Abstract
Background
IL-9 is important for the growth and survival of mast cells. IL-9 is produced by T cells, NKT cells, mast cells, eosinophils, and innate lymphoid cells, although the cells required for mast cell accumulation during allergic inflammation remain undefined.
Objective
To elucidate the role of Th9 cells in promoting mast cell accumulation in models of allergic lung inflammation.
Methods
Adoptive transfer of OVA-specific Th2 and Th9 cells was used to assess the ability of each subset to mediate mast cell accumulation in tissues. Mast cell accumulation was assessed in wild type mice and mice with PU.1-deficient T cells subjected to acute and chronic models of allergic inflammation.
Results
Adoptive transfer experiments demonstrated that recipients of Th9 cells had significantly higher mast cell accumulation and expression of mast cell proteases as compared to control or Th2 recipients. Mast cell accumulation was dependent on IL-9, but not IL-13, cytokine required for many aspects of allergic inflammation. In models of acute and chronic allergic inflammation, decreased IL-9 levels in mice with PU.1-deficient T cells corresponded to diminished tissue mast cell numbers and expression of mast cell proteases. Mice with PU.1-deficient T cells have defects in IL-9 production from CD4+ T cells, but not NKT cells or innate lymphoid cells, suggesting a T helper cell-dependent phenotype. Rag1−/− mice subjected to a chronic model of allergic inflammation displayed reduced mast cell infiltration comparable to accumulation in mice with PU.1-deficient T cells, emphasizing the importance of IL-9 produced by T cells in mast cell recruitment.
Conclusion
Th9 cells are a major source of IL-9 in models of allergic inflammation and play an important role in mast cell accumulation and activation.
Keywords: Th9 cells, Th2 cells, mast cells, allergic inflammation, PU.1
INTRODUCTION
IL-9, originally considered a Th2-cytokine, is now ascribed to a specialized subset of CD4+ T helper cells, the Th9 subset (1, 2). IL-9 plays an important role in human and murine atopic disease (3–5), anti-tumor immunity (6), anaphylaxis to allergen challenge at mucosal surfaces (7), immunity to intestinal parasites (8, 9), antiviral immunity (10) and autoimmune inflammation (11, 12). IL-9 is a pleiotropic cytokine with direct and indirect effects on multiple cell types. IL-9 acts as a growth factor for T cells (13, 14), enhances the production of IgE from B cells (15), induces mucus production by epithelial cells (16, 17) and promotes the proliferation and differentiation of mast cells (18, 19). In mast cells, IL-9 enhances expression of MC proteases, increases expression of the high-affinity IgE receptor (FcεR1α), and induces IL-6 production (18–20). Transgenic expression of IL-9 in the lungs of mice results in mast cell hyperplasia, and studies with IL-9-deficient mice demonstrated a role for IL-9 in pulmonary mastocytosis (21). In allergic airway inflammation, mast cells are increased in both the acute and chronic models of disease (22–24). Mast cell accumulation is a critical aspect of the pathology of immediate hypersensitivity responses (25).
IL-9 is mainly produced by T cells but can also be produced by mast cells (26), NKT cells (27), eosinophils (28) and type 2 innate lymphoid cells (ILC2s) (29). The transcription factors important for Th9 lineage determination and IL-9 regulation include IRF4, BATF and the ETS family transcription factor, PU.1 (5, 30, 31). Increased PU.1 expression leads to a decrease in Th2 cytokines and increased phenotypic characteristics of Th9 cells including production of IL-9. Mice with a T-cell specific deletion of PU.1 have decreased IL-9 in vivo and do not develop allergic inflammation despite the presence of normal Th2 and Th17 responses. Recent studies with Il9 fate reporter mice that evaluated the IL-9-producing cell types established innate lymphoid cells (ILCs) as a major source of IL-9 in an in vivo model of lung inflammation (29). Thus, the functional relevance for Th9 cells specifically in mast cell accumulation and the relative role of cell types that produce IL-9 in allergic inflammation need further investigation.
We hypothesized that Th9 cells play an important role in mast cell recruitment and activation in acute and chronic models of allergic inflammation. In this report, we evaluated the effects of Th9 cells on mast cell recruitment in adoptive transfer experiments and models of acute and chronic allergic inflammation. We demonstrate that Th9 cells are an important source of IL-9 and promote mast cell accumulation through IL-9-dependent mechanisms in vivo.
METHODS
Mice
BALB/c, Rag1−/− and DO11.10 TCR transgenic mice were purchased from Jackson Laboratories. Female C57BL/6 mice were purchased from Harlan Bioscience. Mice with conditional deletion of the gene encoding PU.1 (Sfpi1fl/fl) on the C57BL/6 background have been described (32) and were mated to mice carrying a transgene encoding Cre recombinase under control of the Lck promoter (B6(CBA)-Tg(Lck-cre)I540Jxm/J). Mice were maintained in pathogen-free conditions and all studies were approved by the Animal Care and Use Committee of the Indiana University School of Medicine.
Adoptive Transfer Experiments and Cytokine Neutralization
Briefly, differentiated OVA-specific Th2 or Th9 cells were adoptively transferred intravenously into wild-type recipient mice (33). Twenty-four hours after cell transfer, mice were challenged intranasally with 100 μg OVA plus 500 ng TSLP for 5 days. Mice were then sacrificed 24 h after the last challenge for further analysis. To neutralize cytokine in recipients of Th2 or Th9 cells, we injected mice via tail vein with anti-IL-9 (10 μg/dose), anti-IL-13 (10 μg/dose), or IgG2b control Ab (10 μg/dose, R&D Systems) on days 1, 3, and 5.
