Abstract
Monocyte and dendritic cell (DC) development was evaluated using in vivo BrdU pulse-chase analyses in rhesus macaques and phenotype analyses of these cells in blood also were assessed by immunostaining and flow cytometry for comparisons between rhesus, cynomolgus, and pigtail macaques as well as African green monkeys and humans. The nonhuman primate species and humans have three subsets of monocytes, CD14+CD16−, CD14+CD16+, and CD14−CD16+ cells that correspond to classical, intermediate, and non-classical monocytes, respectively. In addition, there exist presently two subsets of DC, BDCA-1+ myeloid DC and CD123+ plasmacytoid DC that were first confirmed rhesus macaque blood. Following BrdU inoculation, labeled cells first appeared in CD14+CD16− monocytes, then in CD14+CD16+ cells, and finally in CD14−CD16+ cells, thus defining different stages of monocyte maturation. A fraction of the classical CD14+CD16− monocytes gradually expressed CD16+ to become CD16+CD14+ cells and subsequently matured into the non-classical CD14−CD16+ cell subset. The differentiation kinetics of BDCA-1+ myeloid DC and CD123+ plasmacytoid DC were distinct from the monocyte subsets, indicating differences in their myeloid cell origins. Results from studies utilizing nonhuman primates provide valuable information about the turnover, kinetics and maturation of the different subsets of monocytes and DC using approaches that cannot readily be performed in humans and support further analyses to continue examining the unique myeloid cell origins that may be applied to address disease pathogenesis mechanisms and intervention strategies in humans.
INTRODUCTION
Blood monocytes and dendritic cells (DC) are bone marrow-derived leukocytes involved in innate immune responses to infection (1). Monocytes arise from myeloid progenitors within bone marrow, migrate into the blood circulation and may be induced to leave the circulation for differentiation into tissue macrophages and DC. In humans, three subsets of monocytes have been identified by differential expression of CD14 and CD16 (2, 3). Classical monocytes constitute the majority of monocytes in healthy individuals, and are strongly positive for CD14 and negative for CD16 (CD14+CD16−). Intermediate monocytes express high levels of both CD14 and CD16 (CD14+CD16+), and the non-classical monocytes express low levels of CD14 and high levels of CD16 (CD14−CD16+). Monocytes expressing CD16 account for only 5–15% of all monocytes during homeostasis but increase significantly during infectious diseases and inflammatory disorders (4–6).
Two functional populations of blood DC have been described and include myeloid DC (mDC) and plasmacytoid DC (pDC) based on precursor cells of origin (7, 8). Blood DC and monocytes express HLA-DR and are distinct from the leukocyte lineage cell fraction, but there is still confusion in clearly delineating DC subsets from monocytes due to a lack of specific cell surface markers (9). CD11c, for example, is often considered one of the myeloid DC markers, but it is also expressed at highest density on blood monocytes and at moderate levels on granulocytes in humans and mice (10, 11). In addition, the CD14−CD16+ monocytes in humans are currently classified as non-classical monocytes but this population overlaps with CD16+ myeloid DC (mDC) using a previously-reported blood DC gating strategy (12). Currently, human blood DC populations are defined by their lineage and expression of Blood Dendritic Cell Antigens (BDCA) (3). The pDC are identified by expression of BDCA-2 (CD303) while the mDC can be further subdivided by differential expression of either BDCA-1 (CD1c) or BDCA-3 (CD141) (3).
Nonhuman primates (NHP) are genetically and physiologically closely related to humans and thus serve as valuable models of human diseases and immune responses (13). An added advantage is that many antibodies to human monocytes, macrophages, and DC exhibit cross-reactivity to these cells from rhesus macaques (14, 15). In earlier studies, we successfully demonstrated that in vivo 5-bromo-2’-deoxyuridine (BrdU) pulse-chase experiments could be applied to monitor changes in the turnover rates of blood monocytes during viral and bacterial infections in rhesus macaques that were predictive for disease outcomes (16, 17). BrdU, a thymidine analogue, incorporates into hematopoietic progenitor cells possessing proliferating capacity in bone marrow and thus can be used as a tool to characterize differentiation of myeloid lineage cells in vivo. The purpose of this study therefore was to characterize the phenotype, turnover, and differentiation of monocytes and DC in rhesus macaques to expand our knowledge of the biology of myeloid cell development in humans. Such information is difficult to monitor longitudinally in humans and the results from studying rhesus macaques will contribute to better understanding mechanisms of disease pathogenesis and development of immune responses produced by monocytes, macrophages, and dendritic cells in humans.
MATERIALS AND METHODS
Nonhuman primates
Rhesus macaques (Macaca mulatta), cynomolgus macaques (Macaca fascicularis), pigtail macaques (Macaca nemestrina), and African green monkeys (Chlorocebus sabaeus) were housed at the Tulane National Primate Research Center were sampled for blood samples used in this study. In addition, blood was obtained from a set of rhesus macaques infected with 300 TCID50 units of SIVmac239 intravenously and treated with subcutaneous inoculations of combination anti-retrovirus therapy (cART) that comprised 20 mg/kg of tenofovir dispoproxil fumarate (PMPA) once daily and 40 mg/kg Lamivudine (3TC) once daily, a gift from Gilead, for antigen-specific cell proliferation assay. Animal facilities, husbandry, and procedures were approved by the Institutional Animal Care and Use Committee of Tulane University in compliance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Research Council.
Blood specimens and BrdU administration to measure cell turnover
Rhesus macaques were injected with the thymidine analogue, BrdU intravenously at a dose of 60 mg/kg body weight, and EDTA anti-coagulated blood samples were collected at varying times after administration as indicated in the results. BrdU incorporation to measure cell turnover rate was determined by antibody staining and flow cytometry. Blood from cynomolgus macaques, pigtail macaques, and African green monkeys were only used for phenotype analyses in this study.
Healthy human EDTA anti-coagulated blood samples were obtained from Research Blood Components (Boston, MA).
Flow cytometry
Blood specimens collected in EDTA anti-coagulant were washed once with at least five volumes of PBS containing 2% of FBS in PBS (2% FBS-PBS) and resuspended in the original volume of 2% FBS-PBS. Fresh blood specimens were stained for the evaluations in this study. There was negligible non-specific background staining, so the live/dead marker stain was not used here. Two hundred microliters of washed blood were stained for expression of surface markers by incubation with antibodies for 20 min at room temperature (see Table I for list of antibodies). Red blood cells were lysed with 1× FACS lysing solution (BD Biosciences) and the remaining cells were permeabilized using a three-step incubation procedure with Cytofix/Cytoperm (BD Biosciences) for 20 min, Perm/Wash buffer (BD Biosciences) supplemented with 10% dimethyl sulfoxide to boost permeabilization for 10 min (18) and Cytofix/Cytoperm for 5 min. For analysis of BrdU incorporation, cells were incubated with anti-BrdU antibody for 20 min at room temperature after one hr incubation at 37° C with DNase I (Roche diagnostics). After washing, all sets of cells were fixed with PBS containing 1% paraformaldehyde (Electron Microscopy Systems), acquired with an LSR II flow cytometer (BD Biosciences), and analyzed for cell surface phenotype using FlowJo software version 9.6 (TreeStar Inc). Live/dead marker was not used in this study
Table I.
