Abstract
Previous work demonstrates that spatial (explicit) and non-spatial (implicit) elements of place learning in the Morris water maze (MWM) task can be dissociated and examined in the context of experimental traumatic brain injury (TBI). Providing non-spatial cognitive training (CT) after injury can improve place learning compared to untrained controls. In the present study, we hypothesized that brief exposure to extra-maze cues, in conjunction with CT, may further improve MWM performance and extra-maze cue utilization compared to CT alone. Adult male Sprague-Dawley rats (n=66) received controlled cortical impact (CCI) injury or sham surgery. Beginning D8 post-surgery, CCI and Sham rats received 6 days to no training (NT) or cognitive training with/without brief, non-contextualized exposure to extra-maze cues (BE and CT, respectively). Acquisition (D14-D18), Visible Platform (VP; D19), Carryover (CO; D20-D26), and periodic probe trials were performed. Platform latencies, peripheral and target zone time allocation, and search strategies were assessed. CCI/BE rats had shorter acquisition trial latencies than CCI/NT (p<0.001) and tended to have shorter latencies than CCI/CT rats (p<0.10). Both BE and CT reduced peripheral zone swimming for CCI rats vs. CCI/NT. CCI/BE animals increased spatial swim strategies from D14 to D18 relative to CCI/CT and showed similar swim strategy selection to the Sham/NT group. These data suggest that visual priming improves initial place learning in the MWM. These results support the visual priming response as another clinically relevant experimental rehabilitation construct, to use when assessing injury and treatment effects of behavioral and pharmacological therapies on cognition after TBI.
Keywords: Traumatic Brain Injury, Cognitive Training, Visual Priming, Implicit Learning
1. Introduction
Traumatic brain injury (TBI) is a prominent health issue that results in significant physical and cognitive deficits.1 Many survivors have impaired memory, which can have profound effects on daily function,2,3 and place emotional and financial strain on individuals with TBI, their families, and society.4 The controlled cortical impact (CCI)5 injury model of TBI creates reproducible damage to the hippocampus, a subcortical structure critical for memory formation.6 As such, it is often used to study memory deficits in rodents, and more recently, to design and evaluate rehabilitation paradigms to treat memory problems.7,8,9
TBI results in explicit and implicit memory impairments. Implicit memory does not require conscious recollection and is associated with automatic processing, while explicit memory necessitates conscious recall of previous experiences and is related to controlled processing.4,10 These two systems are distinct, but interact cooperatively for normal functioning.4 Implicit memory tends to be preserved post-injury, while explicit memory and controlled processing tend to be impaired.11 Utilizing implicit processing during a learning and memory task may reflect how attention is preferentially allocated to implicit or explicit components of solving the task.4
The Morris water maze (MWM) is widely used to assess learning and memory, particularly hippocampal-dependent spatial (explicit) memory.12 Learning the platform location, or place learning, in the MWM requires behavioral components associated with implicit processing: thigmotaxis suppression, search strategy formulation, and recognizing the platform as the solution to the task. However, to solve the maze effectively, animals can use explicit processing of distal spatial cues to acquire spatial maps important for place learning.13 Rats with fimbria fornix lesions in the hippocampal formation, can learn behavioral strategies to achieve some ability for place learning, but cannot match the performance of controls in acquiring an accurate spatial map.13
Previously, we tested the effect of an implicit training paradigm, administered before and after CCI injury, on implicit and explicit aspects of learning and memory during the MWM (Wagner et.al., 2013; Brayer et.al., in press). Although trained rats exhibited shorter platform latencies compared to untrained controls, injured rats exhibited deficits incorporating extra-maze cues into their search strategy relative to shams.14,15 Here, we extend previous work to investigate if cue utilization can be further improved through specific implicit training procedures.
Priming is a technique used to train and test implicit memory by providing non-contextualized information designed to elicit a guided response.10,16,17 Graf and Schacter showed visual word-stem priming improved subsequent recall of paired words in amnesic patients.10 These results indicate that task completion does not require intentional retrieval of information, or explicit processing. Rather, tasks can be effectively completed using implicit memory capacity related to previous associations or experience.
