Abstract
B-lineage cells (B lymphocytes and plasma cells) predominate in the inflammatory infiltrate of human chronic periodontitis. However, their role in disease pathogenesis and the factors responsible for their persistence in chronic lesions are poorly understood. In this regard, two cytokines of the TNF ligand superfamily, namely a proliferation-inducing ligand (APRIL) and B-lymphocyte stimulator (BLyS), are important for the survival, proliferation, and maturation of B cells. We thus hypothesized that APRIL and/or BLyS are upregulated in periodontitis and contribute to induction of periodontal bone loss. This hypothesis was addressed in both human and mouse experimental systems. We show that, relative to healthy controls, the expression of APRIL and BLyS mRNA and protein was upregulated in natural and experimental periodontitis in humans and mice, respectively. The elevated expression of these cytokines correlated with increased numbers of B cells/plasma cells in both species. Moreover, APRIL and BLyS partially colocalized with kappa light chain-expressing B lineage cells at the epithelial-connective tissue interface. Ligature-induced periodontitis resulted in significantly less bone loss in B cell-deficient mice compared to wild-type controls. Ab-mediated neutralization of APRIL or BLyS diminished the number of B cells in the gingival tissue and inhibited bone loss in wild-type but not in B cell-deficient mice. In conclusion, B cells and specific cytokines involved in their growth and differentiation contribute to periodontal bone loss. Moreover, APRIL and BLyS have been identified as potential therapeutic targets in periodontitis.
Introduction
Periodontitis is a prevalent chronic inflammatory disease typified by destruction of the tooth-supporting tissues (gingiva, periodontal ligament and alveolar bone) and is associated with increased risk for certain systemic disorders (e.g., atherosclerosis and rheumatoid arthritis) (1). Although the pathogenesis of periodontitis involves polymicrobial synergy and dysbiosis, the bacterial communities of the tooth-associated biofilm (dental plaque) are not sufficient to cause disease; it is actually the host inflammatory response to this polymicrobial challenge that ultimately induces tissue damage and bone loss, the hallmark of periodontitis (2). A number of inflammatory and stromal cell types have been implicated in this destructive process (3–6). However, the role of plasma cells and their precursors, B lymphocytes, remains quite enigmatic despite their high abundance in the advanced periodontal lesions (7–9). Indeed, plasma and B cells together comprise approximately 60% of the total leukocytes present in periodontal lesions associated with bone loss (8).
Through their role in humoral immunity, B cells/plasma cells could–in principle–have a protective function in periodontitis. For instance, the Ab response could contribute to the control of the dysbiotic microbiota in periodontal pockets and prevent bacterial invasion into the gingival connective tissue, thereby limiting inflammation and disease. However, it is well established that periodontitis progresses despite the induction of specific Ab responses to periodontal bacteria in affected individuals. This is likely due to the low affinity of the Abs and/or their unfavorable functional characteristics (e.g., poor opsonophagocytic capacity) (5, 10, 11). In fact, it is thought that the B cell-mediated immune response, and specifically an IgG-dominant response, might contribute to the pathogenesis of periodontitis. In this regard, advanced periodontitis is associated with increased deposition of IgG immune complexes along with complement activation fragments (12, 13). Most importantly, B cells constitute a major source of membrane-bound and secreted receptor activator of NF-κB ligand (RANKL) in the bone resorptive lesions of human periodontitis (14). In this regard, it has been shown that greater than 90% of B cells in periodontitis lesions express RANKL, whereas the percentage of RANKL-positive B cells in healthy gingiva is negligible (14). Given that RANKL plays a key role in osteoclast differentiation and activation (15), it is plausible that B cells participate in the induction of pathological bone loss in periodontitis.
The notion that B cells mediate destructive effects in periodontitis is consistent with findings from previous studies in animal models. Adoptive transfer of bacterial antigen-specific B cells into rats followed by local oral exposure to the same bacterium (Aggregatibacter actinomycetemcomitans) leads to RANKL-dependent periodontal bone loss (16, 17). Despite the artificial priming approach involved, these studies indicated that specific antigen-triggered B cells can potentially provoke bone resorption. The role of B cells in periodontitis was addressed more recently in studies utilizing a model of the disease induced by oral gavage with Porphyromonas gingivalis in wild-type (WT) and B-cell knockout (KO) mice. An investigation by Zhu and coworkers did not support a role for naturally occurring B cells in periodontal disease pathogenesis, unless the mice were fed a high-fat diet (18). This study, however, did not detect B cells in the periodontal tissue of normal (non-obese) mice (18), suggesting that the model used or the specific experimental conditions do not readily support the development of a B cell infiltrate that is characteristic of human periodontitis. In contrast, Oliver-Bell et al. showed that B-cell KO mice are protected from P. gingivalis-induced bone loss relative to WT controls (19). In sum, correlative human studies and certain animal model-based studies provide suggestive evidence for B cell involvement in plaque-induced periodontal bone loss.
Two cytokines of the TNF superfamily, namely B-lymphocyte stimulator (BLyS; also known as B cell-activating factor, TNFSF13B, or TALL-1) and a proliferation-inducing ligand (APRIL; also known as TNFSF13 or TALL-2) are important contributors to the survival and maturation of B cells (20, 21). APRIL and BLyS are type II transmembrane proteins that are proteolytically cleaved, either intracellularly (APRIL) or at the cell membrane (BLyS), to generate active secreted forms (21, 22). Upon secretion, APRIL and BLyS can interact with two common receptors found on B cells and plasma cells, the B-cell maturation antigen (BCMA) and transmembrane activator and cyclophilin ligand interactor (TACI) (21, 23). In addition, BLyS interacts with a third receptor, the so-called B cell-activating factor receptor (BAFF-R) (21, 23). BCMA promotes the survival of plasma cells, TACI supports T cell-independent B cell Ab responses and isotype switching, and BAFF-R is critical for survival and maturation of immature B cells. Accordingly, APRIL-deficient mice exhibit impaired mucosal humoral immunity (24) and BLyS-deficient mice display impaired B cell maturation (25).
While APRIL and BLyS have been implicated in autoimmunity and neoplastic B cell transformation (23, 26), their role in periodontal disease pathogenesis has not been addressed. We reasoned that APRIL and/or BLyS are required for the maintenance of the large number of B cells and plasma cells typically found in periodontitis lesions. We therefore hypothesized that these B-cell stimulatory cytokines are upregulated in periodontitis and play a role in disease pathogenesis. This hypothesis was addressed in both human and mouse experimental systems; the former allowed us to obtain clinically relevant information and the latter enabled us to perform cause-and-effect experiments that cannot be typically addressed in human studies (27). Our study has confirmed the hypothesis and shown that, in the presence of APRIL and BLyS, B cells are causally linked to periodontal bone loss, thereby identifying potential B-cell–targeted treatments for periodontitis.
