Abstract
Claudication is the most commonly recognized peripheral artery disease (PAD) symptom, but not the most prevalent. Only 7.5% to 33% of patients report claudication as being part of their symptom experience. However, there is little evidence supporting atypical symptom reporting. The study purpose was to describe the full spectrum of symptoms experienced by older and younger individuals with PAD. Semistructured interviews were conducted with a purposive sample of 38 commmunity-dwelling adults aged 49 to 83 years; transcripts were analyzed using content analysis. Six themes emerged: symptom descriptors (claudication and atypical), maintaining equilibrium, temporal fluctuations, the role of exercise, perceived impact on quality of life, and disease presence and treatment. Results suggest heavy reliance on claudication can result in mis- or under-diagnosis of PAD. Further research is needed to validate the correspondence of atypical symptoms with ischemic changes during exercise to broaden currently accepted symptom locations and descriptors associated with PAD.
Keywords: peripheral artery disease, symptom experience, claudication
Introduction
Claudication is the symptom most commonly associated with peripheral artery disease (PAD) and is described as an aching, cramping, painful or tired feeling in the calves. However, research has shown that the presentation and progression of PAD-related symptoms can vary by individual and may be influenced by gender, age, and ethnicity.1 Some individuals remain asymptomatic despite disease progression, while others consistently experience discomfort upon exertion that subsides when physical activity ceases. Further, atypical symptom reporting among individuals with PAD may be more common than classic claudication.2–4
PAD Symptom Reporting
Differences in symptom reporting can partially be attributed to the methods used to obtain the symptom experiences of individuals with PAD. Individuals with PAD experience discomfort during exercise primarily due to the presence of ischemia in the calf, thigh, or buttocks.5,6 Subsequently, these exercise-induced ischemic symptoms limit an individual’s ability to exercise and affect oxygen consumption during exercise testing.7,8 Verbal report of PAD symptoms (e.g., location, severity, and descriptors of sensation) can be used to subjectively assess the PAD symptom experience. Currently, the most common way to assess PAD-related symptoms in clinical practice is through claudication questionnaires. However, multiple questionnaires exist and categorization of symptoms vary, with most questionnaires excluding atypical symptoms.1 For example, due to the restricted symptom location and descriptor options on PAD questionnaires, an ache in the calf could be reported (classic claudication), but a burn in the quadriceps could not (atypical symptom).
The variety of PAD questionnaires and the restriction of symptom categories further complicates the ability to present a clear picture of the array of symptoms experienced by individuals afflicted with the disease. One potential solution to obtaining a more comprehensive understanding of PAD symptom experiences is to conduct a semi-structured interview that would allow an individual to describe the symptoms they experience in their own words, using the locations and descriptors of their choice, as opposed to restricting them to the predetermined responses on current PAD questionnaires. Responses from a diverse group of individuals with PAD may provide a deeper understanding of the influence of gender, age, and ethnicity on the PAD symptom experience.
Purpose
The purpose of this study was to describe the symptom experience of individuals diagnosed with PAD (e.g., attitues, beliefs, and perceptions). Participants were asked to report their symptom experience during a semi-structured interview, and subsequently by completing a PAD symptom questionnaire. The primary research question was “How do individuals with PAD describe the PAD symptom experience?”
Methods
Semi-structured, face-to-face, descriptive interviews and a PAD symptom questionnaire were used to measure symptom experiences among people with PAD during a one-time visit to a University of Minnesota clinic site. The completion of both the interview and the questionnaire took approximately 35 minutes per participant.
Sample
Adults were eligible to participate in the study if they were: (a) ≥ 21 years of age, (b) diagnosed with PAD (ankle-brachial index (ABI) ≤ 0.90 or a post-exercise drop in ABI confirming PAD), (c) reporting exercise-limiting claudication or ischemia-related symptoms, (d) cleared for exercise via exercise or pharmacological stress test within one year of study enrollment, and (e) able to read, write, and speak the English language.
Sample size
Sample size guidelines for qualitative research were used to provide an estimate of an appropriate sample size for this study.9 A purposive sample of 38 participants completed a semi-structured interview; it was anticipated that data saturation would be achieved with this number.
