Abstract
Background
Inhaled corticosteroids are the most commonly used controller therapies for asthma, producing treatment responses in six clinical phenotypes; lung function, bronchodilator response, airway responsiveness, symptoms, need for oral steroids and frequency of emergency department visits and hospitalizations. We hypothesize that treatment response in all of these phenotypes is modulated by a single, quantative corticosteroid responsiveness endophenotype.
Objective
To develop a composite phenotype that combines multiple clinical phenotypes to measure corticosteroid responsiveness with high accuracy, high stability across populations, and high robustness to missing data.
Methods
We employed principal component analysis (PCA) to determine a composite corticosteroid responsiveness phenotype that we tested in four replication populations. We evaluated the relative accuracy with which the composite and clinical phenotypes measure the endophenotype using treatment effect area under the receiver operating characteristic curve (AUC).
Results
In the study population, the composite phenotype measured the endophenotype with an AUC of 0.74, significantly exceeding the AUCs of the six individual clinical phenotypes, which ranged from 0.56 (p-value <.001) to 0.67 (p-value 0.015). In four replication populations with a total of 22 clinical phenotypes available, the composite phenotype AUC ranged from 0.69 to 0.73, significantly exceeded the AUCs of 14 phenotypes, and was not significantly exceeded by any single phenotype.
Conclusion
The composite phenotype measured the endophenotype with higher accuracy, higher stability across populations, and higher robustness to missing data than any clinical phenotype. This should provide the capability to model corticosteroid pharmacologic response and resistance with increased accuracy and reproducibility.
Keywords: asthma, corticosteroids, drug therapy, endophenotype, pharmacogenetics, pharmacologic response
Introduction
Inhaled corticosteroids (ICS) are the most commonly used [1] [2] and efficacious controller therapies for asthma. Variation in treatment response to ICS has been well identified, and within-person variation in ICS treatment response is highly repeatable [3]. ICS treatment response has a genetic basis using the change in lung function as the clinical phenotype [4], [5]. Current asthma guidelines characterize treatment response in terms of lung function, symptoms, or exacerbations [2]. ICS also produces significant treatment response in bronchodilator response and airway responsiveness [6].
Non-response to corticosteroids is a common clinical problem with up to 24% of patients with severe asthma who take oral corticosteroids fail to respond with a >15% improvement in prebronchodilator FEV1 [7]. Identifying steroid non-response is clinically difficult, rendering it under-recognized, even though steroid non-response poses a significant patient risk as patients are more likely to experience adverse outcomes. When patients prescribed ICS experience adverse outcomes, clinicians often attribute these problems to environmental triggers or medication non-adherence. In reality, these patients may share a group of common phenotypes that suggest steroid non-response.
For 15 years, asthma researchers have considered these phenotypes individually; however this approach presents significant problems. For example, in pharmacogenetic modeling, researchers aim to define an interaction between a genomic feature or variation and response to a particular pharmacological agent. When studying the pharmacogenetics of corticosteroid response in asthma, there are many potential pharmacologic responsiveness phenotypes to choose from. Choosing a specific phenotype is typically based on the characteristics of the cohort available. Nevertheless, this choice carries with it many repercussions for the researcher. Different clinical phenotypes will have differing statistical power; different phenotypes will have different rates of missing data; most importantly, different phenotypes will be assessed in other cohorts with varying frequencies, leading to difficulties in replication of the pharmacogenetic findings. As a result of such factors, the choice of a single clinical phenotype to characterize ICS treatment response is problematic.
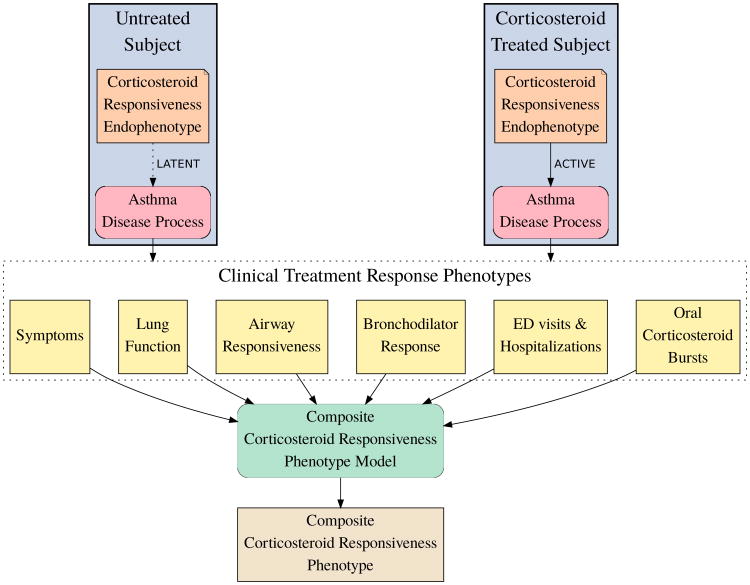
We propose to move away from the focus on single phenotypes to a more holistic approach. We hypothesize that a single, quantative corticosteroid responsiveness endophenotype modulates the asthma disease process (Figure 1). The endophenotype is latent in untreated subjects and active in ICS treated subjects. Under this hypothesis, clinical treatment response phenotypes are not regulated by separate mechanisms but instead by this endophenotype that influences the asthma disease process to produce the treatment effect observed in all clinical phenotypes. If this hypothesis is true, the endophenotype should characterize steroid response better than individual phenotypes. For the purpose of pharmacologic modeling, our objective is to measure this endophenotype as accurately as possible in each subject. We interpret the various clinical phenotypes as indirect measurements of the endophenotype, which display varying accuracy between different phenotypes and across populations. We propose a composite corticosteroid responsiveness model that combines clinical phenotypes to produce a composite phenotype that measures the endophenotype with higher accuracy, more stability across populations, and more robustness to missing data than any single clinical phenotype. Our objective in the current work is to develop such a composite corticosteroid responsiveness phenotype for subjects with mild to moderate asthma.
