Abstract
Complex Regional Pain Syndrome (CRPS) is a major cause of chronic pain after surgery or trauma to the limbs. Despite evidence showing that the prevalence and severity of many forms of chronic pain, including CRPS, differ between males and females, laboratory studies on sex-related differences in animal models of CRPS are not available, and the impact of sex on the transition from acute to chronic CRPS pain and disability are unexplored. Here we make use of a tibia fracture/cast mouse model that recapitulates the nociceptive, functional, vascular, trophic, inflammatory and immune aspects of CRPS. Our aim is to describe the chronic time course of nociceptive, motor and memory changes associated with fracture/cast in male and female mice, in addition to exploring their underlying spinal mechanisms. Our behavioral data shows that, compared to males, female mice display lower nociceptive thresholds following fracture in the absence of any differences in ongoing or spontaneous pain. Furthermore, female mice show exaggerated signs of motor dysfunction, deficits in fear memory, and latent sensitization that manifests long after the normalization of nociceptive thresholds. Our biochemical data show differences in the spinal cord levels of the glutamate receptor NR2b, suggesting sex differences in mechanisms of central sensitization that could account for differences in duration and severity of CRPS symptoms between the two groups.
Keywords: Complex regional pain syndrome, chronic pain, pain-related memory deficits, sex differences in pain, central sensitization
1. INTRODUCTION
The sex and gender of clinical patients are important variables in the pain equation since the prevalence and severity of many forms of chronic pain differ between males and females. Examples include the female preponderance of patients suffering from migraine headache, fibromyalgia, irritable bowel syndrome (Racine et al., 2012a, 2012b), various forms of neuropathic pain (Butler, Jonzon, Branting-Ekenback, Wadell, & Farahmand, 2012), and chronic postoperative pain (van Gulik et al., 2011). Furthermore, sex- and gender-related differences to experimental pain in humans have been reported (Alabas, Tashani, Tabasam, & Johnson, 2012; Dannecker et al., 2012; Racine et al., 2012a, 2012b). Despite the clinical evidence, only a limited number of preclinical studies have addressed these differences, and with varying results. For example, the severity of neuropathic pain was shown to be less in female rats after sciatic nerve lesion (Wagner, DeLeo, Coombs, & Myers, 1995), although allodynia was shown to be greater in female rats after spinal nerve transection (DeLeo & Rutkowski, 2000) and in female mice after chronic nerve constriction injury (Vacca et al., 2014). Likewise, inflammatory stimuli have different pain-related effects in male and female rats (Tall & Crisp, 2004). Both intrinsic differences in nociceptive neurons and estrogen effects may be responsible for these sex-related responses (Chaban, Li, McDonald, Rapkin, & Micevych, 2011; Dina, Aley, Isenberg, Messing, & Levine, 2001; Hendrich et al., 2012). For instance, estradiol receptors are expressed in dorsal root ganglion neurons and interact with metabotropic glutamate receptors to mediate intracellular signaling (Dewing et al., 2007), a mechanism that is very relevant to nociception.
Complex regional pain syndrome (CRPS) is a debilitating condition characterized by severe pain usually confined to one limb. It encompasses a wide range of signs and symptoms including the sensory, motor and autonomic nervous systems, bone demineralization, skin growth changes and vascular dysfunction. Additionally, CRPS patients often display deficits in executive functioning, memory, and even global cognitive impairment (Libon et al., 2010) that could further exacerbate overall patient well-being. With an estimated 50,000 new cases in the US annually, CRPS exhibits a high prevalence in female patients, with females affected at least 3 times more than males (de Mos et al., 2007). Despite the need for studies targeting sex differences in CRPS, data from animal models of CRPS is not available, and the impact of sex on the transition from acute to chronic CRPS pain and disability are unexplored, thereby limiting patient-specific selection of treatments and the rational design of new therapies.
For the present studies, we made use of the tibia fracture/cast model of CRPS. Characterized both in mice and rats, this model recapitulates the nociceptive, functional, vascular, trophic, inflammatory, and immune aspects of CRPS observed in patients, including unilateral limb warmth, edema, allodynia, unweighting, and the associated peripheral (e.g. neurogenic inflammation) and central (brain neuroplasticity) alterations (Gallagher et al., 2013; Guo, Offley, Boyd, Jacobs, & Kingery, 2004; Li, Guo, Li, Kingery, & Clark, 2010; Tajerian et al., 2014; Wei, Sabsovich, et al., 2009). Our main goal was to describe the timecourse of nociceptive, motor, and memory changes associated with fracture/cast in male and female mice, in addition to exploring their underlying spinal mechanisms. It is our hope that studies such as ours exploring disease etiology and mechanism in both sexes will identify gender specific mechanisms contributing to the development and maintenance of CRPS, and ultimately explain the preponderance of female CRPS patients.
2. MATERIALS AND METHODS
2.1 Animals
A total of 3 cohorts of mice were used. Cohort 1 was used for the longitudinal measurements of mechanical sensitivity and physiological measures of hindpaw edema and temperature, in addition to measures of rotarod and latent sensitization. Cohort 2 was used for conditioned place preference and fear memory, while cohort 3 was used for tissue collection. Male and female C57/B6J mice aged 12–14 weeks were purchased from the Jackson Laboratory (Sacramento, CA, USA) and were allowed to habituate to the animal facility for a minimum of 10 days prior to the experiments. Mice were housed in groups of 4 on a 12-hr light/dark cycle and an ambient temperature of 22 ± 3°C, with food and water available ad libitum. All animal procedures and experimental designs were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, CA, USA) and followed the “animal subjects” guidelines of the International Association for the Study of Pain.
2.2 Limb fracture and cast immobilization
Mice were randomly allocated to the control or the fracture/cast group. Mice were anesthetized with 1.5% isoflurane and underwent a distal tibia fracture in the right leg. Briefly, a hemostat was used to make a closed fracture of the right tibia just distal to the middle of the tibia. Then the hindlimb was wrapped in casting tape (Scotchcast™ Plus) so the hip, knee and ankle were all fixed. The cast extended from the metatarsals of the hindpaw up to a spica formed around the abdomen. A window was left open over the dorsal paw and ankle to prevent constriction when post-fracture edema developed.(Guo et al., 2012) After the procedure, the mice were given subcutaneous buprenorphine (0.05 mg/kg) and enrofloxacin (5 mg/kg) for the next two days, as well as normal saline (1.5 ml once) for post-operative analgesia, prevention of infection and prevention of dehydration. Mice were inspected daily to ensure the cast was positioned properly through the 3-week period of cast immobilization. Mice were provided with chow pellets postoperatively ad libitum; dietary gels were also made available on the cage floor for mice having undergone surgery. At 3 weeks after surgery, the mice were briefly anesthetized using isoflurane and the casts were removed.
A summary of the allocation of cohorts in addition to the experimental timeline is illustrated in Fig. 1.