Induction of Allergic inflammation
Acute Model: Wild type (WT) and Sfpi1lck−/− mice were sensitized by intraperitoneal injection of OVA (Sigma) adsorbed with alum (Sigma) on days 0 and 7 and subsequently challenged with intranasal OVA for 5 days as described previously (5). Where specified, mice were given 20 μg control antibody or anti-IL-9 (222622; R&D Systems) intravenously 30 min before the first, third and fifth challenges. Mice were sacrificed 48 h after the final intranasal challenge. Chronic model: WT and Sfpi1lck−/− mice were sensitized by intranasal injection of 40 μg HDM extract (Dermatophagoides pteronyssinus in phosphate-buffered saline, PBS) from Greer Laboratories (Lenoir, NC) or PBS 3 days per week for 5 weeks. Mice were sacrificed 24 h after the final intranasal challenge. Cells from mediastinal lymph nodes were stimulated with HDM for 5 days, and cytokine production measured by ELISA.
Bronchoalveolar lavage and lung histology
The trachea was cannulated and lungs were lavaged three times with 1 ml PBS to collect bronchoalveolar lavage (BAL) cells. Cells recovered in BAL fluid were counted with a hemocytometer. Eosinophils, neutrophils, T cells, B cells and mononuclear cells in the BAL fluid were distinguished by cell size and by expression of CD3, B220, CCR3, CD11c and major histocompatibility complex class II and analyzed by flow cytometry as described (34).
Cytokine concentrations in cell-free BAL fluid were measured with Multiplex reagents (Millipore).
After the lavage, lung tissues were fixed in neutral buffered Formalin. Paraffin-embedded lung tissue sections were stained with hematoxylin and eosin (H & E), Periodic Acid-Schiff (PAS) or toluidine blue to evaluate the infiltration of inflammatory cells, mucus-producing cells and mast cells, respectively, by light microscopy.
Intracellular cytokine staining (ICS)
BAL cells were stimulated with PMA and ionomycin for 5 h with the addition of monensin during the last 3h of stimulation for cytokine analysis by intracellular cytokine staining (ICS). Cells were stained for surface CD4, fixed with 4% neutral buffered paraformaldehyde and permeabilized with saponin. Cells were then stained with fluorochrome-conjugated anti-mouse IL-9 and anti-IL-4. Before transfer, a sample of Th2 and Th9 cultures were activated with PMA/ionomycin and stained with fluorochrome-conjugated anti-mouse IL-9, IL-13, CD4, and KJ1.26 (eBioscience).
Intranasal administration of IL-33 and culture of ILCs
WT C57BL/6 and Sfpi1lck−/− mice were challenged intranasally with 500 ng recombinant mouse IL-33 (Biolegend) on days 0, 1 and 2. Control mice received PBS only. Mice were sacrificed on day 3 and BAL cells were isolated for analysis. Briefly, ILCs were sorted by gating on the lin− Thy1+ and CD4− cell population. CD4+ T cells were identified as Thy1+CD4+. Sorted ILCs and CD4+ cells were cultured for 24h in 96 well plates (concentration of 5×103 cells/30 μl) with IL-2 (100 U/ml) and IL-9 expression determined by quantitative RT-PCR.
Alpha galactosylceramide administration
WT C57BL/6 and Sfpi1lck−/− mice were challenged intranasally with 2 μg alpha galactosylceramide or PBS and 24 h after challenge the expression of cytokines were determined in the lung tissues by quantitative RT-PCR.
Quantitative RT-PCR
Lung tissues were homogenized in a tissue lyser (Qiagen) and RNA isolated with an RNeasy kit (Qiagen) was used for synthesis of cDNA for subsequent analysis. Quantitative PCR was performed with Taqman Fast Universal PCR Master Mix and commercially available primers for genes Mcpt1, Mcpt2, Il9, Ifng, Muc5ac, and Clca3 with the 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). RNA was normalized to expression levels of β2-microglobulin and relative expression was calculated by the change-in-threshold (−ΔΔ CT) method.
Mouse MCPT-1 (mMCP-1) in serum
mMCP-1 is a mouse mast cell protease that can be detected in the serum of mice after activation of mast cells (35). Mouse mast cell protease-1 kit was purchased from eBioscience and used according to the manufacturer’s protocol.
Immunohistochemistry
Sections were deparaffinized with xylene and rehydrated from ethanol to water. Antigen retrieval was performed by high pH antigen retrieval using the proprietary DAKO buffer in the DAKO PT Link. Endogenous peroxidase activity was quenched by incubating the specimen for 10 minutes with 3% hydrogen peroxide. Tissue sections were incubated with primary antibodies recognizing CD3 (DAKO, IR 503) and CD117 (c-kit, Santa Cruz, sc-168) for 30 minutes. C-KIT staining was detected with LSAB2 (DAKO; HRP labeled streptavidin followed by donkey -anti–goat-biotin) and DAB, CD3 staining followed by Rabbit-on-mouse-AP (Biocare) with Vulcan Red, and sections were counterstained with hematoxylin (DAKO).