Figure 1.
Measuring corticosteroid responsiveness. We hypothesize a corticosteroid responsiveness endophenotype that modulates the asthma disease process, is latent in untreated subjects, and active in ICS treated subjects. We propose a composite corticosteroid responsiveness phenotype model that combines six clinical phenotypes into a composite phenotype to measure the endophenotype.
Methods
Study Design
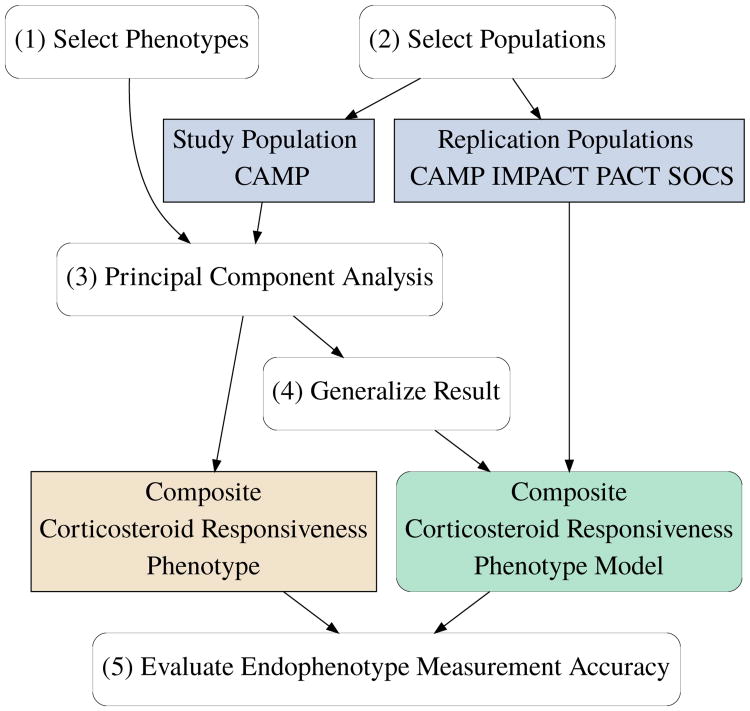
We designed this study (Figure 2) to have the following steps: (1) Select clinical phenotypes known to exhibit significant treatment response. (2) Select a well-powered, representative study population and suitable replication populations. (3) Use PCA to determine the composite corticosteroid responsiveness phenotype in the study population. (4) Generalize the study population result to a composite corticosteroid responsiveness phenotype model and test in replication populations. (5) Evaluate & compare the relative endophenotype measurement accuracy of all phenotypes.
Figure 2.
Study design. We selected clinical phenotypes known to exhibit significant treatment response and representative study and replication populations. We used PCA to determine the composite corticosteroid responsiveness phenotype in the study population. We generalized this result to produce the composite phenotype model for testing in replication populations. We evaluated the relative measurement accuracy of all phenotypes.
Clinical Phenotypes
We selected six clinical phenotypes that display statistically significant ICS treatment response in mild to moderate asthma: symptoms, lung function, airway responsiveness, bronchodilator response, emergency department (ED) visits/hospitalizations, and oral corticosteroid bursts. We defined these phenotypes and determined values for each subject phenotype by performing simple linear regression of clinical observations as described in Online Repository.
Populations
We selected study and replication populations from among the cohorts of the Single-Nucleotide Polymorphism Health Association-Asthma Resource Project (SHARP). SHARP consolidates clinical trial data from three National Heart, Lung, Blood Institute (NHLBI)-sponsored asthma clinical research networks: the Childhood Asthma Management Program (CAMP) [6], the Childhood Asthma Research and Education (CARE) network [8] [9], and the Asthma Clinical Research Network (ACRN) [10] [11] [12].