Materials and Methods
Human subjects and gingival tissue specimens
Research was performed under an Institutional Review Board–approved protocol. Signed informed consent was obtained to procure normally discarded gingival tissue from individuals treated in the post-graduate clinic of the Department of Periodontics, University of Pennsylvania School of Dental Medicine. All subjects were 18 years of age or older with no serious medical illness contraindicative of surgical treatment. Diseased tissue was obtained from 14 patients undergoing pocket reduction surgery to treat chronic periodontitis. Control tissue specimens were obtained from 14 clinically healthy subjects who required crown-lengthening surgery. The mean demographic and clinical parameters of the two groups are shown in Table 1. The inclusion criteria for chronic periodontitis were as follows. Diseased sites with probing pocket depth ≥ 5 mm associated with clinical attachment loss of ≥ 4 mm, presence of bleeding on probing, and radiographic evidence of bone loss. Sites with PD ≤ 3 mm, no clinical attachment loss, absence of BOP and normal radiographic bone levels were considered as healthy controls. All of the periodontitis patients underwent scaling and root planing one to two months prior to the day of surgery.
Table 1.
Demographic and clinical parameters of periodontally involved and healthy control groups.
| Health (n = 14) | Periodontitis (n = 14) | |
|---|---|---|
| Age (years) | 49.9 ± 17.1 | 58.4 ± 16.6 |
| Gender (M/F) | 7/7 | 8/6 |
| Bleeding on probing | No | Yes |
| Probing pocket depth (mm) | 2.9 ± 0.3 | 6.1 ± 0.9* |
| Bone loss | No | Yes |
P < 0.01 compared to periodontally healthy
Mice
B6.129S2-Ighmtm1Cgn/J mice constitute a model of B cell immunodeficiency and were purchased from The Jackson Laboratories, along with C57BL/6J WT controls. The B6.129S2-Ighmtm1Cgn/J mice, referred to here as B-cell KO, do not express membrane-bound IgM and consequently B cell development is blocked at the pro-B stage (28). WT and B-cell KO mice were housed in a pathogen-free environment and used when they were 10 – 12 wk-old. All animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from human or mouse gingival tissue using the MELT Total Nucleic Acid Isolation System (Life Technologies) or RNeasy Mini Kit (Qiagen) and quantified by spectrometry at 260 and 280 nm. The RNA was reverse-transcribed using the High-Capacity RNA-to-cDNA kit (Life Technologies) and real-time PCR with cDNA was performed using the ABI 7500 Fast System, according to the manufacturer’s protocol (Life Technologies). TaqMan probes, sense primers, and antisense primers for detection and quantification of GAPDH (normalization control) and cytokine genes investigated in this study (see Fig. 1 and Fig. 7A) were purchased from Life Technologies. Data were analyzed using the comparative (ΔΔCt) method.
Figure 1. Upregulation of APRIL and BLyS mRNA expression in gingival biopsy specimens from periodontitis patients relative to healthy controls.
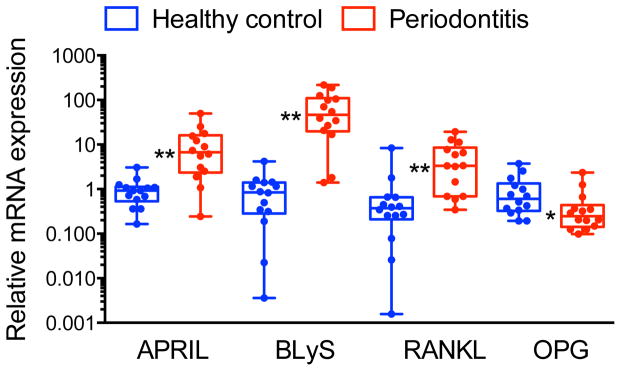
Gingival mRNA expression of APRIL, BLyS, RANKL, and OPG (the latter two molecules were included for comparative reasons) in healthy and diseased sites was determined by qPCR. Results were normalized against GAPDH mRNA and presented as fold change in the transcript levels in diseased sites relative to those of healthy sites, which were assigned an average value of 1. Dots represent individual values (n = 14 subjects per group) and each box in the box and whisker plots extends from the 25th to the 75th percentile. *P < 0.05 and **P < 0.01 compared with healthy control.
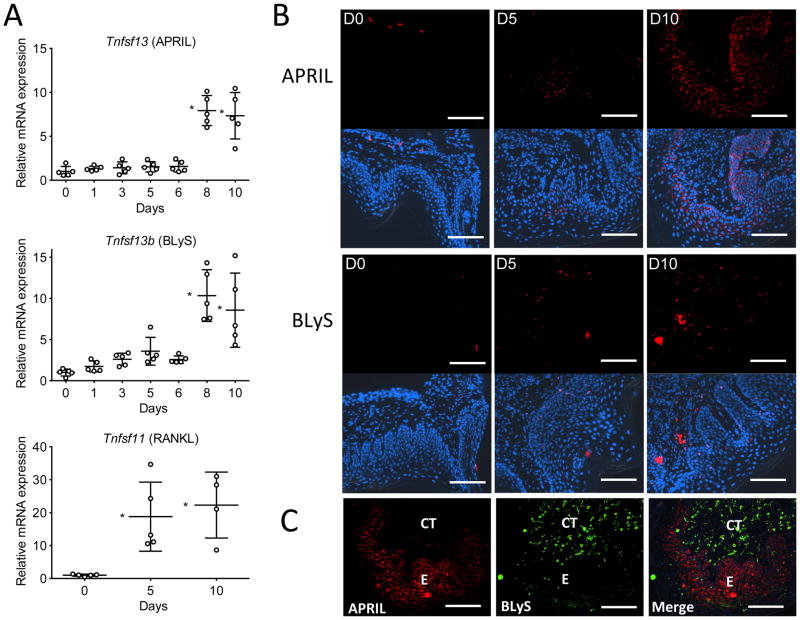
Figure 7. Expression of APRIL and BLyS in the murine gingival tissue.
(A) Timecourse of APRIL (upper), BLyS (middle), and RANKL (lower) mRNA expression in the gingival tissue after ligature-induced periodontitis for 10 d in mice. Results were normalized to GAPDH mRNA and presented relative to those at day 0, set as 1. Scatter dots and mean ± SD were plotted for data from each group of mice (n = 5 mice per timepoint). *P < 0.01 compared to day 0. (B) Gingival tissue sections from ligature-induced periodontitis at days 0, 5, and 10 (D0, D5, and D10) were stained for APRIL (upper panels) or BLyS (lower panels). The APRIL and BLyS fluorescent images were merged with DAPI staining (blue) to reveal nuclei. (C) Staining for APRIL and BLyS in a gingival tissue section at day 10 post-ligation and merged image. E, epithelium; CT, connective tissue. Original magnification 200X. Scale bars, 100 μm.
Immunofluorescence histochemistry
Human tissues
Serial sections (8–10 μm thick) of formaldehyde-fixed OCT-embedded gingival tissue were stained with hematoxylin and eosin to examine the histological features under light microscopy, or processed for immunohistochemistry, as previously described (29), to detect the expression of APRIL, BLyS, and Ig kappa light-chain. To this end, the following Abs were used: goat anti-human APRIL polyclonal IgG (Abcam), goat anti-human BLyS polyclonal IgG (R&D Systems), and FITC-conjugated rabbit anti-human Ig kappa light chain polyclonal IgG (DakoCytomation) that recognizes B cells and Ig-secreting plasma cells (30). Alexa Fluor 594-conjugated donkey anti-goat IgG was used as a secondary reagent for the unconjugated primary antibodies. Human tonsil specimens, purchased from NewComerSupply, were used as positive control for Ig kappa light chain staining. Slides were mounted with cover slips using ProLong Gold anti-fade reagent containing DAPI (Life Technologies).
