Abstract
Sterile particles induce robust inflammatory responses that underlie the pathogenesis of diseases like silicosis, gout and atherosclerosis. A key cytokine mediating this response is IL-1β. The generation of bioactive IL-1β by sterile particles is mediated by the NLRP3 inflammasome, although exactly how this occurs is incompletely resolved. Prior studies have found that the cathepsin B inhibitor, Ca074Me, suppresses this response, supporting a model whereby ingested particles disrupt lysosomes and release cathepsin B into the cytosol, somehow activating NLRP3. However, reports that cathepsin B-deficient macrophages have no defect in particle-induced IL-1β generation have questioned cathepsin B’s involvement. Here, we examine the hypothesis that multiple redundant cathepsins (not just cathepsin B) mediate this process by evaluating IL-1β generation in murine macrophages, singly or multiply deficient in cathepsins B, L, C, S and X. Using an activity-based probe, we measure specific cathepsin activity in living cells, documenting compensatory changes in cathepsin-deficient cells, and Ca074Me’s dose-dependent cathepsin inhibition profile is analyzed in parallel with its suppression of particle-induced IL-1β secretion. Also, we evaluate endogenous cathepsin inhibitors, cystatins C and B. Surprisingly, we find that multiple redundant cathepsins, inhibited by Ca074Me and cystatins, promote pro-IL-1β synthesis, and we provide the first evidence that cathepsin X plays a non-redundant role in non-particulate NLRP3 activation. Finally, we find cathepsin inhibitors selectively block particle-induced NLRP3 activation, independently of suppressing pro-IL-1β synthesis. Altogether, we demonstrate that both small molecule and endogenous cathepsin inhibitors suppress particle-induced IL-1β secretion, implicating roles for multiple cathepsins in both pro-IL-1β synthesis and NLRP3 activation.
Introduction
Sterile particles induce robust inflammatory responses that underlie the pathogenesis of many diseases. These pathogenic particles are diverse, and include silica (1–4), which causes silicosis, monosodium urate (5), the etiologic agent in gout, and cholesterol crystals (CC) (6, 7), which are thought to contribute to the pathogenesis of atherosclerosis. Importantly, the sterile inflammatory response and resultant diseases caused by these particles all involve signaling through the interleukin-1 receptor, IL-1R1 (8, 9). While IL-1R1 can be stimulated by either of two cytokines, IL-1α or IL-1β, it has been shown that IL-1β plays a pivotal role in disease pathogenesis (10) because it not only directly stimulates IL-1R1-dependent inflammatory signaling, but is also needed for the secretion of IL-1α from cells (11). Therefore, it is important to understand the exact mechanisms underlying the generation and secretion of active IL-1β. However, this process is still incompletely understood and the focus of the present report.
The generation of biologically active IL-1β is highly regulated and usually proceeds in two distinct steps (12, 13). The first step (Signal 1 or “priming”) is initiated when cells such as macrophages are stimulated by certain cytokines, pathogen-associated molecular patterns (PAMPs), or danger-associated molecular patterns (DAMPs). Signal 1 leads to the nuclear translocation of NF-κB, which then stimulates the synthesis of biologically inactive pro-IL-1β and, among other things, NOD-like receptor containing a pyrin domain 3 (NLRP3), a protein important for IL-1β activation. The second step (Signal 2 or “activation”) induces the formation of a multimolecular complex, known as the inflammasome. Inflammasomes are composed of a sensor protein, an adaptor protein, apoptosis-associated speck-like protein containing a CARD (ASC), and an executioner protease, caspase-1. Each inflammasome sensor detects distinct stimuli, thereby initiating multimerization and activating caspase-1, which then cleaves pro-IL-1β and facilitates the secretion of bioactive mature IL-1β. Among the known inflammasomes, the NLRP3 inflammasome is unique. While all inflammasomes rely on the availability of a newly-synthesized pool of pro-IL-1β, basal levels of NLRP3 itself are limiting, making priming especially critical for de novo NLRP3 transcription and subsequent activation (14, 15). Moreover, the NLRP3 inflammasome is the exclusive mediator of IL-1β activation in response to sterile particles (1–7).
While the NLRP3 inflammasome is located in the cytosol, how this intracellular complex senses the presence of extracellular particles has been of considerable interest. It has been shown that internalization of particles by phagocytosis is a first essential step in activating the NLRP3 inflammasome (2). Multiple mechanisms have been proposed as to how particles in phagosomes then lead to NLRP3 inflammasome activation, including lysosomal membrane disruption (LMD) (2, 3, 6, 7, 13, 16–29), potassium efflux (1, 4, 7, 21, 29–37), and the generation of reactive oxygen species (ROS) (1, 27, 29, 30, 32, 36, 38–40), among various other mechanisms (Reviewed (12)). All of these pathways may contribute to this process. In support of the LMD model, it has been shown that particles like silica, CC and the adjuvant alum can cause LMD (2, 6, 7), leading to the leakage of the lysosomal cysteine protease cathepsin B into the cytosol, where this protease is thought to activate NLRP3 through an as yet undescribed mechanism. Consistent with this model, particle-induced activation of the NLRP3 inflammasome is blocked by inhibitors of lysosomal acidification (cathepsins are optimally active in acidic conditions) and inhibitors of cathepsin B. However, the requirement for cathepsin B in this process is controversial.
A role for cathepsin B in NLRP3 activation is supported by a number of studies showing that Ca074Me, an inhibitor reported to be specific for cathepsin B, suppresses IL-1β activation induced by particulate and non-particulate stimuli (2, 7, 17, 20, 21, 25–29, 41–46). However, despite a few subsequent studies showing that cathepsin B or L-deficient macrophages show partial impairment of this response (6, 25, 41), several follow-up studies have found that responses are intact in these same mutant cells (31, 42, 47). Thus, it has become unclear whether the efficacy of Ca074Me is really a result of cathepsin B inhibition, or whether this is an off-target effect. Indeed, there are there are several reports demonstrating that Ca074Me inhibits other cathepsins as well (48–52). Therefore, one hypothesis proposed to explain the discrepancy between Ca074Me and genetic models is that multiple cathepsins, which are a highly conserved family of proteases, play redundant roles in NLRP3 activation (53). Redundancy of cathepsins B and L has been demonstrated in a mouse model, where deficiency of both results in neonatal mortality, while deficiency of either alone does not (54). Similar redundancy also been observed in mouse cancer models showing upregulation of cathepsin X when cathepsin B is knocked out (55). However, the role of redundant cathepsins has not been examined in the context of NLRP3 activation and remains an open question.
Here, we utilize genetic inactivation of multiple cathepsins, together with exogenous and endogenous inhibitors of these proteases, and an activity-based probe to investigate the role of cathepsins in NLRP3-dependent particle-induced IL-1β secretion. This analysis reveals that multiple cathepsins indeed contribute to IL-1β secretion. Surprisingly, our data also demonstrate that cathepsins contribute, not only to the inflammasome-mediated cleavage of pro-IL-1β into mature IL-1β (Signal 2), but also, to the priming step of pro-IL-1β synthesis (Signal 1). In addition, we found a unique role for cathepsin X in nigericin-induced NLRP3 activation, a protease not previously implicated in the IL-1 response. Together, these data clarify the contribution of cathepsins to particle-induced IL-1β responses and define a previously unappreciated role for cathepsins and their inhibitors in regulating pro-IL-1β synthesis. In doing so, this study provides insight into the mechanistic regulation of IL-1β production and points to cathepsins as unique therapeutic targets for controlling particle-induced sterile inflammatory responses.
Materials and Methods
Reagents and Antibodies
Antibodies for Western Blots were against mouse IL-1β (R&D Systems), caspase-1 p10 (sc-514; Santa Cruz Biotechnology), NLRP3 (Cryo2; Enzo Life Sciences), β-actin (C4; Santa Cruz Biotechnology) and GAPDH (6C5; EMD Millopore). ELISA kits were purchased for mouse IL-1β (BD Biosciences), pro-IL-1β and TNF-α (eBioscience). Ultrapure LPS was from Salmonella minnesota (Invivogen). Poly(deoxyadenylic-deoxythymidylic) acid and nigericin were purchased from Sigma-Aldrich (St. Louis, MO). Silica crystals (MIN- U-SIL 15) were obtained from U.S. Silica (Frederick, MD). Cholesterol crystals were synthesized by acetone supersaturation and cooling (6), Alum (Imject alum adjuvant; a mixture of aluminum hydroxide and magnesium hydroxide) was from Pierce Biotechnology, and Leu-Leu-OMe-HCl was from Chem-Impex International. ZVAD-FMK and Ca-074-Me were from Enzo Life Sciences and K777 was initially gifted to us by Stephanie A. Robertson and James H. McKerrow at UCSF, and further stocks obtained through services from the NHLBI’s SMARTT Program. Lipofectamine 2000, RNAiMax and all siRNA smart pools were from Life Technologies and Endoporter was from Gene Tools.
Animal and cell lines
Wild-type C57BL/6 mice were purchased from Jackson Laboratories. NLRP3-deficient mice (56) were provided by Millennium Pharmaceuticals. Cathepsin S-deficient mice (57) were provided by Dr. Hal Chapman (UCSF, San Francisco), cathepsin L (58) and B (53) -deficient mice were provided by Dr. Hidde Ploegh (Harvard Medical School), cathepsin C-deficient mice (59) were provided by Dr. Christine Pham (Washington University School of Medicine, St. Louis) and all mice have been backcrossed to C57BL/6 background. Multiple-cathepsin-deficient mice were bred from single-cathepsin-deficient mice at UMMS. All animal protocols were approved by the University of Massachusetts IACUC.
Generation of Bone Marrow Chimeras
Adult wild-type (WT) C57BL/6 mice were lethally irradiated (1100 rads) and reconstituted for at least 8 weeks with bone marrow collected from age-matched WT or mutant donor mice 1–2 wks old. Some recipient mice in each group expressed the leukocyte marker Ly5.1 (CD45.1), while all donors expressed Ly5.2 (CD45.2), allowing confirmation of >90% chimerism to be determined by flow cytometric analysis of peripheral blood samples.