We selected populations from cohorts that contained a treatment group that received ICS and a treatment group that did not receive ICS. In each population, we created two groups: The ICS group contained subjects who received ICS or a combination therapy including ICS. The “not ICS” group contained subjects who received placebo or a non-ICS drug such as a leukotriene antagonist. Clinical observations were taken from the SHARP phs000166.v2.p1 dbGaP[13] dataset, with the exception of CAMP symptoms, which were taken directly from CAMP trial datasets, as the CAMP symptom data was found to be incomplete in the dbGaP dataset.
Endophenotype Measurement Accuracy
We evaluated the relative accuracy with which the composite and clinical phenotypes measure the endophenotype using treatment effect area under the receiver operating characteristic curve (AUC). The rationale for this choice of statistic and methods used are described in the Online Repository.
Composite Corticosteroid Responsiveness Model
We determined a composite corticosteroid responsiveness phenotype from the pattern of treatment response exhibited by the study population. We selected an unsupervised method, principal component analysis (PCA), with the expectation that the endophenotype would primarily modulate the first principal component (PC1) of treatment response, which would comprise a composite phenotype measuring the endophenotype accurately.
We determined clinical phenotypes as described in the Online Repository. After discarding subjects for whom phenotypes were missing we performed PCA on the remaining complete sets of six phenotypes with scaling to unit variance using the R version 3.1.0 base stats package prcomp function. We determined the treatment effect AUC of principal components and clinical phenotypes. We set the comparison direction for clinical phenotypes based on clinical experience. Since the sign of components produced by PCA is arbitrary, we let the pROC package automatically determine the comparison direction for principal components. We analyzed the covariance of PC1 with the covariates typically used in the study of asthma, gender and age.
We generalized the study population result into a population-independent composite corticosteroid responsiveness model that we tested in each replication population. Clinical phenotypes were determined as described in the Online Repository and treatment effect AUCs calculated. Missing phenotypes were imputed by being set equal to the center value of the respective phenotype in the study population. The six phenotypes were then centered and scaled using the study population PCA center and scale coefficients, multiplied by the study population PC1 loading coefficients, and summed to produce the composite phenotype value. Finally, composite phenotype treatment effect AUC was determined.
Sensitivity to Missing Clinical Phenotypes
We investigated the sensitivity of the composite phenotype to missing component phenotypes by forming a pooled replication population comprised of all subjects found in the replication populations that possessed a complete set of clinical phenotypes. We determined the treatment effect AUC of each clinical phenotype for this pooled population. For all possible combinations of 0, 1, 2, 3, or 4 phenotypes missing from this population, we determined the composite phenotype, its treatment effect AUC, and single-sided p-values with respect to each clinical phenotype used in its calculation.
Results
Population Characteristics
The study population consisted of Caucasian children from CAMP. Four replication populations were selected from CAMP, CARE, and ACRN (Table 1). The CAMP replication population consisted of non-Caucasian children. The replication populations included both children (CAMP and PACT) and adults (SOCS and IMPACT). Subjects in the populations had relatively normal lung function. In each population subjects used a single corticosteroid inhaler drug. Collectively the populations represent a variety of different corticosteroid inhalers. Bronchodilator response was relatively consistent across the populations, but airway responsiveness and symptoms were more variable. To increase sample size we included non-ICS drugs in the non-ICS group and ICS combination therapy in the ICS group. In the SOCS population, we grouped long-acting beta2-adrenergic receptor agonist therapy (salmeterol) with placebo. In PACT we grouped salmeterol/ICS combination therapy with ICS-only therapy. In PACT, where placebo treatment was not available, the non ICS group consisted of subjects treated with a leukotriene receptor antagonist (montelukast).
Table 1. Population characteristics.