Mouse tissues
Maxillae with intact surrounding tissue were fixed in 4% paraformaldehyde, decalcified in Immunocal solution (Decal Chemical) for 14 d followed by placement in Cal-Arrest solution (Decal Chemical) to neutralize the pH of the tissue, and then embedded in OCT compound. Coronal sections (6-μm thick) were stained with mAbs to CD19 (6D5; Abcam), CD138 (EPR6454; Abcam), or BLyS (Buffy 2; Abcam) or polyclonal Ab to APRIL (Abcam), followed by appropriate secondary reagents conjugated to AlexaFluor594 or AlexaFluor488 (Life Technologies). Staining for Igs in tissues was performed using AlexaFluor488–conjugated goat anti-mouse IgM (μ chain) and AlexaFluor594–conjugated goat anti-mouse IgG (H+L) (Life Technologies).
The specificity of staining was confirmed using appropriate isotype control or non-immune IgG. Stained images were visualized and captured using a Nikon Eclipse Ni-E automated fluorescent microscope and NIS-Elements software.
Ligature-induced periodontitis and intervention experiments
The placement of ligatures in conventional (but not germ-free) mice accelerates bacteria-mediated inflammation and bone loss (31, 32). To induce bone loss, a 5-0 silk ligature was tied around the maxillary left second molar, as previously described (33). The contralateral molar tooth in each mouse was left unligated to serve as a baseline control for bone loss measurements. The ligatures remained in place in all mice throughout the experimental period. The mice were euthanized at various timepoints (0 to 10 d) after placement of the ligatures and defleshed maxillae were used to measure bone heights (i.e., the distances from the cementoenamel junction [CEJ] to the alveolar bone crest [ABC]) under a Nikon SMZ800 microscope using a 40 × objective. Images of the maxillae were captured using a Nicon Digital Sight DS-U3 camera controller and CEJ-ABC distances were measured at 6 predetermined points on the ligated molar and adjacent regions using NIS-Elements software (33). To calculate bone loss, the 6-site total CEJ-ABC distance for the ligated side of each mouse was subtracted from the 6-site total CEJ-ABC distance of the contralateral unligated side. The results were presented in mm and negative values indicated bone loss relative to the baseline (unligated control). In intervention experiments, blocking mAbs to APRIL (Apry-1-1; IgG2b; Adipogen) or BLyS (Sandy-2; IgG1; Adipogen), or their isotype controls, were microinjected into the palatal gingiva of the ligated second maxillary molar, as previously described (34, 35). Each animal received 5 μg of mAb or isotype control through microinjections performed on day 4 following placement of ligatures and thereafter once daily, until the day before sacrifice performed on day 10.
Statistical analysis
Data were evaluated by ANOVA and the Dunnett multiple comparison test or by two-tailed unpaired t test using the InStat program (GraphPad). When a non-parametric test was warranted (due to non-normality of data in Fig. 1), a two-tailed Mann-Whitney test was used. A p value < 0.05 was taken as the level of significance.
Results
Increased APRIL and BLyS mRNA expression in periodontitis compared to health
To investigate the expression pattern of APRIL (TNFSF13) and BLyS (TNFSF13B) and determine their possible association with periodontitis, we obtained gingival biopsies from periodontally involved and healthy control individuals. Table 1 presents the mean demographic and clinical parameters of the two groups. There were no significant differences in gender or age between periodontitis patients and healthy control individuals, while – as expected – periodontally involved sites exhibited significantly higher readings for probing depth than control sites (p < 0.01; Table 1). APRIL and BLyS mRNA was detected in all gingival samples examined, although the relative expression of APRIL and BLyS was significantly higher in diseased gingival samples than in controls (p < 0.01; Fig. 1). The increased expression of APRIL and BLyS was comparable to that of RANKL (TNFSF11) (Fig. 1), an osteoclastogenic cytokine that serves as a biomarker for periodontal disease activity (36); conversely, the expression of osteoprotegerin (OPG; TNFRSF11b), a natural inhibitor of RANKL (36), was significantly downregulated in diseased gingival samples compared to healthy controls (p < 0.05; Fig. 1).
Detection of Ig kappa light chain, APRIL and BLyS protein in human gingiva
Analysis of haemotoxilyn-eosin stained sections (10 diseased and 2 controls) confirmed minimal inflammatory cell infiltration in healthy control specimens, whilst a moderate-to-heavy inflammatory infiltrate was detected in periodontitis specimens (not shown), as seen in earlier reports (7, 37). To detect B cells/plasma cells, we stained tissue specimens for Ig kappa light chain, which was shown to be a reliable marker for B cells and terminally differentiated Ab-secreting plasma cells (30, 38). As a positive control, we used human tonsil specimens, which revealed the presence of immunopositive cells within the sub-epithelial layer most likely representing a germinal center (Fig. 2A). All periodontally involved specimens examined presented immunoreactivity for kappa light chain. Localization of kappa light chain-positive cells was observed predominantly within connective tissue layers directly adjacent to the gingival epithelium (Fig. 2B). No staining was observed for kappa light chain when FITC-conjugated non-immune IgG was used in lieu of FITC-conjugated anti-human kappa light chain IgG (Fig. 2C).
Figure 2. Ig kappa light chain staining of human mucosal tissues.
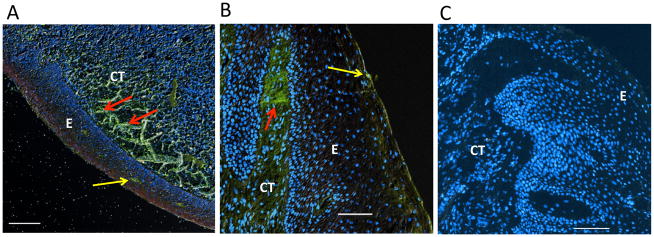
(A) Immunohistochemical analysis of human tonsil shows diffuse staining of kappa light chain (green) within the connective tissue indicating the presence of B cells/plasma cells (red arrows). (B) Immunohistochemical analysis of human gingival tissue from a patient with chronic periodontitis shows prominent staining of kappa light chain (green) within the connective tissue adjacent to the basement membrane (red arrow). In both the tonsil (A) and the gingiva (B), occasional kappa-light-chain–positive cells infiltrate the superficial epithelial layer (yellow arrows). (C) Control staining of human gingival tissue from a patient with chronic periodontitis using FITC-conjugated non-immune IgG. Nuclear staining (blue) by DAPI. E, epithelium; CT, connective tissue. Original magnification 100X. Scale bars, 100 μm.