Antibodies used in this study
| Markers | Clone | Source |
|---|---|---|
| CD1c (BDCA-1) | AD5-8E7 | Miltenyi |
| CD3 | SP34-2 | BD |
| CD8 | SK2 | BD |
| CD11b | ICRF44 | BD |
| CD11c | 3.9 | eBioscience |
| CD11c | S-HCL-3 | BD |
| CD14 | M5E2 | BD |
| CD16 | 3G8 | BD |
| CD20 | B9E9 | Beckman Coulter |
| CD45 | MB4-6D6 | Miltenyi |
| CD56 | B159 | BD |
| CD123 | 7G3 | BD |
| CD141 (BDCA-3) | ADH-14H12 | Miltenyi |
| CD163 | Mac2-158 | Trillium |
| HLA-DR | L243 | BD |
Antigen-specific cell proliferation assay
PBMC were enriched by Ficoll gradient centrifugation of blood obtained from rhesus macaques chronically infected with SIVmac239 and undergoing cART. These animals that controlled plasma viral loads below 1000 copies/ml and exhibited normal monocyte turnover and phenotype (data not shown) were selected for this study since they expressed SIV-specific cell proliferation and immune responses. Monocytes, DC subsets, and CD3+ T cells were sorted by flow cytometry using the BD FACS Aria (Figure 1). Each monocyte and DC population was incubated for 2 hr with SIVmac251 Gag PR55 protein (NIH AIDS Research & Reference Reagent Program) for stimulating antigen-specific CD3+ T cell proliferation ex vivo. After washing with RPMI 1640 supplemented with 10% FBS, 10000, 5000, and 2500 cells of Gag-pulsed monocytes or DC subsets were cultured with 1 × 105 CD3+ T cells in a 96-well U-bottom culture plate for 4.5 days. Another thymidine analogue, 5-ethynyl-2´-deoxyuridine (EdU) was added at a final concentration of 10 µM during the last 18 hr of the culture and cell proliferation was detected as a function of EdU incorporation using the Click-iT EdU Flow Cytometry Assay Kit (Life Technologies). Results were acquired using a FACS Verse flow cytometer (BD Biosciences) and data were analyzed by FlowJo software.
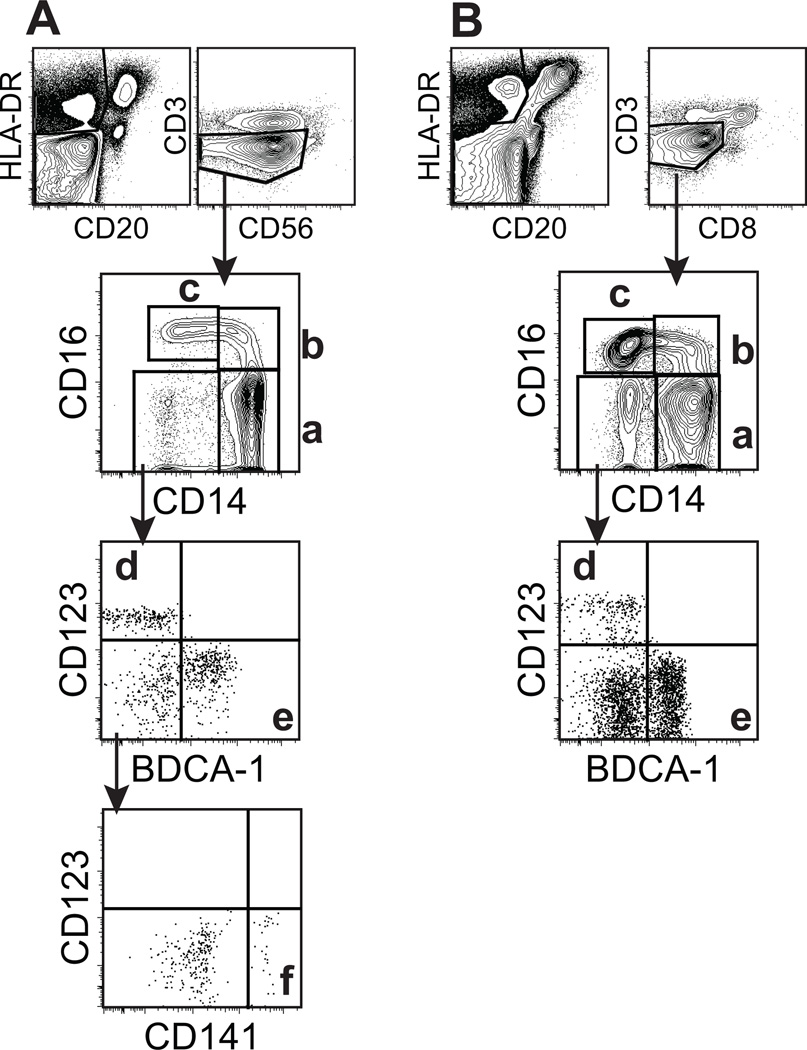
Figure 1. Phenotying of blood monocyte and DC subsets in humans (A) and rhesus macaques (B).
EDTA-treated blood samples were stained with antibodies shown in Table 1 and analyzed by 11-color flow cytometry. (A) In HLA-DR+CD3−CD20−CD56− populations, human monocyte and DC subsets were gated and divided into 4 populations by CD14 and CD16 expression as follows; (a) CD14+CD16− monocytes, (b) CD14+CD16+ monocytes, (c) CD14−CD16+ monocytes, and a CD14−CD16− population that was further divided into (d) CD123+ pDC and (e) BDCA-1+ mDC. In addition, a CD141+ mDC (f) was identified. (B) To analyze rhesus monocyte and DC subsets, HLA-DR+CD3−CD20−CD8−cell populations were similarly gated and further divided as described in panel A with the exception that antibody to human CD141 (BDCA-3) did not cross-react to, or detect this marker on rhesus macaque cells. The populations of cells identified included; (a) CD14+CD16− monocytes, (b) CD14+CD16+ monocytes, (c) CD14−CD16+ monocytes, (d) CD123+ pDC, and (e) BDCA-1+ mDC.
Statistical analyses
Mann-Whitney rank test was used to compare the mean differences between groups using Graphpad Prism version 5.0f for Mac (GraphPad Software, San Diego.CA). P < 0.05 was considered statistically significant.
RESULTS
Blood monocyte and DC subpopulation phenotypes are similar in rhesus macaques and humans
Blood monocytes and DC subsets from rhesus macaques and humans were evaluated by multicolor flow cytometry using previously-described panels of antibodies to phenotypic markers (3, 14, 15) and as shown in Table I and Figure 1. Since monocytes and DC are considered myeloid lineage cells, HLA-DR-positive and lymphocyte /NK marker-negative cells were gated to further characterize monocytes and DC. Although CD56 is a common NK marker in humans, it also is expressed on monocytes in rhesus macaques (19). Thus, CD8 was used instead of CD56 to discriminate NK cells in rhesus macaques. NKG2A or NKp46 were not applied to further exclude NK cells since most NKG2A-positive cells are HLA-DR negative or CD8 positive. Therefore, these HLA-DR-positive and lymphocyte/NK lineage marker-negative cells contained negligible numbers of NK cells (data not shown). As reported previously, four distinct populations of cells could be distinguished based on CD14 and CD16 expression on HLA-DR-positive, lymphocyte/NK lineage marker-negative cells in both humans and rhesus macaques (7, 20) (Figure 1). Of these four populations, three were identified as monocyte subsets corresponding to classical (CD14+CD16−), intermediate (CD14+CD16+), and non-classical (CD14−CD16+) monocytes.