| Population | CAMP Study | CAMP Replication | PACT Replication | SOCS Replication | IMPACT Replication |
|---|---|---|---|---|---|
| Trial | CAMP | CAMP | PACT | SOCS | IMPACT |
| Network | CAMP | CAMP | CARE | ACRN | ACRN |
| N | 388 | 188 | 142 | 56 | 80 |
| Race(s) | Caucasian | African American, Hispanic, Other | Caucasian | Caucasian | Caucasian |
| Age (SD) | 8.8 (2.1) | 9.2 (2.1) | 9.8 (2.2) | 30 (9.8) | 34 (11) |
| Sex (male) | 228 (59%) | 112 (60%) | 92 (65%) | 17 (30%) | 31 (39%) |
| ICS Treatment(s) | Budesonide | Budesonide | Fluticasone, salmeterol/fluticasone combination | Triamcinolone | Budesonide* |
| not ICS Treatment(s) | Placebo | Placebo | Montelukast | Placebo, salmeterol | Placebo* |
| Duration (weeks) | 207 | 207 | 57 | 30 | 60 |
| N ICS Group | 164 | 90 | 92 | 17 | 45 |
| N not ICS Group | 224 | 98 | 50 | 39 | 35 |
| FEVPPB (SD) | 95 (14) | 91 (14) | 98 (12) | 87 (15) | 88 (13) |
| LNPC20B (SD) | 0.048 (1.2) | 0.058 (1.1) | -0.3 (1.3) | 0.61 (0.57) | 0.85 (0.64) |
| BDRB (SD) | 11 (9.9) | 11 (9.8) | 8.4 (7.1) | Not Available | 9.8 (7.7) |
| SYMB (SD) | 0.63 (0.45) | 0.56 (0.43) | 0.59 (0.35) | Not Available | 0.18 (0.28) |
Definitions: CAMP = Childhood Asthma Management Program; PACT = Pediatric Asthma Controller Trial; IMPACT = The Improving Asthma Control Trial; SOCS = Salmeterol Or Corticosteroids Study; CARE = Childhood Asthma Research and Education; ACRN = Asthma Clinical Research Network; N ICS Group = Subjects treated with ICS or a combination therapy including ICS; N not ICS Group = Subjects treated with placebo, or a non-ICS therapy; SD = standard deviation; FEVPPB = FEV1 percent predicted at baseline; LNPC20B = natural log PC20 at baseline; BDRB = bronchodilator percent change at baseline; SYMB = average am symptoms as recorded in daily diary card at baseline.
IMPACT subjects had access to open-label budesonide as part of a symptom-based action plan and were subjected to a 10-to-14-day period of intense combined therapy that included ICS and oral steroids at the end of run-in and treatment phases of the study.
Study Population
We determined clinical phenotypes for the CAMP study population as described in the Online Repository (Figure 3 shows phenotypes for one subject). We discarded subjects with missing phenotypes (Online Repository, Table E1) and performed principal component analysis on 327 (ICS = 141, notICS = 186) subjects. The first principal component (PC1) accounted for 30% of the variance (Online Repository, Table E2).
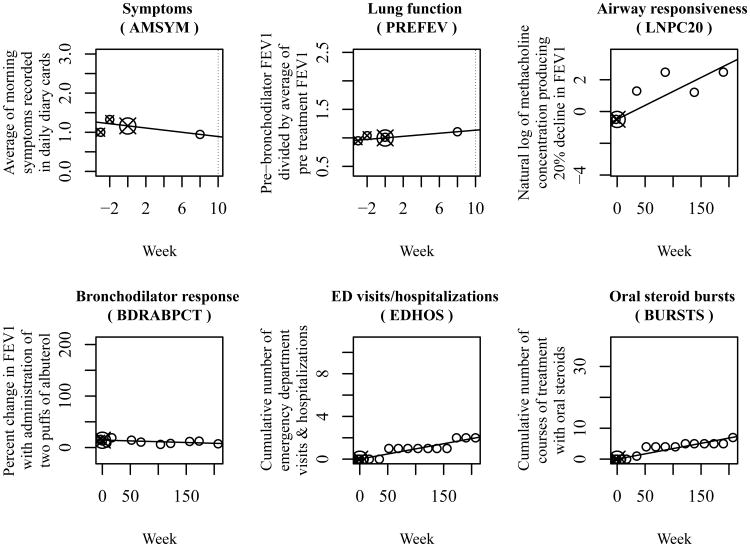
Figure 3.
Clinical treatment response phenotypes for one CAMP study participant. We determined the value of each phenotype by performing linear regression of clinical observations versus time from start of treatment. We constrained the regression lines to pass through the average (target) of pre-treatment values (small targets) at time 0. We interpreted the slopes of these lines as the phenotype values.