Fig. 1. Summary of the experimental timeline.
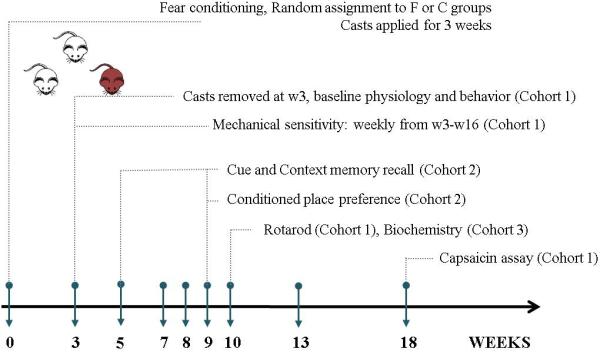
F=fracture, C=control, W=week.
2.3 Physiological measures
All measurements were conducted 3 days prior to fracture and weekly after cast removal at 3 weeks post-fracture.
Hindpaw volume
A laser sensor technique was used to determine the dorsal–ventral thickness of the hindpaw, as we have previously described (Li et al., 2009). The sensor device has a measurement range of 200 mm with a 0.01 mm resolution (cat. #4381-Precicura, Limab, Göteborg, Sweden).
Hindpaw temperature
The temperature of the hindpaw was measured using a fine wire thermocouple (Omega, CT, USA) applied to the paw skin, as described previously (Li et al., 2009). The investigator held the wire using an insulating Styrofoam block. 3 sites were tested over the dorsum of the hindpaw: the space between the 1st and 2nd metatarsals (medial), the 2nd and 3rd metatarsals (central), and the 4th and 5th metatarsals (lateral). After a site was tested in one hindpaw, the same site was immediately tested in the contralateral hindpaw. The testing protocol was medial dorsum right then left, central dorsum right then left, lateral dorsum right then left, medial dorsum left then right, central dorsum left then right, and lateral dorsum left then right. The six measurements for each hindpaw were averaged for the mean temperature.
2.4 Behavioral testing
The experimenter was blind to the identity and experimental condition of the animals throughout behavioral experiments and data analysis. Mice were habituated to handling by the experimenter for a few minutes each day for 7 days before initiation of the behavioral tests. Male and female mice were tested separately.
Mechanical hypersensitivity
Calibrated monofilaments (Stoelting Co., IL, USA) were applied to the plantar surface of the hindpaw and the 50% threshold to withdraw (grams) was calculated as previously described (Chaplan, Bach, Pogrel, Chung, & Yaksh, 1994). The stimulus intensity ranged from 0.004 to 1.7 g, corresponding to filament numbers (1.65, 2.36, 2.44, 2.83, 3.22, 3.61, 3.84, 4.08, 4.17, and 4.31). For each animal, the actual filaments used within the aforementioned series were determined based on the lowest filament to evoke a positive response followed by 5 consecutive stimulations using the up-down method. The filament range and average interval were then incorporated along with the response pattern into each individual threshold calculation. Mechanical sensitivity was assessed on the plantar surface of the hindpaw (response = flexion reflex).
Conditioned place preference
To assess ongoing pain associated with fracture, a single trial counter balanced conditioned place preference (CPP) test was employed starting at 8 weeks after fracture; similar to techniques described previously (Sahbaie, Sun, Liang, Shi, & Clark, 2014; Sun et al., 2012; van der Kam, Vry, Schiene, & Tzschentke, 2008). The CPP assay is based on the concept that pain relief is an unconditioned reward, and as such, analgesic agents that are not rewarding in the absence of pain (such as low-dose morphine used in this paradigm) should become rewarding only in the presence of ongoing pain (King et al., 2009). The CPP experiments were done using standard conditioning chambers placed inside sound attenuating chambers with controlled lighting (visual cues = different chamber colors, tactile cues = floors: bars vs. rods). Video recordings were analyzed by TopScan (Clever Sys., Reston, VA) for time spent in each of the two active association compartments. Each experiment started with 2 pre-conditioning (PC) days where the mice were placed in the middle chamber and given free access to the three chambers for 30 minutes of exploration. Mice were tested at the end of the 8 weeks post fracture or sham surgery. On PC day 3, mice had free chamber exploration for 15 minutes and were video recorded. Any mouse that spent more than 80% or less than 20% of the total experiment time in either of the association compartments was excluded. On day 4 (preconditioning) mice received saline injections and assigned to either one of the association compartments for 50 min. Following a 4-hour interval, mice were injected with ultra-low dose of morphine (0.3 mg/kg) and immediately placed in the opposite compartment. The side chosen for drug administration was the less preferred side on PC day 3. On Day 5, mice were placed in the middle neutral compartment of the apparatus and were assessed for time spent in outer compartments for the duration of 15 minutes.
Rotarod
10 weeks after fracture, locomotor capacity was measured by the use of an accelerating rotarod (cat. #47600, Ugo Basile, Comerio, Italy). The task includes a speed ramp from 0 to 30 rotations per minute over 60 seconds, followed by an additional 240 seconds at the maximal speed. Latency and rotation speed at fall were determined. The latency to fall was reported for the initial exposure to the rotarod, in addition to 5 consecutive trials (30-minute inter-trial interval).
Fear memory
Fear conditioning was used to evaluate both hippocampal-dependent and hippocampal-independent learning and memory. Briefly, mice were placed into the conditioning chamber and allowed to explore for four minutes in order to habituate the animal to the environment and collect baseline freezing levels using FreezeScan (CleverSys, Inc). Operant conditioning chambers (26×32×21 cm) were housed in sound attenuating chambers (43.2×45.7×43.2 cm). All chambers were cleaned using a 70% ethanol solution before each session.
After habituation, mice were then presented with a tone and cue light for 30 seconds (conditioned stimulus - CS). A mild foot shock (0.6 mA) was paired for the last 2 seconds of the tone/cue light presentation (unconditioned stimulus - US). The mice were then allowed to investigate the environment for an additional 90 seconds before being exposed to the CS-US pairing for the 2nd, 3rd, and 4th times. 90 seconds after the 4th CS-US pairing, animals were removed to their home cages. Mice were trained in the fear conditioning operant five days prior to the induction of tibia fracture.
Recall of fear memory was tested twice: once at 5 weeks after fracture, and again 9 weeks after fracture. The hippocampal-dependent contextual fear response was scored as percent time spent freezing during a 3-minute period in which mice were placed in the conditioning chamber without the CS or US. Roughly 24 hours after the hippocampal-dependent contextual fear test, mice were tested for hippocampus-independent fear memory. To change the context of the conditioning chamber, opaque, white floor and wall inserts were added, and the chamber was scented with 4% acetic acid. Animals were placed in the modified chambers for 3 minutes with just the room light, followed by 3 minutes with CS only (cue light and sound), followed by one minute with room light only before returned to the home cage. Freezing behavior was scored for the first 3 minutes (pre-cue freezing), second 3 minutes (cued freezing), and the final one minute (post cue freezing).