Blood DC populations are commonly identified from within the population of cells that are positive for HLA-DR and negative for lymphocyte/NK/monocyte markers. MacDonald et al. (12) described four distinct sets of DC populations were identified in humans as CD11c+CD16+, CD11c+BDCA-1+, CD11c+CD141+, and CD11c−CD123+. In rhesus macaques, however, three populations of cells expressing DC markers are identified by Autissier et al.(14) as CD11c+CD16+, CD11cdimBDCA-1+, and CD11c−CD123+ but not CD141+ DC. Using these reported gating strategy, the CD14−CD16+ monocytes and CD11c+CD16+ DC populations completely overlapped and identified the same cell subsets in humans and rhesus macaques (Supplement figures 1 and 2). The CD16+ population previously identified as a DC subset is recently classified instead as a subset of monocytes (21). Therefore, antibodies to BDCA-1, CD141, and CD123 markers were used to identify three DC subsets in humans (BDCA-1+, CD141+ and CD123+ DC) (Figure 1A) and only two DC subsets have been identified in rhesus macaques due to the lack of a reliable rhesus CD141-specific antibody (Figure 1B and Table II). By careful analysis of the three distinct DC subsets in human blood, we observed a CD141+ DC subset that expressed CD11c but was negative for CD16 and BDCA-1 and did not overlap with any of the other positive subsets of DC (Figure 1A).
Table II.
Phenotype comparison of monocyte and DC subsets from human and rhesus macaque blood.
| Human | Rhesus macaques | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monocyte | DC | Monocyte | DC | |||||||||
| CD14+ CD16− |
CD14+ CD16+ |
CD14− CD16+ |
CD1c+ | CD141+ | CD123+ | CD14+ CD16− |
CD14+ CD16+ |
CD14− CD16+ |
CD1c+ | CD123+ | ||
| CD3 | − | − | − | − | − | − | − | − | − | − | − | |
| CD8 | − | − | − | − | − | − | − | − | − | − | − | |
| CD20 | − | − | − | − | − | − | − | − | − | − | − | |
| CD11b | + | + | −/+ | − | − | − | + | + | −/+ | − | − | |
| HLA-DR | + | + | + | + | + | + | + | + | + | + | + | |
| CD14 | + | + | − | − | − | − | + | + | − | − | − | |
| CD16 | − | + | + | − | − | − | − | + | + | − | − | |
| CD163 | + | −/+ | − | + | − | − | + | −/+ | − | + | − | |
| CD1c | − | − | − | + | − | − | − | − | − | + | − | |
| CD11c | + | + | + | + | + | − | − | −/+ | + | − | − | |
| CD141 | − | − | − | − | + | − | − | − | − | − | − | |
| CD123 | − | − | − | − | − | + | − | − | − | − | + | |
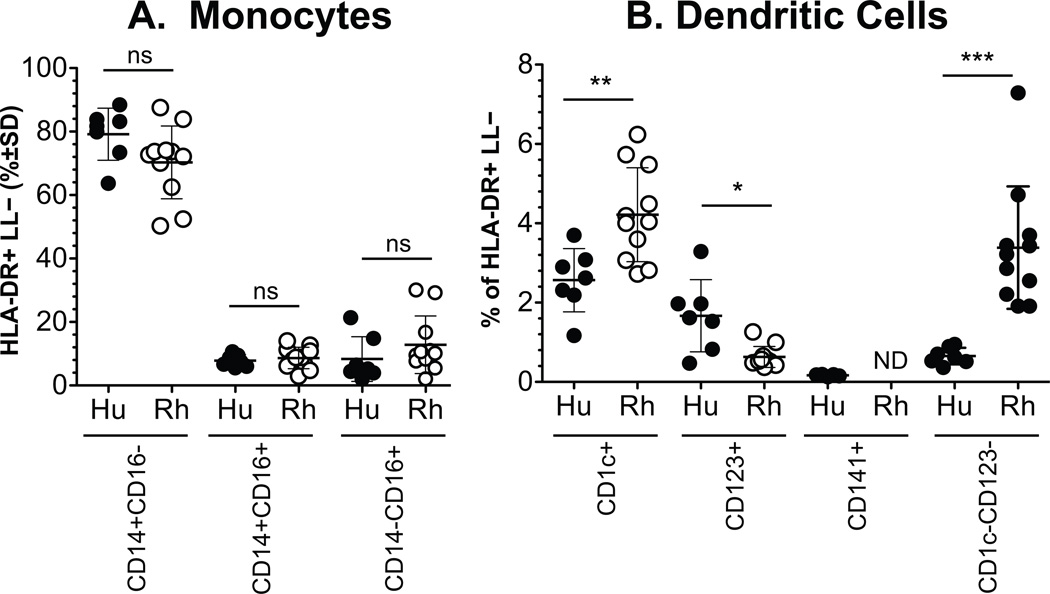
The rhesus cell subset that was negative for CD16 and BDCA-1 within the HLA-DR-positive, but lineage marker-negative cell fraction of cells (i.e. negative for CD3, CD20, CD8, CD16 and CD14) may include the rhesus homologous CD141+ cells. Therefore, the CD123−BDCA-1− fraction of rhesus cells and the equivalent fraction from human samples were compared to assess proportions of monocyte and DC subsets (Figure 2). In both humans and rhesus macaques, similar patterns were observed. Over 70% of the HLA-DR-positive, lymphocyte-lineage marker-negative cells were CD14+ monocytes and less than 10% of the cells comprised a subset of DCs. Statistically significant differences in the mean percent of HLA-DR-positive, lymphocyte lineage-negative subsets, however, were observed between human and rhesus macaque blood DC subsets. Specifically, levels of BDCA-1+DC and BDCA-1−CD123− DC cell populations were higher in rhesus macaques while CD123+ pDC numbers were lower in rhesus macaques compared to humans. No significant difference was observed in the monocyte subset levels between humans and rhesus macaques (Figure 2 A).
Figure 2. Comparison in the proportion of blood monocyte and DC subset populations between humans and rhesus macaques.
Blood from 7 humans and 11 rhesus macaques were gated and analyzed as described in the materials and methods and in Figure 1. The proportions of monocytes (A) and DC (B) subsets were calculated from the total number of HLA-DR+ lymphocyte lineage (LL) negative cells. Comparisons between cell subsets in human and rhesus macaque blood were calculated for statistically significant differences by the Mann Whitney rank test. Not detected, ND; P<0.05,*; P<0.01,**; P < 0.001,***.