Production and measurement of IL-1β, pro-IL-1β and TNF-α from in vitro cultures
Peritoneal exudate cells were elicited by i.p. injection of 3 mL 1% thioglycollate and collected after 72 h by peritoneal lavage. Prior to experimentation, non-adherent cells were decanted, leaving primarily macrophages behind. Bone marrow–derived macrophages were generated as described (60). Bone marrow neutrophils were isolated from whole bone marrow, following RBC lysis, using the anti–Ly-6G Microbead Kit from Miltenyi Biotec. Purity was assessed to be 95–98% by flow cytometry. Murine bone marrow-derived mast cells were derived from whole bone marrow using murine rIL-3 (PeproTech), and purity was assessed to be 95% by toluidine blue (61). Cells were plated overnight in 96-well plates (ELISA), or 12-well plates (SDS-PAGE, cathepsin activity labeling with BMV109, and western blotting). Unless otherwise stated, the “Standard Protocol” followed herein is as follows: Priming in RPMI 1640 (or MC/9 medium for mast cells (61)) for 3h with LPS (200 ng/mL), with or without the addition of inhibitors after 2h of priming, followed by 6h of stimulation. Inhibitors were added in a final concentration of ≤0.1% DMSO, which has no effect on readouts compared to media alone. Supernatants were collected, with or without addition of Promega’s 10x lysis solution for measuring intracellular cytokines or LDH measurement by plate reader at OD490 using Promega’s Cytox96 Non-radioactive cytotoxicity assay, and cytokine levels were analyzed by ELISA.
siRNA Knockdowns
Each siRNA was used at a 50 nM final concentration (or control siRNA at 100 nM for double-knockdowns) after complexation in a mixture of Endoporter (GeneTools) and RNAiMax (Life Technologies) at a ratio of 0.11 μL:0.15 μL in OptiMEM (Gibco), respectively, per 0.1 mL final volume (in 10% FCS). Complexes were combined with 10% FCS-containing RPMI at a ratio 1:10 (complexes:10% FCS) and added to cells for 96h. Media was supplemented with 2 mM L-glutamine, 1 mM Na-pyruvate, 0.2 mM β-Me, 1x NEAAs and 100 μg/mL ciprofloxacin.
Immunoblotting and Live-cell intracellular cathepsin activity labeling with BMV109
In 12-well plates, adherent macrophages were washed with RPMI, and incubated with or without LPS as indicated for 3h (inhibitors added after 2h) at which time BMV109(62) was added at a final concentration of 1 μM. After 1h with BMV109, supernatants were collected, cells were washed with PBS and lysates made with Cell Extraction Buffer from Life Technologies with complete protease inhibitor cocktail from Roche. Supernatants were precipitated with chloroform/methanol and lysate protein concentration was equalized using the Pierce BCA Assay. At least 15 μg was loaded for each sample and separated by 15% (or 8% for NLRP3 blots) SDS-PAGE, and gels were analyzed with a Typhoon Trio phosphor-imager from GE, and protein transferred onto nitrocellulose membranes. Densitometry was performed using ImageJ. Images of gels or blots were cropped for the bands of interest and any contrast enhancement applied evenly throughout using iPhoto.
Real-time measurement of lysosomal integrity
In black high-binding 96-well clear-bottom plates, 50 μL of acridine orange (LifeTechnologies) in warm HBSS (with Ca2+ & Mg2+) was added to cells in 100 μL of RPMI containing 10% FCS to reach a final concentration of 3.75 μg/mL and then incubated at 37°C for 15 min prior to washing 1x with 200 μL HBSS. Cells were then treated and stimulated as indicated in phenol red-free CO2-independent Leibovitz’s medium. Fluorescence was measured in each well every 1–3 min using an incubated VictorX5 plate reader. Background fluorescence was subtracted from wells treated with dye-free HBSS.
Results
Genetic and biochemical analysis of the impact of individual cathepsin deficiency on particle-induced IL-1β secretion
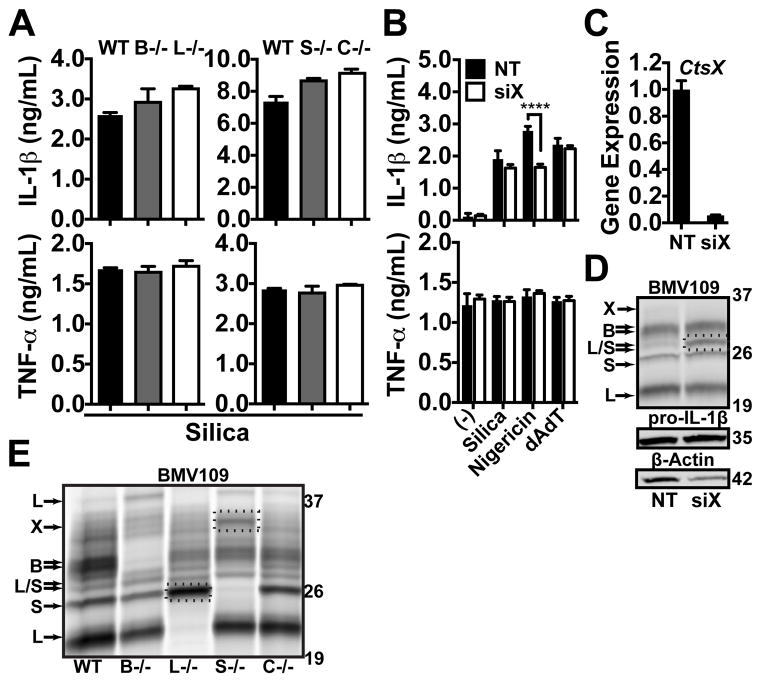
The role of cathepsins in NLRP3 activation remains controversial. Some studies describe a role for cathepsin B or L (6, 25, 41), while others show no role for either cathepsin in particle-induced NLRP3 activation and IL-1β secretion (31, 42, 47). One interpretation of these data suggests that other cathepsins, besides B or L, may be the key players in this response. Therefore, we examined the impact that genetic deficiency of five closely-related individual cathepsins has on particle-induced IL-1β secretion. Unless noted otherwise, IL-1β secretion was induced with various stimuli following 3h of LPS priming. First, we examined peritoneal macrophages (PMs) elicited from mice lacking cathepsins B, L, S or C. However, these cathepsin-deficient PMs displayed no difference in IL-1β secretion in response to silica compared to PMs derived from wild-type (WT) mice (Fig. 1a). To examine the role of cathepsin X, we silenced cathepsin X in PMs by siRNA knockdown, and then these cells were stimulated with silica, the soluble NLRP3 activator nigericin, or the Absent In Melanoma 2 (AIM2) inflammasome activator poly(deoxyadenylic-deoxythymidylic) acid (dAdT) (Fig. 1b). We confirmed a 90–95% knockdown of cathepsin X mRNA by quantitative PCR (qPCR) (Fig. 1c) and noted a similar loss of cathepsin X activity using the fluorescent activity-based probe BMV109 (Fig. 1d), which binds covalently to active cathepsins inside live cells (62). In lysates generated from these cells, the proteins were separated by SDS-PAGE, and then the activity of specific cathepsins was assessed in the gels using a laser phosphor-imager to analyze the degree of fluorescence for each cathepsin at the appropriate m.w.’s. Again, we noted no significant difference in IL-1β secretion between cathepsin X-sufficient and cathepsin X-deficient PMs in response to either silica or dAdT. Strikingly, cathepsin X deficiency significantly reduced IL-1β secretion in response to nigericin. In contrast, LPS-induced TNF-α secretion was unaffected by the loss of any of the cathepsins tested. Therefore, the individual cathepsins B, L, S, C and X are dispensable for silica-induced IL-1β secretion, but we found, unexpectedly, that in the response induced by nigericin, cathepsin X plays a non-redundant role.
Figure 1. Sterile particle-induced IL-1β secretion does not require cathepsins B, L, C, S or X, but nigericin is partially dependent on cathepsin X.
(A) LPS-primed PMs from WT mice or mice deficient for cathepsins B (B−/−), L (L−/−), S (S−/−) or C (C−/−), were stimulated with silica (40 μg/mL). (B) PMs were treated with non-targeting (NT) control siRNA or siRNA targeting cathepsin X (siX) before priming with LPS and stimulating with media control (−), silica (80 μg/mL), nigericin (1.5 μM) or dAdT (0.5 μg/mL). IL-1β & TNF-α were measured in supernatants by ELISA. (C) PMs from “B” were analyzed for cathepsin X (CtsX) expression by qPCR following siRNA (siX) treatment and LPS priming; data are normalized to GAPDH expression and plotted relative to NT siRNA, or (D) cathepsin X activity was probed with BMV109; lysates were processed and pro-IL-1β & β-Actin analyzed by western blot; dashed box highlights upregulated cathepsin L/S activity; m.w. markers are on the right in kDa. (E) LPS-primed PMs from WT or cathepsin-deficient mice in “A” were probed for cathepsin activity with BMV109; dashed boxes highlight upregulated cathepsin S or X activity in L−/− and S−/− macrophages; m.w. markers are on the right in kDa. Error bars represent (A) range bars of technical duplicates, (B) S.D. of technical quadruplicates, and (C) S.D. of technical triplicates. (B) Statistical analysis was performed by Two-way ANOVA and Sidak’s multiple comparisons test; ****P<0.0001. All data are representative of at least three independent experiments.
Using cathepsin knockout animals to study IL-1β secretion could potentially be confounded if some cathepsins are upregulated in order to compensate for the deficiency of others (54, 63, 64). Using BMV109, we examined the activity of specific intracellular cathepsins in the LPS-primed WT and cathepsin-deficient PMs that were tested above in Fig. 1a and b. Indeed, knockdown of cathepsin X with siRNA resulted in an upregulation of cathepsin L and S activity (Fig. 1d). Moreover, PMs lacking cathepsin L showed increased cathepsin S activity, while those deficient in cathepsin S upregulated cathepsin X activity (Fig. 1e). Together, these data indicate that the cathepsins examined, including cathepsins B, L, S and X, are not essential for particle-induced IL-1β secretion, and they cannot be readily studied using genetic methods due to compensation issues upon knockdown.
Analysis of small molecule cathepsin inhibitors
The absence of a phenotype in cathepsin B-deficient macrophages, shown here and reported by others, contradicts the results reported with cathepsin B inhibitors (31, 42, 47). Despite several reports demonstrating that the cathepsin inhibitor Ca074Me inhibits multiple cathepsins in biochemical and cellular assays (48–52), Ca074Me is cited as a cathepsin B-specific inhibitor and used to implicate cathepsin B in NLRP3 activation in many studies (2, 7, 17, 20, 21, 25–29, 41–46). The non-selective pro-drug methyl ester, Ca074Me, is processed in lysosomes into the highly cathepsin B-selective free acid, Ca-074. However, this processing occurs slowly and allows time for Ca074Me to inhibit multiple cathepsins (48–52). Therefore, in the context of NLRP3 activation, Ca074Me’s targets in intact cells have not yet been verified and closely examined as a function of inhibitor concentration. Here, we re-examine both Ca074Me and a newly described broad cathepsin inhibitor, K777 (N-methyl-piperazine-phenylalanyl-homophenylalanyl-vinylsulfone-phenyl), whose anti-inflammatory properties have not yet been tested. K777 inhibits cathepsins B, L, S, C, V and K in cell-free assays (65). Using Ca074Me or K777 in combination with the active site probe allowed us to correlate their effects on IL-1β secretion with the extent of inhibition of specific cathepsins as a function of concentration.