Participant Recruitment
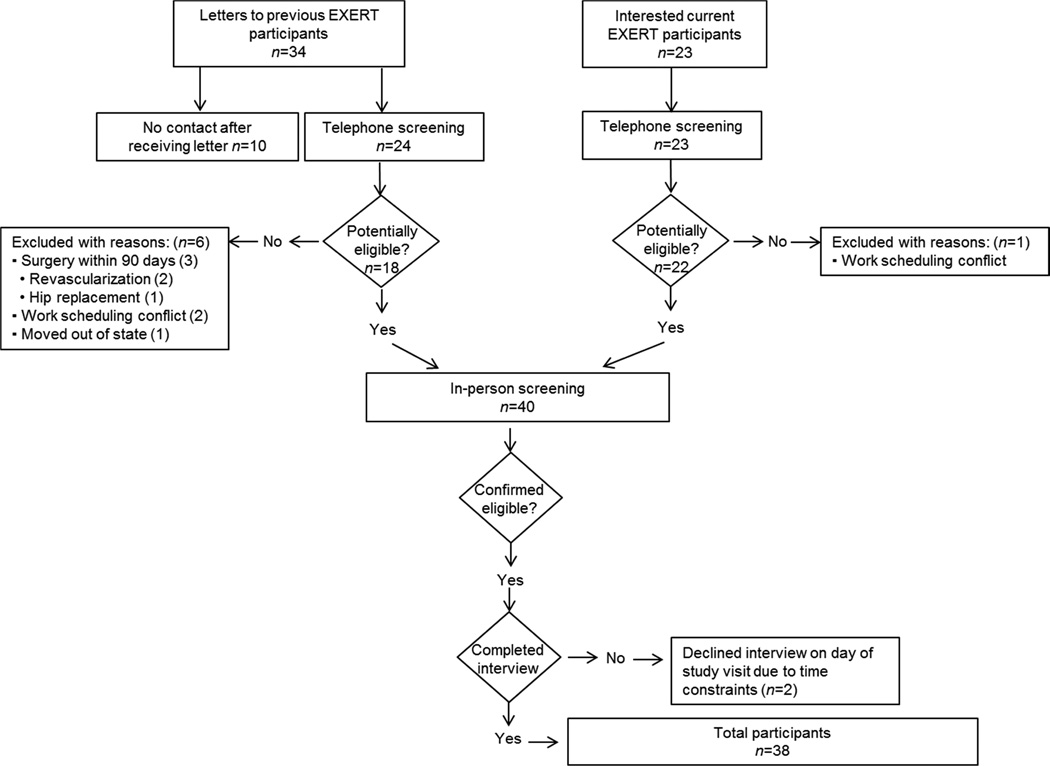
This study was approved by the University of Minnesota Institutional Review Board. Potential study participants were recruited from the EXercise Training to Reduce Claudication: Arm ERgometry versus Treadmill Walking (EXERT) study. This study was funded by the National Heart, Lung, and Blood Institute (NHLB) (R01 HL 090854-03, PI: Dr. Diane Treat-Jacobson). The study examined the efficacy of two forms of supervised exercise compared to the usual care provided by a physician for the treatment of PAD. Specifically, the EXERT study compared aerobic arm exercise and treadmill walking, to determine which, if any, form of supervised exercise reduced the symptoms of claudication and improved the walking ability in patients with PAD. Study procedures included a screening visit, baseline testing (cardiac exercise stress test, confirmatory ABI, etc.), supervised exercise training three times per week or a weekly control visit, and follow-up testing at six week increments, until 24 weeks of study participation had been completed. All current and previous EXERT study participants were contacted for potential participation in this study. After determining eligibility of EXERT study participants, 38 out of 40 individuals agreed to participate in this ancillary study. Figure 1 provides the specific details of study recruitment and enrollment.
Figure 1.
Flow diagram illustrating the details of study recruitment.
Measures
A standard demographic form was given to participants to obtain the following information: age, gender, marital status, race, ethnicity, education, and employment status. Next, a semi-structured interview was conducted using an interview guide (Table 1) to obtain a detailed account of the PAD symptom experience in a participant’s own words. Each question on the interview guide was designed to be clear, open-ended, sensitive, neutral, and targeted towards the participants’ PAD symptom experience. Questions focused on the PAD symptoms experienced at rest and with exercise. Participants were encouraged to provide the location, sensation, timing, and estimated duration of each symptom reported. Participants were also asked about “other pain or discomfort” during exercise or rest in an effort to understand symptoms that may not be related to PAD. All participant interviews were audio recorded and transcribed verbatim. Field notes were be taken by the PI as necessary, but kept to a minimum to avoid participant distraction.
Table 1.