Capsaicin-evoked behaviors
This assay was performed 18 weeks following fracture. The assay is the measurement of the duration of total behaviors (biting, scratching, licking, and shaking) during the 5 minutes after the local subcutaneous injection of capsaicin (2.5 μg in 5 μL, cat. # MT028, Sigma-Aldrich, MO, USA), or vehicle (0.25% DMSO, 0.25% ethanol, 0.125% Tween-80 in saline), into the plantar surface of the right hind paw (Millecamps, Tajerian, Naso, Sage, & Stone, 2012). Quantification was carried out in real time by a blind observer using a stopwatch.
Each animal was tested with both capsaicin and vehicle with a 5-day washout between treatments. To examine the long-term effects of capsaicin, mechanical hypersensitivity was measured on the plantar aspect of the right and left hindpaws 1 hour after injection as described above.
2.5 RNA isolation and quantitative real-time polymerase chain reaction (PCR)
At 10 weeks post-fracture, the mice were euthanized and the spinal cord (SC) tissue was immediately harvested by extrusion and ipsilateral lumbar SC segments were dissected on a chilled surface. Dissected tissue was stored at −80°C until required for analysis. Total RNA was isolated from SC using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The purity and concentration were determined spectrophotometrically. The messenger RNA samples were reverse transcribed into complementary DNA using a First Strand complementary DNA Synthesis Kit (Invitrogen, Carlsbad, CA). Real-time PCR was performed in an ABI prism 7900HT system (Applied Biosystems, Foster City, CA). All PCR experiments were performed using the SYBR Green I master kit (Applied Biosystems). The primer sets for 18S messenger RNA (mRNA) and the amplification parameters were described previously (Sun et al., 2012). Melting curves were performed to document single product formation and agarose electrophoresis confirmed product size. The Grin2b primers were purchased from SABiosciences (Valencia, CA). As negative controls, RNA samples that were not reverse-transcribed were run. Data were normalized to 18S mRNA expression. The following primers were used: Grin2b: forward: ATGAAGAGGGGCAAGGAGTT, reverse: CGATGATGGAGGAGACTTGG; 18S: forward: AAGACGATCAGATACCGTCGTAG, reverse: TCCGTCAATTCCTTTAAGTTTCA.
2.6 Two-color fluorescent western blot analysis
To assess the protein levels of NR2b in our lumbar SC preparation 10 weeks post-fracture, Western blot analysis was performed according to standard procedures. Tissues were homogenized using T-PER Protein Extraction Reagent (Thermo Scientific 87793) in the presence of proteinase and phosphatase inhibitors (04906837001; Roche Applied Science, San Francisco, CA) and centrifuged at 12,000 g for 4 min at 4°C. Supernatant fractions were then frozen at −80°C until use. An aliquot was subjected to protein assay (500-0001; Bio-Rad, Hercules, CA) to normalize protein levels. In brief, after sodium dodecyl sulfate polyacrylamide gel electrophoresis and blotting, proteins on the membranes were detected by overnight incubation at 4°C with the primary antibody (rabbit polyclonal anti NR2b, 1:500, cat. #ab65783, Abcam, MA, USA) followed by incubation with an IRDye 800CW goat anti-rabbit IgG (H+L) (1:20,000; cat. #925-32211, LI-COR biosciences; NA, USA). β-Actin was used as an internal control and was detected with the mouse monoclonal anti-β-actin antibody (1:5,000, ab6276; Abcam, Cambridge, MA) followed by incubation with an IRDye 680CW goat anti-mouse IgG (H + L) (1:20,000, 926–32220; LI-COR Biosciences). The signals were detected using Odyssey (LICOR Biosciences, NA, USA) and quantified using the Image J software (MD, USA).
2.7 Statistical analysis
All data are expressed as mean ± SEM. Analysis of repeated parametric measures was accomplished using a two-way repeated measures analysis of variance followed by Bonferroni post-test (Figs. 1,5,6). For fig. 2, the time spent in the drug associated chamber, post conditioning (ttest) and time spent in the same chamber at baseline (tpreconditioning ) were compared by paired 2-tailed t-tests. To evaluate the overall differences between treatment groups in the 2 sexes, the difference time scores (ttest-tpreconditioning) were first calculated and one-way ANOVAs were used to determine significant score differences between treatment groups followed by post-hoc Tukey’s tests. One-way analysis of variance was used to compare the 4 groups in Fig. 3,4, and 7 and was followed by Bonferroni testing. Significance was set at p < 0.05. (Prism 5; GraphPad Software, La Jolla, CA). Sample sizes are indicated in the figure legends.
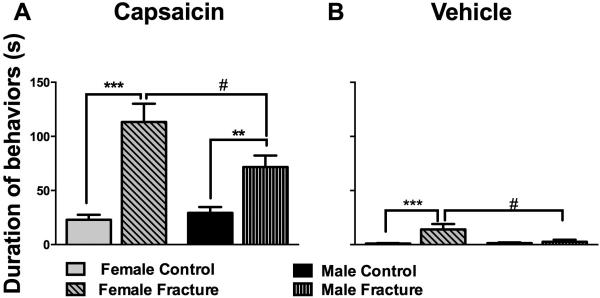
Fig. 5. Fracture female mice had increased hypersensitivity to subcutaneous capsaicin and vehicle injections.
At 18 weeks after fracture, both female and male fracture mice spent more time engaging in capsaicin-evoked behaviors (licking, biting, scratching, and shaking) compared to control mice (A). Furthermore, female fracture mice had significantly increased duration of evoked nociceptive behavior, compared to males, both after capsaicin (A) and vehicle (B) injections. #p<0.05, ** p<0.005, *** p<0.001. n=8-10/group.
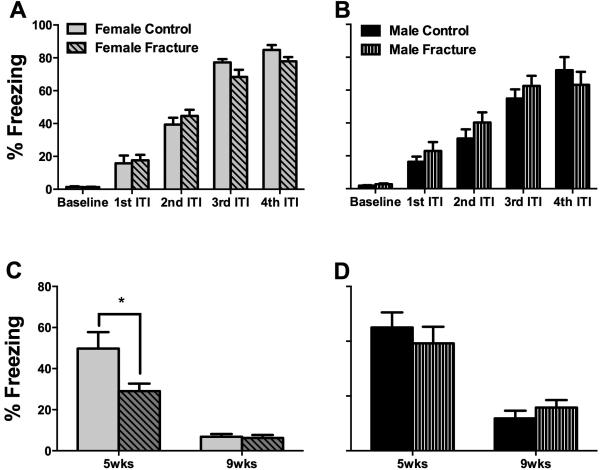
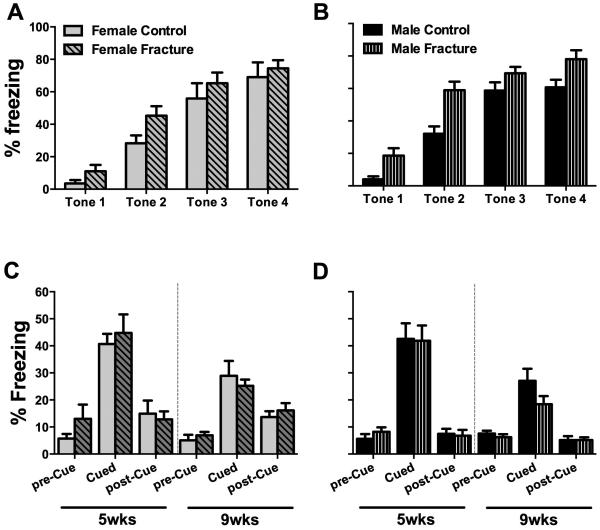
Fig. 6. Fracture female mice had deficits in context fear memory 5 weeks after fracture.