We paired visual priming with our previously reported implicit training paradigm using non-contextualized extra-maze cues. Animals were “visually primed” through exposure to extra-maze cues, on the first two days of a 6-day training regimen. We hypothesized that adding visual priming would 1) decrease platform latency, 2) increase target zone time allocation, and 3) increase spatial swim strategy selection. Results suggest that priming increases the initial incorporation of spatial cues to locate the platform. These findings extend our experimental model for implicit cognitive training after TBI, and demonstrate translational relevance to clinical cognitive rehabilitation paradigms.
2. Materials and Methods
2.1 Animals & Experimental Groups
Sixty-six male Sprague-Dawley rats were pair-housed in 12/12 light-dark cycle with ad libitum access to food and water. Animals were divided into 6 groups based on surgery (CCI or Sham) and training treatment (Cognitive training (CT), Brief Cue Exposure (BE), or No Treatment (NT)); CCI/BE (n=14), CCI/CT (n=14), CCI/NT (n=12), Sham/BE (n=8), Sham/CT (n=8), Sham/NT (n=10). All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
2.2 Controlled Cortical Impact Surgery
The CCI model was used as previously.5,7,14 Rats underwent either a unilateral parasagittal CCI or sham surgery, with an impact depth of 2.8mm and velocity of 4.0m/s. Shams underwent identical procedures except the impact. Post-operative flexion and righting reflexes were monitored as previously described.14 No differences in reflexes existed among CCI or Sham groups (data not shown).
2.3 Behavioral testing
Behavioral testing occurred daily between 7:00-11:00 AM by experimenters blinded to treatment groups.
2.3.1 Motor Testing
Beam balance and beam walk tasks were used as previously described to assess gross and fine motor function following injury.8,18 Animals were pre-trained on each task one day prior to surgery. Beam-balance (BB) and beam-walk (BW) testing occurred on post-injury days 1-6. BB was tested in three 60-second trials by placing the rat on an elevated narrow wooden beam and recording the time that the rat maintained balance on the elevated beam.
The beam-walk pre-training began with a 60-second habituation period, during which the animal was placed in a dark, enclosed goal box and was exposed to adverse stimuli (white noise, bright light) upon exit from the box. Training trials were conducted until the average latency to traverse the beam was 5 seconds. Rats were pre-assessed the morning of surgery to determine baseline performance for both tasks. On post-surgery testing days, each task was assessed for 3 consecutive trials, with a maximum balance time or latency of 60 seconds for BB and BW, respectively.
2.3.2 Morris Water Maze Training
We used the MWM previously to assess place learning and the effects of training on place learning performance with and without extra-maze cues affixed to the walls.14,15 The MWM pool (180 cm diameter) was filled with water (26±1°C) and contained a platform, submerged 2cm under the water surface unless otherwise noted. Extra-maze cues were affixed to the walls around the pool and consisted of high-contrast 2-dimensional geometric shapes. All trials were recorded using ANY-maze video-tracking software (Stoelting).
Cognitive Training Protocol
Cognitive Training (CT) was conducted on D8-D13 post-surgery (Figure 1A). CT exposed rats to implicitly learned task components, including exploratory behavior/suppression of thigmotaxis, mounting the platform, and search strategy formation, as reported previously (Brayer et.al., in press). During CT, rats entered the pool at a static entry point with dynamic, randomized platform locations for 4 trials/day. Extra-maze cues were covered during training, and efforts were made to eliminate incidental cues (e.g. shadows, corners).
Figure 1.

(A) Graphic explanation of interventions and testing for the 3 treatment groups. One injured and one sham group was assigned to each treatment profile. (B) Schematic of the Morris water maze with specific zones indicated. (A) Target quadrant (Southwest) for acquisition and visual platform days, (B) peripheral zone used to determine percent time allocation (TA), (C) target zones utilized to determine target zone TA for acquisition and VP (Southwest) and carryover trials (Northeast), which do not include peripheral zone portion of each specific quadrant as indicated. (TA=time Allocation)
Visual priming via brief exposure (BE) to extra-maze cues occurred on D8-D9 (Figure 1A). During each of the 4 trials/day provided on these days included the presence of extra-maze cues. When paired with a CT protocol that uses a dynamic randomized platform location for each trial, this additional priming component provided non-contextualized extra-maze cue exposure during pool training to acclimate rats to these cues while precluding spatial map formulation about platform location. BE animals otherwise received the same treatment as the CT groups on D10-D13. Rats that did not receive CT or BE were handled and placed in warming boxes used between trials for 10 minutes on each training day.