Semi-Structured Symptom Interview Guide
| 1. Please describe in as much detail as possible the symptoms you experience while exercising. This can include activities such as walking, gardening, shoveling, etc. |
| 2. When does this symptom come? |
| 3. How does it feel? What words would you use to describe it? |
| 4. If you rest (stand still, sit or lie down) how long does it take to go away? |
| 5. Do you experience any other pain or discomfort while exercising or resting? |
| 6. If you got up to walk right now what would happen? |
| 7. Does your ability to exercise change from day to day? |
| Sample Interviewer Prompts: |
| …Tell me more about that. |
| …Can you elaborate a little on that? |
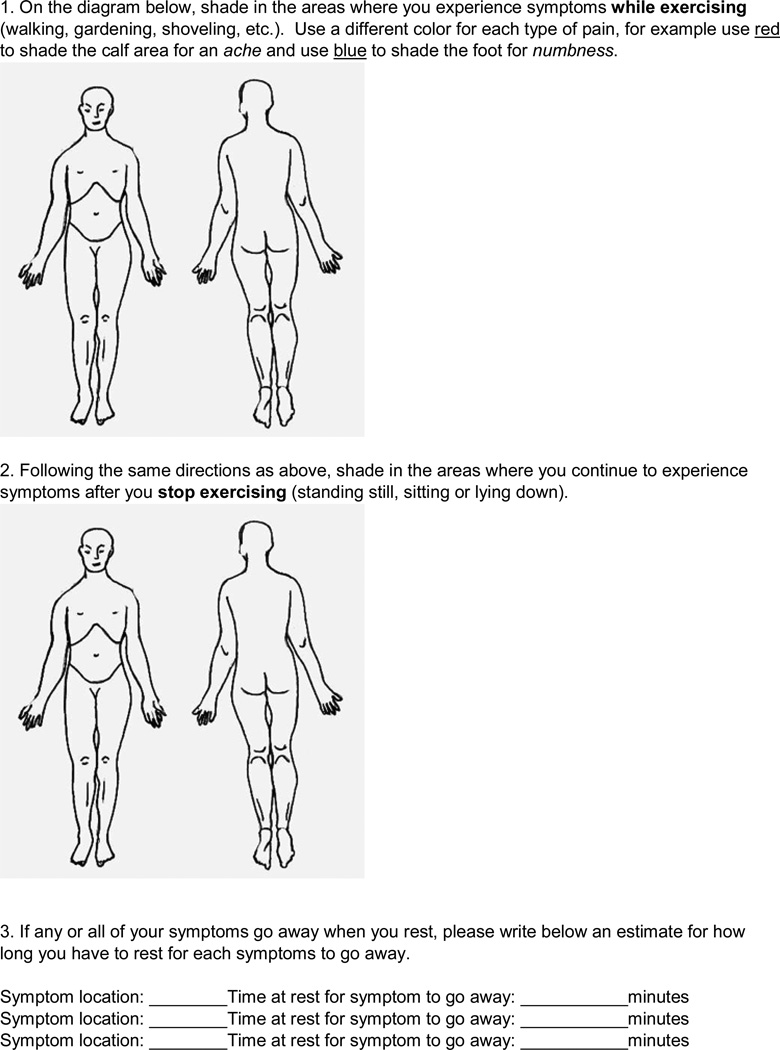
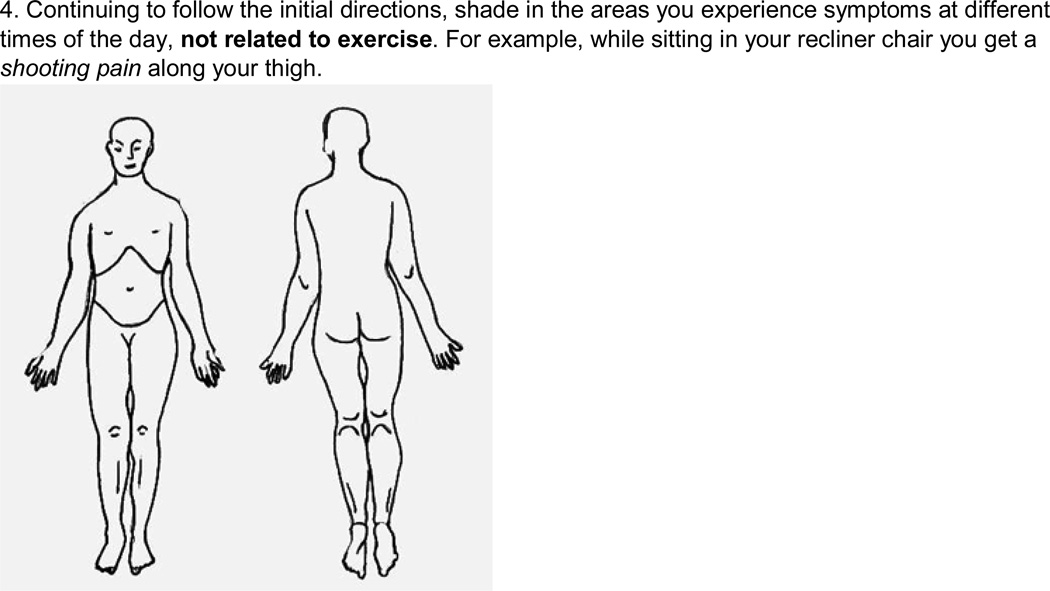
The PAD symptom questionnaire (Figure 2) was developed by the PI and pilot tested among four individuals with PAD prior to introduction in this study (2 men and 2 women, aged 48–79 years). It allowed for a visual representation of the individuals’ symptom location and description; it also served to verify congruence with symptom information that was provided during the interview. It was designed to be a comprehensive assessment of all the symptoms a participant experienced during exercise and rest, related or unrelated to PAD. The questionnaire utilized a body diagram and color coding to differentiate the location and sensation of each symptom, and to follow its course from rest to onset and time to relief. The primary goal was for participants to first describe all of their symptoms in detail, and then identify symptoms they believed to be directly related to their PAD. Questionnaire descriptions included location, sensation, and duration, for symptoms thought to be related to PAD, as well as those thought to be unassociated with the disease.