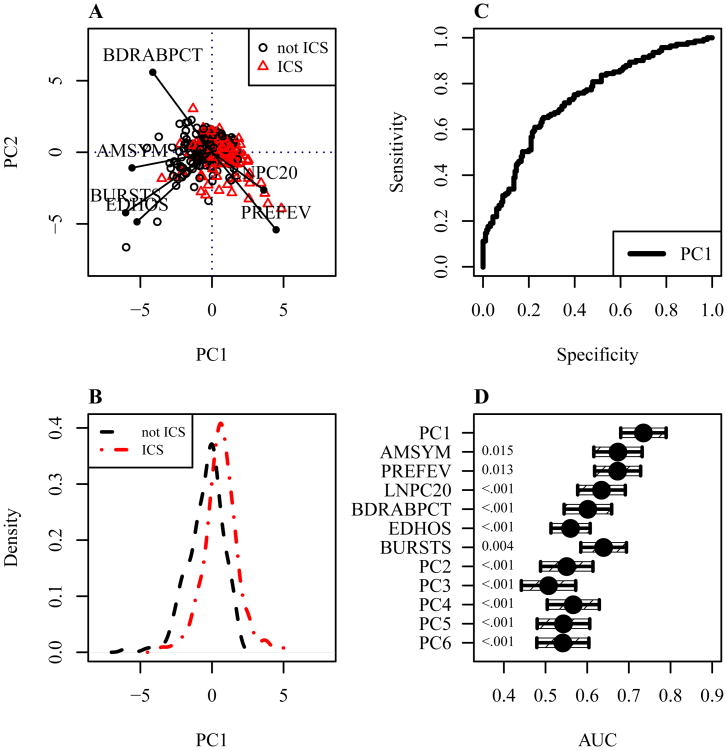
Figure 4A shows the first (PC1) and second (PC2) principal components of ICS treatment response for subjects in the ICS treatment group (red triangle) and not ICS treatment group (black circles). Lines indicate the direction and strength with which each clinical phenotype contributed to PC1 and PC2. Increasing corticosteroid responsiveness, as measured by PC1, was most closely aligned with decreasing symptoms (AMSYM), and also associated with decreasing bronchodilator response (BDRABPCT), decreasing cumulative ED visits/hospitalizations (EDHOS), decreasing cumulative oral corticosteroid bursts (BURSTS), increasing lung function (PREFEV), and decreasing airway responsiveness (increasing LNPC20).
Figure 4.
Learning the composite phenotype in the study population. A. The first (PC1) and second (PC2) principal components of ICS treatment response. B. The distribution of PC1 in treatment groups. C. PC1 receiver operating characteristic curve (AUC = .74). D. AUCs of PC1, clinical phenotypes, and other components, with 95% confidence intervals (single-sided p-values are shown along the left axis).
Figure 4B shows the distribution of the composite phenotype, PC1, in ICS treatment group (red dash dot line) and not ICS treatment group (black dashed line). The distribution of PC1 in the treatment groups was reasonably normal.
Figure 4C shows the treatment effect receiver operating characteristic curve for PC1. ROC analysis of PC1 determined a treatment effect AUC of 0.74, which significantly exceeded the AUC of all clinical phenotypes (Figure 4D), which ranged from 0.56 for ED visits/hospitalizations (p-value <.001) to 0.67 for symptoms (p-value 0.015), and significantly exceeded the AUC of all other principal components, which ranged from 0.51 for PC3 to 0.57 for PC4 (all with p-value <.001). The PCA center, scale, and loading coefficients associated with PC1 (Online Repository Table E3) were saved for use in the composite phenotype model. An analysis of the covariance of PC1 with gender and age indicated no significant effects (Online Repository Tables E4 and E5).
Replication Populations
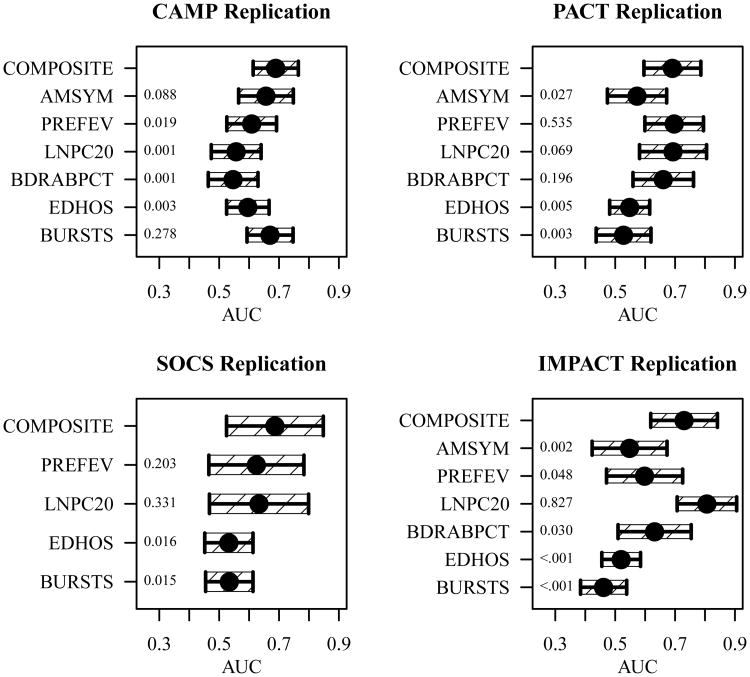
In replication populations, missing clinical observations were generally minimal (Online Repository, Table E1), with the following exceptions; the CAMP replication population was missing 23.9% of symptoms, and the SOCS population did not include symptoms or bronchodilator observations. We determined clinical phenotypes as described in the Online Repository, with the exception of the lung function window for IMPACT being increased to 18 weeks to include observations from the 15-week follow up visit. Composite phenotypes were determined as described above. The composite phenotype AUC was stable across the replication populations, ranging from 0.69 to 0.73, whereas the clinical measure phenotype AUCs were more variable, ranging from 0.46 to 0.81 (Figure 5). The composite phenotype AUCs significantly exceeded the AUCs of 14 of the 22 available clinical phenotypes (single-sided p-values are shown along the left axes of Figure 5), exceeding airway responsiveness in 1 of 4, lung function in 2 of 4, symptoms in 2 of 3, bronchodilator response in 2 of 3, Oral corticosteroid bursts in 3 of 4, and ED visits/hospitalizations in 4 of 4 replication populations. Conversely, no clinical phenotype in any population measured the endophenotype with significantly higher relative accuracy.