2.3.3 Morris Water Maze Testing
All rats underwent acquisition trials on D14-D18 with extra-maze cues with a static platform location (SW quadrant) and pseudo-randomized entry point (N,S,E,W) (Figure 1B). On D19 rats underwent visible platform (VP) trials. Conditions were identical to acquisition trials, except the platform was raised 2cm above the water surface and made visible by white tape placed around its edge. All rats underwent carryover (CO) trials on D20-26. CO trials assessed the ability to generalize platform search strategies from the acquisition trials to a novel situation. Test conditions were identical to acquisition trials, except for a new static platform location (NE quadrant) and novel extra-maze cues (Figure 1B). Novel extra-maze cues were similar to acquisition cues, but different in geometric shape.
For each of the four daily acquisition and CO trials, rats were placed in the water hindquarters first, facing the wall of the pool. Maximum trial duration was 120 seconds. Upon location of the platform a 30s platform habituation period was observed, after which rats were placed in a warming box for a 4 minute inter-trial interval. Animals unable to find the platform within 120s were manually guided there prior to the 30s habituation period. After CO trial completion, rats also completed 30s probe trial to evaluate short term retention on D24. The platform was removed from the pool, and rats were placed in the pool at the north entry point.
Latency to find the platform was recorded for all platform trials. For both platform and probe trials, we assessed peripheral zone time allocation (TA), which was defined as the percentage of each trial that rats spent in the outer 29% of the pool. We also recorded average percent TA in the target zone, which comprised the area of the pool containing the platform location, but did not include the peripheral zone associated with that quadrant (Figure 1B&C).
2.4 Qualitative Swim Strategy Analysis
Qualitative swim strategy analysis was completed for D14, D15, D18, D19, D20, D21 and D26 using ANY-maze software. Swim strategy categories were assigned as thigmotaxic, non-spatial, or spatial for each trial.19,14 For ambiguous runs, a video of the trial itself was utilized. An investigator blinded to treatment group determined the predominant swim strategy exhibited during each trial using a classification schema previously described.14,15
2.5 Statistical Analysis
Statistical analyses were completed using SPSS Statistics Version 22 and SAS Version 9.4. Data are presented as mean±standard error of the mean (SEM) and as TA percent. We examined treatment effects (BE/CT/NT) and injury status on platform latencies, average peripheral zone TA, target zone TA, and swim strategy utilization.
Group differences in quantitative motor and MWM performance due to injury status were assessed using repeated measures analysis of variance (ANOVA). Sphericity was evaluated for within-subject comparisons, and main effects were adjusted using Greenhouse-Geisser correction where appropriate. When significant main effects were found (p<0.05), post-hoc pair-wise analyses were completed to examine differences between specific injury/treatment groups using Fisher's least significant difference (LSD). Single factor ANOVA with Fishers LSD was used to assess behavioral performance among groups within each trial day to further describe pairwise comparisons that account for post-hoc comparisons conducted with RMANOVA
For swim strategy, trials were categorized into thigmotaxic, spatial, or non-spatial, and percentages for each category were calculated for each testing day by group. Generalized estimating equations (GEE) were used to examine the effects of injury and treatment on swim strategy, while accounting for repeated measures (i.e. four trials/day). Swim strategy was treated ordinally, assuming thigmotaxis was the “least spatial” strategy, followed by non-spatial and spatial. GEE methods modeled the probability of selecting a more spatial strategy. GEE analysis examined treatment effects (NT/CT/BE) on swim strategy during acquisition, VP, and CO trials, stratifying by injury status. Additional GEE analyses examined group effects on swim strategy for specific testing days, accounting for repeated measures. Odds ratios were calculated using GEE results.
All post-hoc analyses were adjusted for multiple comparisons using FDR.