Comparative expression of CD11c on monocytes and DC in human and nonhuman primate blood
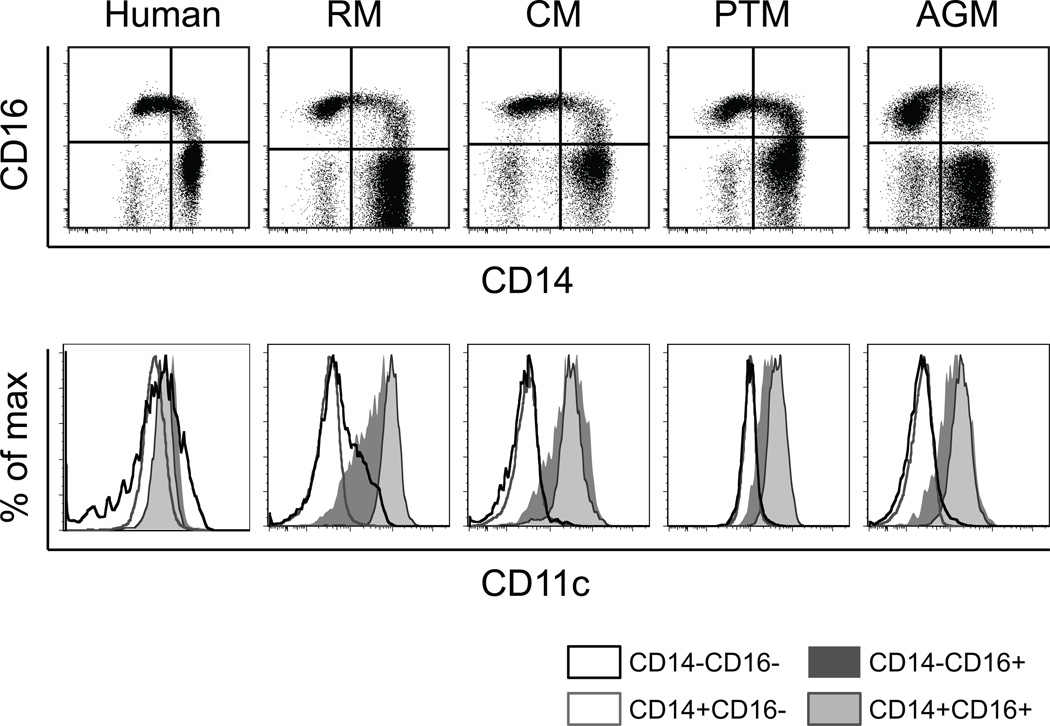
CD11c is considered a mDC marker in humans and mice and also has been widely used to identify mDC in rhesus macaques (3, 12, 22). After detailed characterization of the different DC subsets shown in Figure 1 and Supplement Figure 1, it became apparent that myeloid DC subsets, as well as monocyte subsets, were positive for CD11c expression in humans, whereas only monocytes expressing CD16 (non-classical monocytes) were positive for CD11c expression in rhesus macaques. Thus, relatively few DC were included within the CD11c+ blood cell fraction in rhesus macaques. Since anti-human antibodies are generally used for NHP studies, these differences in staining patterns could result from differences in levels of cross reactivity. Therefore, we tested rhesus macaque blood specimens with two different monoclonal antibody clones (S-HCL-3 and 3.9) for detecting CD11c and observed that cells incubated with clone 3.9 did not exhibit staining by S-HCL-3 in some rhesus macaques (data not shown). To further confirm whether this CD11c staining pattern is specific for rhesus macaques, we analyzed blood from other nonhuman primate species including cynomolgus macaques (n =11), pigtail macaques (n =8) and African green monkeys (n =4) for comparison to human and rhesus macaque blood (Figure 3). To maintain consistency, the same staining and flow cytometer setting conditions were used for all NHP samples analyzed in these studies. PBMC of pigtail macaques demonstrated CD11c staining of some cells within the CD14+ subset, but the rhesus macaque staining pattern for CD11c was similar to that of cells from cynomolgus and African green monkeys (Figure 3).
Figure 3. Comparison of CD11c expression on monocytes and DC among primate species.
Blood from human, rhesus macaque (RM), cynomolgus macaque (CM), pigtail macaque (PTM), and African green monkey (AGM) was gated for HLA-DR+ lymphocyte lineage-negative cells and divided into four populations based on CD14 and CD16 expression. CD11c staining on cell subsets CD14−CD16−, CD14+CD16−, CD14+CD16+ and CD14−CD16+ was plotted.
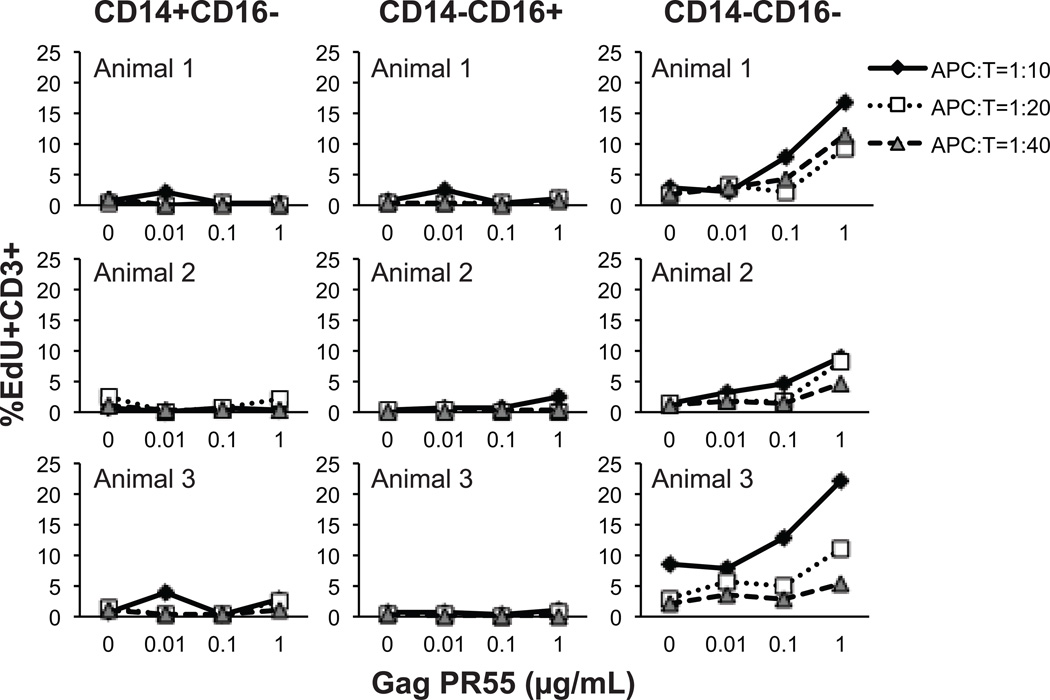
To confirm that the monocyte/DC classification using CD14 and CD16 expression in rhesus macaques is comparable to humans, with the exception of CD11c staining patterns, we performed a functional assay to compare the ability of the three rhesus cell populations, CD14+CD16−, CD14−CD16+ and CD14−CD16−, to present a recall protein antigen to T cells as reported earlier for human monocyte/DC subsets (23). The cell populations obtained from immune-competent rhesus macaques (n =5) infected with SIVmac239 and administered cART were evaluated for their ability to respond to SIV Gag PR55. These animals exhibited normal monocyte turnover rate and phenotype of monocytes and DC when the assay was performed. Similar to the reported data in humans(23), macaque CD14−CD16− cells consisting BDCA-1+ and CD123+ DC promoted Gag protein-specific T-cell proliferation, while CD14+CD16− and CD14−CD16+ cells did not induce a recall proliferative response by T cells (Figure 4).
Figure 4. Cell proliferation induced by antigen presentation on rhesus monocyte and DC subsets.
CD14+CD16− classical monocytes, CD14−CD16+ non-classical monocytes, and the CD14−CD16− fraction that includes DC subsets were sorted by flow cytometry of blood samples obtained from SIV-infected and ART-treated rhesus macaques. The subset populations were then pulsed with Gag pr55 protein at indicated concentrations for 2 hr. The antigen-pulsed effector cells were added to wells at numbers ranging from 2500 - 10000 per well and incubated with 1×105 autologous CD3+ T cells per well for 4.5 days resulting in antigen presenting cell to T cell (APC:T) ratios of 1:40-1:10. The thymidine analogue, EdU, was added during the last 18 hr of incubation and EdU incorporation in CD3+ T cells was detected by immunostaining and flow cytometry. Data from three animals are shown.