Figure 5.
Composite phenotype performance in the replication populations. A comparison of the relative accuracy (AUC) with which the corticosteroid responsiveness endophenotype was measured by the composite (COMPOSITE), symptoms (AMSYM), lung function (PREFEV) airway responsiveness (LNPC20) bronchodilator response (BDRABPCT), ED visits/hospitalizations (EDHOS) and oral corticosteroid bursts (BURSTS) phenotypes with 95% confidence interval (single-sided p-values are shown along the left axis).
In the IMPACT replication population, airway responsiveness AUC (0.81) exceeded the composite phenotype AUC (0.73) approaching statistical significance (p-value 0.18). This may reflect outliers or confounding by the IMPACT trial's somewhat atypical design (Table 1). In the SOCS replication population, which contained the smallest ICS treatment group (N=17) and was missing 100% of symptoms and bronchodilator observations, the composite phenotype (AUC = 0.69) demonstrated excellent robustness to missing data and small population size.
Sensitivity to Missing Clinical Phenotypes
We created a pooled replication population as described earlier using all subject with complete sets of clinical phenotypes found in the replication populations (Online Repository, Table E6). For all possible combinations of up to 0, 1, 2, 3, or 4 phenotypes missing, we determined the composite phenotype AUC and the single-sided p-values (Online Repository, Table E7). The composite phenotype AUC was found to be significantly greater than the individual component clinical phenotype AUC in 144 of 186 cases, and in no case did a component clinical phenotype AUC exceed the composite phenotype AUC.
Discussion
The composite phenotype measured the corticosteroid responsiveness endophenotype significantly more accurately than any of the six clinical phenotypes individually. This should allow the study of corticosteroid pharmacology with increased accuracy and reproducibility. As an example, the composite phenotype should enable asthma pharmacogenetic studies that possess more power for a given sample size, or, for a given power, require a smaller sample. The composite phenotype also captured virtually the entire corticosteroid responsiveness signal, as evidenced by the low AUCs of the other principal components. This result is consistent with the hypothesis of a single endophenotype modulating corticosteroid response in all six clinical phenotypes.
In the replication populations, the composite phenotype demonstrated higher accuracy, higher stability, and robustness to missing data than any clinical phenotype alone. This suggests that the endophenotype is stable across the asthma populations explored in this study, and that studies performed using the composite phenotype will replicate more readily.
Our primary goal in selecting replication populations was to create ICS and non-ICS groups on which to apply our methods. Other equivalence between the replication populations was not an objective or requirement and that is why we did not present p-values in Table 1. The consistent performance of the composite phenotype despite the variability between the replication populations is a strength of the study result.
In the pooled replication population with 0, 1, 2, 3, and 4 clinical phenotypes assumed missing the composite phenotype performed as well as, and usually significantly better than, the individual clinical phenotypes. This suggests that there may be no penalty to using the composite phenotype in general and that it offers benefit when as few as two clinical phenotypes are available.
These results indicate that, for the purpose of corticosteroid pharmacogenetic study design, the addition of phenotypes can increase AUC. Since, for a given power, increases in AUC reduce sample size, such studies could be cost-optimized by trading off the costs of additional subjects and additional phenotypes.
The analysis of covariates indicated no significant gender or age effect in the CAMP study population subjects that ranged in age from 5.2 to 13.2 years at randomization and were followed for 4 years. This suggests that the corticosteroid responsiveness endophenotype is stable from age 5 to 17 years, and that an estimate of the endophenotype made at one point in childhood might reasonably be used later in childhood. This could be confirmed by an analysis of subjects tracked over a longer period of time, and ideally into adulthood.