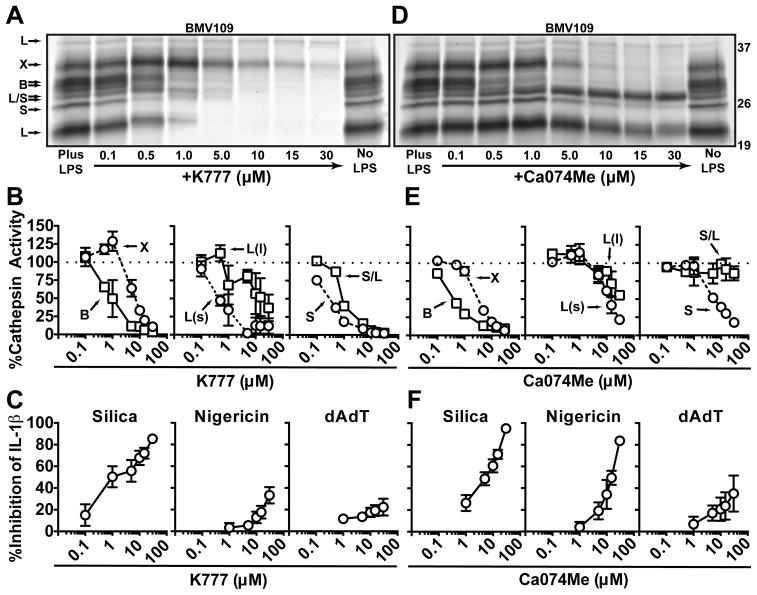
To examine the inhibition profile of K777, we treated PMs with K777 or solvent control (DMSO) for 1h (after 2h of LPS priming, unless stated otherwise), after which we probed for cathepsin activity in the intact cells with BMV109. As previously reported, K777 inhibited cathepsins B, L and S (65) over a titration range from 0.1 – 30 μM (Fig. 2a,b). Interestingly, we also found that K777 inhibited cathepsin X at high concentrations, but unexpectedly increased cathepsin X activity at lower concentrations. These paradoxical effects can be explained by K777’s greater potency towards cathepsin S, which fits with our data, in Fig. 1e above, showing that cathepsin S deletion causes an increase in cathepsin X activity. Therefore, K777 inhibits cathepsin S at low concentrations, which likely causes a compensatory increase in cathepsin X activity.
Figure 2. Both Ca074Me and K777 inhibit multiple cathepsins at concentrations needed to block IL-1β secretion.
(A) PMs were given media control (No LPS) or LPS-primed (Plus LPS; +K777) and subsequently treated with media control (No LPS; Plus LPS) or the indicated concentrations of K777 (+K777), after which cathepsin activity was labeled with BMV109 in live cells before lysates were processed by SDS-PAGE and phosphor imaged; m.w. markers are on the right in kDa. (B) Concentration-dependent inhibition of cathepsin activity by K777 analyzed by densitometry of “A”: cathepsin B (square) and X (circle), large (L(l); square) and small (L(s); circle) m.w. isoforms of cathepsin L, cathepsin S (circle) and overlapping m.w. isoforms of S and L (S/L; square). (C) LPS-primed PMs were treated with media control or the indicated concentrations of K777 and stimulated with silica (40 μg/mL), nigericin (2 uM) or dAdT (0.5 μg/mL); data shows percent inhibition of IL-1β secretion measured in supernatants compared to no inhibitor treatment. (D–F) Same as “A–C”, but with Ca074Me instead of K777. Error bars represent (B) S.E. of means from three independent experiments, (C) S.D. of means from four independent experiments (0.1–15 μM) or S.D. of means from three independent experiments (30 μM), (E) range bars of the means from two independent experiments, or (F) S.D. of the means from three independent experiments (0.5–15 μM), or range bars of the means from two independent experiments (30 μM).
In parallel to examining its effects on cathepsin activity, we also tested the effect of K777 on IL-1β secretion (Fig. 2c). PMs were primed with LPS and treated with K777 as done above (2h after LPS priming and 1h prior to stimulation) at which point they were exposed to various stimuli for an additional 6h of incubation; this is the “Standard Protocol” used for the rest of this study, unless stated otherwise. At concentrations where multiple cathepsins were inhibited, K777 suppressed silica-induced IL-1β secretion. In contrast to silica, K777 was much less effective at suppressing IL-1β secretion induced by nigericin. Presumably, this is because K777 has opposing effects on cathepsin X, which is uniquely required for the nigericin response, shown in Fig. 1b. Moreover, K777 had a negligible affect on the IL-1β response induced by dAdT. We also confirmed that K777 is similarly selective and/or efficacious at suppressing IL-1β secretion induced by other particles, including alum and CC, and in other primary myeloid cell lines, including bone marrow-derived macrophages, mast cells and neutrophils (Supp. Fig. 1a,b). Importantly, K777 did not affect LPS-induced TNF-α production within the tested concentration range, suggesting specific inhibition of IL-1β secretion.
We performed similar analyses for Ca074Me (Fig. 2d–f). While Ca074Me was selective for cathepsin B at concentrations below 1 μM, at higher concentrations (typically used in previous studies) it inhibited cathepsins broadly (Fig. 2d,e). Moreover, >10 μM of Ca074Me was required to completely inhibit cathepsin B. Unlike K777, Ca074Me suppressed nigericin and silica-induced IL-1β secretion with similar potency, presumably because Ca074Me inhibits cathepsin X more potently than K777 (Fig. 2f). Interestingly, the concentration required to achieve and maximize these effects exceeds the range in which Ca074Me is selective for cathepsin B. In reviewing previous studies examining Ca074Me’s effects on IL-1β responses, the concentrations used were also in the range that would inhibit multiple cathepsins (10–200 μM)(2, 7, 17, 20, 21, 25–29, 41–46). Therefore, our findings likely explain the difference in results seen for the genetic loss of cathepsin B compared to small-molecule inhibitors of this protease. In summary, although both K777 and Ca074Me inhibit multiple cathepsins at concentrations required to suppress IL-1β secretion, K777 blocks particle-induced NLRP3 activation more selectively than Ca074Me.
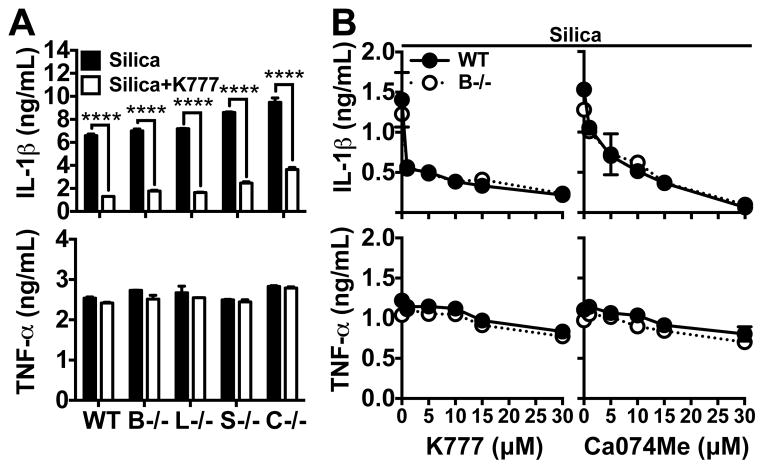
To further investigate whether Ca074Me or K777 can inhibit IL-1β secretion in PMs from cathepsin-deficient mice, these cells were LPS primed and treated with inhibitors prior to stimulation. Indeed, K777 inhibited IL-1β secretion to the same extent in WT cells as in cathepsins B, L, S, or C-deficient cells (Fig. 3a). Moreover, across a titration range for both K777 and Ca074Me, the extent to which they suppressed IL-1β secretion was the same in both WT and cathepsin B-deficient PMs (Fig. 3b). Again, LPS-induced TNF-α secretion was relatively unaffected by cathepsin B deficiency or inhibitor treatments. Together, these data indicate that the individual cathepsins examined, including cathepsin B, are not essential for the activation of particle-induced IL-1β secretion or as targets for cathepsin inhibitors that suppress this response.
Figure 3. Cathepsin inhibitors suppress particle-induced IL-1β secretion independently of individual cathepsins.
IL-1β (upper graphs) and TNF-α (lower graphs) were measured in supernatants. (A) LPS-primed WT PMs or those lacking cathepsins B (B−/−), L (L−/−), S (S−/−) or (C−/−) were treated with silica (black bars; 40 μg/mL) or silica plus K777 (white bars; 15 μM). (B) LPS-primed WT (closed circles, solid line) or cathepsin B-deficient (open circles, dashed line) PMs were treated silica (50 μg/mL) or silica plus a range of K777 or Ca074Me concentrations (1, 5, 10, 15 or 30 μM). Error bars represent range bars of technical duplicates. Statistical analysis was performed by Two-way ANOVA and Sidak’s multiple comparisons test; ****P<0.0001. Data are representative of two (“B” for Ca074Me) or three (“A”; “B” for K777) independent experiments.
Analysis of compound cathepsin deficiencies
The analyses above suggest that multiple cathepsins likely play compensatory roles in particle-induced IL-1β secretion. This is in line with some genetic evidence that has shown partial or conditional involvement for cathepsin B or L in NLRP3 activation (6, 25, 41). In fact, these two cathepsins have been shown to compensate for one another in a study demonstrating that combined cathepsin B and L deficiency is neonatal lethal in mice, but deficiency of either protease alone is non-lethal (54). Therefore, a dual-deficiency of cathepsins B and L may have a greater effect on the IL-1β response (6).
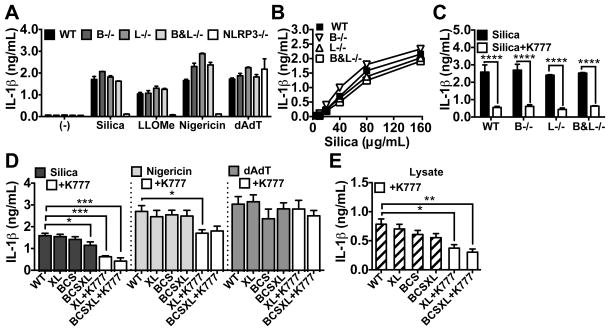
To test this hypothesis, we bred mice lacking both cathepsins B and L. Since combined cathepsin B and L deficiency is neonatal lethal (54), we could not analyze responses directly in these animals. Instead, we harvested bone marrow from neonates and used it to reconstitute lethally irradiated adult WT mice. In these chimeric mice, cells of hematopoietic origin lack cathepsin B and L (B&L−/−). For comparison, we made similar chimeras with WT, cathepsin B−/− and cathepsin L−/− bone marrow. Then we elicited PMs from these chimeric mice, and treated them as above. We verified that the PMs collected from these chimeric mice lacked activity for the appropriate cathepsins using BMV109 (Supp. Fig. 2a). Again, we observed upregulation of cathepsin S activity upon loss of cathepsin L. However, cathepsin B−/−, L−/− or B&L−/− PMs showed no attenuation of IL-1β secretion in response to the lysosome-disrupting agent Leu-Leu-OMe (LLOMe), silica, nigericin or dAdT (Fig. 4a). Moreover, there was no defect in IL-1β secretion over a broad titration of silica (Fig. 4b). Interestingly, K777 still suppressed silica-induced IL-1β secretion in the absence of cathepsins B and/or L (Fig. 4c), suggesting that other cathepsins potentially contribute to this response.
