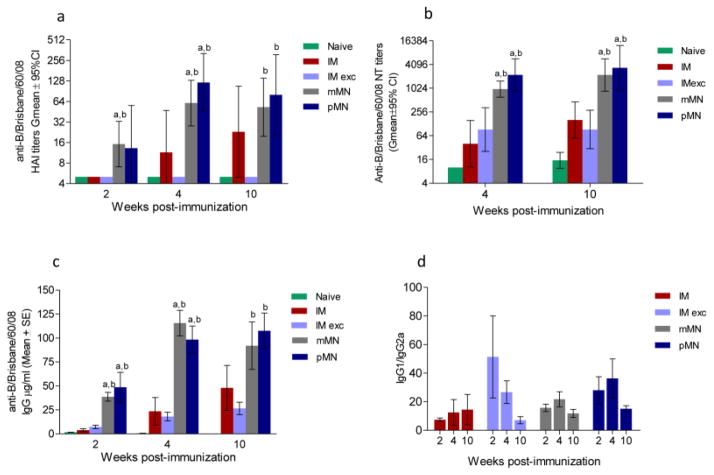
Humoral responses to influenza B vaccine
Influenza B strains are known to induce the lowest HAI titers of the antigens in TIV vaccine, especially in children [57–59]. Similarly, in intramuscularly vaccinated mice we observed lower HAI titers and delayed appearance of protective antibodies to influenza B/Brisbane/60/2008 antigen compared to both influenza A antigens (compare Fig. 2a and 3a with Fig. 4). Remarkably, a single vaccine dose delivered with either pMN or mMN resulted in HAI titers above 40 as early as week 4, while IM immunization failed to induce similar titers (Fig. 4a). The HAI titers in the group that received the vaccine mixed with excipients were not above those measured in unimmunized controls at any time point. At week 4, the titers measured in the pMN group reached GMT 120, two-fold higher than the mMN group, and 11-fold higher than the IM group (p=0.005). Despite a decrease by week 10, skin immunization with pMN resulted in 4-fold higher titers when compared to the IM group (Fig. 4a) The GMT HAI titers in the pMN and mMN groups remained as high as 80 and 53, respectively, at week 10 post-immunization (Fig. 4a). Similarly, the levels of neutralizing antibodies were 5 to 6-fold higher in the mMN and pMN groups when compared with the IM (p=0.001) or the IM exc. cohorts (p<0.001) at weeks 4 and 10 (Fig. 4b). The pattern of influenza-specific IgG antibody production resembled that of functional (HAI and neutralizing) antibodies; approximately 2-fold difference was observed between skin and systemically vaccinated mice (mMN vs. IM exc. p=0.03; or pMN vs. IM exc. p=0.003) (Fig. 4c). The IgG1/IgG2a ratios did not show significant differences between IM and both MN groups particularly at week 10 (Fig. 4d). Similarly to H3N2 vaccine, the highest IgG1/IgG2a ratio was observed in the IMexc group as early as week 2 post-vaccination (Fig. 4d) although not as pronounced as in the H3N2 vaccinated group. These data demonstrate that skin immunization, particularly with dissolving microneedle patches encapsulating influenza B subunit vaccine, provides superior immunogenicity over IM vaccination.
Fig. 4. Humoral immune responses in mice immunized with B/Brisbane/60/08 vaccine.
(a) Anti-B/Brisbane/60/08 HAI titers; (b) Neutralizing antibody titers; (c) Total IgG titers; (d) IgG1 to IgG2a titer ratio. Abbreviations are as listed in the legend for Fig. 3. ELISA antibody data for ratios represent the mean ± SEM, HAI titers and NT represent the geometric mean ± 95%CI. ap ≤ 0.05 mMN or pMN compared to the IM group. bp ≤ 0.05 mMN or pMN compared to the IM exc. group at the same post-immunization time points.
Stability of polymer microneedle patch
A critical component in vaccine manufacturing is the need for refrigeration during storage and transport that warrantees full vaccine potency and safety. The vaccine expiration date is estimated using stability testing under good manufacturing practices as determined by the Food and Drug Administration (FDA). Many medications though do not need refrigeration and their storage takes place at this temperature. For countries with limited resources it is important to design and manufacture vaccines that can be stored at ambient temperatures. For the proof of concept we tested each vaccine component formulated in pMN for stability at 25 °C up to 3 months by measuring HA activity with SRID.