At 5 days prior to tibia fracture the female (A) and male (B) mice were successfully trained in the fear memory paradigm. Subsequent testing for fear memory demonstrated deficits in context memory in female mice at 5 weeks post-fracture (C). No such deficits were observed in male mice (D). ITI= Inter-trial interval. *p<0.05. n=8-12/group.
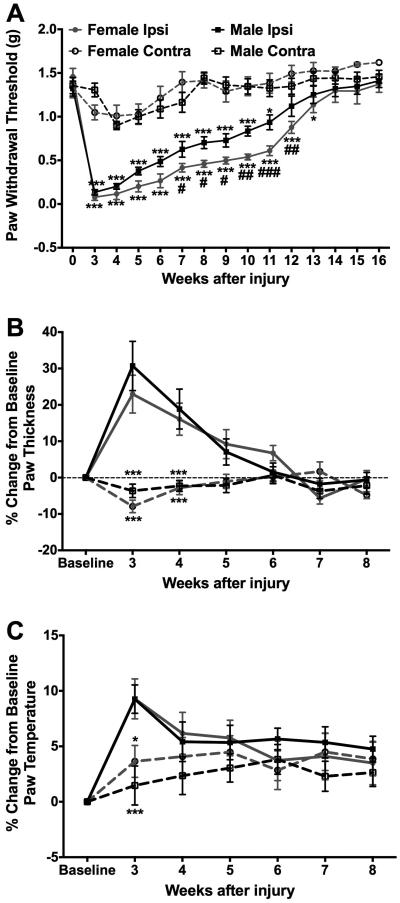
Fig. 2. Compared to males, female mice displayed increased and persistent mechanical allodynia in response to tibia fracture.
While both male and female fracture mice displayed persistent mechanical allodynia (A), transient edema (B), and transient warmth (C) in the ipsilateral hindpaw, female mice exhibit greater mechanical allodynia compared to the male mice (A), with no differences between the sexes in measures of edema (B) or temperature (C) at any time point. #*p<0.05, ###*** p<0.001. (* Compared to contralateral measurements, # compared to males). n=8-10/group.
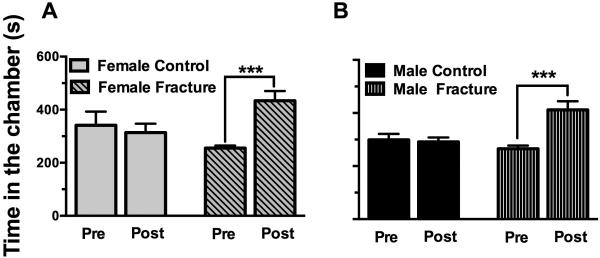
Fig. 3. Both female and male mice exhibited signs of ongoing pain after fracture.
At 9 weeks after fracture, both female and male fracture mice spent more time in a chamber that was previously paired with morphine administration (A, B), a preference indicative of ongoing pain. No such preference was observed in control mice of either sex. *** p<0.001. n=8-12/group.
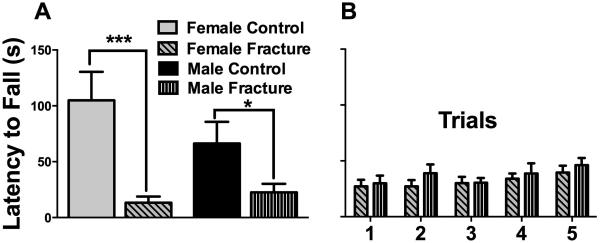
Fig. 4. Female and male mice both exhibited chronic signs of motor disturbance after fracture.
At 10 weeks after fracture, female mice had a greater impairment in their motor performance (87.3% reduction in fall latency), compared to males (66% reduction in fall latency), but this difference did not reach statistical significance. (A). Subsequent training sessions in fracture mice (5 consecutive sessions separated by 30 minutes) did not show differences between the male and female groups (B). *p<0.05, *** p<0.001. n=8-10/group.
Fig. 7. No deficits in cued fear memory were observed after fracture.
At 5 days prior to tibia fracture female (A) and male (B) mice were successfully trained in the fear memory paradigm. Subsequent testing of cued fear memory demonstrated no deficits in either sex in cued memory at both the 5 and 9 week time points (C, D). *p<0.05. n=8-12/group.
3. RESULTS
3.1 Female mice displayed more intense and persistent mechanical allodynia in response to tibia fracture
In order to measure the timecourse of the development and resolution of nociceptive sensitization in addition to the physiological correlates of fracture in male and female mice, measurements of ipsilateral and contralateral hindpaw allodynia, edema, and temperature were performed starting at 3 weeks post-fracture. Consistent with our previous publications using this mouse model, fracture mice displayed persistent mechanical hypersensitivity (Fig. 2A) in addition to transient edema (Fig. 2B) and transient increase in temperature (Fig. 2C) in the ipsilateral hindpaw. Additionally, our data shows that, between 7 and 12 weeks post-fracture, female mice exhibit significantly lower mechanical thresholds on the ipsilateral hindpaw compared to the male mice (Fig. 2A), with no differences between the 2 sexes being present in measures of ipsilateral hindpaw edema (Fig. 2B) or temperature (Fig. 2C) at any of the timepoints.
3.2 Conditioned place preference demonstrated ongoing pain in male and female mice after fracture
In addition to reflexive measures of nociception assessed using von Frey filaments, we evaluated ongoing pain using conditioned place preference. Our data shows that 9 weeks after fracture both female and male fracture mice spend more time in a chamber that was previously paired with morphine administration, but no gender differences were observed in this spontaneous pain measurement (Fig. 3 A, B). No such preference was observed in control mice of either sex indicating that the low dose of morphine (0.3 mg/kg) was not itself reinforcing. Furthermore, similar to our previously published findings (Sahbaie et al., 2014), our pilot studies showed no changes in mechanical sensitivity thresholds 1 hour following the administration of morphine (data not shown), indicating that the low dose of morphine used in the CPP paradigm is not anti-allodynic.