Figure 4. Compound cathepsin-deficiency causes a minor reduction in particle-induced IL-1β secretion.
IL-1β was measured in supernatants. (A–C) Lethally irradiated WT mice were reconstituted with bone marrow from WT, cathepsin B (B−/−), L (−/−), B and L (B&L−/−), or NLRP3 (NLRP3−/−)–deficient donor mice. LPS-primed PMs elicited from these mice were stimulated with (A) media control (−), silica (40 μg/mL), LLOMe (0.75 mM), nigericin (2 μM), or dAdT (0.4 μg/mL), (B) a range of silica concentrations, (C) silica plus media (black bars) or silica plus K777 (white bars; 20 μM). (D) PMs elicited from WT or mice deficient in the three cathepsins B, C and S (BCS) were treated with non-targeting siRNA (WT) or siRNA targeting both cathepsins X and L (“XL” when given to WT, or “BCSXL” when given to BCS) and subsequently LPS-primed and stimulated with media control (−), silica (80 μg/mL), nigericin (1.5 μM), or dAdT (0.5 μg/mL). XL and BCSXL macrophages were also treated with K777 (XL+K777 and BCSXL+K777; white bars; 15 μM). Error bars represent (A–C) range bars of technical duplicates, or (D) S.E. of means from either five independent experiments (WT, XL, BCS, BCSXL) or three independent experiments (+K777). Statistical analysis was performed by (A–C) Two-way ANOVA and Sidak’s multiple comparisons test, or (D–E) Two-tailed Student’s t-test *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. All data are representative of at least three independent experiments.
Since both K777 and Ca074Me inhibit more cathepsins than just B and L at the concentrations required to block particle-induced IL-1β secretion, we examined the particle-induced responses of macrophages genetically deficient in up to five cathepsins (Fig. 4d,e). To do this, we elicited PMs from WT mice or mice deficient in the three cathepsins B, C and S (BCS−/−), which are viable with no obvious physical or behavioral pathology. In addition, in both WT and BCS−/− macrophages, we silenced cathepsins X and L with siRNA (siXL; XL), or treated cells with non-targeting siRNA (WT). This resulted in a 90–95% reduction in mRNA of each targeted gene and reduction in enzyme activity, as assayed with BMV109 (Supp. Fig. 2b,c). Finally, PMs were primed with LPS, with media or K777 treatment, and stimulated with silica, nigericin or dAdT, as done above. Indeed, macrophages deficient in the five cathepsins B, C, S, X and L (BCSXL) showed a significant, though small, reduction in IL-1β secretion in response to silica, but not nigericin or dAdT (Fig. 4d). However, K777 was still effective at further suppressing IL-1β secretion in these macrophages. Interestingly, in the lysates of samples treated with LPS only, we observed a similar decrease in intracellular IL-1β levels, suggesting that lower levels of IL-1β synthesis may be contributing to the reduction in IL-1β secretion seen for both BCSXL deficiency and K777 treatment (Fig. 4e). Again, we observed a compensatory upregulation of cathepsin activity, with cathepsin B and S activity upregulated in the cathepsin XL knockdown and increased cathepsin X activity in the cathepsin BCS−/− PMs (Supp. Fig. 2c). This may explain why nigericin was not significantly affected by knockdown of cathepsin X in combination with these other cathepsin deficiencies. As above, TNF-α secretion was unaffected, suggesting that compound cathepsin deficiency specifically impacts the IL-1β pathway (Supp. Fig. 2d–h). Thus, compound deficiency of cathepsins B, C, S, X and L demonstrates a reproducible, albeit minor, attenuation of particle-induced IL-1β secretion. However, the fact that cathepsin inhibitors have shown, yet again, more profound effects on IL-1β secretion than that caused by genetic deficiency, suggests that additional cathepsins or possibly other targets affected by the inhibitors might be involved in particle-induced IL-1β secretion.
Analysis of endogenous cathepsin inhibitors
While technical limitations prevent us from genetically deleting all potentially relevant cathepsin activity, these proteases are specifically inhibited by a family of endogenous regulators called cystatins (66). Therefore, we examined the effects of genetically disabling the activity of the endogenous cathepsins inhibitors, cystatin C and B, on particle-induced IL-1β secretion.
We used siRNA to silence cystatin C, B or C and B in PMs to investigate their role in IL-1β secretion (Fig. 5). In all cases, we achieved ~95% knockdown of cystatin expression (Supp. Fig. 3a). Indeed, cystatin C deficiency alone caused a significant increase in silica and nigericin-induced IL-1β secretion, but not following stimulation with dAdT (Fig. 5a). Moreover, combined deficiency of cystatin B and C synergistically enhanced IL-1β secretion for all stimuli tested. In the absence of these cystatins, K777 selectively reduced silica-induced IL-1β secretion. Therefore, cystatins C and B appear to non-specifically regulate the level of IL-1β secretion, while cystatin C preferentially affects particulate and NLRP3-activating stimuli.
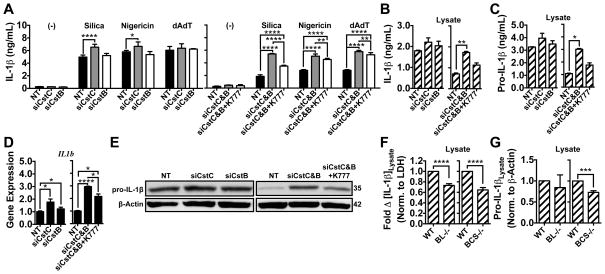
Figure 5. Endogenous cathepsin inhibition by Cystatins C & B regulates particle-induced IL-1β secretion and LPS-induced pro-IL-1β synthesis.
In all experiments, PMs were LPS-primed and treated with media control or K777 (+K777; white bars; 15 μM) prior to stimulation or analysis. (A–E) PMs were transfected with non-targeting (NT), cystatin C (siCstC), cystatin B (siCstB), or both cystatin C and B (siCstC&B) siRNA. (A) PMs were stimulated with media control (−), silica (80 μg/mL), nigericin (1.5 μM) or dAdT (0.5 μg/mL), and IL-1β measured in supernatants. (B–E) After priming, PMs were treated with media control for 6h. (B) IL-1β or (C) Pro-IL-1β were measured in cell lysates by ELISA. (D) IL-1β (IL1b) expression was analyzed by qPCR; data are normalized to GAPDH expression and plotted relative to NT siRNA. (E) Lysates were processed, then pro-IL-1β and β-Actin analyzed by western blot; m.w. markers are on the right in kDa. (F,G) PMs from WT mice and cathepsin BCS−/− mice, or chimeric WT mice lethally irradiated and reconstituted with WT or cathepsin BL−/− bone marrow. PMs were treated with media for 6h after LPS priming. (F) IL-1β (hatched bars) was measured in lysates by ELISA; data are normalized to LDH (OD490) and plotted as fold-change in IL-1β relative to WT controls. (G) lysates were processed and analyzed for pro-IL-1β and β-Actin by western blot (measured by densitometry); data are plotted as pro-IL-1β levels normalized to β-Actin and relative to WT controls. Error bars represent (A) S.D. of technical quadruplicates, (B,C) range bars of technical duplicates, (D) S.D. of technical triplicates, (F) S.E. of means from nine (WT vs. BCS−/−) or twelve (WT vs. BL−/−) independent experiments, (G) S.E. of means from five (WT vs. BCS−/−) or four (WT vs BL−/−) independent experiments. Statistical analysis was performed by (A) Two-way ANOVA and Dunnett’s multiple comparisons test, (B–D) One-way ANOVA and Sidak’s multiple comparisons test, or (F,G) Two-tailed Student’s t-test; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. All data are representative of at least three independent experiments.
Surprisingly, knockdown of cystatin C and/or B caused an upregulation of pro-IL-1β transcript levels induced by LPS priming, and an increase in the level of mature IL-1β and pro-IL-1β detected in lysates; mature IL-1β detected in lysates by ELISA after LPS priming directly reflects levels of pro-IL-1β (Fig. 5b–e). While this effect is more prominent with cystatin C deficiency, knockdown of both cystatin C and B synergistically enhances pro-IL-1β synthesis. Assessment of cellularity by detergent-induced LDH release (OD490) indicated that the elevation in pro-IL-1β levels was not a result of enhanced proliferation during knockdown (Supp. Fig. 3b). Interestingly, the observed elevation in pro-IL-1β synthesis was proportional to increases observed in IL-1β secretion following stimulation with silica, nigericin or dAdT. Moreover, K777 suppressed the increase in pro-IL-1β synthesis and IL-1β secretion resulting from cystatin C and B knockdown, specifically for silica. The fact that K777 reduced pro-IL-1β synthesis more effectively than it reduced IL-1β secretion induced by nigericin and dAdT may reflect that intracellular levels of pro-IL-1β were not limiting for these stimuli and/or that there are kinetic differences in pro-IL-1β induction with the different stimuli. Alternatively, cathepsins may also play a selective role in particle-induced NLRP3 activation (Signal 2) as originally proposed.
Given that cathepsins are not known to play a role in Signal 1 (LPS priming), our finding that cystatins regulate pro-IL-1β synthesis is surprising. However, this is consistent with our observation that the multiply-deficient BCSXL PMs have a lower level of IL-1β detected in the lysate that seems proportional to the reduction in IL-1β secretion. In fact, this indicates that previous findings of lower IL-1β secretion from cathepsin-deficient macrophages may be a direct result of depressed pro-IL-1β synthesis. Indeed, careful examination revealed that cathepsin B&L−/− or BCS−/− macrophages have partial but significant reductions in intracellular IL-1β/pro-IL-1β detected in lysates after LPS priming by either ELISA or western blot (Fig. 5f,g). The fact that we did not see a significant reduction in secreted IL-1β corresponding to the reduction in intracellular IL-1β/pro-IL-1β is presumably because the reduced pro-IL-1β levels were not below the threshold required to limit the response. Importantly, no single-cathepsin deficiency significantly reduced intracellular IL-1β levels (Supp. Fig. 3c,d). Therefore, this effect was not responsible for the reduction in the response to nigericin after silencing cathepsin X (Supp. Fig. 3d and Fig. 1d). In any case, our data indicate that cathepsins do indeed play a role in pro-IL-1β synthesis. Notably, LPS-induced TNF-α secretion is relatively unaffected, suggesting that the impact of cystatin deficiency or K777 treatment on pro-IL-1β synthesis does not apply to all NF-κB-dependent cytokines (Supp. Fig. 3e). Also, knockdown of cystatins B and C also enhanced NLRP3 transcript levels and NLRP3 protein synthesis, but to a lesser extent than for IL-1β (Supp. Fig. 3f,g). Our data indicate a previously unreported and significant role for cathepsins and their endogenous inhibitors in pro-IL-1β synthesis and that cystatins B and C regulate particle-induced IL-1β secretion by suppressing multiple cathepsins involved in mediating pro-IL-1β synthesis.