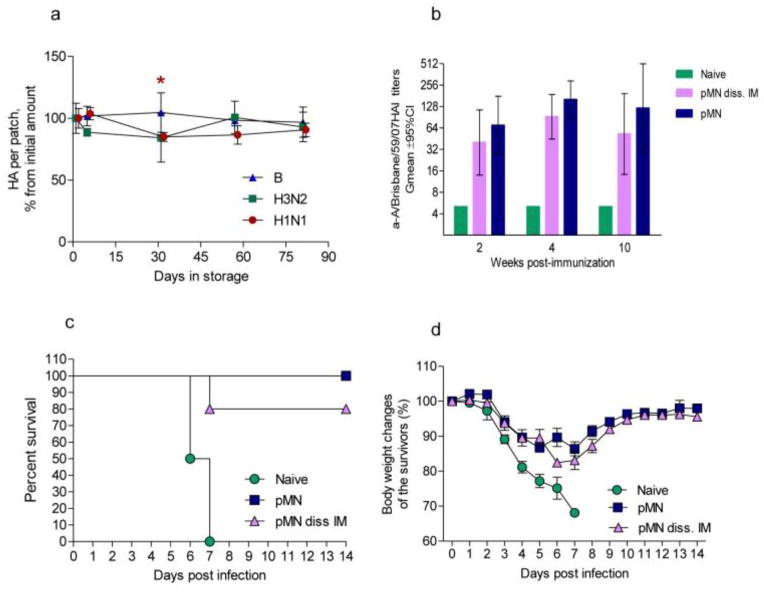
The only statistically significant drop of HA activity (15%) was noted at day 31 in H1N1 pMN. There was no statistically significant difference of hemagglutinin activity for any strain at the end of the storage period (Fig. 5a). Although the patches became slightly more brittle, they retained structural integrity. Mice immunized with the 3 month-old A/Brisbane H1N1 patch reached HAI titers of 160 at week 4 that decreased to 77 by week 10. Mice intramuscularly injected with the extract of stored patches showed maximum HAI titers of 92 at week 4 that decreased to 53 by week 10; there was no statistically significant difference between the two groups (Fig. 5b). Eighty percent of mice vaccinated intramuscularly with the extract of stored patch and 100% of mice vaccinated with the stored patch survived challenge with homologous virus (Fig. 5c). The overall weight loss of the surviving mice was similar between the two groups and did not exceed 14 % (Fig. 5d). In conclusion, the vaccine formulated in pMN remained stable and effective for at least 3 months storage at room temperature. Importantly, stored pMN vaccines were not less protective that the freshly prepared ones (see Fig. 2 e, f).
Fig. 5. Antigen preservation in pMN formulated with the individual vaccines within 3 month period of storage at 25°C.
(a) Hemagglutinin content relative to the day of manufacturing as measured by SRID. The data are presented as percent of the initial load. The values are averages of three measurements of three patches ± SD, except two H3N2 patches were analyzed at day 5. Star indicates statistically significant difference in A/Brisbane/59/07 (H1N1) pMN content at day 31 compared to day one. (b) HAI titers in the groups of mice immunized with A/Brisbane H1N1 pMN stored for 3 month and systemically with the extract of the stored pMN; (c) Survival of the immunized and naïve mice upon challenge with 5 LDx50 of the homologous virus (n = 2 in Naïve group); (d) body weight loss upon challenge.
DISCUSSION
In our initial published studies we used metal microneedle arrays (mMN) ranging between 650–700 μm in length for skin immunization with influenza viral antigens. Antigens were mixed with excipients, coated onto solid microneedles and air-dried [21, 60, 22]. A second generation of microneedles was further developed, composed of water-soluble polymers that dissolve and release the antigen upon penetration into the skin. Using this approach, immunization with polyvinylpyrrolidone (PVP) microneedle patches encapsulating inactivated influenza virus induced significantly higher humoral and cellular immune responses than intramuscular immunization [54].