Non-classical CD14−CD16+ monocytes differentiate from classical CD14+CD16− monocytes that originated from bone marrow
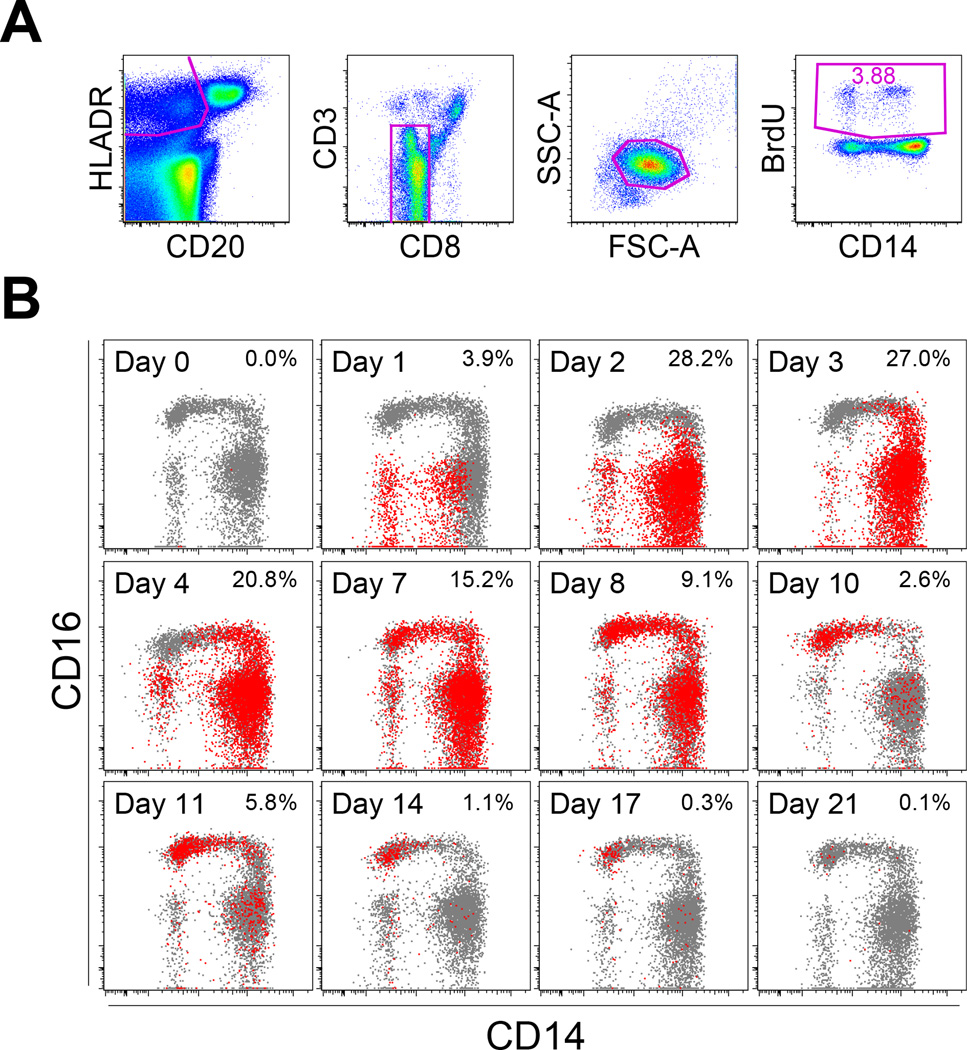
Incorporation of BrdU following injection has been used to successfully track the fate of CD14+ (classical monocytes) in rhesus macaques (16). To further investigate the origin and differentiation of the monocyte and DC subsets, blood samples were collected from four rhesus macaques between day 0 and day 21 after BrdU injection to follow the kinetics of BrdU incorporation by myeloid cells using flow cytometry (Figure 5). BrdU-positive cells appeared as early as day 1 in the CD14−CD16− cell fraction, and then peaked between days 2 to 4 primarily in the CD14+CD16− cells (Figure 5B). BrdU incorporation was subsequently detected in the CD14+CD16+ cell fraction on day 3 and then in the CD14−CD16+ monocytes on day 7 (Figure 5B). The percentage of BrdU-positive cells gradually decreased after day 7 until no BrdU-staining cells were observed 21 days after injection (Figure 5B).
Figure 5. Kinetics of BrdU incorporation by monocyte and DC populations.
Normal healthy rhesus macaques were injected with BrdU intravenously at a dose of 60 mg/kg body weight. EDTA-treated blood was collected at varying time intervals as indicated. The data shown are representative for four animals. (A) BrdU incorporation within the HLA-DR+CD3−CD20−CD8− cell population was examined by immunostaining and flow cytometry. Since monocytes and DC displayed slightly distinct scatter plots, the cell population first was gated on HLA-DR, CD20, CD3, and CD8, and then the scatter plot was confirmed. (B) BrdU-staining cells, as identified in Panel A, were indicated here in red with the percent of HLA-DR+CD3−CD20−CD8− cells noted on the top right corner of each time point after BrdU injection, and results were overlaid onto plots of cells stained for CD14 and CD16 expression shown in gray.
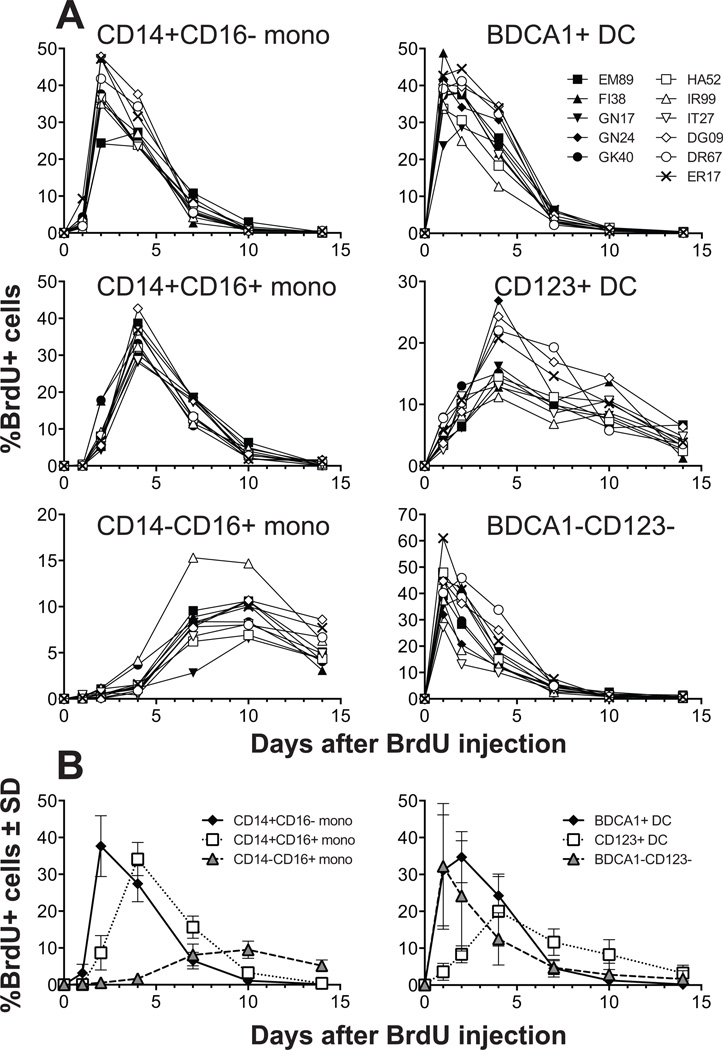
We also followed the kinetics of BrdU incorporation into each subset of monocytes and DCs based on the gating strategy described in Figure 1 and the results chronicle the development of each cell subset after emigration from the bone marrow to blood and subsequent transition to tissues (Figure 6). The initial peak in BrdU incorporation occurred in CD14+CD16− classical monocytes within 2 days. Then CD14+CD16+ intermediate monocytes were observed to preferentially stain for BrdU incorporation within 4 days and finally, CD14−CD16+ non-classical monocytes exhibited peak BrdU uptake approximately 10 days after BrdU injection (Figure 6A). At that time, no BrdU staining was observed in the classical or intermediate monocyte subsets (Figure 6). These results indicate that CD14+CD16− classical monocytes are continuously generated from bone marrow and that there is a steady-state differentiation from intermediate to non-classical monocytes.