Analyzing the effect of small molecule cathepsin inhibitors on pro-IL-1β synthesis
We demonstrated that cathepsin deficiency attenuates pro-IL-1β synthesis, while cathepsin deregulation by cystatin C and B knockdown enhances pro-IL-1β synthesis. These data indicate that cathepsin inhibitors may suppress IL-1β secretion by affecting pro-IL-1β synthesis. However, if this is true, it is surprising that K777 and Ca074Me did not similarly suppress dAdT-induced IL-1β secretion in previous experiments. However, the kinetics of LPS priming is an important variable when considering the effect of inhibitors on IL-1β secretion, and influences on priming seem to be selective for NLRP3-dependent stimuli compared to those activating other inflammasomes (15). Therefore, we examined whether cathepsin inhibitors affect pro-IL-1β synthesis and how the timing of inhibitor treatment affects their specificity.
To test the effect of cathepsin inhibitors on pro-IL-1β synthesis, we varied the timing of inhibitor treatment relative to LPS priming using an “Early versus Late Inhibitor Treatment Protocol” (Fig. 6a–c). First, we treated PMs with K777, Ca074Me or the pan-caspase inhibitor ZVAD immediately prior to LPS priming. In a parallel sample set, we added these inhibitors just prior to stimulation, 3h after LPS priming, and examined how treatment with inhibitors at this time point compares with the former. K777 or Ca074Me treatment prior to LPS priming suppressed both pro-IL-1β in macrophage lysates (Fig. 6a) and IL-1β secretion by silica, nigericin and dAdT (Fig. 6b). Moreover, these effects were greater for inhibitor treatment just prior to priming. K777 or Ca074Me treatment 3h after LPS priming (just before stimulation) had no effect on dAdT and, as shown earlier, Ca074Me had a more potent effect on nigericin-induced IL-1β secretion (Fig. 6b).
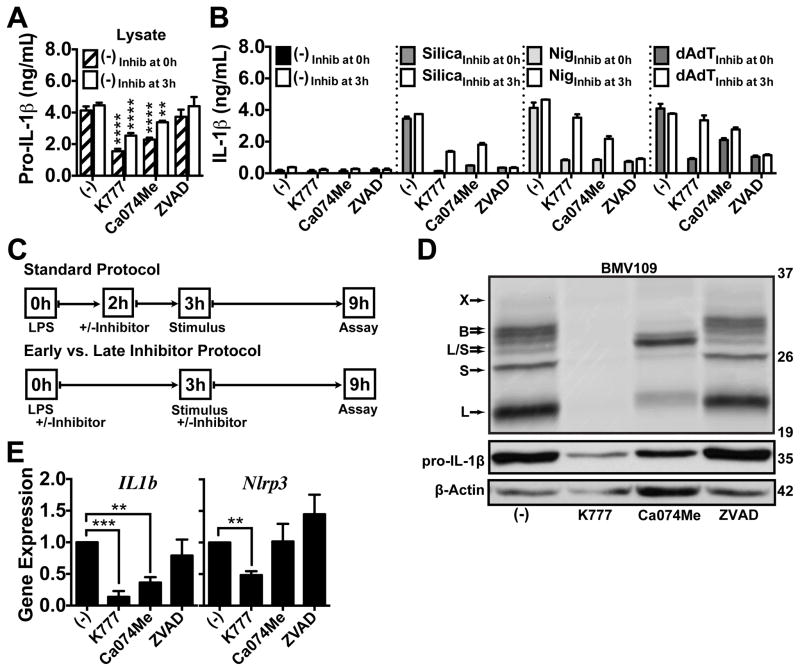
Figure 6. Small-molecule cathepsin inhibitors suppress pro-IL-1β synthesis.
In all experiments, PMs were primed with LPS for 3h, and then treated with media control (−), K777 (15 μM), Ca074Me (15 μM), or ZVAD (10 μM) at the indicated time points. (A,B) Inhibitors were added at the same time as LPS (Inhib at 0h; hatched or filled bars) or 3h after LPS (Inhib at 3h; white bars) prior to the addition of media control (−), silica (80 μg/mL), nigericin (1.5 μM) or dAdT (0.5 μg/mL) for an additional 6h, at which point (A) pro-IL-1β was measured in lysates, or (B) IL-1β was measured in supernatants by ELISA. (C) Comparison of the inhibitor protocol followed in prior figures and “D and E” (Standard Protocol) with the protocol used in “A” and “B” (Early vs. Late Inhibitor Protocol). (D,E) Inhibitors were added 2h after LPS priming for 1h, as in the Standard Protocol, then cells were treated with media for 4h. (D) Cathepsin activity was probed with BMV109 in live cells; lysates were processed by SDS-PAGE and phosphor imaged, or analyzed for pro-IL-1β and β-Actin by western blot; m.w. markers are on the right in kDa. (E) IL-1β (IL1b) or NLRP3 (Nlrp3) expression was analyzed by qPCR; data are normalized to GAPDH expression and plotted relative to media controls (−). Error bars represent (A,B) S.D. of technical triplicates (−), (B) duplicates (silica, nigericin, dAdT), (E) S.E. of means from three independent experiments. Statistical analysis was performed by (A) Two-way ANOVA and Dunnett’s multiple comparisons test, or (E) Two-tailed Student’s t-test; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. All data are representative of at least three independent experiments.
To determine whether the reductions in IL-1β secretion that we previously observed were also a reflection of reduced pro-IL-1β levels, we re-tested K777 and Ca074Me using the “Standard Protocol” described for these earlier experiments and examined their effects on pro-IL-1β synthesis (Fig. 6c–e). Indeed, treatment with K777 or Ca074Me after only 2h of LPS priming reduced pro-IL-1β levels in lysates (Fig. 6d) and also reduced pro-IL-1β transcript levels (Fig. 6e). In fact, K777 even suppressed NLRP3 transcript levels, although the reduction in NLRP3 transcript caused by Ca074Me was not significant. In contrast to the near complete inhibition of IL-1β secretion by all stimuli, ZVAD treatment had no effect on intracellular IL-1β or pro-IL-1β levels detected in LPS-primed macrophage lysates (Fig. 6a–e, Supp. Fig. 4a). Moreover, ZVAD did not suppress pro-IL-1β and NLRP3 transcript levels or cathepsin activity. Again, under all these conditions above, TNF-α secretion remained unaffected (Supp. Fig. 4b). Therefore, cathepsin inhibitors suppressed the synthesis of pro-IL-1β and not TNF-α. When added just prior to LPS priming, cathepsin inhibitors also attenuated NLRP3-independent IL-1β secretion, yet they maintained some selectivity for NLRP3-dependent IL-1β secretion (Fig. 6b). These finding are consistent with a previous study finding that several inhibitors, which also affect Signal 1, preferentially affect NLRP3-dependent stimuli (15). Indeed, the persistent selectivity of cathepsin inhibitors for NLRP3-dependent stimuli may reflect a unique dependence of these responses on Signal 1, based on the requirement for de novo NLRP3 transcription or some other factor yet to be defined. While this is less likely a reflection of differences in Signal 2 kinetics, which are similar for silica and dAdT (Supp. Fig. 4c,d), the ultimate reason for this difference is unclear since the effect of K777 and Ca074Me on pro-IL-1β protein levels are more pronounced their effects NLRP3 protein levels (Supp. Fig. 4e).
Analyzing the effect of cathepsin inhibitors on Signal 2 of NLRP3 activation
We found that cathepsin inhibition by both small molecules and endogenous regulators suppresses pro-IL-1β synthesis. However, we expected that these effects on pro-IL-1β synthesis would affect all stimuli equally, but cathepsin inhibition had a greater impact on silica-induced IL-1β secretion compared to nigericin or dAdT. Moreover, this selectivity cannot be completely explained by kinetics. Therefore, it was important to determine whether cathepsin inhibitors suppress IL-1β secretion by blocking NLRP3 activation, independently of their effects on pro-IL-1β synthesis.
To determine whether cathepsin inhibition blocks NLRP3-dependent IL-1β secretion (Signal 2) independently of suppressing pro-IL-1β synthesis, we examined the effect of K777 or Ca074Me treatment on IL-1β responses in macrophages with a pool of preexisting pro-IL-1β (Fig. 7a–c). Following an extended priming protocol, we primed PMs with LPS for 5.5h to build up an intracellular pool of pro-IL-1β, at which time we added K777, Ca074Me, cycloheximide (CHX), or CHX combined with K777 or Ca074Me, and stimulated 30 min later with silica, nigericin or dAdT for an additional 3h; CHX blocked new IL-1β synthesis so that we could isolate and analyze the effect of the protease inhibitors on the processing of pro-IL-1β. K777 and Ca074Me had minimal effect on IL-1β or pro-IL-1β protein levels in LPS-primed macrophage lysates at this late time point, and also had no additional effect when combined with CHX compared to CHX alone (Fig. 7a, Supp. Fig. 4f). Importantly, K777 and Ca074Me still attenuated silica-mediated IL-1β secretion, both alone and in the presence of CHX, while only Ca074Me affected nigericin-induced activation of the pathway. Again, neither K777 nor Ca074Me blocked dAdT-induced IL-1β secretion, and TNF-α secretion was unaffected (Fig. 7b, Supp. Fig. 4g).
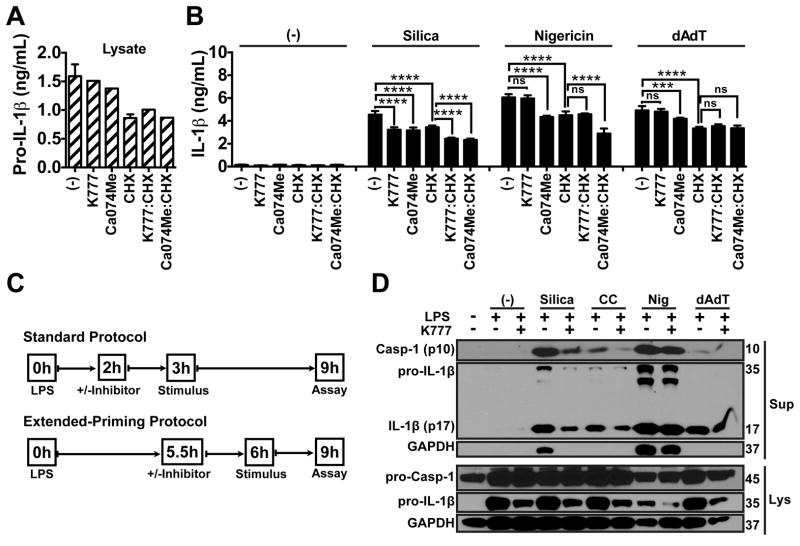
Figure 7. Cathepsin inhibitors also suppress NLRP3 activation independently of effects on pro-IL-1β synthesis.