Here we demonstrate that H1N1, H3N2 and B subunit influenza vaccines can be effectively formulated into a dissolving microneedle patch fabricated from gelatin. By analyzing the specific HA content per total protein we found that vaccine concentration and buffer exchange procedures, which represent the initial steps in dissolving microneedle patch manufacturing, did not negatively affect the antigenic properties of vaccine monobulks. Our data on antigen preservation in highly concentrated vaccines are consistent with a previous report by Kommareddy et al [48]. Importantly, all vaccine strains retained activity after incorporation into pMN. Since our pMN formulation did not contain carboxymethylcellulose which increases the viscosity of patch extracts [43], we were able to use SRID to measure vaccine potency.
Using the BALB/c mouse model we found that subunit influenza vaccines delivered with pMN outperformed intramuscular injection in the induction of immune responses. Mice immunized with pMN encapsulating influenza A/Brisbane/59/07 (H1N1) vaccine demonstrated superior titers of functional antibodies when compared to intramuscular injections and were fully protected against lethal challenge by homologous mouse-adapted influenza virus. These data are in agreement with our previous reported study in which mice immunized with mMN coated with the same subunit H1N1 vaccine survived the same lethal dose of live homologous virus [24].
H3N2 influenza viruses cause the majority of hospitalization cases especially in the elderly during seasonal epidemics [56, 61] and the seasons dominated by H3N2 viruses are generally worse than other seasons. Our data suggest that skin immunization has the potential to improve immunogenicity of H3N2 vaccine as well. The absence of significant improvement with pMN vaccination over IM injection of the H3N2 vaccine strain may be attributed to the effect of excipients added to the vaccine prior to incorporation into pMN. Metal microneedle arrays that did not contain the same excipients as pMN outperformed the IM group in HAI titers as seen in Fig. 3a. For instance, the tracer dye incorporated routinely into pMN to visualize microneedle insertion pattern in the mouse skin (which will be omitted in the pMN developed for human use) may have impacted the levels of humoral immune responses otherwise preserved in dry vaccine formulation as previously seen in mMN and pMN [54]. H3N2 and B liquid vaccines may be more sensitive to the excipients than the H1N1 subtype as seen in the IMexc. groups that exhibited the lowest immune response when compared to the other vaccinated groups. The addition of excipients in H3N2 and B subtypes affected the isotype class switching, yielding a high IgG1/IgG2a ratio in the IMexc groups at week 2 indicative of a Th-2 shift. Future experiments studying systematic elimination of excipients and their effects on pMN-induced immune responses may address the quantitative and qualitative differences among subtypes.
The enhancement of the immune responses due to cutaneous vaccine delivery was particularly impressive for the influenza B vaccine strain. A single vaccine dose with microneedle patches resulted in HAI titers above 40 as early as week 4, while IM immunization failed to produce similar titers. Similarly, the binding antibody titers were 2.5-fold higher in the skin-immunized groups than in the intramuscularly vaccinated cohorts. Although the neutralizing antibody titers were above 40 for all influenza B vaccinated groups (Fig. 4a), the differences between skin and systemically induced immunization were impressive, clearly demonstrating the superiority of microneedle patches.
Mice lack pre-existing immunity to influenza, and certain features of their immune responses may resemble those observed in young children who are mostly naïve to influenza. In young children even after two vaccinations functional antibody titers were reported to be higher for influenza A strains and lower for the influenza B strain [59]. Thus, our data suggest that skin vaccination with dissolving microneedle patches may potentially improve influenza B-specific immune responses in children who are especially susceptible to influenza B infection.
Human adults are not naïve to influenza antigens. Prior exposure of the population to influenza viruses or influenza vaccines elicits recall responses upon immunization. Clinical studies in older adults have demonstrated that by quadrupling the vaccine dose it is possible to significantly increase post-vaccination HAI titers for influenza A components of the TIV vaccine, but not for the influenza B component. Thus, our finding of improved immunogenicity of influenza B strain by use of a dissolving MN patch may potentially benefit both the young and the aged population groups.