Figure 6. Kinetics of BrdU incorporation by each monocyte and DC population in rhesus macaque blood.
(A) Eleven healthy normal adult rhesus macaques were administrated BrdU intravenously and blood was drawn and analyzed at indicated time points. The percent of BrdU+ cells within each of the monocyte subsets (CD14+CD16−, CD14+CD16+, and CD14−CD16+ monocytes) and two DC subsets (BDCA-1+ mDC, and CD123+ pDC), as well as the non-BDCA-1 and CD123 populations identified in Figure 1B were plotted as a percent of the HLA-DR+ lymphocyte lineage-negative cells. (B) Mean values (%±SD) of BrdU incorporation by the monocyte (left panel) and DC plus BDCA-1− and CD123+ populations (right panel) were plotted from the 11 animals.
In contrast to the monocyte subsets, two of the DC subsets identified in rhesus macaques, BDCA-1+ mDC and CD123+ pDC, showed distinct kinetics of BrdU incorporation (Figure 6). The BDCA-1+ mDC showed the fastest turnover and exhibited peak BrdU labeling within the first 24 hr. BrdU incorporation peaked after four days in the CD123+ pDC subset and the decline or loss of BrdU occurred slower in than the decline observed for the other monocyte or DC subsets (Figure 6A and B). These results imply that the two DC subpopulations of cells originate from distinct cells in the bone marrow and develop independently from monocyte differentiation.
Discussion
Nonhuman primates are physiologically similar to humans and thus serve as important animal models to better understand the human immune system. In this study, we compared the phenotype of blood monocytes and DC in nonhuman primates, particularly in rhesus macaques, to the phenotype of equivalent cells in human blood. Using our results and recommendations of the Nomenclature Committee of the International Union of Immunological Societies, we attempted to develop concordant classifications (3) that can be assigned to the cell subpopulations in rhesus macaques and determined the proportions of monocyte and DC subsets that reside in the HLA-DR-positive and lymphocyte (T, B and NK cells) lineage-negative cell fractions (Figure 1 and 2). We confirmed that the three subsets of monocytes, CD14+CD16−, CD14+CD16+, and CD14−CD16+ corresponding to classical, intermediate, and non-classical monocytes, respectively, and two subsets of DC, BDCA-1+ mDC and CD123+ pDC described for humans were also represented in rhesus blood (Figure 1). Despite the lack of clearly identifiable CD141+ mDC in rhesus blood, our overall results are consistent with previous reports (4, 14, 15).
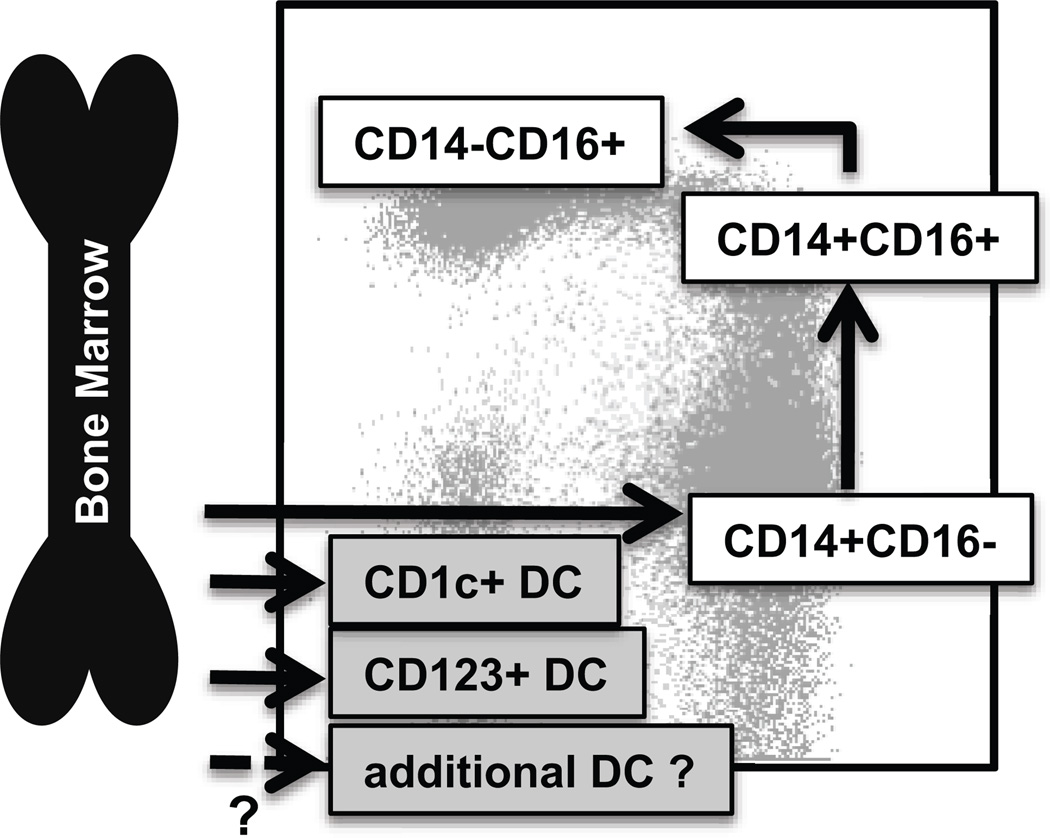
The results in this study further examined additional relationships between human and rhesus macaque blood myeloid cell phenotypes (Figure 1). For example, mDC in rhesus macaques have been analyzed by the use of the CD11c marker on HLA-DR+ lymphocyte lineage (CD3, CD20, CD8, and CD14) marker-negative cells (Supplementary Figure 2). This conventional mDC population includes a large proportion of non-classical monocytes defined by the expression of CD16. This is likely attributed to the differences between humans and nonhuman primates regarding expression of CD11c (Figure 3 and Supplementary Figure 2). CD11c was expressed only by CD16+ monocyte populations including CD14+CD16+ and CD14−CD16+ monocytes in NHP, whereas in humans, all of the monocyte and DC subsets, except for the CD123+ pDC, express CD11c. This may have led to the belief that CD11c+ HLA-DR+ lymphocyte lineage (CD3, CD20, CD8, and CD14) marker-negative cells in nonhuman primate blood include an mDC subset. Further, the antigen presentation assay comparing the monocyte and DC subsets in rhesus macaques were functionally similar to results reported for these subset populations in human; namely that the CD14−CD16− fraction containing BDCA-1+ mDC and CD123+ pDC induced Gag pr55 protein specific T cell proliferation while the CD14+CD16− (classical monocytes) and CD14−CD16+ (that had been classified as CD11c+ mDC) did not present antigen to induce T cell proliferation (Figure 4) (23). These results thus suggest that the expression of CD11c for characterization of mDC in NHP needs to be reconsidered. A concern, however, is that we cannot conclude that CD14+CD16− monocytes and BDCA-1+ mDC in NHP do not express CD11c because the human-specific antibody to CD11c either does not cross react well to rhesus CD11c or that this marker is not expressed by nonhuman primate blood cells. Examination of CD11c expression by nonhuman primate monocytes using gene expression analysis may help answer this question. CD141+ DC in humans were identified within the CD123/BDCA-1 double-negative fraction of the HLA-DR+ lineage-marker negative (CD3−, CD20−, CD8−, CD16− and CD14−) cells (Figure 1) and the unidentified CD123/BDCA-1 double-negative cells within the CD14−CD16− fraction in rhesus macaques exhibited distinct kinetics of BrdU incorporation from other DC subsets (Figure 6). These results imply that additional DC subsets homologous to the CD141+ DC in human blood exist in nonhuman primate blood (Figure 7).