3. Results
3.1 Motor Testing
Group, day, and group*day interaction effects were observed for beam balance latency, beam walk latency, and beam walk score (p<0.05 all comparisons).As expected, there were significant differences between injured and sham groups for beam balance latency, beam walk latency, and beam walk score (p<0.05) on all test days with the exception of D5. By D5 of motor testing, CCI rats were not significantly different than sham animals on beam balance latency (data not shown).
3.2 Platform latencies
3.2.1. Acquisition Latencies
There was a main effect of group, day, and group*day interaction on hidden platform latencies during acquisition trials (p<0.001, all effects). The CCI/NT group performed worse than all other groups (p<0.001, all comparisons); (Figure 2A).
Figure 2.
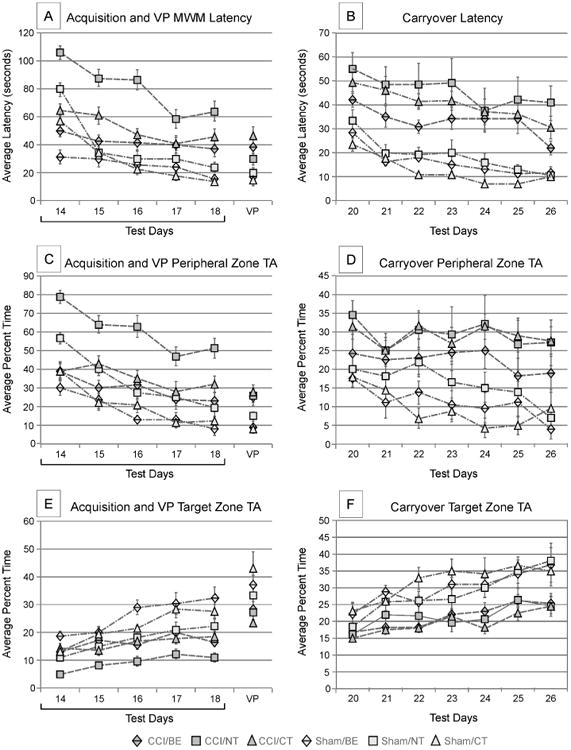
(A) Acquisition and visual platform trial latencies - CCI/NT group performed worse than all other groups (p<0.001, all comparisons). CCI/BE had shorter latencies than both CCI/CT (p<0.10) and CCI/NT (p<0.001). Sham/BE has shorter latencies than Sham/NT (p<0.05). CCI/BE and CCI/CT significantly differed on D14 and D15 (p<0.05). CCI/BE performed similarly to Sham/NT on acquisition latencies. During the visual platform trials, CCI/NT was similar to CCI/BE and marginally different from CCI/CT (p<0.10). Error bars designate standard error of the mean. (B) Carryover trial latencies – Sham animals performed better than injured animals (p<0.05). CCI/BE had significantly shorter latencies than Sham/NT in CO (p<0.05). Error bars designate standard error of the mean. (C) Peripheral Zone TA during acquisition and visible platform trials – CCI/NT rats spent the greatest time in the peripheral zone (p<0.001, all comparisons). CCI/BE and CCI/CT did not differ on Peripheral Zone TA. Sham animals spent less time in the periphery than injured animals during the VP trials for CT and BE treatments (p<0.05). CCI/CT and CCI/BE did not differ on Peripheral Zone TA in VP trials. CCI/NT animals did not differ from other injury groups during VP. Error bars designate standard error of the mean. (D) Carryover trial Peripheral Zone TA – No differences in Peripheral Zone TA between any CCI groups or among sham groups. CCI/BE and Sham/NT performed similarly. Error bars designate standard error of the mean. (E) Target Zone TA during acquisition and visual platform trials – CCI/NT rats had lower Target Zone TA than all other groups (p<0.001, all comparisons). The CCI/CT and CCI/BE groups did not differ on acquisition Target Zone TA. CCI/BE and CCI/CT performed similarly to Sham/NT on acquisition Target Zone TA. Sham/BE performed marginally better than Sham/CT in Target Zone TA during acquisition trials (p<0.10). During VP trials, all injured groups performed similarly as did all uninjured groups. Error bars designate standard error of the mean. (F) Carryover trial Target Zone TA – All sham groups spent more time in the target zone than injured groups with similar treatment (p<0.05, all comparisons). No differences existed among the injured rats in CO Target Zone TA. Error bars designate standard error of the mean. (CCI=controlled cortical impact; NT=no training; CT=cognitive training; BE=brief exposure; VP=visible platform; TA=time allocation; CO=carryover)