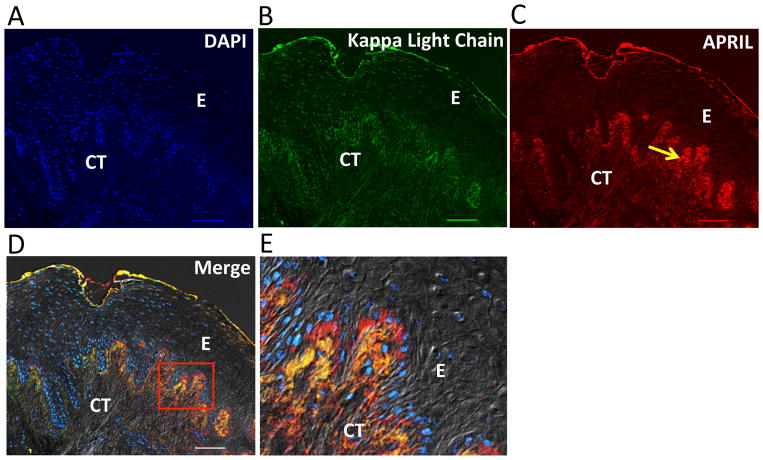
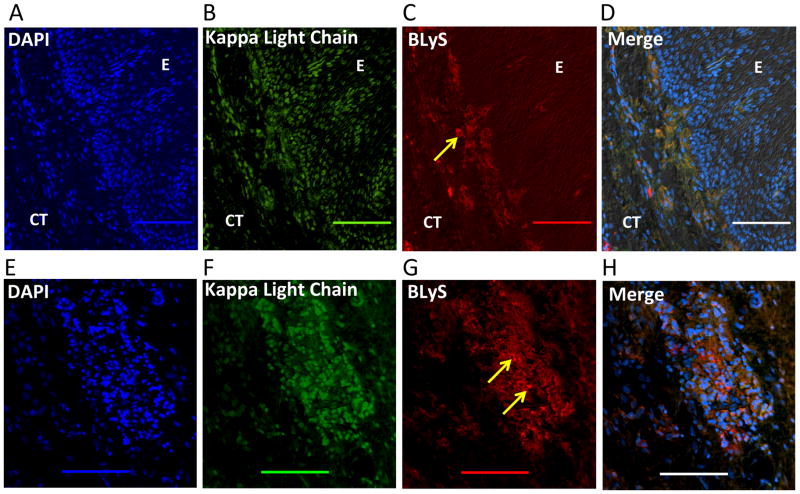
In diseased samples stained for cell nuclei, kappa light chains, and APRIL (Fig. 3), APRIL immunoreactivity was positive within the gingival epithelium and underlying connective tissue adjoining the basement membrane (Fig. 3C). In the gingival connective tissue, APRIL was detected dispersed in the extracellular matrix and co-localized with Ig kappa light chain (yellow/orange staining) in cellular areas adjacent to the basement membrane (Fig. 3, D and E). Similarly, BLyS was detected in diseased samples in the connective tissue adjacent to the basement membrane (Fig. 4, A–D). However, stronger BLyS immunostaining was observed in gingival granulomatous tissue (Fig. 4, E–H). The variability of staining at different layers of the diseased gingival samples seemed to be related to the abundance of cell populations (infiltrating inflammatory cells and resident basal epithelial cells). In contrast to diseased samples, control specimens displayed relatively poor staining for APRIL, BLyS, and Ig kappa light chain (Fig. 5).
Figure 3. APRIL expression in human periodontitis.
Immunohistochemical analysis of diseased gingival tissue stained as follows: (A) DAPI to indicate cell nuclei. (B) Anti-kappa light chain mAb indicating the presence of Ig-expressing/secreting cells (B cells/plasma cells) predominantly within the gingival connective tissue directly adjacent to the basement membrane. (C) Anti-APRIL mAb showing immunoreactivity (yellow arrow) within the gingival epithelium and underlying connective tissue adjacent to the basement membrane. (D) Fluorescence and differential interference contrast merged image showing positive co-localization (orange/yellow) of APRIL and kappa light chain expression within the gingival connective tissue. (E) The demarcated area in D showing co-localized APRIL and kappa light chain surrounding DAPI-stained cells likely representing B cells/plasma cells. E, epithelium; CT, connective tissue. Original magnification 100X. Scale bars, 100 μm.
Figure 4. BLyS expression in human periodontitis.
(A–D) Immunohistochemical analysis of diseased gingival tissue stained with as follows: (A) DAPI to indicate cell nuclei. (B) Anti-Ig kappa light chain mAb indicating the presence of Ig-expressing/secreting cells (B cells/plasma cells) predominantly within the gingival connective tissue. (C) Anti-BLyS mAb showing immunoreactivity (yellow arrow) within the gingival connective tissue and to a lesser extent within the epithelium. (D) Fluorescence and differential interference contrast merged image showing positive co-localization (orange) of BLyS and Ig kappa light chain expression within the gingival connective tissue. (E–H) Similar analysis as above using granulomatous tissue taken from the deepest aspect of an osseous defect. (E) DAPI staining to indicate cell nuclei. (F) Staining with anti-Ig kappa light chain mAb indicating the presence of Ig-expressing/secreting cells (B cells/plasma cells). (G) Staining with anti-BLyS mAb showing intense immunoreactivity (yellow arrows). (H) Fluorescence and differential interference contrast merged image showing intense positive co-localization (orange) of BLyS and Ig kappa light chain at heavily populated area of DAPI-stained cells likely representing B cells/plasma cells. E, epithelium; CT, connective tissue. Original magnification 200X. Scale bars, 100 μm.
Figure 5. APRIL and BLyS expression in healthy human gingival tissue.
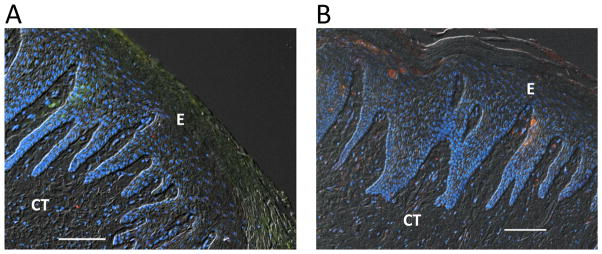
Immunohistochemical analysis of healthy gingiva stained for (A) APRIL or (B) BLyS (both red). In both A and B, the tissue was stained with DAPI to indicate cell nuclei (blue) and anti-Ig kappa light chain (green). The fluorescent images (merged with differential interference contrast) show little if any positive staining for APRIL, BLyS, or Ig kappa light chain (compare with diseased tissue in Figures 3 and 4). E, epithelium; CT, connective tissue. Original magnification 100X. Scale bars, 100 μm.
Detection of increased levels of APRIL and BLyS in gingival tissue obtained from patients with chronic periodontitis prompted us to determine their functional significance relative to alveolar bone loss using an appropriate animal model.
B cells are temporally and causally linked to alveolar bone loss in experimental murine periodontitis
The ligature-induced periodontitis model involves placement of silk ligature around posterior teeth (e.g., the maxillary left second molar in mice); this procedures causes local accumulation of bacteria and enhances bacteria-mediated inflammation and bone loss at the ligated site relative to the contralateral unligated site (‘zero baseline’) (31, 33). In this model, there is robust complement activation and expression of high levels of IL-6 (35, 39), which are factors shown to promote B cell activation and differentiation (40, 41). We therefore reasoned that this may be an appropriate model to investigate the role of B cells and their stimulatory cytokines APRIL and BLyS in periodontal bone loss. We first determined the time-course of appearance of B cells/plasma cells in the murine gingiva following initiation of periodontitis. Moreover, in view of conflicting results in the literature (18, 19), we examined whether the B-lineage cells are indeed involved in periodontal bone loss.