RESULTS
Transfer of Th9 cells increased the activation of mast cells and their accumulation
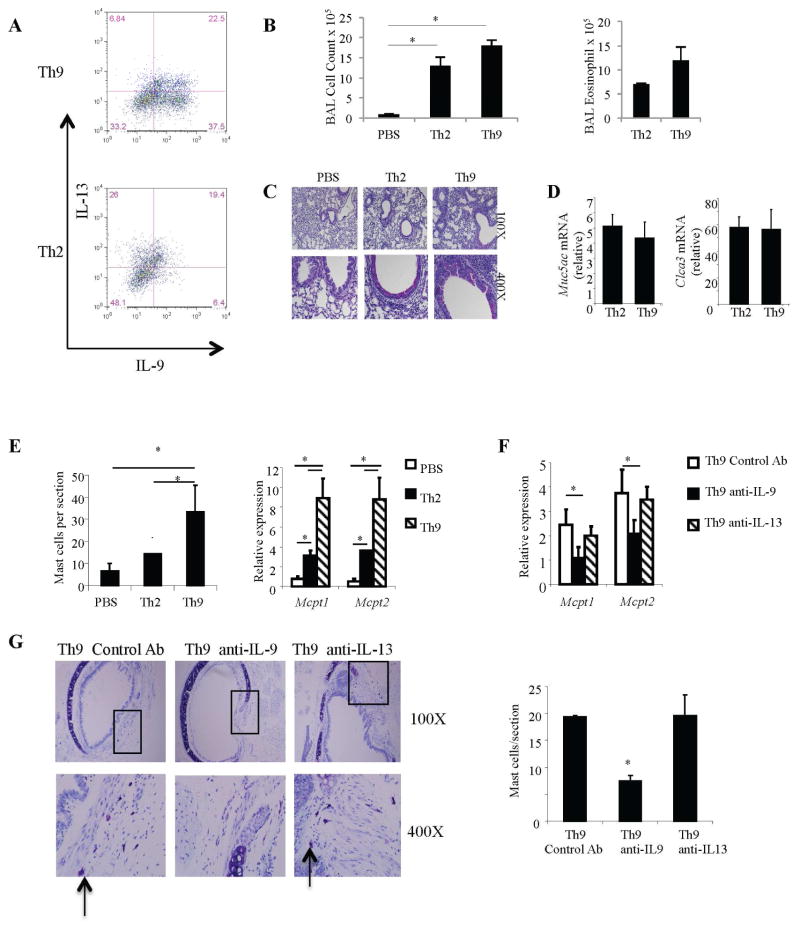
To compare the ability of Th9 and Th2 cells to promote mast cell accumulation in situ, a hallmark of allergic inflammation, we adoptively transferred differentiated DO11.10 Th9 or Th2 cells to BALB/c mice followed by intranasal allergen (OVA+TSLP) challenge of the recipient mice as recent studies from our laboratory have shown that TSLP results in enhanced Th9-mediated pathology, compared to mice given OVA alone (33). Before transfer, we assessed the polarization of differentiated Th2 (high IL-13, low IL-9) and Th9 (high IL-9, low IL-13) cells by intracellular staining and observed patterns of cytokine secretion (Fig 1, A) similar to our previous report (33). Recipients of Th2 or Th9 cells demonstrated similar inflammatory cell infiltration in the bronchoalveolar fluid (BAL) that was predominantly eosinophilic (Fig 1, B). There was a trend toward increased inflammation in recipients of Th9 cells and this was similar to our previous report (33). Mucus production, assessed by histological analyses of lung tissues (Fig 1, C) or expression of genes associated with mucus metaplasia (Fig 1, D) was indistinguishable between recipients of Th2 and Th9 cells. The effects of Th2 and Th9 cells on mast cell accumulation/activation indicate that although Th2 cells modestly increased mast cell numbers and mast cell protease expression, Th9 cells were significantly more potent in increasing mast cell numbers and the expression of mast cell proteases (Fig 1, E). The increased expression of the mast cell proteases is likely due to both increased numbers of mast cells, and increased gene expression per cell. Thus, Th9 cells have a significantly greater ability to promote mast cell accumulation during allergic inflammation.
Figure 1.
Adoptive transfer of Th9 cells increases mast cell numbers. A, Naive CD4+ T cells from DO11.10 mice were differentiated under Th9 / Th2 conditions with OVA323–339 peptide and mitomycin C treated antigen presenting cells. Before transfer, the percentage of IL-13+ and IL-9+ cells were measured in Th9 / Th2 cell cultures by intracellular cytokine staining. B, Total number of inflammatory cells were determined in BAL fluid and numbers of eosinophils determined by flow cytometry. C and D, Mucus production was assessed in the lung tissues by PAS staining and expression of mucus genes Muc5ac and Clca3 quantified by qPCR. E, PBS, Th2 or Th9 DO11.10 cells were intravenously transferred to BALB/c mice that were subsequently challenged with OVA+TSLP for 5 days. Mast cells were counted after staining tracheal sections with toluidine blue and expression of mast cell proteases was measured in lung tissues by quantitative PCR. F and G, Th9 cell recipients received 10 μg of anti-IL-9, anti-IL-13, or IgG2b control mAb on days 1, 3 and 5 before challenge with OVA+TSLP. Mice were sacrificed 24 h after the last challenge and mast cell numbers were quantitated in tracheal tissue sections by toluidine blue staining and expression of mast cell proteases was determined by quantitative PCR. Boxes on low magnification micrographs indicate the region for the higher magnification panel. Arrow: mast cells. Data are the mean ± SEM of 3–5 mice per group and representative of 2 independent experiments with similar results. *p < 0.05.
To determine if mast cell accumulation following adoptive transfer of Th9 cells required IL-9, Th9 cells were transferred into BALB/c mice and the recipient mice challenged intranasally with allergen for 5 days. Before the first, third, and fifth challenge, mice received anti-IL-9, anti-IL-13 or control antibody. Th9 cell recipient mice that received neutralizing anti-IL-9 had significantly reduced infiltration of mast cells in tracheal sections concomitant with reduced mast cell protease expression as compared to the mice given control antibody (Fig 1, F and G). In contrast, blocking IL-13, which diminishes airway inflammation (33) did not affect mast cell accumulation or activation (Fig 1, F and G). These data indicate that Th9 cells are more efficient than Th2 cells in orchestrating mast cell accumulation and inducing mast cell gene expression in vivo.