Figure 7. Summary of blood monocyte and DC differentiation in rhesus macaques.
Myeloid precursor cells in the bone marrow migrate into blood and give rise to CD14+CD16− classical monocytes. A large proportion of these classical monocytes rapidly disappeared from the circulation to become tissue macrophages. A fraction of the classical monocytes differentiated into CD14+CD16+ intermediate monocytes and then into CD14−CD16+ non-classical monocytes in the circulation. The populations of mDC, pDC and perhaps other unidentified DC appeared to differentiate from various DC precursors directly in the bone marrow prior entering the blood circulation.
BrdU, a thymidine analogue, is incorporated during DNA synthesis at the S-phase of cell cycle and is considered a reliable marker of dividing cells (24–26). In vivo BrdU pulse-chase studies thus are useful for tracing the development, kinetics, trafficking, and turnover of replicating cells of the immune system in animal models (17, 27–30). Results presented here indicate that division of monocytes occurs at the hematopoietic stem cell-derived progenitor stage with myeloid-restricted differentiation potential in bone marrow, and that monocytes are then released into the circulation at the end of S-phase (31). While less information has been reported about the development and maturation of blood DC, our results shown in figures 5 and 6 directly support previous findings that the CD14−CD16+ cells derived from a fraction of monocyte subsets was clearly distinct from the mDC subsets (23, 32). BrdU-positive CD14+CD16− monocytes were observed within the first 24 hr, rapidly increased to peak levels at day 2, and BrdU-uptake was then detected sequentially in CD14+CD16+ and then CD14−CD16+ fractions of cells. It is interesting to note that the BrdU+CD14+CD16− monocytes observed 24 hr after BrdU injection expressed lower levels of CD14 antigen suggesting that newly-recruited monocytes from bone marrow gradually contribute to increasing the level of CD14 expression in the blood. These data also strongly suggest that non-classical monocytes differentiate from CD14+CD16− (classical monocytes) by gradually expressing CD16+ to become CD16+CD14+ (intermediate monocytes) cells and subsequently mature to the non-classical CD14−CD16+ cell subset as they gradually exhibit decreased expression of CD14 protein while circulating in blood. Our studies using BrdU pulse-chase analyses in rhesus macaques thus directly demonstrated a link in the differentiation and turnover among the three monocyte subsets and corroborates previous studies linking the developmental relationship among three monocyte subsets using macrophage colony-stimulating factor treatment in humans and NHP (33, 34)
In vivo BrdU labeling of monocytes also has contributed to better understanding mechanisms of pathogenesis in infectious diseases (17, 27, 28, 30). Since BrdU is considered carcinogenic, it has only rarely been applied in human studies (35) so its use is more common in laboratory animals. For example, SIV infections in NHP have served as useful models of HIV/AIDS and we reported that an increasing monocyte turnover based on kinetics of BrdU labeling directly correlated with and predicted onset of terminal disease progression (27, 30, 36). Similar observations regarding increased CD14+ monocyte turnover kinetics are lacking in HIV-infected humans despite expression of clinical signs (37). This discrepancy, however, possibly could be investigated by applying continuous BrdU exposure in drinking water for humans instead of applying a single iv injection of BrdU as used in the NHP that would still enable labeling of the replicating cells to monitor turnover rate kinetics. Currently, however, these findings emphasize the usefulness of nonhuman primate models to study the kinetics of myeloid lineage cells as well as infectious diseases pathogenesis in humans.
In this study, we also described distinct kinetics of BrdU labeling between BDCA-1+ mDC and CD123+ pDC that also differed from those of the monocyte subsets (Figure 6). BrdU positive BDCA-1+ mDC peaked within the first 24 hr and rapidly disappeared by 10 days suggesting that they were derived rapidly from the bone marrow. By contrast, BrdU incorporation in CD123+ pDC only gradually increased during the first four days and slowly decreased over the subsequent six days, indicating a slower cell turnover than exhibited by the monocyte and BDCA-1+DC subsets. The common DC progenitors (CDP) in the bone marrow are strongly committed to produce both mDC and pDC, but they produce fewer pDC than mDC (38, 39). Recently, a novel distinct DC-restricted precursor that produces pDC predominantly has been reported (8) and the differences in kinetics and cell turnover between BDCA-1+ mDC and CD123+ pDC described in this study also suggest that they may originate from distinct progenitors. CD14+ monocytes are known to differentiate into monocyte-derived DC (Mo-DC) with many characteristics similar to mDC, so monocytes have been considered to be DC precursors. Our data suggest, however, that monocyte subsets did not give rise to any known DC subsets in vivo based on the BDCA-1+ mDC exhibiting the highest turnover kinetics among the monocyte and DC subset populations of cells.
In summary, our results using in vivo BrdU pulse-chase analyses demonstrate a link between the three subsets of monocytes and the two major DC subsets based on immune-staining and flow cytometry using the rhesus macaque nonhuman primate model (Figure 7). We observed that myeloid precursors gave rise to CD14+CD16− classical monocytes and that a large proportion of these classical monocytes rapidly disappeared from the circulation to become tissue macrophages. A fraction of the classical monocytes differentiated into CD14+CD16+ intermediate monocytes and then into CD14−CD16+ non-classical monocytes during approximately 10 days in circulation. It remains to be determined whether the monocytes in the intermediate fraction are simply classical monocytes differentiating into non-classical monocytes or if they comprise an independent cell subset that exists in the circulation. It also appears likely that mDC and pDC differentiate from different DC precursors and directly migrate from the bone marrow to the blood circulation. These findings could significantly enhance understanding of innate immune responses to infectious pathogens and the role of myeloid cells in infections and inflammation.
Supplementary Material
Acknowledgments
The authors thank Jason Dufour for veterinary support, Julie Bruhn and Calvin Lanclos for flow cytometry expertise, Erin Haupt, Ashley Leach, Toni Penney, Desiree Waguespack, and Faith Schiro for technical support.
This research was supported by grants from the National Institutes of Health to MJK (AI097059, AI087302, AI110163 and AI091501), to the Tulane National Primate Research Center (OD011104), to WKK (Virginia’s Commonwealth Health Research Board #11-09) as well as AIDS research grants from the Health Sciences Research Grants and from the Ministry of Health, Labor, and Welfare of Japan.