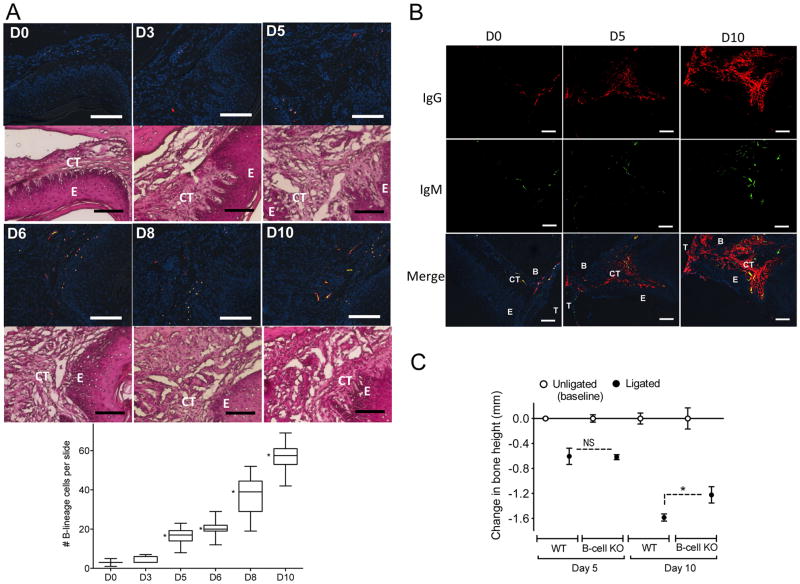
B cells and plasma cells were readily detected in mouse gingiva 5 d after initiation of experimental periodontitis in WT mice and their numbers increased in the following days (Days 6 to 10; Fig. 6A). B cells/plasma cells were predominantly located in the connective tissue in proximity to the interface with the epithelium (Fig. 6A). Consistent with this, IgG and IgM were more abundant at day 10 relative to day 5 or earlier (Fig. 6B). WT mice displayed substantial bone loss by day 5 following a similar time course to that observed previously (35, 42). This bone loss in WT mice at day 5 was comparable to that experienced by B-cell KO mice at the same time interval (Fig. 6C). In stark contrast, WT mice developed significantly more bone loss than B-cell KO mice at day 10 (p < 0.01; Fig. 6C). These data suggest that B cells contribute to a late phase of ligature-induced alveolar bone loss. Temporally, this occurred in parallel with the increase in the number of B-lineage cells within gingival connective tissue initiated 5 d after ligature placement.
Figure 6. Presence and impact of B cells in murine periodontitis.
(A and B) Tissue sections from ligature-induced periodontitis at the indicated timepoints were stained for CD19 (B cells; red) and CD138 (plasma cells; green) (A) or for mouse Igs as indicated (B). All immunofluorescent sections in A and the bottom row in B were stained with DAPI to indicate cell nuclei. E, epithelium; CT, connective tissue; T, tooth; B, bone. Scale bars, 100 μm. Original magnification 200X (A) and 100X (B). In A (left panel), H&E staining of the same tissue sections used for immunofluorescence analysis confirmed that the B-lineage cells are predominantly found in the connective tissue in proximity to the interface with the epithelium. In A (right panel), B-lineage cells were enumerated from 50 random sections (10 from each of five mice/time point) and data are presented as box-and-whisker plots. The median is marked inside each box, and the ends of each box correspond to the interquartile range from the 25th to the 75th percentile. *P < 0.01 as compared to D0. *P < 0.01 as compared to D0. (C) Periodontal bone loss was induced in groups of WT or B-cell KO mice for 5 or 10 d by ligating a maxillary second molar and leaving the contralateral tooth unligated (baseline control). Data are means ± SD (n = 5 mice per group). *P < 0.01 between indicated groups. NS, not significant.
APRIL and BLyS are required for B cell/plasma cell involvement in alveolar bone loss
Consistent with our observations of elevated APRIL and BLyS in human periodontitis, mice subjected to ligature-induced periodontitis displayed a gradual increase in APRIL and BLyS expression which reached statistical significance after day 6 (p < 0.01; Fig. 7A). On the other hand, RANKL expression was significantly upregulated as early as day 5 (p < 0.01; Fig. 7A), consistent with the induction of B-cell–independent bone loss at the same time interval (Fig. 6C). Consistent with the pattern of elevated APRIL and BLyS mRNA expression over time, immunofluorescent staining of gingival tissue for APRIL and BLyS protein revealed increased expression of these proteins at day 10 post-ligation as compared to day 5 or the baseline (Fig. 7B). APRIL staining seemed to be predominantly within the epithelium, consistent with previous reports on the capacity of mucosal epithelia to produce APRIL (43). In contrast, BLyS staining appeared to be compartmentalized within the connective tissue in close proximity to the epithelium (Fig. 7B). Although APRIL and BLyS co-localized partially, both proteins were detected at the boundaries of the epithelium and the underlying connective tissue (coinciding with the basement membrane) (Fig. 7C), consistent with our observations in the human gingival tissue.
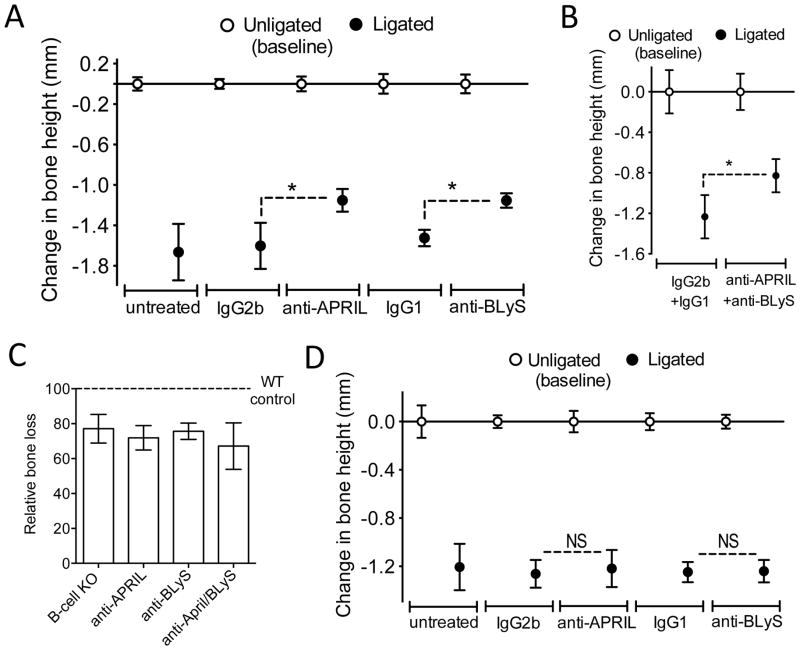
To determine whether APRIL and/or BLyS are involved in mediating alveolar bone loss, we microinjected the gingiva adjacent to ligated sites with specific neutralizing mAbs (i.e., that block binding of these cytokines to their receptors) or isotype controls. Treatment with either anti-APRIL mAb or anti-BLyS mAb caused comparable and significant inhibition of bone loss relative to their respective isotype controls (p < 0.01; Fig. 8A). Untreated mice (i.e., not microinjected at the ligated sites) developed comparable bone loss to that experienced by isotype control-treated mice (Fig. 8A). In a similar experiment, microinjection of WT mice with a combination of anti-APRIL and anti-BLyS mAbs resulted in inhibition of bone loss relative to the combined isotype control treatment (p < 0.01; Fig. 8B). The relative bone loss was then calculated for each treatment in figures 8A and 8B and compared to that observed in ligated B-cell KO and WT mice (set as 100%) (Fig. 8C). Inhibition of alveolar bone loss mediated by the combined treatment with both mAbs was not significantly different from treatments with either mAb alone. Furthermore, bone loss in the presence of anti-APRIL and/or anti-BLyS was comparable to that seen in B cell KO mice. It therefore appears that APRIL and BLyS are each required for B cells to mediate a late phase of alveolar bone loss.