3.3 Male and female mice had reduced motor function and coordination after fracture
Motor impairment and functional deficits are frequently observed in CRPS patients (Schwartzman & Kerrigan, 1990), prompting us to evaluate motor performance on the rotarod in male and female mice at 10 weeks after fracture. While fracture is associated with deteriorated motor function (compared to control animals) both in male (66% reduction in fall latency) and female mice (87.3% reduction in fall latency) at 10 weeks after fracture, female mice show a trend towards a more dramatic reduction in motor performance (Fig. 4A). Subsequent training sessions in fracture mice (5 consecutive sessions separated by 30 minutes) did not identify differences between the male and female groups (Fig. 4B).
3.4 Female fracture mice displayed increased hypersensitivity to subcutaneous capsaicin and vehicle injections
Having monitored reflexive nociceptive thresholds, spontaneous pain measures, and motor performance in male and female mice, we then proceeded to examine whether there exist differences in latent sensitization in these mice, long after normalization of the nociceptive thresholds. Eighteen weeks after fracture, both male and female fracture mice spent more time engaging in capsaicin-evoked behaviors (licking, biting, scratching, and shaking) compared to control mice (Fig 5A, B). This is indicative of persistent hindpaw sensitization in the absence of notable differences in mechanical thresholds at this timepoint. Furthermore, female fracture mice showed significantly increased duration of evoked nociceptive behavior compared to males both after capsaicin (Fig. 5A) and even after vehicle (Fig. 5B) injections (no vehicle-evoked behaviors were observed in males). No changes were observed in mechanical thresholds one hour after capsaicin or vehicle injections in the ipsilateral and contralateral hindpaws (data not shown).
3.5 Female mice demonstrated deficits in context fear memory at 5 weeks after fracture
Both female (Fig. 6,7A) and male (Fig. 6,7B) mice were successfully trained in the fear memory paradigm 5 days prior to inducing the tibia fractures. Subsequent testing of fear memory demonstrated deficits in context memory in female mice at 5 weeks post-fracture (Fig. 6C). No such deficits were observed in male mice (Fig. 6D). Furthermore, no deficits were observed in either of the sexes in cue memory both at the 5 and 9-week timepoints (Fig. 7B, C).
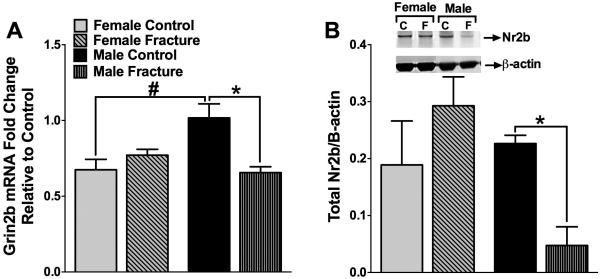
3.6 Spinal levels of Grin2b mRNA and corresponding NR2b protein are decreased following fracture in male mice only
In order to assess the molecular and biochemical correlates associated with the sex differences in nociceptive thresholds, we evaluated the Grin2b mRNA and NR2b protein levels in the ipsilateral lumbar spinal cord at 10 weeks following fracture. NR2b is a subunit of the NMDA receptor widely implicated in central sensitization and chronic pain (Baron, Hans, & Dickenson, 2013; D'Mello & Dickenson, 2008). Our results show that male mice displayed a significant decrease in mRNA levels of Grin2b in the ipsilateral lumbar spinal cord. No such decrease was observed in female mice (Fig 8A). Consistent with the mRNA findings, protein levels of NR2b were decreased in male mice only (Fig 8B).
Fig. 8. Spinal levels of Grin2b and NR2b were decreased following fracture in only male mice.
At 10 weeks after fracture levels of Grin2b mRNA and it’s constitutively expressed protein NR2b were examined in lumbar spinal samples. Both Grin2b RNA levels (A) and NR2b protein levels (B) were decreased after fracture in males, but not in females. *#p<0.05. n=4-5/group.
4. DISCUSSION
The current study addresses crucial differences between male and female animal subjects often overlooked in preclinical pain research in general, and CRPS research in particular. Using the tibia fracture/cast immobilization model of CRPS in both sexes, we have shown that female mice display lower nociceptive thresholds following fracture in the absence of any differences in ongoing or spontaneous pain. Furthermore, female mice show exaggerated signs of motor dysfunction, deficits in fear memory, and latent sensitization that manifests long after the normalization of nociceptive thresholds. These behavioral phenotypes are associated with a decrease in NR2b NMDA receptor subunit levels in the spinal cords of male, but not female, mice. Importantly, not all CRPS-like sequelae of tibia fracture differ between sexes in the mouse model. Overall, these results are consistent with the sex-related differences in CRPS prevalence (de Mos et al., 2007).
Our results showing lower nociceptive thresholds in females after fracture are consistent with previous publications generally reporting lower mechanical thresholds in female rodents (Hurley & Adams, 2008; Wiesenfeld-Hallin, 2005). The extended time course of sensitization of the rodent CRPS model provided us the opportunity to more carefully characterize these differences, however. Our data suggests that, while the two sexes do not show differences in allodynia at the onset of the syndrome, female mice show both an increase in the magnitude and a delay in the resolution of allodynia that are detected between weeks 7-12 post fracture .In our model of CRPS, it is during the more chronic phases of the condition that the sex-related differences are most important. In clinical terms, the correlate might be of a more severe and longer lasting course of CRPS in females. On the other hand, reflexive measures such as von Frey mechanical testing may not be the best proxy indicators of ongoing pain; operant conditioning paradigms may prove to be more useful in this regard (Mogil et al., 2010; Vierck & Yezierski, 2015). Our conditioned place preference assay was conducted at the 9 week post-fracture timepoint where differences in nociceptive thresholds between the 2 sexes were evident. Using this operant measure, we were able to detect signs of spontaneous/ongoing pain after fracture in both male and female mice, although no differences between the 2 sexes were observed. The distinction is important as the mechanisms and pharmacological sensitivities of allodynia and spontaneous pain may be distinct (King et al., 2009). In fact, the very low dose of morphine used to provide place preference in the CRPS model mice was not sufficient to reduce allodynia, similar to our observations when using an incisional rodent pain model (Sahbaie et al., 2014).
CRPS is often accompanied by functional limitations and motor disturbances including impairment in the initiation and execution motor tasks and limited range of motion (Veldman, Reynen, Arntz, & Goris, 1993) and fear of pain associated with such movement (de Jong, Vlaeyen, de Gelder, & Patijn, 2011). Our data in fracture mice is in agreement with these clinical signs, where both male and female fracture mice show reduced latency to fall off the rotarod, indicative of overall motor disturbance, lack of motor coordination, and/or fear of movement. Although the differences did not achieve statistical significance, there was a trend towards increased deficits in female mice in the fracture group, mainly due to better motor performance in control female mice (compared to control male mice). More detailed testing of motor coordination and the utilization of the measures at additional time points might improve our understanding of these possible differences.