(A,B) PMs were primed with LPS for 5.5h and treated with either media control (−), K777 (15 μM), Ca074Me (15 μM), CHX (1 μM), K777 combined with CHX, or Ca074Me combined with CHX for another 0.5h, and then treated with media control (−), silica (80 μg/mL), nigericin (1.5 μM), or dAdT (0.5 μg/mL) for another 3h. (A) Pro-IL-1β (hatched bars) was measured in lysates, or (B) IL-1β (filled bars) was measured in supernatants by ELISA. (C) Comparison of the inhibitor protocol followed in prior figures (Standard Protocol) with the protocol used in “A” and “B” (Extended-Priming Protocol). (D) PMs were either unprimed or primed with LPS and treated with K777 (20 μM) 2h after LPS priming, as in the Standard Protocol, and cells were treated 1h later with media control (−), silica (40 μg/mL), CC (100 μg/mL), nigericin (2 μM) or dAdT (0.4 μg/mL) for an additional 6h, then lysates were processed by SDS-PAGE and analyzed for pro-caspase-1, active caspase-1 (p-10), pro-IL-1β, active IL-1β (p-17) and GAPDH by western blot; m.w. markers are on the right in kDa. Error bars represent (A) S.D. of technical triplicates, (B) S.D. of technical triplicates (media or CHX), duplicates (K777 & Ca074Me ±CHX), sextuplicates (silica, nigericin, dAdT ±CHX), or triplicates (silica, nigericin, dAdT with K777 & Ca074Me ±CHX). Statistical analysis was performed by (B) Two-way ANOVA and Dunnett’s multiple comparisons test; ***P<0.001, ****P<0.0001. Data are representative of two (A,B) or at least three (D) independent experiments.
To determine whether K777 selectively attenuates particle-induced NLRP3 activation, we examined caspase-1 cleavage in response to silica, CC, nigericin or dAdT (Fig. 7c,d). Following our standard protocol, we treated PMs with media or K777, 2h after LPS priming and 1h prior to stimulation with silica, CC, nigericin or dAdT (Fig. 7c). After 6h of stimulation, we examined caspase-1 cleavage by western blot. Interestingly, while K777 reduced pro-IL-1β levels in lysates of LPS-primed macrophages, K777 also suppressed caspase-1 activation and mature IL-1β secretion only after stimulation with silica or CC, and not with nigericin or dAdT (Fig. 7d). Importantly, K777 and Ca074Me did not prevent particle-induced lysosomal disruption (Supp. Fig. 4h). K777 and Ca074Me did suppress LLOMe-dependent lysosomal disruption (Supp. Fig. 4i), but this was most likely a result of inhibiting of cathepsin C (a known target of K777) activity required for the activation of LLOMe’s membrane disruptive properties in the lysosome (65, 67, 68). Therefore, in addition to suppression of pro-IL-1β synthesis, both K777 and Ca074Me can also independently suppress NLRP3 activation, while K777 does so selectively for particles without blocking lysosomal disruption.
Taken together, our data suggests a hitherto unrecognized role for cathepsins in inflammasome-mediated IL-1β responses to sterile particles. Furthermore, our study implicates a complex role for cathepsins and their endogenous regulators, cystatins, in regulating not only IL-1β secretion but also IL-1β induction, highlighting the potential for a multi-step involvement of this family of proteases during particle-induced inflammation.
Discussion
Cathepsin B has been implicated in the activation of NLRP3 inflammasomes by particulate stimuli. In this report, we show that contrary to earlier suggestions, multiple cathepsins are involved redundantly in the production of IL-1β induced by sterile particles. These data address and potentially reconcile earlier controversies on the role of cathepsins. Surprisingly, our data are consistent with a role for cathepsins not only in the NLRP3-dependent maturation of pro-IL-1β, but also suggest that they play a substantial role in the priming phase of this response.
Given the controversial role of cathepsins in NLRP3-dependent IL-1β responses (2, 7, 17, 20, 21, 25–29, 31, 41–47), it was important to clarify their contribution by performing a rigorous analysis of two confounding variables that have likely influenced prior results and caused confusion. First, we found that the loss of certain cathepsins causes a compensatory upregulation in the activity of other cathepsins. Since the cysteine cathepsin family shares considerable homology and broad substrate specificities (69), functional redundancy may obscure the contribution of any one cathepsin. Therefore, the lack of a phenotype in any single cathepsin knockout does not rule out the involvement of that cathepsin or other cathepsins.
Second, as we show here, the inhibitor Ca074Me actually inhibits multiple cathepsins in living cells at the concentrations used in prior studies of NLRP3 activation (2, 7, 17, 20, 21, 25–29, 41–46). In fact, we found that, at doses where Ca074Me is cathepsin B-specific, it does not block NLRP3-dependent IL-1β secretion; at higher doses where it inhibits multiple cathepsins, its blockade of IL-1β secretion increases. Indeed, Ca074Me suppresses IL-1β secretion in cathepsin B-deficient macrophages, and we found similar results with the other cathepsin knockouts as well. Concomitant testing with K777, an orally bioavailable broad spectrum inhibitor of cathepsins (65, 70–75), yielded comparable results to Ca074Me. Given this new evidence, its is now clear that the broad specificity of cathepsin inhibitors (Ca074Me and K777) is concordant with a role for multiple cathepsins in particle-induced IL-1β secretion. Moreover, even if it plays an important role in NLRP3 activation under some conditions, our data indicate that cathepsin B is not essential for this response.
Importantly, we document these two confounding variables above using a recently developed activity-based probe, BMV109(62). Although a separate report has shown that Ca074Me can inhibit cathepsins B, S and L in live cells with a similar probe (48), this is the first time that the concentration-dependent inhibition of these cathepsins, or the compensatory upregulation of cathepsin activity, has been demonstrated in parallel with an examination of IL-1β secretion. Moreover, BMV109 labels cathepsin X, which allowed us to investigate the role of this cathepsin in IL-1β secretion.
It is critical to note that, of the five cathepsins tested herein, cathepsin X was the only one that played a non-redundant role in IL-1β secretion. Cathepsin X appeared to be uniquely required for the IL-1β response to nigericin. In fact, we show that Ca074Me potently inhibits cathepsin X, and this likely accounts for its ability to strongly suppress nigericin-induced IL-1β secretion. Unlike Ca074Me, K777 inhibits cathepsins S at low concentrations, and deficiency of cathepsins S upregulates cathepsin X activity. Thus, this may explain why K777 is less effective against nigericin than Ca074Me, and how its broader specificity for cathepsins paradoxically makes it a more selective inhibitor of particle-induced responses. Therefore, pharmacological suppression of IL-1β secretion induced by particular stimuli likely depends on, not only on how many but, which cathepsins are inhibited and at what concentrations.
While Ca074Me and K777 could have non-cathepsin off-target effects responsible for their suppression of particle-induced IL-1β secretion, we strongly favor the interpretation that they are achieving this effect by inhibiting multiple functionally redundant cathepsins. Although we observed a minor but insignificant reduction of particle-induced IL-1β secretion in the cathepsin BL−/− PMs, and a small but significant reduction in the pentuple cathepsin BCSXL−/− PMs, we believe that the residual cathepsin activity in these cells, as shown by BMV109 labeling, could be sufficient to mediate NLRP3 activation. In fact, a recent study demonstrated that inflammasome activation is an “all-or-none” response (76), which gives credence to earlier proposals that only a few molecules of active cathepsins may be sufficient to reach a minimum threshold for inflammasome activation (47). Whether this is true or not remains to be demonstrated. However, we did find more robust genetic evidence supporting an unexpected role for cathepsins in regulating the priming phase of IL-1β secretion.
Because we could not genetically suppress cathepsin activity to the same extent as inhibitors, which further reduced IL-1β secretion by these genetically deficient cells, we adopted an alternative strategy. Instead of examining cathepsin deficiency, we evaluated the effect of cathepsin deregulation by silencing two broadly active endogenous cathepsin inhibitors, cystatins C and B. Like the cathepsin family (69), the cystatin family is large (66), as might be expected of regulators of a large family of proteases. Moreover, individual cystatins specifically regulate multiple cysteine cathepsin proteases, including B, L and S (66). Indeed, knockdown of cystatin C and B synergistically enhanced IL-1β secretion, but did so for all stimuli tested. Further analyses revealed that the increase in IL-1β secretion we observed was directly proportional to the upregulation of pro-IL-1β transcript and synthesis. In fact, reexamination of the compound cathepsin knockouts (BL−/− & BCS−/−) also showed that multiple redundant cathepsins play a partial, but significant, role in LPS-induced pro-IL-1β synthesis. As far as we know, these findings are among the first to implicate and clarify the role of endogenous cathepsin inhibitors, cystatins, in regulating IL-1β responses.
While an association between cystatins and inflammation has been widely reported, the mechanism underlying this association has not been established. Given this context, our evidence that both cystatin B and especially cystatin C play a role in the IL-1β response is enlightening. In fact, lower serum levels of cystatin C, considered the “dominant” cystatin (77), are associated with numerous inflammatory conditions (66), including sterile inflammatory arterial disease (78). Furthermore, cystatin B deficiency in mice exacerbated LPS-induced sepsis and elevated IL-1β levels in the serum (79). This latter study demonstrated higher caspase-1 and/or -11 activity and mitochondrial ROS, suggesting that loss of cystatin B increased inflammasome activation (79). However, the authors noted that there were no signs of LMD or elevated cathepsin activity in the cytosol, and effects on pro-IL-1β were not measured. Thus, our data demonstrating that cystatin deficiency increases pro-IL-1β and NLRP3 synthesis offers a different perspective that may help to explain these results. In this context, it is interesting that other studies have shown that cystatin B interacts with cathepsin L in the nucleus (80), and that cathepsin L can play a role in NF-κB activation (81). Moreover, cystatin B-deficient macrophages have lower IL-10 expression (82), and IL-10 transcriptionally downregulates IL-1β synthesis (83).
While unexpected, our data with cystatins shed further light on the mechanism by which small molecule cathepsin inhibitors may impact IL-1β secretion by modulating pro-IL-1β synthesis. Indeed, we directly demonstrated that exogenous cathepsin inhibitors also suppress LPS-induced pro-IL-1β synthesis, and that this effect contributes substantially to their suppression of IL-1β secretion by inflammasome-activating particulates and non-particulates. Importantly, K777 and Ca074Me reduce pro-IL-1β synthesis in response to LPS priming alone, prior to any IL-1β being secreted, and they do not affect TNF-α secretion. Thus, it is unlikely that inhibitors are reducing the autocrine-like priming of pro-IL-1β synthesis simply by suppressing TNF-α or IL-1β secretion upon stimulation. Together, these findings reiterate the importance of examining both Signal 1 and 2 when interpreting inflammasome studies. In fact, a recent paper emphasized this point by demonstrating that several ROS inhibitors thought to suppress NLRP3 activation actually affect Signal 1(15). We also find that the timing of inhibitor treatment relative to LPS priming can confirm this phenomenon. If inhibitors are added earlier with respect to LPS priming, effects on priming become more pronounced and less NLRP3-specific. In some contexts, this may actually be a therapeutically advantageous characteristic.