Figure 8. Ab-mediated neutralization of APRIL or BLyS inhibits ligature-induced periodontal bone loss in a B cell-dependent manner.
(A) Periodontal bone loss in WT mice locally microinjected (or not) with 5 μg anti-APRIL mAb or anti-BLyS mAb (or equal amounts of their isotype controls), 4 d after placing the ligatures and every day thereafter until the day before sacrifice (day 10). (B) Similar bone loss experiment in which mice were microinjected with a combination of anti-APRIL mAb and anti-BLyS mAb or a combination of their isotype controls (each mAb or control was used at 5 μg). (C) The bone loss for each treatment in A and B was calculated relative to their unligated controls (set as 100%). Also included for comparison is the bone loss of B-cell KO relative to WT controls from the experiment in figure 6C. (D) Periodontal bone loss in B-cell KO mice locally microinjected (or not) with 5 μg anti-APRIL mAb or anti-BLyS mAb (or equal amounts of their isotype controls), 4 d after placing the ligatures and every day thereafter until the day before sacrifice (day 10). Data are means ± SD (n = 5 mice per group). Data are means ± SD (n = 5 mice per group). *P < 0.01 between indicated groups. NS, not significant.
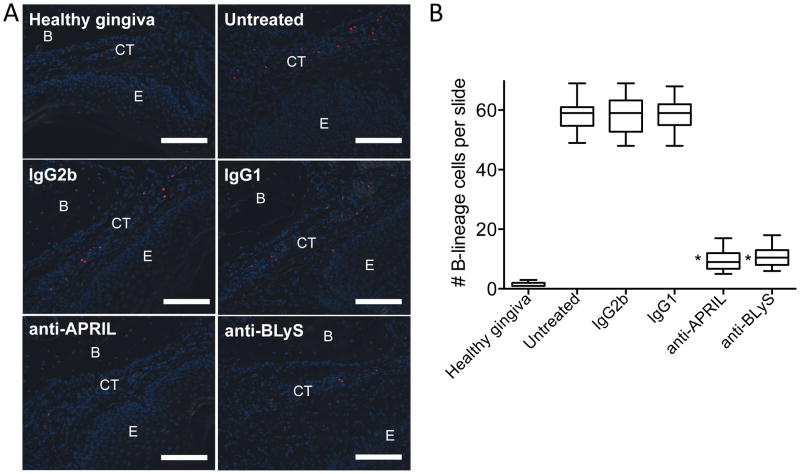
To conclusively demonstrate that the observed effects of anti-APRIL and anti-BLyS are mediated through action on B cells, the following experiments were performed. First, in contrast to their protective effects in WT mice, anti-APRIL and anti-BLyS lost their ability to inhibit bone loss in B-cell KO mice (Fig. 8D). Second, using the same protocol as in the bone loss study with WT mice (Fig. 8A), we showed that treatments with either anti-APRIL mAb or anti-BLyS mAb (but not with their isotype controls) diminished the number of B cells in the gingival tissue (p < 0.01; Fig. 9). Together, these data show unequivocally that the involvement of APRIL and BLyS in ligature-induced bone loss requires the presence of B cells.
Figure 9. Ab-mediated neutralization of APRIL or BLyS diminishes the numbers of B cells in the gingival tissue of mice subjected to ligature-induced periodontitis.
(A) Mice undergoing ligature-induced periodontitis were locally microinjected or not (“untreated”) with 5 μg anti-APRIL mAb or anti-BLyS mAb (or equal amounts of their isotype controls, IgG2b and IgG1, respectively), 4 d after placing the ligatures and every day thereafter until the day before sacrifice (day 10). Gingival tissue sections from these groups of mice as well as from mice not subjected to ligature-induced periodontitis (“healthy gingiva”) were stained for CD19 (B cells; red) and DAPI (blue) to reveal nuclei. E, epithelium; CT, connective tissue; B, bone. Scale bars, 100 μm. Original magnification 200X. (B) B cells were enumerated from 30 random sections (10 from each of three mice/group) and data are presented as box-and-whisker plots. The median is marked inside each box, and the ends of each box correspond to the interquartile range from the 25th to the 75th percentile. *P < 0.01 as compared to untreated and corresponding isotype control.
Discussion
This study has shown for the first time that (i) the expression of APRIL and BLyS mRNA and protein is enhanced in gingival tissue from patients with chronic periodontitis (relative to samples from healthy individuals) and (ii) Ab-mediated neutralization of APRIL and/or BLyS in experimental mouse periodontitis inhibits the late phase of alveolar bone loss that is mediated by B-lineage cells.
APRIL immunostaining was detected in diseased human gingiva within both the epithelium and the directly adjacent connective tissue, where it co-localized with Ig kappa light chain. APRIL co-localization with kappa light chain likely represents APRIL bound to either B cells or plasma cells. It has been shown that intestinal epithelial cells secrete APRIL in response to TLR-mediated stimulation by intestinal bacteria (43). Thus, it is likely that APRIL detected in the gingival epithelium was locally produced in response to dental plaque bacteria. An additional possibility is that APRIL might be in part systemically derived, although the serum levels of APRIL in patients with chronic periodontitis are not significantly higher than those of healthy controls (44). The APRIL-rich zone within the gingiva was similar to that previously described in mucosa-associated lymphoid tissues (24). The authors showed that APRIL secretion is upregulated upon infection and the cytokine is retained by heparan sulfate proteoglycans in subepithelial zones, thereby forming APRIL-rich niches that promote the survival and accumulation of IgG-producing plasma cells (24). In contrast to APRIL, BLyS expression within the diseased gingiva was limited to the connective tissue. This is consistent with the production of BLyS by myeloid cells including dendritic cells and macrophages (45). Nevertheless, similar to APRIL, BLyS was readily detected near the epithelial-connective tissue interface, an area rich in kappa light chain-expressing B lineage cells.
RANKL is produced by activated lymphocytes and stromal/osteoblastic cells (46) and is associated with periodontal disease activity in humans (36). Consistently, we detected increased RANKL mRNA expression in diseased gingival specimens compared to controls, thus correlating with the increased expression of APRIL and BLyS. Given that B cells constitute a major cellular source of RANKL in the bone resorptive lesions of human periodontitis (14), it is reasonable to suggest that APRIL and BLyS support the survival of RANKL-expressing B cells, thereby contributing to bone loss in the course of chronic periodontitis. Our intervention study (with neutralizing mAbs to APRIL and/or BLyS) in the ligature-induced periodontitis model is consistent with this hypothesis. Indeed, treatments with either anti-APRIL mAb or anti-BLyS mAb diminished the number of B cells in the gingival tissue and inhibited bone loss. Moreover, in contrast to their protective effects in WT mice, the treatments with anti-APRIL and anti-BLyS failed to inhibit bone loss in B-cell KO mice, hence conclusively indicating that their effects require the presence of B cells.