Decreased IL-9 corresponds to reduced mast cell numbers in mice with PU.1-deficient T cells in an acute model of allergic airway inflammation
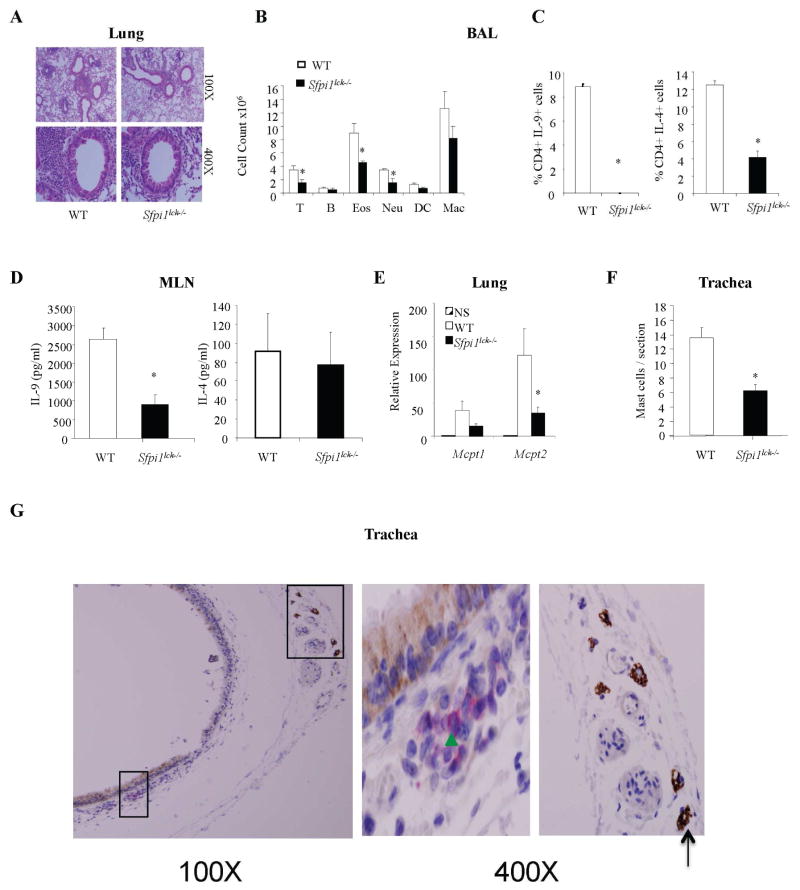
To determine if mast cell accumulation in mice subjected to the OVA/Alum protocol for the development of acute allergic inflammation is dependent on IL-9, we administered anti-IL-9 before the first, third, and fifth challenge (5). As shown in Fig 2A, neutralizing IL-9 dramatically reduced the numbers of infiltrating mast cells in WT mice. We previously demonstrated that mice with PU.1-deficient T cells (Sfpi1fl/fl Lck-Cre mice, hereafter referred to as Sfpi1lck−/− mice) subjected to an OVA/Alum protocol have significantly lower inflammation (total and eosinophil cell counts) that corresponded to lower amounts of IL-9, compared to WT mice (5). To determine if diminished IL-9 levels correlate with decreased mast cell numbers, we examined mast cell infiltration in Sfpi1lck−/− mice sensitized and challenged with OVA. Diminished IL-9 in Sfpi1lck−/− mice (5) corresponded to reduced mast cell numbers and decreased expression of mast cell proteases (Fig 2, B and C). These studies suggest that Th9 cells mediate mast cell accumulation in an acute model of allergic inflammation.
Figure 2.
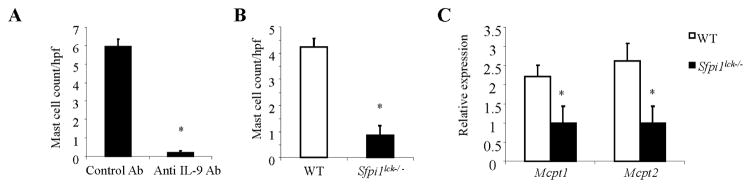
Th9-dependent accumulation of mast cells in an acute model of allergic inflammation. A, Wild-type mice were sensitized and challenged using the OVA/alum protocol. Mice received control immunoglobulin (control Ab) or anti-IL-9, 30 min before the first, third and fifth intranasal challenge. Mice were sacrificed 48 h after the last challenge and tracheal tissues were evaluated for the presence of mast cells. B and C, Wild-type and Sfpi1lck−/− mice were sensitized and challenged with the OVA/Alum protocol and mast cells were counted in toluidine blue stained tracheal tissue sections and quantitative PCR was performed for mast cell protease gene expression in lung tissues. Data shown represent the mean ± SEM of 5 to 6 mice per group from 2 independent experiments with similar results. *p < 0.05.
Reduced mast cell numbers in mice with PU.1-deficient T cells correlate with less IL-9 in a chronic house dust mite model of allergic pulmonary inflammation
Having shown that IL-9 is required for mast cell accumulation in an acute model of allergic inflammation we next examined its role in a house dust mite (HDM) model of chronic allergic inflammation without adjuvant. WT and Sfpi1lck−/− mice were challenged intranasally with HDM extract for 5 weeks and the development of pulmonary inflammation was examined. Consistent with our results for the OVA-induced acute model, inflammation in the lungs of Sfpi1lck−/− mice was lower than WT mice (Fig 3, A). Moreover, the cellular composition of BAL revealed significantly reduced numbers of eosinophils, neutrophils and T cells in PU.1-deficient mice (Fig 3, B). Analysis of cytokine responses indicated that approximately 10 % of BAL CD4+ T cells in WT mice stained positive for IL-9 in contrast to 0.1% positive cells in Sfpi1lck−/− mice (Fig 3, C). Similarly, IL-4 positive BAL cells were decreased in Sfpi1lck−/− mice (Fig 3, C). We next assessed the generation of helper T cell responses in the lung draining lymph nodes by stimulating mediastinal lymph node cells with HDM extract. We observed that IL-9 production was significantly reduced in cultures from Sfpi1lck−/− mice compared with those from control mice, although there was no difference in the production of IL-4 (Fig 3, D). These results suggest that in the chronic HDM model, inflammation in Sfpi1lck−/− mice is also dependent on IL-9 as there were reduced Th9 responses in the draining lymph nodes and BAL, consistent with our earlier studies (7). We then assessed mast cell accumulation in situ. Mast cell numbers in tracheal sections of PU.1-deficient mice were also reduced concomitant with decreased expression of mast cell proteases compared to WT mice (Fig 3, E and F). To determine if T cells and mast cells co-localize in the tracheal sections, we performed immunohistochemistry using CD3 and c-kit antibodies on paraffin embedded sections from the chronic HDM model. We observed that T cells and mast cells are present in tracheal sections, but were not located directly adjacent to each other (Fig 3, G). Thus, these data indicate that decreased IL-9 production in mice with PU.1-deficient T cells parallels reduced mast cell numbers/ and mast cell protease expression in a chronic airway inflammation model.