Seroprotection and seroconversion rates differ between strains in trivalent vaccines given to human populations during seasonal vaccination campaigns [28] and some strains may be especially poorly immunogenic [58]. Differences in magnitude of immune responses may be explained by the antigenic similarity of newer influenza strains to previously encountered ones [62], as well as by antigenic properties of particular strains. By using mice naïve to influenza we show that specific antigenic properties of each strain define the kinetics of immune response. The most important outcome of this study is that each of the three strains induced elevated functional antibody levels when administered through skin compared with those delivered by systemic immunization.
Besides clear immunological advantages, the long-term stability of pMN at room temperature offers major practical advantages since dry stabilized vaccine in ready-to use dissolving patches offers easier storage and distribution eliminating the requirement for cold chain. As a prove of concept, our patches were as protective after three months of storage at + 25 °C as freshly prepared (Fig. 5c, d). The dissolving formulation provides safety by elimination of sharps waste, and the simplistic design enables self-administration by the end-user without the need for trained healthcare professionals to administer the vaccine. Further, since the patches are thermostable, they cut down the costs associated with storage in the cold-chain and allows easy transportation to remote areas and rapid distribution thereby significantly improving vaccination rates on a global scale. Thus, better vaccination logistics combined with the improved immune response may ultimately lead to enhanced population immunity.
In conclusion, we demonstrated that for each separate strain of influenza vaccine the novel dissolving pMN performed similarly to the coated mMN in terms of improved humoral immune response over intramuscular injection but offered major practical advantages such as vaccine stability during storage at room temperature.
Animal study statement
All institutional and national guidelines for the care and use of laboratory animals were followed.
Supplementary Material
Acknowledgments
We thank Dahnide Taylor-Williams for her valuable technical support. We thank Derek O’Hagan, Sushma Kommareddy and their colleagues at Novartis Vaccines and Diagnostics for providing influenza vaccine monobulks.
Research Funding
The work was supported by U.S. National Institutes of Health grant EB012495.
Footnotes
Conflict of Interest
Mark Prausnitz is an inventor of patents that have been licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products, and is a founder/shareholder of companies developing microneedle-based products. The terms of this arrangement have been reviewed and approved by Georgia Tech and Emory University in accordance with their conflict of interest policies.
Devin McAllister is an inventor of patents that have been or may be licensed to companies developing microneedle-based products and a founder/shareholder of a company developing microneedle-based products. The terms of this arrangement have been reviewed and approved by Georgia Tech in accordance with its conflict of interest policies.
Elena Vassilieva, Haripriya Kalluri, Misha Taherbhai, E. Stein Esser, Winston Pewin, Joanna Pulit-Penaloza, Richard Compans and Ioanna Skountzou declare that they have no conflict of interest.
References
- 1.del Pilar Martin M, Weldon WC, Zarnitsyn VG, Koutsonanos DG, Akbari H, Skountzou I, et al. Local response to microneedle-based influenza immunization in the skin. mBio. 2012;3(2):e00012–12. doi: 10.1128/mBio.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Fernando GJ, Raphael AP, Yukiko SR, Fairmaid EJ, Primiero CA, et al. Rapid kinetics to peak serum antibodies is achieved following influenza vaccination by dry-coated densely packed microprojections to skin. Journal of controlled release : official journal of the Controlled Release Society. 2012;158(1):78–84. doi: 10.1016/j.jconrel.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Pulit-Penaloza JA, Esser ES, Vassilieva EV, Lee JW, Taherbhai MT, Pollack BP, et al. A protective role of murine langerin(+) cells in immune responses to cutaneous vaccination with microneedle patches. Scientific reports. 2014;4:6094. doi: 10.1038/srep06094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn HL, Kearney MC, Courtenay AJ, McCrudden MT, Donnelly RF. The role of microneedles for drug and vaccine delivery. Expert opinion on drug delivery. 2014;11(11):1769–80. doi: 10.1517/17425247.2014.938635. [DOI] [PubMed] [Google Scholar]