Figure 2.
Peripheral artery disease (PAD) symptom questionnaire completed with each participant at the end of the semi-structured interview.
Data Analysis
A qualitative content analysis approach for data analysis, adapted from the work of Hsieh and Shannon,10 Van Manen,11 and Sandelowski,12 was used to identify themes from the semi-structured interviews. First, a preliminary analysis was performed. This consisted of reading through each transcript once to understand each interview in its entirety, writing memos throughout as necessary to note emerging themes.10,11 The goal of this preliminary analysis was to get a sense of the whole and understand the essential features of each interview.12
Next, the emerging codes (i.e., thematic units) were listed and additional memos were taken throughout the rest of the analysis.11,13 Then, connections between the codes were identified, clustering codes together as necessary to capture the essence of the PAD symptom experience. The final result was multiple categories reflecting topics in all of the data. Verbatim quotes representative of each theme were extracted from the transcripts, as appropriate. Finally, the content of each category was analyzed to ensure that there were no subcategories that could be merged into a higher category. Memos were part of the data analyzed; they enabled the steps in the analysis process to be auditable by another person.12
Analysis was conducted by two human coders with experience in qualitative research methods. The first coder was the PI and the second coder was an expert in qualitative research methodology who reviewed the transcripts and verified the codes. Inter-coder reliability was further increased by providing the second coder with the PI’s content analysis steps with ambiguous words being clearly and consistently defined throughout, in addition to providing memos and field notes that detailed reactions, biases, and thought processes throughout data collection and analysis. Accuracy was assessed by test coding pieces of text extracted from the transcripts. For example, the second coder was provided with the following extract and asked to provide a code: “I don’t walk at a pace that’s enough.” This extract was classified by the first and second coders as “pace,” which was merged into a higher category “control pace,” and ultimately fell under the thematic unit “equilibrium tactics.” After completing this process, no new themes emerged.
Lincoln and Guba’s14 five concepts of trustworthiness were used to establish rigor in this study. Credibility is the first criterion which refers to confidence in the truth of the data and interpretations of them.14 The following enhancement strategies were used to maintain credibility: reflective journaling, comprehensive field notes, audiotaping and verbatim transcription, data saturation, transcription rigor/data cleaning, and inter-coder checking. An example of transcription rigor was listening to the audio recording while reviewing each transcript three times to verify the authenticity and to capture any emotional content not evident in the written transcripts.
The next criterion, dependability, is the stability (i.e., reliability) of data over time and over conditions.14 A carefully documented decision trail that would provide for an inquiry audit was developed to increase dependability of the study findings. Confirmability refers to the congruence between two or more independent people about the data’s accuracy or meaning (i.e., objectivity).14 To ensure confirmability, the decision trail was carefully documented, intercoder checks were performed, and a codebook was developed.
The transferability criterion is the extent to which the findings can be transferred to other settings or like groups (i.e., generalizability).14 Transferability strategies included taking comprehensive field notes, ensuring saturation of data, and providing thick, vivid descriptions of the symptom experience. The last criterion, authenticity is the extent to which the researchers fairly show a range of different realities and convey the tone of participants’ lives as they are lived.14 Strategies to maintain authenticity included: reflective journaling, audiotaping and verbatim transcription, thick, vivid descriptions, and impactful, evocative writing.
Results
Sample
Thirty-eight participants experiencing lower extremity symptoms during exercise due to underlying PAD were recruited and enrolled in this study over an eight month period. Following the content analysis procedures previously outlined, data saturation was reached after analyzing 27 out of 38 interviews (i.e., all transcripts were reviewed, but no new themes emerged after the 27th transcript).
Demographics
Characteristics of the 38 participants appear in Table 2. The average age of participants was 67.58 years (SD 9.13). Participants were predominately Caucasian males (79%). Thirty-two percent of participants were aged 65 years and older, retired, and living with a spouse or partner at the time of study participation. Overall, it was a highly educated group, with nearly 85% of the participants completing some college or graduate school.
Table 2.
Characteristics of the Study Sample (N=38)
| Characteristic | Category | n | (%) |
|---|---|---|---|
| Age | 49 – 64 years | 16 | (42.1) |
| 65 – 83 years | 22 | (57.9) | |
| Gender | Male | 33 | (86.8) |
| Female | 5 | (13.2) | |
| Ethnicity | Caucasian | 34 | (89.5) |
| African American | 2 | (5.3) | |
| Native American | 2 | (5.3) | |
| Education | High school diploma | 8 | (21.1) |
| Some college | 16 | (42.1) | |
| College degree | 10 | (26.3) | |
| Graduate school | 4 | (10.5) | |
| Employment | Full time | 5 | (13.2) |
| Homemaker | 1 | (2.6) | |
| Part time | 8 | (21.1) | |
| Retired | 24 | (63.2) | |
| Marital Status | Married/Living with partner | 23 | (60.5) |
| Divorced | 8 | (21.1) | |
| Widowed | 2 | (5.3) | |
| Single | 5 | (13.2) | |
Note. Percentages do not add up to 100% due to rounding.