Figure 3.
Th9-dependent accumulation of mast cells in an HDM-induced chronic model of allergic inflammation. A–F, Wild-type mice were immunized intranasally with HDM for 5 weeks and A, Cellular infiltration in the lungs of wild-type and Sfpi1lck−/− mice was evaluated by hematoxylin and eosin (H&E) staining. Magnification is indicated. B, Inflammatory cells in the BAL fluid (T cells, B cells, Eosinophils (Eos), Neutrophils (Neu), Dendritic cells (DC) and Macrophages (Mac) were evaluated by flow cytometry. C, BAL CD4+ T cells were stimulated with PMA and ionomycin for 5 hours to assess cytokine production by intracellular staining. D, Cells from mediastinal lymph nodes were stimulated with HDM for 5 days. Cell-free supernatant was used to assess cytokine production using ELISA. E, Expression of mast cell proteases was determined by quantitative PCR analysis of lung RNA. F, Mast cell numbers were evaluated in tracheal sections. Data shown represent mean ± SEM of 5 to 7 mice per group from 2 independent experiments with similar results. *P < 0.05. G, Immunohistochemical staining of T cells (CD3) and mast cells (c-kit, CD117) with Vulcan Red and DAB in paraffin embedded tracheal tissue sections. Green arrowhead indicates T cells and black arrow shows mast cells. Boxes on low magnification micrographs indicate the region for the higher magnification panel.
CD4+ T cells are a major source of IL-9 in vivo in a model of allergic pulmonary inflammation
IL-9 can be produced by a number of cell types. Although CD4+ T cells from Sfpi1lck−/− mice have decreased IL-9 production, it is not known whether T helper cells are the only cells affected by Lck-Cre-dependent PU.1-deficiency, and this is an important point in being able to link the defects in mast accumulation in Sfpi1lck−/− mice specifically to Th9 cells. As NKT cells are also reported to produce IL-9 in vivo (27), we examined the effect of alpha-galactosylceramide instillation in Sfpi1lck−/− mice. As shown in Supplementary Fig E1A, expression of IL-9 and IFN-γ were unimpaired in mice with PU.1 deficiency in T cells indicating that PU.1 is not required for the production of IL-9 in NKT cells.
Studies from IL-9 fate reporter mice established ILCs as major producers of IL-9 in an in vivo model of papain- and IL-33-induced lung inflammation (29). To determine if ILC production of IL-9 is affected by Lck-Cre mediated deletion of PU.1, we intranasally challenged WT and Sfpi1lck−/− mice with IL-33 on days 0, 1 and 2. One day after the last challenge, populations of ILCs and CD4+ T cells were sorted as described in Materials & Methods. After stimulation with IL-2, we observed that PU.1-deficient CD4+ T cells had reduced IL-9 expression, compared with control cells, but that IL-9 expression by ILCs was not decreased (Supplementary Fig E1B). Thus our data indicate that Lck-Cre-mediated deletion of PU.1 affects IL-9 production primarily in conventional CD4+ T cells.
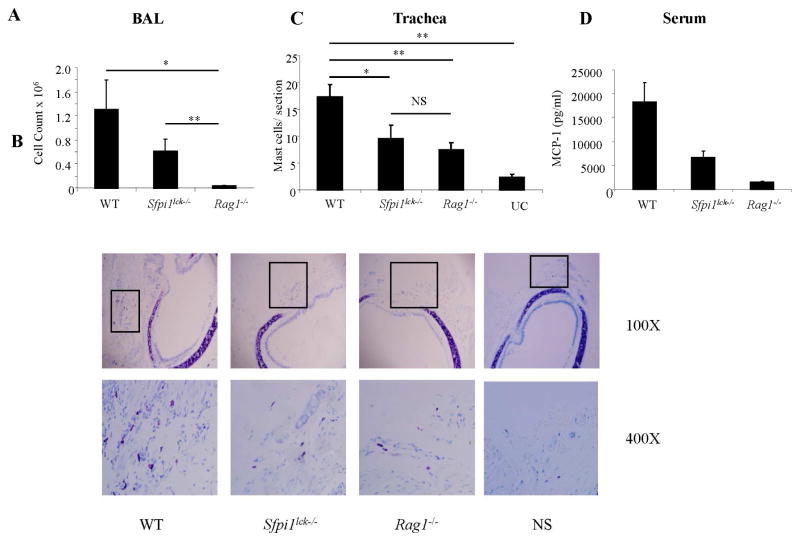
Knowing that ILC production of IL-9 is not affected in Sfpi1lck−/− mice and that ILCs are an important source of IL-9, we wanted to test the relative role of Th9 cells and ILCs in mast cell accumulation in a chronic HDM induced allergic inflammation model. For these studies we compared WT and Sfpi1lck−/− mice to Rag1−/− mice that lack T and B cells but have no defect in ILCs (29). Quantification of inflammatory cells in BAL revealed a drastic reduction in cell numbers in Rag1−/− mice in comparison to WT or Sfpi1lck−/− mice, supporting a requirement for T cells in maximal inflammation in this model (Fig 4, A). Analysis indicated a similar and significant reduction of mast cell numbers in the tracheal sections of Sfpi1lck−/− and Rag1−/− mice compared to WT mice (Fig 4, B and C). In accordance with decreased numbers of mast cells, serum levels of mast cell protease 1 were also decreased in Rag1−/− and Sfpi1lck−/− mice, compared to WT mice (Fig 4, D). Thus, although Rag1−/− mice had dramatically reduced overall inflammation, the reduction in mast cell accumulation is similar to that observed in mice with PU.1-deficient T cells, but greater than unchallenged mice. These data suggest although ILCs clearly contribute some IL-9 to promote mast cell accumulation, Th9 cells have an obligate role in mast cell accumulation during the development of allergic inflammation.