Our findings are consistent with a prior study demonstrating that a cathepsin B inhibitor, Z-FA-fmk, suppresses LPS signaling (84). Finding discordant results with cathepsin B-deficient cells, the authors suggested this was a non-cathepsin off-target effect. Similarly, we cannot completely exclude the possibility that the various exogenous and endogenous cathepsin inhibitors are reducing IL-1β responses through off-target effects. However, given our results, it is likely that redundant cathepsins compensated for the loss of cathepsin B, and even more likely that Z-FA-fmk is non-specific for cathepsin B. Moreover, since we observed concordant results with two chemically distinct cathepsin inhibitors, Ca074Me and K777, as well as the endogenous cathepsin inhibitors, we favor the idea that the common effect of these inhibitors on pro-IL-1β synthesis is attributable to their common cathepsin targets.
Importantly, Ca074Me and K777 were consistently more effective against NLRP3-mediated IL-1β secretion compared to that mediated by AIM2 via dAdT, and the effects of cystatin deficiencies were similarly biased. Therefore, it appeared that cathepsins may indeed have a role in mediating stimulus-specific/priming-independent NLRP3 activation. While this is one interpretation, others would predict that NLRP3-mediated IL-1β secretion is particularly sensitive to the levels of pro-IL-1β or that the levels of NLRP3 itself are significantly impacted by inhibitor treatment. Given the importance of LPS priming kinetics, deducing priming-independent effects on IL-1β secretion can be achieved via prolonged priming and/or concomitant inhibition of protein synthesis. Indeed, by inhibiting further pro-IL-1β synthesis with CHX following a prolonged period of LPS priming, we showed that subsequent treatment with K777 and Ca074Me affects Signal 2, independently of Signal 1. This is consistent with our finding that cathepsin inhibition suppressed inflammasome activation, as assayed by examining cleavage of caspase-1, indicating that cathepsins may also play a role in NLRP3 activation, as originally proposed. Importantly, our data showed that cathepsins are not necessary for particle-induced lysosome disruption, although this has been suggested previously (42, 85). Recently, cathepsins have also been implicated in inducing particle-stimulated K+ efflux (K+ efflux is thought to be an absolute requirement for NLRP3 activation)(37) in LPS-primed macrophages. Whether cathepsins affect K+ efflux by influencing a K+ channel or the integrity plasma membrane (secondary to inducing LMD-dependent cell death) (42, 48, 85) is not yet clear.
Whether cathepsins play a role in Signal 1 or Signal 2, it is likely that the proteolytic activity of cathepsins is necessary, given the efficacy of inhibitors; if true, the substrate involved remains to be elucidated. Importantly, both TLR4 and NLRP3, which sequentially mediate the priming and activation of IL-1β secretion, respectively, have large leucine-rich repeats (LRRs). It is presumed the LRRs act as autoinhibitory motifs that block activation until induction of structural changes or ligand binding. In fact, cathepsin inhibitors have been used to demonstrate that cleavage of the LRRs for TLRs 3, 7 and 9 is necessary for optimal activation (86, 87). Moreover, it has also been shown that NLRP1 activation can be directly mediated by proteolytic cleavage of its LRR (88, 89), and that expression of a transgenic NLRP3 protein lacking an LRR motif makes it constitutively active (90). Although this is still all speculation, LRR-targeted cleavage of TLR4 and NLRP3 by cathepsins remains an intriguing possibility that might explain our findings.
Together, this study identifies a previously unappreciated role for cathepsins and cystatins in the regulation of pro-IL-1β synthesis (as well as IL-1β secretion), and provides compelling evidence that cathepsins play redundant and compensatory roles in these processes. Furthermore, we have re-confirmed that Ca074Me inhibits multiple cathepsins and demonstrate conclusively that cathepsin B is not the sole target of this agent that mediates its effect on IL-1β secretion. Moreover, we identified cathepsin X as a previously unappreciated player in nigericin-induced NLRP3 activation, and raised important questions as to the relative importance of cathepsins in mediating Signal 1 and 2 during particle-induced NLRP3 activation and IL-1β secretion. Finally, we have characterized a cathepsin inhibitor, K777, which selectively reduces particle-induced induced IL-1β responses and possesses pharmacological properties warranting its investigation as a potential anti-inflammatory therapeutic (65, 70–75). Indeed, cathepsins are tractable targets for the development of small molecule inhibitors. Our data predict that inhibitors that broadly inhibit cathepsins, like K777, might have potential as therapeutic inhibitors of particle-induced sterile inflammation.
Supplementary Material
Acknowledgments
We thank Havisha Karnam from the Brown and Khvorova labs as well as Myriam Aouadi from the Czech lab at UMMS for help in the optimization of siRNA knockdowns, as well as Kate Fitzgerald, Douglas Golenbock and Eicke Latz for advice and reagents.
Grant Support
This work was supported by an R01 (Grant# 5R01AI078287-05) to K.L.R., a T32 (Grant# T32AI095213-01), and services from the NHLBI, NIH, DHHS, and the Science Moving Towards Research Translation and Therapy (SMARTT) program via the following contract HHSN268201100015C.
Abbreviations
- AIM2
Absent in melanoma 2
- CC
cholesterol crystals
- CHX
cycloheximide
- DAMPs
danger-associated molecular patterns
- LLOMe
Leu-Leu-OMe
- LRR
leucine-rich repeat
- LMD
lysosomal membrane disruption
- NLRP3
NOD-like receptor containing a pyrin domain 3
- PAMPs
pathogen-associated molecular patterns
- PM
peritoneal macrophage
- dAdT
poly(deoxyadenylic-deoxythymidylic) acid
- qPCR
quantitative PCR
- ROS
reactive oxygen species
- WT
wild-type
References
- 1.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 6.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kono H, Orlowski GM, Patel Z, Rock KL. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J Immunol. 2012;189:3734–3740. doi: 10.4049/jimmunol.1200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J Immunol. 2010;184:4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fettelschoss A, Kistowska M, LeibundGut-Landmann S, Beer HD, Johansen P, Senti G, Contassot E, Bachmann MF, French LE, Oxenius A, Kundig TM. Inflammasome activation and IL-1beta target IL-1alpha for secretion as opposed to surface expression. Proc Natl Acad Sci U S A. 2011;108:18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol. 2010;40:620–623. doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Castejon G, Theaker J, Pelegrin P, Clifton AD, Braddock M, Surprenant A. P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol. 185:2611–2619. doi: 10.4049/jimmunol.1000436. [DOI] [PubMed] [Google Scholar]
- 17.Hentze H, Lin XY, Choi MS, Porter AG. Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ. 2003;10:956–968. doi: 10.1038/sj.cdd.4401264. [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa A, Kambe N, Saito M, Nishikomori R, Tanizaki H, Kanazawa N, Adachi S, Heike T, Sagara J, Suda T, Nakahata T, Miyachi Y. Disease-associated mutations in CIAS1 induce cathepsin B-dependent rapid cell death of human THP-1 monocytic cells. Blood. 2007;109:2903–2911. doi: 10.1182/blood-2006-07-033597. [DOI] [PubMed] [Google Scholar]
- 19.Morishige T, Yoshioka Y, Tanabe A, Yao X, Tsunoda S, Tsutsumi Y, Mukai Y, Okada N, Nakagawa S. Titanium dioxide induces different levels of IL-1beta production dependent on its particle characteristics through caspase-1 activation mediated by reactive oxygen species and cathepsin B. Biochem Biophys Res Commun. 392:160–165. doi: 10.1016/j.bbrc.2009.12.178. [DOI] [PubMed] [Google Scholar]
- 20.Duncan JA, Gao X, Huang MT, O’Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu J, Thomas LM, Watkins SC, Franchi L, Nunez G, Salter RD. Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J Leukoc Biol. 2009;86:1227–1238. doi: 10.1189/jlb.0309164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barlan AU, Danthi P, Wiethoff CM. Lysosomal localization and mechanism of membrane penetration influence nonenveloped virus activation of the NLRP3 inflammasome. Virology. 412:306–314. doi: 10.1016/j.virol.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 24.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 25.Terada K, Yamada J, Hayashi Y, Wu Z, Uchiyama Y, Peters C, Nakanishi H. Involvement of cathepsin B in the processing and secretion of interleukin-1beta in chromogranin A-stimulated microglia. Glia. 2009;58:114–124. doi: 10.1002/glia.20906. [DOI] [PubMed] [Google Scholar]
- 26.Meixenberger K, Pache F, Eitel J, Schmeck B, Hippenstiel S, Slevogt H, N’Guessan P, Witzenrath M, Netea MG, Chakraborty T, Suttorp N, Opitz B. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1beta, depending on listeriolysin O and NLRP3. J Immunol. 2009;184:922–930. doi: 10.4049/jimmunol.0901346. [DOI] [PubMed] [Google Scholar]
- 27.Barlan AU, Griffin TM, McGuire KA, Wiethoff CM. Adenovirus membrane penetration activates the NLRP3 inflammasome. J Virol. 2010;85:146–155. doi: 10.1128/JVI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rintahaka J, Lietzen N, Ohman T, Nyman TA, Matikainen S. Recognition of cytoplasmic RNA results in cathepsin-dependent inflammasome activation and apoptosis in human macrophages. J Immunol. 2011;186:3085–3092. doi: 10.4049/jimmunol.1002051. [DOI] [PubMed] [Google Scholar]
- 29.Kankkunen P, Teirila L, Rintahaka J, Alenius H, Wolff H, Matikainen S. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol. 2010;184:6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 30.Said-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One. 5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, Corradin G, Tschopp J. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 33.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 35.Petrovski G, Ayna G, Majai G, Hodrea J, Benko S, Madi A, Fesus L. Phagocytosis of cells dying through autophagy induces inflammasome activation and IL-1beta release in human macrophages. Autophagy. :7. doi: 10.4161/auto.7.3.14583. [DOI] [PubMed] [Google Scholar]