Description of the PAD Symptom Experience
Information obtained from the semi-structured interviews provided a foundational description of the PAD symptom experience. A total of 32 thematic units were identifed from 38 individiual interviews and these were merged into 6 main themes: Symptom descriptors; Maintaining equilibrium; Temporal fluctuations; The role of exercise; The perceived impact on quality of life; and Disease presence and treatments.
Theme 1: Symptom descriptors
Twenty-four symptom descriptors in 10 lower extremity locations were provided during the semi-structured interviews (Table 3). Some individuals used the same word (e.g., ache) to describe symptoms that were present in multiple locations, while others had distinct vocabulary for each lower extremity location (e.g., aching calf and burning buttock). Even when symptoms were restricted to one location, participants used different vocabulary to describe their discomfort as it progressed and subsided.
Table 3.
Symptom Descriptors and Locations provided during Interviews (N=27)
| Descriptors | Locations |
|---|---|
| Ache | Ankle |
| Burn | Buttock |
| Charley horse | Calf |
| Cramp | Foot |
| Grinding/Rubbing | Groin |
| Gripping | Hamstring |
| Hard | Hip |
| Heavy | Knees |
| Hurt/Tender | Quadriceps |
| Jammed | Shin |
| Knot | |
| Numb | |
| Pain | |
| Pressure | |
| Prickly | |
| Pulling | |
| Sore | |
| Stiff | |
| Tight | |
| Tingling | |
| Tired/Fatigue | |
| Twinge | |
| Warm | |
| Weak |
Note. Descriptors and locations in bold are those commonly associated with classic claudication.
Some participants reported symptoms that were consistent with the descriptors associated with classic claudication (e.g., aching, cramping, painful, and tired). Participants provided the following ‘classic’ descriptions during interviews:
“I get a cramping in the left calf.”
“My legs get tired. I can feel it in my thighs.”
“I get a strong pain in the right buttock, it’s a painful throbbing.”
Other participants provided symptom descriptors in ‘atypical’ locations, symptom descriptors that differed by location, and symptom descriptors that extended beyond the aching, cramping, painful, and tired description of the classic and most commonly recognized symptom, claudication.
“My feet get kind of tingly…like they’re sleeping.”
“It’s like a knot, and it’s in my calf. If I’m walking up the stairs, it goes to my shin, and that’s a burning sensation.”
“My left calf and heel get sore. Even after a couple hours, it feels like I’ve already put a full day on it.”
Additionally, symptom descriptors varied based on the intensity and duration of exercise, as well as the treadmill grade. Participants reported a more rapid onset of symptoms with an increase in treadmill grade and symptoms that didn’t present themselves until after walking for a long period of time or increasing the pace while walking.
Theme 2: Maintaining equilibrium
Participants described different tactics that enabled them to prevent symptoms from occurring or to maintain symptoms at a tolerable level once they had begun. This theme consisted of three sub-categories, all with a common element of control: controlling the pace, controlling the environment, and controlling the elements of recovery.
Controlling the pace
Participants described deliberately controlling the pace of their walking to prevent or delay the onset of ischemic symptoms and/or prevent further symptom progression.
“It depends completely on how hard I walk. If I walk very slowly, I can go many, many blocks, more than a dozen, or so. If I walk aggressively, I can start to feel something in maybe two blocks.”
“[My husband] will walk with me, but then we have to hold hands so he doesn’t get ahead.”
Controlling the environment
Maintaining equilibrium for some individuals involved manipulating the environment in some way to avoid symptom onset and/or progression (e.g., driving short distances or parking closer). For others, this meant avoiding situations in which they knew they would have to walk faster or further than they were comfortable doing (e.g., the Mall of America) or a situation in which they knew there wouldn’t be a place to sit down and rest (e.g., no benches walking around a lake).
“I’ll drive a block and a half over to the Y. I can’t get there without taking a time-out on the way.”
“I usually shy away from places where I won’t be able to sit down and rest.”
Many individuals identified self-imposed limits and activities that were avoided because of having PAD. However, several individuals described being able to walk through the discomfort in certain situations.
“I just tend to ignore the pain. I just keep going and attempt to push through it. I’m used to the pain, so I don’t stop.”
Controlling the elements of exercise recovery
The last element of control involved manipulating the recovery position and utilizing specific techniques participants believed to aid in recovery, such as massaging or stretching the affected leg(s). Some described achieving complete symptom relief from stopping the activity and simply standing still. A few stated that walking around or leaning against a wall would provide quicker relief from symptoms. However, the vast majority of participants preferred to recover in a seated position.
“I prefer to sit down, but if I stand it will eventually go away. It goes away faster if I’m sitting.”
Theme 3: Temporal fluctuations
Participants described the influence of time on the symptom experience and described day to day fluctuations in their symptoms. This included fluctuations based on the time of day, any recent physical activity, current exercise duration, and the amount of time spent in recovery.