Figure 4.
T cell-dependent mast cell accumulation in HDM-induced chronic allergic airway inflammation. A, Numbers of inflammatory cells in the BAL fluid of wild-type, Sfpi1lck−/−, Rag1−/−, and unchallenged wild-type mice (UC) were determined. B and C, Mast cell infiltration in tracheal toluidine blue stained sections. Original magnification is indicated. Mast cell numbers were determined by counting in at least 5 hpfs. Boxes on low magnification micrographs indicate the region for the higher magnification panel. D, Serum mMCP-1 levels were determined by ELISA. Data are mean ± SEM of 5–7 mice per group and representative of 2 independent experiments with similar results. *P <0.05.
DISCUSSION
Previous studies to define a relevant function for Th9 cells in vivo have utilized IL-9- or IL-9R-deficient mice or neutralizing IL-9 antibodies (1, 36). Recent reports with models of allergic inflammation and helminthic parasite infection have shown that IL-9 can be produced not only by IL-9-producing CD4+ T cells but also by other cells including, ILCs (29, 37). Therefore, studies blocking cytokine or cytokine receptor function do not definitively link IL-9 function to Th9 function in vivo. To evaluate the biological source of IL-9 in vivo, we employed mice with a conditional deficiency of PU.1 in T cells. We have previously shown that these mice have diminished lung inflammation and decreased IL-9 in the lung with no impairment of peripheral Th2/Th17/Th1 responses (5, 38). In this report, we identified Th9 cells as a major source of IL-9 in the context of allergic pulmonary inflammation in mice and demonstrated their ability to induce mast cell accumulation and activation.
Our demonstration that adoptive transfer of polarized Th9 cells promotes mast cell accumulation, is similar to a previous report showing mast cell accumulation following adoptive transfer of HDM-sensitized and polarized Th9 cells (39), and that transfer of Th9 cells caused rapid worm expulsion and increased numbers of mast cells (37, 40). However, our data carries those observations further by showing that Th9 cells are more potent than Th2 cells in promoting mast cell accumulation and mast cell gene expression. This is a critical point as IL-4 was previously demonstrated to have some compensatory effects on mastocytosis in the absence of IL-9 (40). We further demonstrated that IL-9 was required for Th9 cells to mediate mast cell accumulation, whereas IL-13 was not required. This is in contrast to the ability of Th9 cells to mediate eosinophilic inflammation, where blockade of either IL-9 or IL-13 diminished allergic inflammation (33). Thus, mast cell accumulation during allergic inflammation might be a specific outcome of Th9- and IL-9-induced inflammation.
IL-9 has been reported to regulate mast cell numbers in the lung during chronic allergic inflammation (22). However, the cellular source of IL-9 that contributes to this regulation is still unclear, and ILCs are a significant source of IL-9 in parasitic infections and some models of allergic inflammation (29, 37, 41). We took the approach of examining mast cell accumulation in mice that have diminished IL-9 production specifically in Th9 cells following deletion of PU.1. Critical for drawing conclusions from this system was demonstrating that conditional deletion of PU.1 by an Lck-Cre transgene did not affect IL-9 production in NKT cells or in ILCs. As we observed normal induction of IL-9 in alpha galactosylceramide challenged mice and normal IL-9 production from ILCs in IL-33 challenged mice, it strongly suggests that the effects of Lck-Cre-dependent PU.1-deficiency on IL-9 production are restricted to conventional T cells. NKT cells were linked to regulation of mast cell precursors (MCp) in one model of allergic airway disease through an IL-9-dependent mechanism, although transgenic IL-9 expression in the intestine did not induce alterations in MCp populations (27, 42). Whether Th9 cells also impact MCp requires further investigation. Our observations correlate decreased IL-9 in the lungs of mice with PU.1-deficient T cells with diminished mast cell recruitment and activation in both the acute and chronic models of allergic inflammation suggesting a major contribution of Th9 cells to this process.
Our report, in combination with other recent work, suggests that Th9 cells, as a source of IL-9, are both necessary and sufficient for mast cell accumulation. Adoptive transfer of Th9 cells in wild type or IL-9-deficient recipients results in increased mast cell accumulation in the context of allergen-induced inflammation or parasite infection (37, 39). We further demonstrated that Th9 cells are more potent than Th2 cells in mediating this function. We show that in mice lacking PU.1 expression in T cells there is decreased tracheal mast cell accumulation in both acute and chronic inflammation models, consistent with the accumulation of tracheal mast cells in IL-9 transgenic mice (43). Moreover, the decrease in mast cell numbers in the chronic model in the absence of Th9 cells is similar to that observed in Rag1−/− mice that lack T cells but have normal ILC development (29). Thus, PU.1-dependent regulation of IL-9 is critical for the ability of T cells to mediate mast cell accumulation.
It is not clear if Th9 cells and mast cells need to physically interact during the development of allergic inflammation and co-localization has not been examined in detail. In the stroma of the lung, it is likely that T cells and mast cells can interact, and some studies have supported a role for mast cells as antigen presenting cells (44). However, IL-9 can have both local and regional effects by virtue of its release into the microenvironment. We analyzed tracheal sections of mice sensitized and challenged with HDM extract, and observed the presence but not the co-localization of T cells and mast cells. This result does not exclude the possibility that T cells interact directly with mast cells, but it does suggest that T cells are not required at the site of mast cell accumulation.
IL-9 is pleiotropic and has many functions in vivo. Among the more recently identified functions, studies have demonstrated that IL-9 is an amplifier of ILC2 function and survival and IL-9R-deficient mice have reduced numbers of ILC2s in a helminth infection model (41). However, we did not observe a decrease in the ILC2 numbers in mice with a conditional deletion of PU.1 in T cells (data not shown). This would suggest that the pro-survival function of IL-9 on ILCs may be autocrine, or at least that Th9 cells do not contribute to this activity. We further tested whether ILCs were required for Th9-induced mast cell accumulation using an anti-CD25 depleting antibody in the adoptive transfer recipients and observed no differences in mast cell accumulation between control antibody or anti-CD25 injected mice (data not shown). However, as the depleting antibody also had effects on adoptively transferred effector cells, these studies have several caveats in their interpretation.