Despite the fact that our tibia fracture/cast immobilization model of CRPS is characterized by a reduction in nociceptive thresholds that gradually resolves over time, we were able to identify signs of latent sensitization in male and female fracture mice long after the normalization of mechanical thresholds. This is an important phenotype to study since it is further evidence of ongoing neuroplasticity in the chronic phases of our CRPS model, and could help link the previous painful injury to consequent chronic pain and even predict the response to further injuries (Reichling & Levine, 2009). Interestingly, compared to males, female mice showed a significantly greater nociceptive response to the challenging stimulus (sub-dermal capsaicin) and even to the vehicle injections that failed to elicit any response in male fracture mice. It needs to be kept in mind that vehicle injections are somewhat noxious in that they involve needle trauma and the administration of solvent chemicals (DMSO, ethanol, and tween 80). Our results suggest that post-fracture central nociceptive sensitization persists even after resolution of hindpaw allodynia, and these chronic central neuroplastic changes are more robust in female than in male mice.
In addition to ongoing pain and motor dysfunction, CRPS is associated with various cognitive and memory deficits both in patients (Libon et al., 2010) and preclinical animal subjects (Tajerian et al., 2014). These pain-associated co-morbidities can significantly impair both patient quality of life and response to treatment, and as such, measures of cognitive function and changes in emotion are increasingly used in animal models of pain. While some of the cognitive testing performed in the CRPS model revealed similar results between the sexes, we observed a sex-specific deficit in spatial/fear memory at 5 weeks after fracture in female mice. This particular memory test is believed to reflect hippocampal function (Phillips & LeDoux, 1992). In fact, we have performed biochemical and structural analyses of the hippocampus in CRPS model mice and found fracture-related decreases in levels of synaptophysin and brain-derived neurotrophic factor in the absence of significant changes in dendritic spine density (Tajerian et al., 2014). In humans, imaging studies have found decreased hippocampal volume in CRPS patients (Mutso et al., 2012), and recent imaging data in female CRPS patients found an association between pain severity and gray matter hypertrophy in the posterior hippocampus (Barad, Ueno, Younger, Chatterjee, & Mackey, 2014). Future investigations into the structural and biochemical changes in male and female fracture mice might help inform the design of comparative imaging studies and provide a mechanistic basis for the differences observed between male and female CRPS patients.
The chronicity of pain after fracture, as well as the presence of latent sensitization in our model, prompted us to study the spinal markers of central sensitization associated with the detected phenotype. Central sensitization is a mechanism common to most chronic pain syndromes (Latremoliere & Woolf, 2009), including CRPS, where increased glutamate levels in the cerebrospinal fluid of patients are observed (Alexander, Perreault, Reichenberger, & Schwartzman, 2007). For our molecular and biochemical studies, the Nr2b subunit was chosen based on our preliminary data, on a recent study showing that NR2b-containing receptors play a predominant role in synaptic transmission in the superficial lamina of the rodent spinal cord (Hildebrand et al., 2014),on the observation that NMDA receptor antagonists can sometimes be effective CRPS treatments (Finch, Knudsen, & Drummond, 2009; Schwartzman et al., 2009; Sigtermans et al., 2009), and on our own study demonstrating that the NMDA receptor antagonist ketamine can reverse allodynia during the chronic phase of CRPS-like symptoms in the fracture mice (manuscript under review). We postulated that higher levels of Nr2b would be associated with greater levels of nociceptive sensitization. Our results show a decrease in Grin2b mRNA and Nr2b protein levels in the ipsilateral lumbar spinal cord after fracture in male mice only. Our interpretation of these results is that compensatory mechanisms targeting synaptic transmission could be at play during the more chronic phase of the syndrome, and that the absence of such compensatory mechanisms in female animals may be responsible for the increased duration and severity of allodynia that we observed in the female fracture mice. This lack of adequate compensatory pain suppression hypothesis is somewhat novel and distinct from most investigations focused on biological factors that exacerbate rather than moderate pain after injuries. It is important to note that, in the current study, we did not distinguish between the inhibitory and excitatory laminae of the spinal cord dorsal horn when measuring Nr2b levels. Lamina II GABAergic interneurons express both Nr2a,b and Nr2c,d subunits while lamina II excitatory neurons express mainly Nr2a,b (Shiokawa, Kaftan, MacDermott, & Tong, 2010). Additionally, loss of inhibitory control (e.g. through the loss of GABAergic inhibition or even the loss of the interneurons themselves) has been shown to contribute to spinal cord hyper-excitability and increased nociception [for example, see (Braz, Wang, Guan, Rubenstein, & Basbaum, 2015)]. It is therefore possible that the observed changes (or lack thereof) of Nr2b levels in our male and female mice simply reflect a shift of balance between the excitatory and inhibitory drives in the spinal cord following tibia fracture.
There are many factors that could account for the sex differences observed in our model. One possibility is the presence of faster regenerative mechanisms in males compared to females; for instance, proteomic analysis in control male vs. female CD1 mice demonstrates higher levels of myosin and actin protein expression in males, which could be implicated in differing rates of axonal transport and sensory plasticity (Vacca et al., 2014). Another possibility is the sex-dependent differential excitation of primary afferents; for example, in the formalin model of pain, female mice show increase in SP release during phase 2 of the formalin response compared to males (Nazarian, Tenayuca, Almasarweh, Armendariz, & Are, 2014). This could be an interesting target to pursue, since SP has been shown to be an important signaling molecule in the development of many of the signs and symptoms observed in our rodent fracture model of CRPS (Guo et al., 2012; Wei et al., 2012; Wei, Li, et al., 2009).
Additionally, the peripheral autoimmune responses observed during the acute post-fracture period, including mast cell accumulation (Li et al., 2012) and autoantibody production (Li et al., 2014) could differ between the 2 sexes, especially since autoimmune diseases commonly affect females at a rate significantly exceeding males. Finally, gonadal hormones could be key players in chronic pain, since both testosterone (and/or its metabolites) (Calabrese et al., 2014) and estrogen can be involved in the perception and chronification of pain (Arevalo, Santos-Galindo, Bellini, Azcoitia, & Garcia-Segura, 2010; Garcia-Segura & Melcangi, 2006; Papka et al., 2001; Shughrue, Lane, & Merchenthaler, 1997; Vanderhorst, Gustafsson, & Ulfhake, 2005), despite that fact that no association has been observed between estrogen exposure and CRPS in female patients (de Mos, Huygen, Stricker, Dieleman, & Sturkenboom, 2009). Nonetheless, the use of overiectomized mice could be useful in evaluating CRPS-related sex differences in future investigations.