“It changes not only day to day, but hour to hour. I’m not really sure what controls it, but some days I just feel much better than other days.”
“I have a longer recovery if I’ve pushed harder.”
Participants also reported fluctuations in symptoms based on the walking surface or incline, as well as the influence of various medical conditions and medications. Some described a harder surface (e.g., tile or concrete) as being more painful, while a softer surface (e.g., carpet or a treadmill) allowed them to walk further distances. Others indicated that the unevenness of the surface (e.g., grass) was the culprit for bringing symptoms about quicker. All participants agreed that the higher the incline, the sooner they had to stop walking. Individuals with diabetes described an “ache” or “soreness” in the legs when blood sugar levels were higher than normal. Another participant noticed a difference symptoms based on variations in hemoglobin levels.
“You have more resistance with hard pavement. You have more absorption with a softer grass or something like that. The treadmill seems to be in the middle of all that. Most of my walking around the home is on tar, so I’m stuck with that. I can’t walk on people’s lawns…”
“I could walk as much as 14 minutes at one time, but I was ‘cheating’ because the platform was flat. So when they lifted it to 0.6%, then the best I could do was three to four minutes.”
Participants described a blurring of symptoms that made it difficult to decipher the cause of their discomfort at certain points in time. Some individuals believed the symptoms they were experiencing during exercise were a direct result of PAD, a few people thought the symptoms were a result of another medical condition (e.g., multiple sclerosis, neuropathy, bursitis), and for others, it was thought to be a combination of both.
“For me it’s hard to distinguish. Is it the PAD totally or is it the medication? As soon as they took me off of the Effexor is when I saw a major improvement in my leg symptoms.”
“I can’t run. That’s PAD and knee related, at the same time. It hurts a lot. I have weak ankles too.”
Theme 4: The role of exercise
Participants described the effect of multiple bouts of walking within the same exercise session.
“One thing I have noticed is that if I’ve walked hard enough to develop pain and then have it go away, I don’t have to go as far to have it come back. It will start coming back sooner than the first time.”
Some individuals also reported experiencing a post-exercise pain spike after stopping activity.
“After I get off of the treadmill and I come to sit down, I’ve got maybe 5 to 15 seconds, then I’ll just hit a streak of it, it just goes up.”
A separate, but related sub-category in the role of exercise theme was the progress that individuals perceived they were making because of their commitment to an ongoing exercise program.
“In the beginning, it was about two minutes [of recovery time] after seven or eight minutes of walking. Now I can walk up to 15 minutes, but then I need about four minutes of recovery. If I only go seven or eight minutes, then two minutes [of recovery] is fine.”
Theme 5: The perceived impact on quality of life (QOL)
Having a diagnosis of PAD and living with the disease on a daily basis impacted participants in different ways. For some, the biggest impact was personal. Having PAD negatively affected their self-esteem and their outlook on life.
“I get angry, at myself, I guess, because I don’t like being restricted. I used to walk for miles and miles.”
“It’s definitely affected walking and anything that associated with walking more than a block or two. And I’m embarrassed, so I don’t tell anybody, and then I just say, ‘No, I can’t do that. No, I’m not up to that,’ and I don’t say why.”
For others, the impact of the disease on their QOL was relative to their advanced age or to a medical condition that they perceived as being more severe (e.g., cancer). Many individuals explained that they were more accepting of physical limitations they were experiencing because they expected that as they aged they wouldn’t be able to do as much as they did when they were younger. Despite the perceived personal and relative impact on QOL, most individuals had defined limits in place and spoke fondly of their “old life” and described a variety of activities they used to enjoy.
“I can’t go for a walk or go on a picnic and take a hike, and I used to like doing that stuff. I’m embarrassed that I don’t have the energy or the ability to do it and I don’t want to hold them back.”
“I can’t run, just run because it felt good. I can’t do it anymore. I can’t go hill climbing or anything…or hiking is something I would like to do.”
“I think it’s just a matter of becoming limited in what you’re going to do. You just don’t move around as much as you used to. And that’s basically the whole extent of what it’s done to me.”
Theme 6: Disease presence and treatment
Participants described their awareness of the disease and subsequent treatment to prevent further disease progression to varying degrees. Some individuals provided accurate physiological descriptions of the disease and appropriate lifestyle modifications in hopes of preventing disease progression and avoiding an increased chance of having a heart attack or stroke.
“It’s like a cramp. The legs are hollering because they’re not getting sufficient oxygen.”
“I always visualize a closure of a path that there’s not enough blood circulating and then the body is signaling starvation.”
Other participants expressed having knowledge of what they needed to do or should be doing, but acknowledged that they were unable or unwilling to change their behavior (e.g., quitting smoking).
“The doctor said ‘If you keep smoking, you’re going to have to redo [the surgery]’ so I started wearing support hose. But being a blockhead, I decided not to give up smoking yet.”