Abbreviations
- DC
dendritic cells
- mDC
myeloid DC
- pDC
plasmacytoid DC
- NHP
nonhuman primates
- cART
combination anti-retrovirus therapy
- BrdU
5-bromo-2’-deoxyuridine
- EdU
5-ethynyl-2´-deoxyuridine
References
- 1.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 2.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJM, Liu Y-J, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 4.Kim W-K, Sun Y, Do H, Autissier P, Halpern EF, Piatak M, Lifson JD, Burdo TH, McGrath MS, Williams K. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J Leukoc Biol. 2010;87:557–567. doi: 10.1189/jlb.0209082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moniuszko M, Bodzenta-Lukaszyk A, Kowal K, Lenczewska D, Dabrowska M. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin Immunol. 2009;130:338–346. doi: 10.1016/j.clim.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Nockher WA, Scherberich JE. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun. 1998;66:2782–2790. doi: 10.1128/iai.66.6.2782-2790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim W-K, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, González RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onai N, Kurabayashi K, Hosoi-Amaike M, Toyama-Sorimachi N, Matsushima K, Inaba K, Ohteki T. A clonogenic progenitor with prominent plasmacytoid dendritic cell developmental potential. Immunity. 2013;38:943–957. doi: 10.1016/j.immuni.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogg N, Takacs L, Palmer DG, Selvendran Y, Allen C. The p150,95 molecule is a marker of human mononuclear phagocytes: comparison with expression of class II molecules. Eur J Immunol. 1986;16:240–248. doi: 10.1002/eji.1830160306. [DOI] [PubMed] [Google Scholar]
- 11.Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJM. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald KPA, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DNJ. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler-Heitbrock L. Monocyte subsets in man and other species. Cell Immunol. 2014;289:135–139. doi: 10.1016/j.cellimm.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Autissier P, Soulas C, Burdo TH, Williams KC. Immunophenotyping of lymphocyte, monocyte and dendritic cell subsets in normal rhesus macaques by 12-color flow cytometry: clarification on DC heterogeneity. J Immunol Methods. 2010;360:119–128. doi: 10.1016/j.jim.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown KN, Barratt-Boyes SM. Surface phenotype and rapid quantification of blood dendritic cell subsets in the rhesus macaque. J Med Primatol. 2009;38:272–278. doi: 10.1111/j.1600-0684.2009.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, Vinet-Oliphant H, Kim W-K, Williams KC, Ribeiro RM, Lackner AA, Veazey RS, Kuroda MJ. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehra S, Golden NA, Stuckey K, Didier PJ, Doyle LA, Russell-Lodrigue KE, Sugimoto C, Hasegawa A, Sivasubramani SK, Roy CJ, Alvarez X, Kuroda MJ, Blanchard JL, Lackner AA, Kaushal D. The mycobacterium tuberculosis stress response factor SigH is required for bacterial burden as well as immunopathology in primate lungs. J Infect Dis. 2012;205:1203–1213. doi: 10.1093/infdis/jis102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raue HP, Slifka MK. Pivotal advance: CTLA-4+ T cells exhibit normal antiviral functions during acute viral infection. J Leukoc Biol. 2007;81:1165–1175. doi: 10.1189/jlb.0806535. [DOI] [PubMed] [Google Scholar]
- 19.Carter DL, Shieh TM, Blosser RL, Chadwick KR, Margolick JB, Hildreth JE, Clements JE, Zink MC. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry. 1999;37:41–50. [PubMed] [Google Scholar]
- 20.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 22.Coates PTH, Barratt-Boyes SM, Zhang L, Donnenberg VS, O'Connell PJ, Logar AJ, Duncan FJ, Murphey-Corb M, Donnenberg AD, Morelli AE, Maliszewski CR, Thomson AW. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102:2513–2521. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- 23.Cros J, Cagnard N, Woollard K, Patey N, Zhang S-Y, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais J-P, D'Cruz D, Casanova J-L, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray JW, Dolbeare F, Pallavicini MG, Beisker W, Waldman F. Cell cycle analysis using flow cytometry. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49:237–255. doi: 10.1080/09553008514552531. [DOI] [PubMed] [Google Scholar]
- 25.Cremer C, Gray JW. Application of the BrdU/thymidine method to flow cytogenetics: differential quenching/enhancement of Hoechst 33258 fluorescence of late-replicating chromosomes. Somatic Cell Genet. 1982;8:319–327. doi: 10.1007/BF01538890. [DOI] [PubMed] [Google Scholar]
- 26.Trent JM, Gerner E, Broderick R, Crossen PE. Cell cycle analysis using bromodeoxyuridine: comparison of methods for analysis of total cell transit time. Cancer Genet Cytogenet. 1986;19:43–50. doi: 10.1016/0165-4608(86)90370-5. [DOI] [PubMed] [Google Scholar]
- 27.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J Immunol. 2014;192:2821–2829. doi: 10.4049/jimmunol.1302269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goto Y, Hogg JC, Suwa T, Quinlan KB, van Eeden SF. A novel method to quantify the turnover and release of monocytes from the bone marrow using the thymidine analog 5'-bromo-2'-deoxyuridine. Am J Physiol Cell Physiol. 2003;285:C253–259. doi: 10.1152/ajpcell.00035.2003. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa A, Moriya C, Liu H, Charini WA, Vinet HC, Subbramanian RA, Sen P, Letvin NL, Kuroda MJ. Analysis of TCRalphabeta combinations used by simian immunodeficiency virus-specific CD8+ T cells in rhesus monkeys: implications for CTL immunodominance. J Immunol. 2007;178:3409–3417. doi: 10.4049/jimmunol.178.6.3409. [DOI] [PubMed] [Google Scholar]
- 31.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins SH, Walzer T, Dembélé D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, Sharp FR, Chan S, Kastner P, Dalod M. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munn DH, Bree AG, Beall AC, Kaviani MD, Sabio H, Schaub RG, Alpaugh RK, Weiner LM, Goldman SJ. Recombinant human macrophage colony-stimulating factor in nonhuman primates: selective expansion of a CD16+ monocyte subset with phenotypic similarity to primate natural killer cells. Blood. 1996;88:1215–1224. [PubMed] [Google Scholar]
- 34.Weiner LM, Li W, Holmes M, Catalano RB, Dovnarsky M, Padavic K, Alpaugh RK. Phase I trial of recombinant macrophage colony-stimulating factor and recombinant gamma-interferon: toxicity, monocytosis, and clinical effects. Cancer Res. 1994;54:4084–4090. [PubMed] [Google Scholar]
- 35.Kovacs JA, Lempicki RA, Sidorov IA, Adelsberger JW, Herpin B, Metcalf JA, Sereti I, Polis MA, Davey RT, Tavel J, Falloon J, Stevens R, Lambert L, Dewar R, Schwartzentruber DJ, Anver MR, Baseler MW, Masur H, Dimitrov DS, Lane HC. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. The Journal of experimental medicine. 2001;194:1731–1741. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuroda MJ. Macrophages: do they impact AIDS progression more than CD4 T cells? J Leukoc Biol. 2010;87:569–573. doi: 10.1189/jlb.0909626. [DOI] [PubMed] [Google Scholar]
- 37.Mohri H, Perelson AS, Tung K, Ribeiro RM, Ramratnam B, Markowitz M, Kost R, Hurley A, Weinberger L, Cesar D, Hellerstein MK, Ho DD. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J Exp Med. 2001;194:1277–1287. doi: 10.1084/jem.194.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naik SH, Sathe P, Park H-Y, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, Kwak J-Y, Wu L, Shortman K. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 39.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.