- 5.Skountzou I, Compans RW. Skin immunization with influenza vaccines. Current topics in microbiology and immunology. 2015;386:343–69. doi: 10.1007/82_2014_407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin Y, Kochba E, Hung I, Kenney R. Intradermal vaccination using the novel microneedle device MicronJet600: past, present and future. Human vaccines & immunotherapeutics. 2015 doi: 10.1080/21645515.2015.1010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkam S, Saraf S, Seal S. Fabricated micro-nano devices for in vivo and in vitro biomedical applications. Wiley interdisciplinary reviews Nanomedicine and nanobiotechnology. 2013;5(6):544–68. doi: 10.1002/wnan.1236. [DOI] [PubMed] [Google Scholar]
- 8.Jin J, Reese V, Coler R, Carter D, Rolandi M. Chitin microneedles for an easy-to-use tuberculosis skin test. Advanced healthcare materials. 2014;3(3):349–53. doi: 10.1002/adhm.201300185. [DOI] [PubMed] [Google Scholar]
- 9.Paliwal S, Hwang BH, Tsai KY, Mitragotri S. Diagnostic opportunities based on skin biomarkers. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2013;50(5):546–56. doi: 10.1016/j.ejps.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Koutsonanos DG, Compans RW, Skountzou I. Targeting the skin for microneedle delivery of influenza vaccine. Advances in experimental medicine and biology. 2013;785:121–32. doi: 10.1007/978-1-4614-6217-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu Y, Guo L, Zhang S, Xu B, Gao Y, Hu Y, et al. DNA-based vaccination against hepatitis B virus using dissolving microneedle arrays adjuvanted by cationic liposomes and CpG ODN. Drug delivery. 2015:1–8. doi: 10.3109/10717544.2014.992497. [DOI] [PubMed] [Google Scholar]
- 12.Carey JB, Vrdoljak A, O’Mahony C, Hill AV, Draper SJ, Moore AC. Microneedle-mediated immunization of an adenovirus-based malaria vaccine enhances antigen-specific antibody immunity and reduces anti-vector responses compared to the intradermal route. Scientific reports. 2014;4:6154. doi: 10.1038/srep06154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Maaden K, Trietsch SJ, Kraan H, Varypataki EM, Romeijn S, Zwier R, et al. Novel hollow microneedle technology for depth-controlled microinjection-mediated dermal vaccination: a study with polio vaccine in rats. Pharmaceutical research. 2014;31(7):1846–54. doi: 10.1007/s11095-013-1288-9. [DOI] [PubMed] [Google Scholar]
- 14.Prow TW, Chen X, Prow NA, Fernando GJ, Tan CS, Raphael AP, et al. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small. 2010;6(16):1776–84. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 15.Suh H, Shin J, Kim YC. Microneedle patches for vaccine delivery. Clinical and experimental vaccine research. 2014;3(1):42–9. doi: 10.7774/cevr.2014.3.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirobe S, Azukizawa H, Matsuo K, Zhai Y, Quan YS, Kamiyama F, et al. Development and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device. Pharmaceutical research. 2013;30(10):2664–74. doi: 10.1007/s11095-013-1092-6. [DOI] [PubMed] [Google Scholar]
- 17.Gill HS, Prausnitz MR. Pocketed Microneedles for Drug Delivery to the Skin. The Journal of physics and chemistry of solids. 2008;69(5–6):1537–41. doi: 10.1016/j.jpcs.2007.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomedical microdevices. 2009;11(1):35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 19.Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. Journal of controlled release : official journal of the Controlled Release Society. 2011;154(2):148–55. doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: Usability and acceptability for self-vaccination against influenza. Vaccine. 2014 doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(19):7968–73. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, Jacob J, Prausnitz MR, Compans RW, et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. The Journal of infectious diseases. 2011;204(4):582–91. doi: 10.1093/infdis/jir094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weldon WC, Martin MP, Zarnitsyn V, Wang B, Koutsonanos D, Skountzou I, et al. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clinical and vaccine immunology : CVI. 2011;18(4):647–54. doi: 10.1128/CVI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koutsonanos DG, Vassilieva EV, Stavropoulou A, Zarnitsyn VG, Esser ES, Taherbhai MT, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Scientific reports. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song JM, Kim YC, OE, Compans RW, Prausnitz MR, Kang SM. DNA vaccination in the skin using microneedles improves protection against influenza. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20(7):1472–80. doi: 10.1038/mt.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84(15):7760–9. doi: 10.1128/JVI.01849-09. JVI.01849-09 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernando GJ, Chen X, Primiero CA, Yukiko SR, Fairmaid EJ, Corbett HJ, et al. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. Journal of controlled release : official journal of the Controlled Release Society. 2012;159(2):215–21. doi: 10.1016/j.jconrel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Xie H, Jing X, Li X, Lin Z, Plant E, Zoueva O, et al. Immunogenicity and cross-reactivity of 2009–2010 inactivated seasonal influenza vaccine in US adults and elderly. PloS one. 2011;6(1):e16650. doi: 10.1371/journal.pone.0016650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belongia EA, Sundaram ME, McClure DL, Meece JK, Ferdinands J, VanWormer JJ. Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season. Vaccine. 2015;33(1):246–51. doi: 10.1016/j.vaccine.2014.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang SM, Song JM, Kim YC. Microneedle and mucosal delivery of influenza vaccines. Expert review of vaccines. 2012;11(5):547–60. doi: 10.1586/erv.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. The Journal of infectious diseases. 2008;198(5):650–8. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 32.Duggan ST, Plosker GL. Intanza 15 microg intradermal seasonal influenza vaccine: in older adults (aged >or=60 years) Drugs & aging. 2010;27(7):597–605. doi: 10.2165/11203880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Sugimura T, Ito Y, Tananari Y, Ozaki Y, Maeno Y, Yamaoka T, et al. Improved antibody responses in infants less than 1 year old using intradermal influenza vaccination. Vaccine. 2008;26(22):2700–5. doi: 10.1016/j.vaccine.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Wong YL, Sampson S, Germishuizen WA, Goonesekera S, Caponetti G, Sadoff J, et al. Drying a tuberculosis vaccine without freezing. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(8):2591–5. doi: 10.1073/pnas.0611430104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtake S, Martin RA, Yee L, Chen D, Kristensen DD, Lechuga-Ballesteros D, et al. Heat- stable measles vaccine produced by spray drying. Vaccine. 2010;28(5):1275–84. doi: 10.1016/j.vaccine.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Chen D, Kapre S, Goel A, Suresh K, Beri S, Hickling J, et al. Thermostable formulations of a hepatitis B vaccine and a meningitis A polysaccharide conjugate vaccine produced by a spray drying method. Vaccine. 2010;28(31):5093–9. doi: 10.1016/j.vaccine.2010.04.112. [DOI] [PubMed] [Google Scholar]
- 37.Soema PC, Willems GJ, van Twillert K, van de Wijdeven G, Boog CJ, Kersten GF, et al. Solid bioneedle-delivered influenza vaccines are highly thermostable and induce both humoral and cellular immune responses. PloS one. 2014;9(3):e92806. doi: 10.1371/journal.pone.0092806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frolov VG, Seid RC, Jr, Odutayo O, Al-Khalili M, Yu J, Frolova OY, et al. Transcutaneous delivery and thermostability of a dry trivalent inactivated influenza vaccine patch. Influenza and other respiratory viruses. 2008;2(2):53–60. doi: 10.1111/j.1750-2659.2008.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raphael AP, Prow TW, Crichton ML, Chen X, Fernando GJ, Kendall MA. Targeted, needle- free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small. 2010;6(16):1785–93. doi: 10.1002/smll.201000326. [DOI] [PubMed] [Google Scholar]
- 40.Kommareddy S, Baudner BC, Oh S, Kwon SY, Singh M, O’Hagan DT. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. Journal of pharmaceutical sciences. 2012;101(3):1021–7. doi: 10.1002/jps.23019. [DOI] [PubMed] [Google Scholar]
- 41.Matsuo K, Hirobe S, Yokota Y, Ayabe Y, Seto M, Quan YS, et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. Journal of controlled release : official journal of the Controlled Release Society. 2012;160(3):495–501. doi: 10.1016/j.jconrel.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Sharma M, Brahmne H, Bafna P. Potency testing of novel DTP group of vaccines. International Journal of Comprehensive Pharmacology. 2011;11(2):1–9. [Google Scholar]