Some individuals were able to articulate the symptoms they experienced better than others. A few individuals struggled to describe their symptoms beyond “pain” or “hurt.” However, most of the individuals interviewed were able to provide very specific symptom descriptors regardless of their knowledge of the disease.
Discussion
The purpose of this study was to describe the symptom experience of individuals with PAD. Although a variety of vocabulary and phrases were provided during interviews, responses were categorized into six main themes. The six themes identified were closely aligned with the themes identified in a previous study by Treat-Jacobson and colleagues which was a broader exploration of the PAD experience and its perceived effects on health-related QOL.15 Participant characteristics of the present study are similar to this previous study, but Treat-Jacobson and colleagues included a larger percentage of females and conducted open-ended interviews with participants from two geographical locations.15 Participants provided similar descriptions of the symptom experience (e.g. cramping, aching, burning, cramping, and tired/fatigue), but provided a less exhaustive list compared to the participants in this study. Additionally, participants in the Treat-Jacobson et al.15 study only reported claudication in classic locations (e.g., calf, thigh, and buttock), as opposed to the broader list of locations (i.e., typical and ‘atypical’) provided by participants in this study.
Study findings also resembled the themes identified by a qualitative study conducted in 2005 that investigated the experience of living with PAD and the influence of the disease on activities of daily living.16 Participants described PAD as being physically, socially, and emotionally burdensome. Again, participants talked about the consequences of the disease and specific coping strategies designed to relieve the burden and gain control in daily life. This theme closely aligned with the maintaining equilibrium theme of this study and the adaptation theme of the Treat-Jacobson et al. study.15 Participants in the Wann-Hanson et al.16 study also talked about accepting and adapting to the burden, and discussed particular limitations in daily life because of the disease, which again closely aligned with themes identified in both of the aforementioned studies.
Symptom descriptors provided by participants in this study during interviews were consistent with the descriptors that typically indicate classic claudication (e.g., ache, cramp, pain, and tired/fatigue). The calf was the most commonly reported location of pain or discomfort, which is consistent with the classic definition of claudication. Many described the sensation as a pain, which is also consistent with classic claudication. However, nearly half described calf discomfort as tight (an atypical descriptor). In fact, this was the most frequently reported descriptor among men, women, and older participants, and the second most frequently reported descriptor among younger participants. Aside from the other classic claudication descriptors reported by participants (e.g., cramp, ache, and tired/fatigue), calf discomfort was described as sore, a Charley horse, weak, burning, hard, heavy, knotting, and pressure.
During interviews, participants reported discomfort in the two other commonly recognized locations, the buttock and the quadriceps. However, participants also reported discomfort in ‘atypical’ locations. The second most commonly reported location during interviews was the hip, followed by the foot. Other ‘atypical’ locations included the hamstring, shin, knee, ankle, and groin. Again, classic descriptions in ‘typical’ and ‘atypical’ locations were provided (e.g., ache, pain, and tired), but were not exhaustive. Similar to the calf, participants described the sensation as burning, tight, grinding, tingling, pulling, pressure, numb, prickly, and a twinge. Burning has previously been a descriptor of the PAD symptom experience,15,17,18 as well as tingling, numbness, throbbing, and shooting.1
Interestingly, the descriptions of lower extremity claudication that participants provided were nearly identical to the early descriptions of ischemic heart pain (i.e., angina) from the initial Rose Questionnaire.19 In the Rose study,19 participants described chest pain as, gripping, occasionally sharp, burning, sharp, shooting, kind of pricking, pins and needles, and like something pressure. Even on early surveys, ischemic heart pain descriptors such as tight, heavy, constricting, crushing, numbing, and burning, with the ability to radiate to other locations were recognized as being associated with angina. Lower extremity ischemia is a similar physiological process, restriction in blood supply to tissues causing a shortage of oxygen and subsequent discomfort, but it occurs in a different body location. Even without the provision of further objective evidence, these descriptions and qualities should also be more commonly accepted descriptions of ischemia occurring in the lower extremities.
Strengths and Limitations
Interviews enabled a rich description of the symptoms experienced by individuals with PAD, and the PAD questionnaire provided further details for each symptom described during the interview (e.g., location, duration, etc.). This approach allowed participants to describe PAD symptoms in their own words, as opposed to the restricted descriptions on claudication questionnaires. The descriptors and locations provided by participants extended beyond the commonly accepted language to describe ischemia in this patient population.
This study was limited by the inclusion of a small sample that was part of a larger study testing the impact of several exercise interventions that took place in one geographic location. Collecting data from individuals in a variety of geographical locations with more diverse backgrounds and a spectrum of disease severity would strengthen the generalizability of future studies. Another limitation is that all of the study participants were volunteer research subjects, which could have potentially introduced a response bias. Perhaps individuals with PAD that do not volunteer for research would report distinctly different PAD symptom experiences.