Our results have clinical relevance. The composite corticosteroid responsiveness model produced more accurate measurements of ICS response than individual phenotypes in Caucasian replication populations of mild-moderately severe asthmatic children and adults and, for children, of multiple races. It performed robustly in child populations where budesonide or fluticasone was used and in adult populations where budesonide or triamcinolone was used. It reliably detected corticosteroid response in mono therapy or in combination with salmeterol. It accurately differentiated corticosteroid response from placebo and leukotriene receptor antagonist treatment control groups. Given that the composite model collapses multiple longitudinal clinical observations into an easily interpreted corticosteroid response metric, and that it can be easily implemented in a simple computer program, it may have potential for use in the clinical setting where it might allow the practitioner to more accurately estimate ICS response.
The idea of composite phenotypes is not new: composite indices have been used for 15 years in the assessment of therapeutic efficacy in clinical trials of rheumatoid arthritis [14], [15]. A number of composite score instruments of asthma control have also been proposed, generally focused on treatment responsiveness as a dichotomous outcome [16]. However, the approach used here is novel in several ways. First, we avoided the need for expert-based specification of model parameters or thresholds. Secondly, we developed a continuous composite phenotype that is a linear combination of treatment response phenotypes determined algorithmically from clinical observations. Thirdly, we developed an unbiased phenotype by using an unsupervised dimensionality reduction approach to learn composite phenotype coefficients from the pattern of response in the study population. Fourthly, we assessed the relative accuracy with which different phenotypes measure the corticosteroid responsiveness endophenotype. Finally, we arrived at a composite corticosteroid responsiveness phenotype with demonstrably higher accuracy. We did so using an approach that is free of subjective judgments, other than the choice of study population, selection of clinical measures, and choice of modeling approach.
Recent asthma phenotype research has focused on disease sub-phenotypes [17], [18]. We expect that the composite phenotype can be applied to asthma sub-phenotype populations, with the possible exception of corticosteroid-resistant asthma.
Approximately 30% of asthmatic subjects have been reported to not respond to ICS treatment [19]. The study of such non-responders could identify the cause of non response and lead to new therapies. The composite phenotype could be used to characterize such subjects with higher accuracy. It could be used directly to study variability in corticosteroid response, or, with appropriate thresholds, to dichotomize responders and non-responders. We believe that the success of our composite phenotype approach derives from a study population with good power, a treatment effect that manifests in multiple clinical measures, and the presence of a primary drug responsiveness endophenotype. Our approach could be productively applied in other situations were these conditions are met.
Other groups can apply the composite phenotype concept in two general ways: Asthma researchers can determine the value of the composite phenotype by determining clinical phenotypes using the regression method described and determining the composite phenotype by applying the model coefficients as described. Researchers studying treatment response for other drugs or conditions could determine a custom composite phenotype for a new cohort by determining clinical phenotypes using the regression method described and determining a composite phenotype by applying PCA as described.
Despite the strengths of this study, a few caveats deserve mention. Our study relied on clinical trial populations and hence might not be generalizable to asthmatics on corticosteroids in non-trial settings. Having actual glucocorticoid levels might have enhanced our endophenotype determination. Finally, we need to assess the endophenotype in genomic studies to assess its practical utility.
In conclusion, we developed a composite corticosteroid responsiveness phenotype model that measures the corticosteroid responsiveness endophenotype with higher accuracy and higher stability across populations than any single clinical measure phenotype. This new phenotype should allow the development of asthma pharmacologic models offering increased power and reproducibility.
Supplementary Material
Table E1. Missing Clinical Phenotypes (Percent)
Table E2. Principal Component Importance
Table E3. Composite Corticosteroid Responsiveness Phenotype Model Coefficients
Table E4. Covariates of PC1 in the CAMP Study Population
Table E5. Covariates of PC1 in the CAMP ICS Treatment Group of the CAMP Study Population
Table E6. Pooled replication population characteristics
Table E7. Sensitivity Study p-values
Key Messages.
Inhaled corticosteroids are the most commonly used controller therapies for asthma, producing treatment responses in six individual clinical phenotypes.
We hypothesize that, instead of being regulated by separate mechanisms, these responses are all regulated by a single corticosteroid responsiveness endophenotype.
A composite phenotype combining all clinical phenotypes measures corticosteroid responsiveness with higher accuracy, higher stability across populations, and higher robustness to missing data than the individual clinical phenotypes.
The composite phenotype offers the prospect for greater insight into corticosteroid response and resistance.
Acknowledgments
Source(s) of support: This work is supported by NHLBI via a K08 HL088046 (PI: Wu), U01 HL065899 (PI: Weiss and Tantisira).