In line with the human data, the expression of APRIL and BLyS was also upregulated in ligature-induced mouse periodontitis and correlated with elevated numbers of B cells/plasma cells. In this regard, the induction of expression and release of APRIL and BLyS by stromal and myeloid cells (e.g., epithelial cells and neutrophils, respectively) involves stimulation by several molecules including G-CSF, LPS and other TLR ligands, C5a, and immune complexes (43, 47, 48), which are present in inflamed gingiva in both humans and mice (4, 34, 35, 49, 50). For the human study, our immunohistochemical observations of APRIL and BLyS were performed using keratinized human oral gingival explants. It is possible that the expression of these cytokines may be higher in the sulcular and junctional epithelium, which are in direct or closer contact with proinflammatory bacterial stimuli. It should also be noted that, prior to collecting gingival tissue samples, the patients went through scaling and root planing, a procedure involving the removal of the dental plaque biofilm. This clinical procedure might have contributed to decreased gingival inflammation and hence to an underestimation of APRIL and BLyS expression, although this is not certain. In this regard, it has been reported that scaling and root planing does not significantly affect the inflammatory infiltrate (B cells, plasma cells, and memory T cells) associated with chronic periodontitis (51).
A recent cross-sectional study reported on the presence of APRIL and BLyS in the serum and saliva of patients with chronic periodontitis and healthy controls (44). Although BLyS was detected at significantly higher concentrations in both fluids from patients relative to controls, there were no statistically significant differences in the levels of APRIL between the two groups (44). However, the levels of cytokines in the gingiva, where they can be locally produced, are not necessarily correlated with their concentrations in serum or saliva. Rather, the gingival expression levels of the two cytokines might be better correlated with their concentrations in the gingival crevicular fluid, which includes locally produced inflammatory mediators and bathes the periodontal pockets. In a separate study, the same group has detected both APRIL and BLyS in the gingival crevicular fluid of periodontitis patients with or without systemic disease (rheumatoid arthritis or osteoporosis), although no comparisons were made with periodontally healthy controls (52).
Our data implicating B cells in periodontal bone loss in C57BL/6J mice are consistent with an earlier study using a different model of mouse periodontitis (oral gavage) (19). Although the authors associated the absence of B cells with protection from bone loss, they did not monitor the appearance of B cells in WT C57BL/6J mice (19). Taken together, our observational and interventional studies have shown that B cells and B cell-stimulatory cytokines are temporally and causally linked to periodontal bone loss. Animal models have limitations that do not allow the investigators to faithfully reproduce the complexity of a given human disease. However, different aspects of the disease process can be represented by distinct models, which can thus be used to test specific hypotheses, especially those that cannot be readily addressed in humans (27). By demonstrating increased expression of APRIL and BLyS in the late stages of ligature-induced bone loss in mice, we had a relevant model to test the functional significance of the two cytokines. Our results implicated both APRIL and BLyS in B-cell–dependent bone loss and, interestingly, neutralizing either cytokine was as effective in blocking bone loss as their combined neutralization. This suggests that B cells cannot mediate pathogenic bone loss in the absence of any one of the two cytokines individually. This, in turn, may facilitate a B-cell–targeted therapeutic approach to treat periodontitis as it may not be necessary to neutralize both cytokines at the same time.
Ligature-induced periodontitis in mice represents a model of accelerated disease that allowed us to observe the emergence of B cells and plasma cells in the late stages of the experimental bone-loss period. However, the model has limitations in that it does not represent naturally occurring chronic periodontitis that could lead to the establishment of B cell-dominated periodontitis lesion. Indeed, as the severity of human periodontitis increases, the numbers of B cells and plasma cells also increase and become the predominant cell type in the advanced chronic lesion (7, 53). Therefore, B-lineage cells likely play a more important role in the pathogenesis of human periodontitis than in ligature-induced mouse periodontitis. We therefore think that treatment with anti-APRIL or anti-BLyS mAb is likely to have a more pronounced therapeutic effect in human periodontitis than in the mouse model that we used to demonstrate proof-of-concept. Whether such therapies can have a strong protective effect in human periodontitis or whether they only confer a partial effect, representing a co-therapeutic possibility, can only be determined in future clinical trials.
In summary, the elevated presence of APRIL and BLyS in B cell-rich areas of chronically inflamed gingiva suggests that these cytokines may contribute to bone loss by promoting the survival and persistence of RANKL-expressing B cells/plasma cells. Support for this notion was provided by intervention experiments that implicated APRIL and BLyS in mediating B cell-dependent alveolar bone loss in mice. Our study has therefore identified B-cell–targeted approaches as potential accessory treatments for periodontitis. In this context, several strategies targeting APRIL and/or BLyS are under clinical development and a neutralizing mAb to BLyS (belimumab) was approved by the FDA in 2011 for the treatment of systemic lupus erythematosus (23, 54, 55). Given that periodontitis is a prevalent disease, it would be interesting to determine the effects of systemic anti-APRIL/BLyS therapies on periodontitis. However, dedicated, locally applied treatments with these biologics in future clinical trials of periodontitis likely represent safer and more effective therapeutic options.
Abbreviations
- ABC
alveolar bone crest
- APRIL
a proliferation-inducing ligand
- BLyS
B-lymphocyte stimulator
- BM
bone marrow
- CAL
clinical attachment loss
- CEJ
cement–enamel junction
- PPD
probing pocket depth
- RANK
receptor activator of NF-κB
- RANKL
RANK ligand
- WT
wild-type
Footnotes
Supported by grants from the NIH: DE015254, DE017138, DE021685, DE024716, AI068730 (GH) and DE023836 (DFK). M.A. was supported from funds (# 4/52/146299) from the College of Dentistry Research Center and Deanship of Scientific Research at King Saud University, Saudi Arabia.