Although mast cells are necessary for immediate hypersensitivity responses, their role in mouse models of allergic inflammation is less clear. Allergic lung inflammation can develop through at least two mechanisms requiring eosinophils or mast cells, with a role for the latter being more apparent for adjuvant-independent models (24, 45–48). However, the requirement for mast cells has not been carefully reported in all models examined, might be restricted to specific functions, and vary with the genetic background of the mice (24, 49–51). Indeed, in one model, mast cell accumulation was observed to be IL-9-independent (50). Despite the lack of an absolute requirement for mast cells in the development of inflammation, these models still display accumulation of mast cells that can be used to define the molecular mechanisms of this process.
Th9 cells likely have many functions in the development of allergic inflammation. Although they clearly have a role in mast cell accumulation, Th9 cells promote allergic inflammation in models that are not strictly mast cell-dependent (47). One of the important remaining questions is distinguishing the functions of Th9 cells from those of Th2 cells, which have similar and somewhat overlapping roles in promoting allergic inflammation. This report demonstrates that at least one function that is preferentially associated with Th9 cells is accumulation of mast cells in situ, and in allergic individuals this might be a critical step facilitating type I hypersensitivities.
Supplementary Material
Clinical Implications.
Mast cell accumulation in tissues is required for immediate hypersensitivity. This report suggests that targeting IL-9 or Th9 cells might be an effective approach for limiting mast cell-dependent immunity.
Abbreviations used
- BAL
Bronchoalveolar lavage
- H & E
hematoxylin and eosin
- HDM
House dust mite
- ILC2s
Type 2 innate lymphoid cells
- ILCs
Innate lymphoid cells
- mMCP-1
mouse mast cell protease 1
- OVA
ovalbumin
- PAS
periodic acid-schiff
- PMA
Phorbol 12-myristate 13-acetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goswami R, Kaplan MH. A brief history of IL-9. Journal of immunology. 2011 Mar 15;186(6):3283–8. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan MH. Th9 cells: differentiation and disease. Immunological reviews. 2013 Mar;252(1):104–15. doi: 10.1111/imr.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erpenbeck VJ, Hohlfeld JM, Volkmann B, Hagenberg A, Geldmacher H, Braun A, et al. Segmental allergen challenge in patients with atopic asthma leads to increased IL-9 expression in bronchoalveolar lavage fluid lymphocytes. The Journal of allergy and clinical immunology. 2003 Jun;111(6):1319–27. doi: 10.1067/mai.2003.1485. [DOI] [PubMed] [Google Scholar]
- 4.Shimbara A, Christodoulopoulos P, Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. The Journal of allergy and clinical immunology. 2000 Jan;105(1 Pt 1):108–15. doi: 10.1016/s0091-6749(00)90185-4. [DOI] [PubMed] [Google Scholar]
- 5.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nature immunology. 2010 Jun;11(6):527–34. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nature medicine. 2012 Aug;18(8):1248–53. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld JC, Hogan SP. Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. The Journal of allergy and clinical immunology. 2010 Feb;125(2):469–76. e2. doi: 10.1016/j.jaci.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. European journal of immunology. 1997 Oct;27(10):2536–40. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- 9.Faulkner H, Renauld JC, Van Snick J, Grencis RK. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infection and immunity. 1998 Aug;66(8):3832–40. doi: 10.1128/iai.66.8.3832-3840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd JS, Lum E, Goulding J, Muir R, Van Snick J, Openshaw PJ. IL-9 regulates pathology during primary and memory responses to respiratory syncytial virus infection. Journal of immunology. 2009 Dec 1;183(11):7006–13. doi: 10.4049/jimmunol.0900085. [DOI] [PubMed] [Google Scholar]
- 11.Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, Steiner S, et al. T9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nature immunology. 2014 Jun 8; doi: 10.1038/ni.2920. [DOI] [PubMed] [Google Scholar]
- 12.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. Journal of immunology. 2009 Dec 1;183(11):7169–77. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proceedings of the National Academy of Sciences of the United States of America. 1988 Sep;85(18):6934–8. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Snick J, Goethals A, Renauld JC, Van Roost E, Uyttenhove C, Rubira MR, et al. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40) The Journal of experimental medicine. 1989 Jan 1;169(1):363–8. doi: 10.1084/jem.169.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petit-Frere C, Dugas B, Braquet P, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993 May;79(1):146–51. [PMC free article] [PubMed] [Google Scholar]
- 16.Longphre M, Li D, Gallup M, Drori E, Ordonez CL, Redman T, et al. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. The Journal of clinical investigation. 1999 Nov;104(10):1375–82. doi: 10.1172/JCI6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louahed J, Toda M, Jen J, Hamid Q, Renauld JC, Levitt RC, et al. Interleukin-9 upregulates mucus expression in the airways. American journal of respiratory cell and molecular biology. 2000 Jun;22(6):649–56. doi: 10.1165/ajrcmb.22.6.3927. [DOI] [PubMed] [Google Scholar]
- 18.Hultner L, Moeller J. Mast cell growth-enhancing activity (MEA) stimulates interleukin 6 production in a mouse bone marrow-derived mast cell line and a malignant subline. Experimental hematology. 1990 Sep;18(8):873–7. [PubMed] [Google Scholar]
- 19.Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, Koike K. IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. Journal of immunology. 2003 Apr 1;170(7):3461–7. doi: 10.4049/jimmunol.170.7.3461. [DOI] [PubMed] [Google Scholar]
- 20.Louahed J, Kermouni A, Van Snick J, Renauld JC. IL-9 induces expression of granzymes and high-affinity IgE receptor in murine T helper clones. Journal of immunology. 1995 May 15;154(10):5061–70. [PubMed] [Google Scholar]