Abbreviations
- ACRN
Asthma Clinical Research Network
- AMSYM
a clinical phenotype measuring the trend in average AM symptoms as recorded in daily diary card
- AUC
area under the receiver operating characteristic curve (also abbreviated AUROC, ROC, and aROC elsewhere)
- BDR
relative percent change in FEV1 with administration of two puffs of albuterol
- BDRABPCT
a clinical phenotype measuring the trend in BDR
- BURSTS
a clinical phenotype measuring the trend in cumulative number of courses of oral corticosteroid treatment
- CAMP
The Childhood Asthma Management Program
- CARE
Childhood Asthma Research and Education network
- EDHOS
a clinical phenotype measuring the trend in cumulative number of emergency department visits & hospitalizations
- FEV1
forced expiratory volume in 1 second
- ICS
inhaled corticosteroids
- IMPACT
The Improving Asthma Control Trial
- LNPC20
a clinical phenotype measuring the trend in the natural log of PC20
- PACT
Pediatric Asthma Controller Trial
- PC20
the provocative concentration of methacholine producing a 20% decline in FEV1
- PCA
principal component analysis
- PC1
first principal component
- PREFEV
a clinical phenotype measuring the trend in FEV1
- ROC
receiver operating characteristic
- SHARP
Single-Nucleotide Polymorphism Health Association-Asthma Resource Project
- SOCS
Salmeterol Or Corticosteroids Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global strategy for asthma management and prevention. Technical report, Global Initiative for Asthma (GINA) [Accessed November 10, 2014];2012 Available at http://www.ginasthma.org/local/uploads/files/GINA_Report_2010_1.pdf.
- 2.Technical report. National Heart, Lung, and Blood Institute; 2007. [Accessed November 10, 2014]. Guidelines for the diagnosis and management of asthma (epr-3) Available at http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf. [Google Scholar]
- 3.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56(4):1054–70. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 4.Weiss ST, Lake SL, Silverman ES, Silverman EK, Richter B, Drazen JM, et al. Asthma steroid pharmacogenetics: a study strategy to identify replicated treatment responses. Proc Am Thorac Soc. 2004;1(4):364–7. doi: 10.1513/pats.200409-043MS. [DOI] [PubMed] [Google Scholar]
- 5.Tantisira KG, Lake S, Silverman ES, Palmer LJ, Lazarus R, Silverman EK, et al. Corticosteroid pharmacogenetics: association of sequence variants in crhr1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004 Jul;13(13):1353–9. doi: 10.1093/hmg/ddh149. [DOI] [PubMed] [Google Scholar]
- 6.The childhood asthma management program research group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000 Oct;343(15):1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 7.Chan MT, Leung DY, Szefler SJ, Spahn JD. Difficult-to-control asthma: clinical characteristics of steroid-insensitive asthma. J Allergy Clin Immunol. 1998;101:594–601. doi: 10.1016/S0091-6749(98)70165-4. [DOI] [PubMed] [Google Scholar]
- 8.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005 Feb;115(2):233–42. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the pediatric asthma controller trial. J Allergy Clin Immunol. 2007 Jan;119(1):64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005 Apr;352(15):1519–28. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 11.Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF, Jr, Sorkness CA, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285(20):2583–93. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 12.Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, et al. The predicting response to inhaled corticosteroid efficacy (price) trial. J Allergy Clin Immunol. 2007 Jan;119(1):73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mailman MD, Feolo M, Jin Y, Kimura M, Tryka K, Bagoutdinov R, et al. The ncbi dbgap database of genotypes and phenotypes. Nat Genet. 2007 Oct;39(10):1181–6. doi: 10.1038/ng1007-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The american college of rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. the committee on outcome measures in rheumatoid arthritis clinical trials. Arthritis Rheum. 1993 Jun;36(6):729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde DM, van't Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993 Mar;20(3):579–81. [PubMed] [Google Scholar]
- 16.Cloutier MM, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, et al. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol. 2012 Mar;129(3 Suppl):S24–33. doi: 10.1016/j.jaci.2011.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung KF, Adcock IM. How variability in clinical phenotypes should guide research into disease mechanisms in asthma. Ann Am Thorac Soc. 2013 Dec;10(Suppl):S109–17. doi: 10.1513/AnnalsATS.201304-087AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung KF. Defining phenotypes in asthma: A step towards personalized medicine. Drugs. 2014 May; doi: 10.1007/s40265-014-0213-9. [DOI] [PubMed] [Google Scholar]
- 19.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? the gaining optimal asthma control study. Am J Respir Crit Care Med. 2004 Oct;170(8):836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table E1. Missing Clinical Phenotypes (Percent)
Table E2. Principal Component Importance
Table E3. Composite Corticosteroid Responsiveness Phenotype Model Coefficients
Table E4. Covariates of PC1 in the CAMP Study Population
Table E5. Covariates of PC1 in the CAMP ICS Treatment Group of the CAMP Study Population
Table E6. Pooled replication population characteristics
Table E7. Sensitivity Study p-values