- 43.Kommareddy S, Baudner BC, Oh S, Kwon SY, Singh M, O’Hagan DT. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. Journal of pharmaceutical sciences. 2011 doi: 10.1002/jps.23019. [DOI] [PubMed] [Google Scholar]
- 44.WHO/CDS/CSR/NCS. WHO Manual of Animal Influenza Diagnosis and Surveillance. Department of Communicable Disease Surveillance and Response; 2002. [Google Scholar]
- 45.Skountzou I, Quan FS, Gangadhara S, Ye L, Vzorov A, Selvaraj P, et al. Incorporation of glycosylphosphatidylinositol-anchored granulocyte- macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J Virol. 2007;81(3):1083–94. doi: 10.1128/JVI.01692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reed LJaMH. A simple method of estimating fifty percent endpoints. The American Journal of Hygiene. 1938;27:493–7. [Google Scholar]
- 47.Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74(11):4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kommareddy S, Bonificio A, Gallorini S, Baudner B, Singh M, O’Hagan D. Preparation of highly concentrated influenza vaccine for use in novel delivery approaches. Journal of pharmaceutical sciences. 2013;102(3):866–75. doi: 10.1002/jps.23444. [DOI] [PubMed] [Google Scholar]
- 49.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. Journal of controlled release : official journal of the Controlled Release Society. 2007;117(2):227–37. doi: 10.1016/j.jconrel.2006.10.017. S0168-3659(06)00583-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PloS one. 2009;4(3):e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer K. Neues Verfahren zur maßanalytischen Bestimmung des Wassergehaltes von Flüssigkeiten und festen Körpern. Angewandte Chemie. 1935;48(26):394–6. doi: 10.1002/ange.19350482605. [DOI] [Google Scholar]
- 52.Wood JM, Schild GC, Newman RW, Seagroatt V. An improved single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen: application for potency determinations of inactivated whole virus and subunit vaccines. Journal of biological standardization. 1977;5(3):237–47. doi: 10.1016/s0092-1157(77)80008-5. [DOI] [PubMed] [Google Scholar]
- 53.Williams MS. Single-radial-immunodiffusion as an in vitro potency assay for human inactivated viral vaccines. Veterinary microbiology. 1993;37(3–4):253–62. doi: 10.1016/0378-1135(93)90027-5. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nature medicine. 2010;16(8):915–20. doi: 10.1038/nm.2182. nm.2182 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atkinson WWS, Hamborsky J, editors. Prevention CfDCa. Epidemiology and Prevention of Vaccine-Preventable Diseases The Pink Book: Course Textbook. 12. Washington DC: Public Health Foundation; 2012. Second Printing. [Google Scholar]
- 56.Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(10):1427–36. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gruber WC, Taber LH, Glezen WP, Clover RD, Abell TD, Demmler RW, et al. Live attenuated and inactivated influenza vaccine in school-age children. American journal of diseases of children. 1990;144(5):595–600. doi: 10.1001/archpedi.1990.02150290089035. [DOI] [PubMed] [Google Scholar]
- 58.Kishida N, Fujisaki S, Yokoyama M, Sato H, Saito R, Ikematsu H, et al. Evaluation of influenza virus A/H3N2 and B vaccines on the basis of cross-reactivity of postvaccination human serum antibodies against influenza viruses A/H3N2 and B isolated in MDCK cells and embryonated hen eggs. Clinical and vaccine immunology : CVI. 2012;19(6):897–908. doi: 10.1128/CVI.05726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neuzil KM, Jackson LA, Nelson J, Klimov A, Cox N, Bridges CB, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5–8-year-old children. The Journal of infectious diseases. 2006;194(8):1032–9. doi: 10.1086/507309. [DOI] [PubMed] [Google Scholar]
- 60.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. Journal of controlled release : official journal of the Controlled Release Society. 2010;142(2):187–95. doi: 10.1016/j.jconrel.2009.10.013. S0168-3659(09)00708-1 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kostova D, Reed C, Finelli L, Cheng PY, Gargiullo PM, Shay DK, et al. Influenza Illness and Hospitalizations Averted by Influenza Vaccination in the United States, 2005–2011. PloS one. 2013;8(6):e66312. doi: 10.1371/journal.pone.0066312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skountzou I, Koutsonanos DG, Kim JH, Powers R, Satyabhama L, Masseoud F, et al. Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A (H1N1) influenza virus. Journal of immunology. 2010;185(3):1642–9. doi: 10.4049/jimmunol.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.