References
- 1.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garlet GP. Destructive and protective roles of cytokines in periodontitis: A reappraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 5.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 6.Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 7.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34:235–249. [PubMed] [Google Scholar]
- 8.Thorbert-Mros S, Larsson L, Berglundh T. Cellular composition of longstanding gingivitis and periodontitis lesions. J Periodontal Res. 2014 doi: 10.1111/jre.12236. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 9.Berglundh T, Donati M, Zitzmann N. B cells in periodontitis: friends or enemies? Periodontol 2000. 2007;45:51–66. doi: 10.1111/j.1600-0757.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 10.Schenkein HA. Host responses in maintaining periodontal health and determining periodontal disease. Periodontol 2000. 2006;40:77–93. doi: 10.1111/j.1600-0757.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 11.Whitney C, Ant J, Moncla B, Johnson B, Page RC, Engel D. Serum immunoglobulin G antibody to Porphyromonas gingivalis in rapidly progressive periodontitis: titer, avidity, and subclass distribution. Infect Immun. 1992;60:2194–2200. doi: 10.1128/iai.60.6.2194-2200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolopoulou-Papaconstantinou AA, Johannessen AC, Kristoffersen T. Deposits of immunoglobulins, complement, and immune complexes in inflamed human gingiva. Acta Odontol Scand. 1987;45:187–193. doi: 10.3109/00016358709098858. [DOI] [PubMed] [Google Scholar]
- 13.Toto PD, Lin LM, Gargiulo AW. Immunoglobulins and complement in human periodontitis. J Periodontol. 1978;49:631–634. doi: 10.1902/jop.1978.49.12.631. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashima T, Hayashi M, Takayanagi H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab. 2012;23:582–590. doi: 10.1016/j.tem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Harada Y, Han X, Yamashita K, Kawai T, Eastcott JW, Smith DJ, Taubman MA. Effect of adoptive transfer of antigen-specific B cells on periodontal bone resorption. J Periodontal Res. 2006;41:101–107. doi: 10.1111/j.1600-0765.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 17.Han X, Kawai T, Eastcott JW, Taubman MA. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol. 2006;176:625–631. doi: 10.4049/jimmunol.176.1.625. [DOI] [PubMed] [Google Scholar]
- 18.Zhu M, Belkina AC, DeFuria J, Carr JD, Van Dyke TE, Gyurko R, Nikolajczyk BS. B cells promote obesity-associated periodontitis and oral pathogen-associated inflammation. J Leukoc Biol. 2014;96:349–357. doi: 10.1189/jlb.4A0214-095R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliver-Bell J, Butcher JP, Malcolm J, MacLeod MK, Adrados Planell A, Campbell L, Nibbs RJ, Garside P, McInnes IB, Culshaw S. Periodontitis in the absence of B cells and specific anti-bacterial antibody. Mol Oral Microbiol. 2014;30(1):60–169. doi: 10.1111/omi.12082. [DOI] [PubMed] [Google Scholar]
- 20.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 21.Cancro MP. Signalling crosstalk in B cells: managing worth and need. Nat Rev Immunol. 2009;9:657–661. doi: 10.1038/nri2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Fraga M, Fernandez R, Albar JP, Hahne M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2001;2:945–951. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. 2014;10:365–373. doi: 10.1038/nrrheum.2014.33. [DOI] [PubMed] [Google Scholar]
- 24.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, Frossard C, Chizzolini C, Favre C, Zubler R, Guyot JP, Schneider P, Roosnek E. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest. 2008;118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 26.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244:115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajishengallis G, Lamont RJ, Graves DT. The enduring importance of animal models in understanding periodontal disease. Virulence. 2015;6:229–235. doi: 10.4161/21505594.2014.990806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 29.Damek-Poprawa M, Korostoff J, Gill R, DiRienzo JM. Cell junction remodeling in gingival tissue exposed to a microbial toxin. J Dent Res. 2013;92:518–523. doi: 10.1177/0022034513486807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss LM, Loera S, Bacchi CE. Immunoglobulin light chain immunohistochemistry revisited, with emphasis on reactive follicular hyperplasia versus follicular lymphoma. Appl Immunohistochem Mol Morphol. 2010;18:199–205. doi: 10.1097/PAI.0b013e3181c59a81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiao Y, Darzi Y, Tawaratsumida K, Marchesan JT, Hasegawa M, Moon H, Chen GY, Nunez G, Giannobile WV, Raes J, Inohara N. Induction of bone loss by pathobiont-mediated nod1 signaling in the oral cavity. Cell Host Microbe. 2013;13:595–601. doi: 10.1016/j.chom.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013;394:49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abe T, Hosur KB, Hajishengallis E, Reis ES, Ricklin D, Lambris JD, Hajishengallis G. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 2012;189:5442–5448. doi: 10.4049/jimmunol.1202339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 37.Lappin DF, Koulouri O, Radvar M, Hodge P, Kinane DF. Relative proportions of mononuclear cell types in periodontal lesions analyzed by immunohistochemistry. J Clin Periodontol. 1999;26:183–189. doi: 10.1034/j.1600-051x.1999.260309.x. [DOI] [PubMed] [Google Scholar]
- 38.Magro C, Crowson AN, Porcu P, Nuovo GJ. Automated kappa and lambda light chain mRNA expression for the assessment of B-cell clonality in cutaneous B-cell infiltrates: its utility and diagnostic application. J Cutan Pathol. 2003;30:504–511. doi: 10.1034/j.1600-0560.2003.00102.x. [DOI] [PubMed] [Google Scholar]
- 39.Maekawa T, Abe T, Hajishengallis E, Hosur KB, DeAngelis RA, Ricklin D, Lambris JD, Hajishengallis G. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J Immunol. 2014;192:6020–6027. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37:199–207. doi: 10.1016/j.immuni.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen-Kiang S. Regulation of terminal differentiation of human B-cells by IL-6. Curr Top Microbiol Immunol. 1995;194:189–198. doi: 10.1007/978-3-642-79275-5_23. [DOI] [PubMed] [Google Scholar]
- 42.Abe T, Shin J, Hosur K, Udey MC, Chavakis T, Hajishengallis G. Regulation of osteoclast homeostasis and inflammatory bone loss by MFG-E8. J Immunol. 2014;193:1383–1391. doi: 10.4049/jimmunol.1400970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Gumus P, Nizam N, Lappin DF, Buduneli N. Saliva and serum levels of B-cell activating factors and tumor necrosis factor-alpha in patients with periodontitis. J Periodontol. 2014;85:270–280. doi: 10.1902/jop.2013.130117. [DOI] [PubMed] [Google Scholar]
- 45.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 47.Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, Cassatella MA. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scapini P, Carletto A, Nardelli B, Calzetti F, Roschke V, Merigo F, Tamassia N, Pieropan S, Biasi D, Sbarbati A, Sozzani S, Bambara L, Cassatella MA. Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood. 2005;105:830–837. doi: 10.1182/blood-2004-02-0564. [DOI] [PubMed] [Google Scholar]
- 49.Hajishengallis G, Abe T, Maekawa T, Hajishengallis E, Lambris JD. Role of complement in host-microbe homeostasis of the periodontium. Semin Immunol. 2013;25:65–72. doi: 10.1016/j.smim.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kajiya M, Giro G, Taubman MA, Han X, Mayer MP, Kawai T. Role of periodontal pathogenic bacteria in RANKL-mediated bone destruction in periodontal disease. J Oral Microbiol. 2010;2 doi: 10.3402/jom.v3402i3400.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleinfelder JW, Lange DE, Bocker W. Some effects of non-surgical therapy on gingival inflammatory cell subsets in patients with adult and early-onset periodontitis. J Periodontol. 2000;71:1561–1566. doi: 10.1902/jop.2000.71.10.1561. [DOI] [PubMed] [Google Scholar]
- 52.Gumus P, Buduneli E, Biyikoglu B, Aksu K, Sarac F, Buduneli N, Lappin DF. Gingival crevicular fluid and serum levels of APRIL, BAFF and TNF-alpha in rheumatoid arthritis and osteoporosis patients with periodontal disease. Arch Oral Biol. 2013;58:1302–1308. doi: 10.1016/j.archoralbio.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Page RC, Schroeder HE. Periodontitis in man and other animals- A comparative review. Karger; Basel, Switzerland: 1982. [Google Scholar]
- 54.La Cava A. Targeting the BLyS-APRIL signaling pathway in SLE. Clin Immunol. 2013;148:322–327. doi: 10.1016/j.clim.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Ding HJ, Gordon C. New biologic therapy for systemic lupus erythematosus. Curr Opin Pharmacol. 2013;13:405–412. doi: 10.1016/j.coph.2013.04.005. [DOI] [PubMed] [Google Scholar]