The current study has several limitations, including those inherent to using animal models of pain and the translational value of the data acquired. While potentially important differences in nociception and memory were identified, more rigorous testing will be required to define the scope of those changes and the similarities or differences from human CRPS patients. Our studies do not address the possibility that prevalence of CRPS is higher in women than in men due in part to a higher incidence of limb trauma in women, e.g. a larger number of fractures due to osteoporosis. Still, the overall set of results we have collected are generally consistent with clinical and epidemiological observations. Use of the fracture/cast rodent model of CRPS may provide us opportunities to understand sex-related differences in the intensity and duration of CRPS, an important and debilitating chronic pain condition.
Highlights.
CRPS is characterized by severe pain and associated cognitive dysfunction
CRPS exhibits high female prevalence; females are affected 3 times more than males
Pre-clinically, female mice show lower nociceptive thresholds compared to males
Female mice show deficits in fear memory and signs of latent sensitization
These behavioral differences are associated with changes in spinal Nr2b levels
ACKNOWLEDGEMENTS
This study was supported by National Institute of Health grant NS072168 to WSK and JDC, and Veterans Affairs Merit Review grant BX-0024-71 to TTH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors report no conflict of interest.
REFERENCES
- Alabas OA, Tashani OA, Tabasam G, Johnson MI. Gender role affects experimental pain responses: a systematic review with meta-analysis. European journal of pain. 2012;16(9):1211–1223. doi: 10.1002/j.1532-2149.2012.00121.x. doi: 10.1002/j.1532-2149.2012.00121.x. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Perreault MJ, Reichenberger ER, Schwartzman RJ. Changes in immune and glial markers in the CSF of patients with Complex Regional Pain Syndrome. Brain Behav Immun. 2007;21(5):668–676. doi: 10.1016/j.bbi.2006.10.009. doi: 10.1016/j.bbi.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim Biophys Acta. 2010;1800(10):1106–1112. doi: 10.1016/j.bbagen.2009.10.002. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Barad MJ, Ueno T, Younger J, Chatterjee N, Mackey S. Complex regional pain syndrome is associated with structural abnormalities in pain-related regions of the human brain. J Pain. 2014;15(2):197–203. doi: 10.1016/j.jpain.2013.10.011. doi: 10.1016/j.jpain.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Hans G, Dickenson A. Peripheral input and its importance for central sensitization. Ann Neurol. 2013 doi: 10.1002/ana.24017. doi: 10.1002/ana.24017. [DOI] [PubMed] [Google Scholar]
- Braz JM, Wang X, Guan Z, Rubenstein JL, Basbaum AI. Transplant-mediated enhancement of spinal cord GABAergic inhibition reverses paclitaxel-induced mechanical and heat hypersensitivity. Pain. 2015;156(6):1084–1091. doi: 10.1097/j.pain.0000000000000152. doi: 10.1097/j.pain.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler S, Jonzon B, Branting-Ekenback C, Wadell C, Farahmand B. Predictors of severe pain in a cohort of 5271 individuals with self-reported neuropathic pain. Pain. 2012 doi: 10.1016/j.pain.2012.10.001. doi: 10.1016/j.pain.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Calabrese D, Giatti S, Romano S, Porretta-Serapiglia C, Bianchi R, Milanese M, Melcangi RC. Diabetic neuropathic pain: a role for testosterone metabolites. J Endocrinol. 2014;221(1):1–13. doi: 10.1530/JOE-13-0541. doi: 10.1530/JOE-13-0541. [DOI] [PubMed] [Google Scholar]
- Chaban V, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. Journal of neuroscience research. 2011;89(11):1707–1710. doi: 10.1002/jnr.22718. doi: 10.1002/jnr.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- D'Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101(1):8–16. doi: 10.1093/bja/aen088. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- Dannecker EA, Liu Y, Rector RS, Thomas TR, Fillingim RB, Robinson ME. Sex differences in exercise-induced muscle pain and muscle damage. The journal of pain : official journal of the American Pain Society. 2012;13(12):1242–1249. doi: 10.1016/j.jpain.2012.09.014. doi: 10.1016/j.jpain.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JR, Vlaeyen JW, de Gelder JM, Patijn J. Pain-related fear, perceived harmfulness of activities, and functional limitations in complex regional pain syndrome type I. J Pain. 2011;12(12):1209–1218. doi: 10.1016/j.jpain.2011.06.010. doi: 10.1016/j.jpain.2011.06.010. [DOI] [PubMed] [Google Scholar]
- de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129(1-2):12–20. doi: 10.1016/j.pain.2006.09.008. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- de Mos M, Huygen FJ, Stricker BH, Dieleman JP, Sturkenboom MC. Estrogens and the risk of complex regional pain syndrome (CRPS) Pharmacoepidemiol Drug Saf. 2009;18(1):44–52. doi: 10.1002/pds.1683. doi: 10.1002/pds.1683. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Rutkowski MD. Gender differences in rat neuropathic pain sensitivity is dependent on strain. Neuroscience letters. 2000;282(3):197–199. doi: 10.1016/s0304-3940(00)00880-6. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27(35):9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. The European journal of neuroscience. 2001;13(12):2227–2233. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- Finch PM, Knudsen L, Drummond PD. Reduction of allodynia in patients with complex regional pain syndrome: A double-blind placebo-controlled trial of topical ketamine. Pain. 2009;146(1-2):18–25. doi: 10.1016/j.pain.2009.05.017. doi: 10.1016/j.pain.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Gallagher JJ, Tajerian M, Guo T, Shi X, Li W, Zheng M, Clark JD. Acute and chronic phases of complex regional pain syndrome in mice are accompanied by distinct transcriptional changes in the spinal cord. Mol Pain. 2013;9:40. doi: 10.1186/1744-8069-9-40. doi: 10.1186/1744-8069-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Melcangi RC. Steroids and glial cell function. Glia. 2006;54(6):485–498. doi: 10.1002/glia.20404. doi: 10.1002/glia.20404. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108(1-2):95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Wei T, Shi X, Li WW, Hou S, Wang L, Kingery WS. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Mol Pain. 2012;8:85. doi: 10.1186/1744-8069-8-85. doi: 10.1186/1744-8069-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich J, Alvarez P, Joseph EK, Ferrari LF, Chen X, Levine JD. In Vivo and in Vitro Comparison of Female and Male Nociceptors. The journal of pain : official journal of the American Pain Society. 2012;13(12):1224–1231. doi: 10.1016/j.jpain.2012.09.009. doi: 10.1016/j.jpain.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand ME, Pitcher GM, Harding EK, Li H, Beggs S, Salter MW. GluN2B and GluN2D NMDARs dominate synaptic responses in the adult spinal cord. Sci Rep. 2014;4:4094. doi: 10.1038/srep04094. doi: 10.1038/srep04094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Adams MC. Sex, gender, and pain: an overview of a complex field. Anesth Analg. 