- 21.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000 Oct;13(4):573–83. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 22.Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. American journal of respiratory and critical care medicine. 2011 Apr 1;183(7):865–75. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. The Journal of allergy and clinical immunology. 2000 May;105(5):847–59. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 24.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006 Jun;116(6):1633–41. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999 Nov 25;402(6760 Suppl):B24–30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 26.Stassen M, Arnold M, Hultner L, Muller C, Neudorfl C, Reineke T, et al. Murine bone marrow-derived mast cells as potent producers of IL-9: costimulatory function of IL-10 and kit ligand in the presence of IL-1. Journal of immunology. 2000 Jun 1;164(11):5549–55. doi: 10.4049/jimmunol.164.11.5549. [DOI] [PubMed] [Google Scholar]
- 27.Jones TG, Hallgren J, Humbles A, Burwell T, Finkelman FD, Alcaide P, et al. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restricted NKT cells. Journal of immunology. 2009 Oct 15;183(8):5251–60. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gounni AS, Nutku E, Koussih L, Aris F, Louahed J, Levitt RC, et al. IL-9 expression by human eosinophils: regulation by IL-1beta and TNF-alpha. The Journal of allergy and clinical immunology. 2000 Sep;106(3):460–6. doi: 10.1067/mai.2000.109172. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nature immunology. 2011 Nov;12(11):1071–7. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010 Aug 27;33(2):192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Jabeen R, Goswami R, Awe O, Kulkarni A, Nguyen ET, Attenasio A, et al. Th9 cell development requires a BATF-regulated transcriptional network. The Journal of clinical investigation. 2013 Nov 1;123(11):4641–53. doi: 10.1172/JCI69489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. The Journal of experimental medicine. 2005 May 2;201(9):1487–502. doi: 10.1084/jem.20050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao W, Zhang Y, Jabeen R, Nguyen ET, Wilkes DS, Tepper RS, et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013 Feb 21;38(2):360–72. doi: 10.1016/j.immuni.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rijt LS, Kuipers H, Vos N, Hijdra D, Hoogsteden HC, Lambrecht BN. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. Journal of immunological methods. 2004 May;288(1–2):111–21. doi: 10.1016/j.jim.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Huntley JF, Gooden C, Newlands GF, Mackellar A, Lammas DA, Wakelin D, et al. Distribution of intestinal mast cell proteinase in blood and tissues of normal and Trichinella-infected mice. Parasite immunology. 1990 Jan;12(1):85–95. doi: 10.1111/j.1365-3024.1990.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 36.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nature reviews Immunology. 2010 Oct;10(10):683–7. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Licona-Limon P, Henao-Mejia J, Temann AU, Gagliani N, Licona-Limon I, Ishigame H, et al. Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity. 2013 Oct 17;39(4):744–57. doi: 10.1016/j.immuni.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang HC, Han L, Jabeen R, Carotta S, Nutt SL, Kaplan MH. PU.1 regulates TCR expression by modulating GATA-3 activity. Journal of immunology. 2009 Oct 15;183(8):4887–94. doi: 10.4049/jimmunol.0900363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-beta promote T(H)9 cell-mediated pulmonary allergic pathology. The Journal of allergy and clinical immunology. 2012 Apr;129(4):1000–10. e3. doi: 10.1016/j.jaci.2011.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002 Jul;17(1):7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 41.Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. The Journal of experimental medicine. 2013 Dec 16;210(13):2951–65. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. The Journal of experimental medicine. 2008 Apr 14;205(4):897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godfraind C, Louahed J, Faulkner H, Vink A, Warnier G, Grencis R, et al. Intraepithelial infiltration by mast cells with both connective tissue-type and mucosal-type characteristics in gut, trachea, and kidneys of IL-9 transgenic mice. Journal of immunology. 1998 Apr 15;160(8):3989–96. [PubMed] [Google Scholar]
- 44.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nature reviews Immunology. 2014 Nov;14(11):719–30. doi: 10.1038/nri3754. [DOI] [PubMed] [Google Scholar]
- 45.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. The Journal of experimental medicine. 2000 Aug 7;192(3):455–62. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi T, Miura T, Haba T, Sato M, Serizawa I, Nagai H, et al. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. Journal of immunology. 2000 Apr 1;164(7):3855–61. doi: 10.4049/jimmunol.164.7.3855. [DOI] [PubMed] [Google Scholar]
- 47.Masuda T, Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, et al. Mast cells play a partial role in allergen-induced subepithelial fibrosis in a murine model of allergic asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2003 May;33(5):705–13. doi: 10.1046/j.1365-2222.2003.01588.x. [DOI] [PubMed] [Google Scholar]
- 48.Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, et al. Mast cells, Fc epsilon RI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. Journal of immunology. 2004 May 15;172(10):6398–406. doi: 10.4049/jimmunol.172.10.6398. [DOI] [PubMed] [Google Scholar]
- 49.Kim YS, Ko HM, Kang NI, Song CH, Zhang X, Chung WC, et al. Mast cells play a key role in the development of late airway hyperresponsiveness through TNF-alpha in a murine model of asthma. European journal of immunology. 2007 Apr;37(4):1107–15. doi: 10.1002/eji.200636612. [DOI] [PubMed] [Google Scholar]
- 50.Pae S, Cho JY, Dayan S, Miller M, Pemberton AD, Broide DH. Chronic allergen challenge induces bronchial mast cell accumulation in BALB/c but not C57BL/6 mice and is independent of IL-9. Immunogenetics. 2010 Aug;62(8):499–506. doi: 10.1007/s00251-010-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becker M, Reuter S, Friedrich P, Doener F, Michel A, Bopp T, et al. Genetic variation determines mast cell functions in experimental asthma. Journal of immunology. 2011 Jun 15;186(12):7225–31. doi: 10.4049/jimmunol.1100676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.