- 36.Lindauer M, Wong J, Magun B. Ricin Toxin Activates the NALP3 Inflammasome. Toxins (Basel) 2:1500–1514. doi: 10.3390/toxins2061500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 39.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdul-Sater AA, Said-Sadier N, Padilla EV, Ojcius DM. Chlamydial infection of monocytes stimulates IL-1beta secretion through activation of the NLRP3 inflammasome. Microbes Infect. 12:652–661. doi: 10.1016/j.micinf.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, Kanellopoulos J, Martin F, Rebe C, Apetoh L, Ghiringhelli F. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2012;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 42.Lima H, Jr, Jacobson LS, Goldberg MF, Chandran K, Diaz-Griffero F, Lisanti MP, Brojatsch J. Role of lysosome rupture in controlling Nlrp3 signaling and necrotic cell death. Cell Cycle. 2013;12:1868–1878. doi: 10.4161/cc.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heid ME, Keyel PA, Kamga C, Shiva S, Watkins SC, Salter RD. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol. 2013;191:5230–5238. doi: 10.4049/jimmunol.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L, Wu Z, Hayashi Y, Peters C, Tsuda M, Inoue K, Nakanishi H. Microglial cathepsin B contributes to the initiation of peripheral inflammation-induced chronic pain. J Neurosci. 2012;32:11330–11342. doi: 10.1523/JNEUROSCI.0677-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niemi K, Teirila L, Lappalainen J, Rajamaki K, Baumann MH, Oorni K, Wolff H, Kovanen PT, Matikainen S, Eklund KK. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol. 2011;186:6119–6128. doi: 10.4049/jimmunol.1002843. [DOI] [PubMed] [Google Scholar]
- 47.Newman ZL, Leppla SH, Moayeri M. CA-074Me protection against anthrax lethal toxin. Infect Immun. 2009;77:4327–4336. doi: 10.1128/IAI.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobson LS, Lima H, Jr, Goldberg MF, Gocheva V, Tsiperson V, Sutterwala FS, Joyce JA, Gapp BV, Blomen VA, Chandran K, Brummelkamp TR, Diaz-Griffero F, Brojatsch J. Cathepsin-mediated necrosis controls the adaptive immune response by Th2 (T helper type 2)-associated adjuvants. J Biol Chem. 2013;288:7481–7491. doi: 10.1074/jbc.M112.400655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montaser M, Lalmanach G, Mach L. CA-074, but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol Chem. 2002;383:1305–1308. doi: 10.1515/BC.2002.147. [DOI] [PubMed] [Google Scholar]
- 50.Mihalik R, Imre G, Petak I, Szende B, Kopper L. Cathepsin B-independent abrogation of cell death by CA-074-OMe upstream of lysosomal breakdown. Cell Death Differ. 2004;11:1357–1360. doi: 10.1038/sj.cdd.4401493. [DOI] [PubMed] [Google Scholar]
- 51.Klemencic I, Carmona AK, Cezari MH, Juliano MA, Juliano L, Guncar G, Turk D, Krizaj I, Turk V, Turk B. Biochemical characterization of human cathepsin X revealed that the enzyme is an exopeptidase, acting as carboxymonopeptidase or carboxydipeptidase. Eur J Biochem. 2000;267:5404–5412. doi: 10.1046/j.1432-1327.2000.01592.x. [DOI] [PubMed] [Google Scholar]
- 52.Bogyo M, Verhelst S, Bellingard-Dubouchaud V, Toba S, Greenbaum D. Selective targeting of lysosomal cysteine proteases with radiolabeled electrophilic substrate analogs. Chem Biol. 2000;7:27–38. doi: 10.1016/s1074-5521(00)00061-2. [DOI] [PubMed] [Google Scholar]
- 53.Deussing J, Roth W, Saftig P, Peters C, Ploegh HL, Villadangos JA. Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc Natl Acad Sci U S A. 1998;95:4516–4521. doi: 10.1073/pnas.95.8.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Felbor U, Kessler B, Mothes W, Goebel HH, Ploegh HL, Bronson RT, Olsen BR. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc Natl Acad Sci U S A. 2002;99:7883–7888. doi: 10.1073/pnas.112632299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, Peters C, Reinheckel T. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66:5242–5250. doi: 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- 56.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 57.Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, Riese R, Ploegh HL, Chapman HA. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 1999;10:197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 58.Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, Villadangos JA, Ploegh H, Peters C, Rudensky AY. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–453. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 59.Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci U S A. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Severa M, Coccia EM, Fitzgerald KA. Toll-like receptor-dependent and -independent viperin gene expression and counter-regulation by PRDI-binding factor-1/BLIMP1. J Biol Chem. 2006;281:26188–26195. doi: 10.1074/jbc.M604516200. [DOI] [PubMed] [Google Scholar]
- 61.Jensen BM, Swindle EJ, Iwaki S, Gilfillan AM. Generation, isolation, and maintenance of rodent mast cells and mast cell lines. Curr Protoc Immunol Chapter. 2006;3(Unit 3):23. doi: 10.1002/0471142735.im0323s74. [DOI] [PubMed] [Google Scholar]
- 62.Verdoes M, Oresic Bender K, Segal E, van der Linden WA, Syed S, Withana NP, Sanman LE, Bogyo M. Improved quenched fluorescent probe for imaging of cysteine cathepsin activity. J Am Chem Soc. 2013;135:14726–14730. doi: 10.1021/ja4056068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sevenich L, Schurigt U, Sachse K, Gajda M, Werner F, Muller S, Vasiljeva O, Schwinde A, Klemm N, Deussing J, Peters C, Reinheckel T. Synergistic antitumor effects of combined cathepsin B and cathepsin Z deficiencies on breast cancer progression and metastasis in mice. Proc Natl Acad Sci U S A. 2010;107:2497–2502. doi: 10.1073/pnas.0907240107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedrichs B, Tepel C, Reinheckel T, Deussing J, von Figura K, Herzog V, Peters C, Saftig P, Brix K. Thyroid functions of mouse cathepsins B, K, and L. J Clin Invest. 2003;111:1733–1745. doi: 10.1172/JCI15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen YT, Brinen LS, Kerr ID, Hansell E, Doyle PS, McKerrow JH, Roush WR. In vitro and in vivo studies of the trypanocidal properties of WRR-483 against Trypanosoma cruzi. PLoS Negl Trop Dis. 2009:4. doi: 10.1371/journal.pntd.0000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ochieng J, Chaudhuri G. Cystatin superfamily. J Health Care Poor Underserved. 2010;21:51–70. doi: 10.1353/hpu.0.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thiele DL, Lipsky PE. The action of leucyl-leucine methyl ester on cytotoxic lymphocytes requires uptake by a novel dipeptide-specific facilitated transport system and dipeptidyl peptidase I-mediated conversion to membranolytic products. J Exp Med. 1990;172:183–194. doi: 10.1084/jem.172.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thiele DL, Lipsky PE. Mechanism of L-leucyl-L-leucine methyl ester-mediated killing of cytotoxic lymphocytes: dependence on a lysosomal thiol protease, dipeptidyl peptidase I, that is enriched in these cells. Proc Natl Acad Sci U S A. 1990;87:83–87. doi: 10.1073/pnas.87.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2011;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacobsen W, Christians U, Benet LZ. In vitro evaluation of the disposition of A novel cysteine protease inhibitor. Drug Metab Dispos. 2000;28:1343–1351. [PubMed] [Google Scholar]
- 71.Engel JC, Doyle PS, Hsieh I, McKerrow JH. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J Exp Med. 1998;188:725–734. doi: 10.1084/jem.188.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barr SC, Warner KL, Kornreic BG, Piscitelli J, Wolfe A, Benet L, McKerrow JH. A cysteine protease inhibitor protects dogs from cardiac damage during infection by Trypanosoma cruzi. Antimicrob Agents Chemother. 2005;49:5160–5161. doi: 10.1128/AAC.49.12.5160-5161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doyle PS, Zhou YM, Engel JC, McKerrow JH. A cysteine protease inhibitor cures Chagas’ disease in an immunodeficient-mouse model of infection. Antimicrob Agents Chemother. 2007;51:3932–3939. doi: 10.1128/AAC.00436-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdulla MH, Lim KC, Sajid M, McKerrow JH, Caffrey CR. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Med. 2007;4:e14. doi: 10.1371/journal.pmed.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKerrow JH, Doyle PS, Engel JC, Podust LM, Robertson SA, Ferreira R, Saxton T, Arkin M, Kerr ID, Brinen LS, Craik CS. Two approaches to discovering and developing new drugs for Chagas disease. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):263–269. doi: 10.1590/s0074-02762009000900034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu T, Yamaguchi Y, Shirasaki Y, Shikada K, Yamagishi M, Hoshino K, Kaisho T, Takemoto K, Suzuki T, Kuranaga E, Ohara O, Miura M. Single-cell imaging of caspase-1 dynamics reveals an all-or-none inflammasome signaling response. Cell Rep. 2014;8:974–982. doi: 10.1016/j.celrep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Chapman HA, Jr, Reilly JJ, Jr, Yee R, Grubb A. Identification of cystatin C, a cysteine proteinase inhibitor, as a major secretory product of human alveolar macrophages in vitro. Am Rev Respir Dis. 1990;141:698–705. doi: 10.1164/ajrccm/141.3.698. [DOI] [PubMed] [Google Scholar]
- 78.Schulte S, Sun J, Libby P, Macfarlane L, Sun C, Lopez-Ilasaca M, Shi GP, Sukhova GK. Cystatin C deficiency promotes inflammation in angiotensin II-induced abdominal aortic aneurisms in atherosclerotic mice. Am J Pathol. 2010;177:456–463. doi: 10.2353/ajpath.2010.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maher K, Jeric Kokelj B, Butinar M, Mikhaylov G, Mancek-Keber M, Stoka V, Vasiljeva O, Turk B, Grigoryev SA, Kopitar-Jerala N. A role for stefin B (cystatin B) in inflammation and endotoxemia. J Biol Chem. 2014;289:31736–31750. doi: 10.1074/jbc.M114.609396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ceru S, Konjar S, Maher K, Repnik U, Krizaj I, Bencina M, Renko M, Nepveu A, Zerovnik E, Turk B, Kopitar-Jerala N. Stefin B interacts with histones and cathepsin L in the nucleus. J Biol Chem. 2010;285:10078–10086. doi: 10.1074/jbc.M109.034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang YR, Qin S, Han R, Wu JC, Liang ZQ, Qin ZH, Wang Y. Cathepsin L plays a role in quinolinic acid-induced NF-Kappab activation and excitotoxicity in rat striatal neurons. PLoS One. 2013;8:e75702. doi: 10.1371/journal.pone.0075702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maher K, Zavrsnik J, Jeric-Kokelj B, Vasiljeva O, Turk B, Kopitar-Jerala N. Decreased IL-10 expression in stefin B-deficient macrophages is regulated by the MAP kinase and STAT-3 signaling pathways. FEBS Lett. 2014;588:720–726. doi: 10.1016/j.febslet.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 83.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 84.Schotte P, Schauvliege R, Janssens S, Beyaert R. The cathepsin B inhibitor z-FA.fmk inhibits cytokine production in macrophages stimulated by lipopolysaccharide. J Biol Chem. 2001;276:21153–21157. doi: 10.1074/jbc.M102239200. [DOI] [PubMed] [Google Scholar]
- 85.Brojatsch J, Lima H, Kar AK, Jacobson LS, Muehlbauer SM, Chandran K, Diaz-Griffero F. A proteolytic cascade controls lysosome rupture and necrotic cell death mediated by lysosome-destabilizing adjuvants. PLoS One. 2014;9:e95032. doi: 10.1371/journal.pone.0095032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011;208:643–651. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frew BC, V, Joag R, Mogridge J. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 2012;8:e1002659. doi: 10.1371/journal.ppat.1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C, Bertin J, Gough PJ. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem. 2012;287:25030–25037. doi: 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.