2008;107(1):309–317. doi: 10.1213/01.ane.0b013e31816ba437. doi: 10.1213/01.ane.0b013e31816ba437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12(11):1364–1366. doi: 10.1038/nn.2407. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Guo TZ, Li XQ, Kingery WS, Clark JD. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain. 2010;151(3):843–852. doi: 10.1016/j.pain.2010.09.026. doi: 10.1016/j.pain.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Guo TZ, Liang DY, Sun Y, Kingery WS, Clark JD. Substance P signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology. 2012;116(4):882–895. doi: 10.1097/ALN.0b013e31824bb303. doi: 10.1097/ALN.0b013e31824bb303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Guo TZ, Shi X, Czirr E, Stan T, Sahbaie P, Clark JD. Autoimmunity contributes to nociceptive sensitization in a mouse model of complex regional pain syndrome. Pain. 2014;155(11):2377–2389. doi: 10.1016/j.pain.2014.09.007. doi: 10.1016/j.pain.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Sabsovich I, Guo TZ, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain. 2009;144(3):303–313. doi: 10.1016/j.pain.2009.04.033. doi: 10.1016/j.pain.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Schwartzman RJ, Eppig J, Wambach D, Brahin E, Peterlin BL, Kalanuria A. Neuropsychological deficits associated with Complex Regional Pain Syndrome. J Int Neuropsychol Soc. 2010;16(3):566–573. doi: 10.1017/S1355617710000214. doi: 10.1017/S1355617710000214. [DOI] [PubMed] [Google Scholar]
- Millecamps M, Tajerian M, Naso L, Sage EH, Stone LS. Lumbar intervertebral disc degeneration associated with axial and radiating low back pain in ageing SPARC-null mice. Pain. 2012;153(6):1167–1179. doi: 10.1016/j.pain.2012.01.027. doi: 10.1016/j.pain.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Graham AC, Ritchie J, Hughes SF, Austin JS, Schorscher-Petcu A, Bennett GJ. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol Pain. 2010;6:34. doi: 10.1186/1744-8069-6-34. doi: 10.1186/1744-8069-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32(17):5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Tenayuca JM, Almasarweh F, Armendariz A, Are D. Sex differences in formalin-evoked primary afferent release of substance P. Eur J Pain. 2014;18(1):39–46. doi: 10.1002/j.1532-2149.2013.00346.x. doi: 10.1002/j.1532-2149.2013.00346.x. [DOI] [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Shupnik M. Estrogen receptor-alpha and beta-immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304(2):193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain. 2012a;153(3):602–618. doi: 10.1016/j.pain.2011.11.025. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and pain perception - part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain. 2012b;153(3):619–635. doi: 10.1016/j.pain.2011.11.026. doi: 10.1016/j.pain.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32(12):611–618. doi: 10.1016/j.tins.2009.07.007. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahbaie P, Sun Y, Liang DY, Shi XY, Clark JD. Curcumin treatment attenuates pain and enhances functional recovery after incision. Anesth Analg. 2014;118(6):1336–1344. doi: 10.1213/ANE.0000000000000189. doi: 10.1213/ANE.0000000000000189. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147(1-3):107–115. doi: 10.1016/j.pain.2009.08.015. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology. 1990;40(1):57–61. doi: 10.1212/wnl.40.1.57. [DOI] [PubMed] [Google Scholar]
- Shiokawa H, Kaftan EJ, MacDermott AB, Tong CK. NR2 subunits and NMDA receptors on lamina II inhibitory and excitatory interneurons of the mouse dorsal horn. Mol Pain. 2010;6:26. doi: 10.1186/1744-8069-6-26. doi: 10.1186/1744-8069-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sigtermans MJ, van Hilten JJ, Bauer MC, Arbous MS, Marinus J, Sarton EY, Dahan A. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145(3):304–311. doi: 10.1016/j.pain.2009.06.023. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li XQ, Sahbaie P, Shi XY, Li WW, Liang DY, Clark JD. miR-203 regulates nociceptive sensitization after incision by controlling phospholipase A2 activating protein expression. Anesthesiology. 2012;117(3):626–638. doi: 10.1097/ALN.0b013e31826571aa. doi: 10.1097/ALN.0b013e31826571aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajerian M, Leu D, Zou Y, Sahbaie P, Li W, Khan H, Clark JD. Brain neuroplastic changes accompany anxiety and memory deficits in a model of complex regional pain syndrome. Anesthesiology. 2014;121(4):852–865. doi: 10.1097/ALN.0000000000000403. doi: 10.1097/ALN.0000000000000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall JM, Crisp T. Effects of gender and gonadal hormones on nociceptive responses to intraplantar carrageenan in the rat. Neuroscience letters. 2004;354(3):239–241. doi: 10.1016/j.neulet.2003.09.081. [DOI] [PubMed] [Google Scholar]
- Vacca V, Marinelli S, Pieroni L, Urbani A, Luvisetto S, Pavone F. Higher pain perception and lack of recovery from neuropathic pain in females: a behavioural, immunohistochemical, and proteomic investigation on sex-related differences in mice. Pain. 2014;155(2):388–402. doi: 10.1016/j.pain.2013.10.027. doi: 10.1016/j.pain.2013.10.027. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, Vry JD, Schiene K, Tzschentke TM. Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain. 2008;136(3):373–379. doi: 10.1016/j.pain.2007.07.027. doi: 10.1016/j.pain.2007.07.027. [DOI] [PubMed] [Google Scholar]
- van Gulik L, Janssen LI, Ahlers SJ, Bruins P, Driessen AH, van Boven WJ, Knibbe CA. Risk factors for chronic thoracic pain after cardiac surgery via sternotomy. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011;40(6):1309–1313. doi: 10.1016/j.ejcts.2011.03.039. doi: 10.1016/j.ejcts.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488(2):152–179. doi: 10.1002/cne.20569. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012–1016. doi: 10.1016/0140-6736(93)92877-v. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Yezierski RP. Comparison of operant escape and reflex tests of nociceptive sensitivity. Neurosci Biobehav Rev. 2015;51C:223–242. doi: 10.1016/j.neubiorev.2015.01.022. doi: 10.1016/j.neubiorev.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Wagner R, DeLeo JA, Coombs DW, Myers RR. Gender differences in autotomy following sciatic cryoneurolysis in the rat. Physiology & behavior. 1995;58(1):37–41. doi: 10.1016/0031-9384(95)00037-j. [DOI] [PubMed] [Google Scholar]
- Wei T, Guo TZ, Li WW, Hou S, Kingery WS, Clark JD. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. J Neuroinflammation. 2012;9:181. doi: 10.1186/1742-2094-9-181. doi: 10.1186/1742-2094-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2009;144(3):278–286. doi: 10.1016/j.pain.2009.04.020. doi: 10.1016/j.pain.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. European journal of pain. 2009;13(3):253–262. doi: 10.1016/j.ejpain.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2(3):137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]