Conclusion
Despite these limitations, the findings from this study contribute to the gap in knowledge regarding the presentation of atypical claudication. Study participants provided typical and atypical descriptions of the PAD symptom experience. These findings have several implications for clinical practice. One, is the need for increased emphasis on individualized assessment of symptoms, using descriptive reporting, in conjunction with standard claudication questionnaires. The challenge for providers is to focus on the patient’s own words, despite any apparent deviations from currently accepted descriptions of claudication, in order to detect the potential presence of PAD based on the description provided by the patient. In summary, this study emphasizes the importance of subjective symptom reporting to gain an understanding of the array of symptoms experienced by individuals with PAD in hopes of improving the accuracy and timeliness of diagnosis and treatment of this debilitating disease. Future studies should include a larger more diverse sample and compare patterns of ischemia with patient reporting of symptoms in an attempt to validate the correspondence of atypical symptoms with ischemic changes in calf muscle during exercise to broaden the currently accepted locations and descriptors associated with PAD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schorr EN, Treat-Jacobson D. Methods of symptom evaluation and their impact on peripheral artery disease (PAD) symptom prevalence: A review. Vascular Medicine. 2013;18(2):95–111. doi: 10.1177/1358863X13480001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Naydeck BL, Sutton-Tyrrell K, Polak JF, Kuller LH Cardiovascular Health Study Research G. The role of comorbidity in the assessment of intermittent claudication in older adults. J Clin Epidemiol. 2001;54(3):294–300. doi: 10.1016/s0895-4356(00)00308-5. [DOI] [PubMed] [Google Scholar]
- 4.Collins TC, Petersen NJ, Suarez-Almazor M, Ashton CM. The prevalence of peripheral arterial disease in a racially diverse population. Arch Intern Med. 2003;163(12):1469–1474. doi: 10.1001/archinte.163.12.1469. [DOI] [PubMed] [Google Scholar]
- 5.Zatina MA, Berkowitz HD, Gross GM, Maris JM, Chance B. 31P nuclear magnetic resonance spectroscopy: Noninvasive biochemical analysis of the ischemic extremity. J Vasc Surg. 1986;3(3):411–420. doi: 10.1067/mva.1986.avs0030411. [DOI] [PubMed] [Google Scholar]
- 6.Holm S, Bylund-Fellenius A. Continuous monitoring of oxygen tension in human gastrocnemius muscle during exercise. Clinical Physiology. 1981;1(6):541–542. [Google Scholar]
- 7.Hiatt WR, Nawaz D, Brass EP. Carnitine metabolism during exercise in patients with peripheral vascular disease. J Appl Physiol. 1987;62(6):2383–2387. doi: 10.1152/jappl.1987.62.6.2383. [DOI] [PubMed] [Google Scholar]
- 8.Hiatt WR, Wolfel EE, Regensteiner JG, Brass EP. Skeletal muscle carnitine metabolism in patients with unilateral peripheral arterial disease. J Appl Physiol. 1992;73(1):346–353. doi: 10.1152/jappl.1992.73.1.346. [DOI] [PubMed] [Google Scholar]
- 9.Guba EG, Lincoln YS. Competing paradigms in qualitative research. Handbook of qualitative research. 1994;2:163–194. [Google Scholar]
- 10.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 11.Van Manen M. Researching Lived Experience: Human Science for an Action Sensitive Pedagogy. Albany, NY: Suny Press; 1990. [Google Scholar]
- 12.Sandelowski M. Qualitative analysis: What it is and how to begin. Res Nurs Health. 1995;18(4):371–375. doi: 10.1002/nur.4770180411. [DOI] [PubMed] [Google Scholar]
- 13.Henri F. Computer conferencing and content analysis. In: Kaye A, editor. Collaborative Learning through Computer Conferencing: The Najaden Papers. London: Springer-Verlag; 1991. pp. 117–136. [Google Scholar]
- 14.Lincoln YS, Guba EG. Naturalistic Inquiry. Newbury Park, CA: Sage; 1985. [Google Scholar]
- 15.Treat-Jacobson D, Halverson SL, Ratchford A, Regensteiner JG, Lindquist R, Hirsch AT. A patient-derived perspective of health-related quality of life with peripheral arterial disease. J Nurs Scholarsh. 2002;34(1):55–60. doi: 10.1111/j.1547-5069.2002.00055.x. [DOI] [PubMed] [Google Scholar]
- 16.Wann-Hansson C, Hallberg I, Klevsgård R, Andersson E. Patients’ experiences of living with peripheral arterial disease awaiting intervention: A qualitative study. Int J Nurs Stud. 2005;42(8):851–862. doi: 10.1016/j.ijnurstu.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Ruger LJ, Irnich D, Abahji TN, Crispin A, Hoffmann U, Lang PM. Characteristics of chronic ischemic pain in patients with peripheral arterial disease. Pain. 2008;139(1):201–208. doi: 10.1016/j.pain.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Tomczyk S, Treat-Jacobson D. Claudication symptom experience in men and women: Is there a difference? J Vasc Nurs. 2009;27(4):92–97. doi: 10.1016/j.jvn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Rose G. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]