Acquisition latency times were marginally different between CCI/BE and CCI/CT groups (p<0.10); CCI/BE rats significantly outperformed CCI/NT rats (p<0.001). The Sham/BE group also outperformed Sham/NT rats (p<0.05). However, Sham/BE acquisition latencies did not differ from the Sham/CT group. CCI/BE rats had shorter average latencies compared to the CCI/CT group on the first two days of acquisition, D14-D15 (p<0.05). Similar effects were observed with Sham/CT vs. Sham/BE rats on D14 (p<0.05). These data indicate that BE priming effects contributed to place learning curve reductions, especially during early acquisition days. Notably, there were latency differences for CCI/BE and Sham/BE groups on D14 (p<0.05), which likely represents residual spatial learning deficits associated with CCI. CCI/BE animals did not differ from Sham/NT rats on overall acquisition latencies, while CCI/CT did differ significantly compared to Sham/NT rats (p<0.05), suggesting that BE further alleviated injury-induced place learning deficits relative to CT effects alone.
3.2.2 Visual Platform Latencies
There was a main effect of group with VP latencies (p<0.001; Figure 2A). Pair-wise comparisons showed no differences in VP latency between CCI/BE and CCI/NT groups and only marginal differences between CCI/CT and CCI/NT groups (p<0.10). There were no differences in VP latency between CCI/BE and CCI/CT rats or among Sham groups, suggesting that priming with extra-maze cues did not influence ability to recognize the VP as an egocentric cue.
3.2.3 Carryover Latencies
There was a main effect of group and trial day (p<0.001 each) on carryover latencies (Figure 2B). All Shams had lower latencies than injured rats (p<0.05, all comparisons). No differences were observed between CCI/BE and CCI/CT. However, differences were noted for CCI/BE versus CCI/NT groups in CO trial latency (p<0.05). Also, no differences were observed between CCI/CT and CCI/NT groups. CCI/BE had higher latencies than Sham/NT rats (p<0.05). Together these results indicate that BE improved CO latencies vs. CCI/NT, yet, BE status did not further facilitate new learning during CO trials over CT relative to shams. However, the cue exposure with BE, combined with training provided through acquisition trials, provided CCI/BE rats a significant advantage with CO performance compared to CCI/NT rats
3.3 Peripheral Zone Time Allocation
3.3.1 Acquisition Trials
There was a main effect of group (p<0.001), trial day (p<0.001), and a group*day interaction (p=0.001) on peripheral zone TA during acquisition trials (Figure 2C). CCI/NT rats spent a higher percentage of time in the periphery relative to other groups (p<0.001, all comparisons), indicating both training treatments effectively suppressed thigmotaxis. CCI/BE performed similarly to CCI/CT rats. Also, Sham/BE performed similarly to Sham/CT rats. However, Sham/NT rats had larger peripheral zone TAs relative to Sham/BE (p<0.05) and marginally different peripheral zone TAs compared to Sham/CT rats (p<0.10). There were significant differences between CT injured and sham rats (p<0.05) and marginal differences between BE injured and sham rats (p<0.10). Interestingly, on D14, CCI/CT and CCI/BE groups had lower peripheral zone TA than Sham/NT (p≤0.05).
3.3.2 Visual Platform Trials
There was a main effect of group with VP peripheral zone TA (p=0.001; Figure 2C). Shams spent less time in the periphery than injured rats (p<0.001). CCI/BE rats spent more time in the periphery than Sham/BE (p<0.05), and the same pattern was observed with CCI/CT and Sham/CT (p<0.05). CCI/BE rats performed similarly to CCI/CT and Sham/BE rats performed similarly to Sham/CT rats, suggesting both treatments effectively reduce thigmotaxis during VP trials. Finally, CCI/NT rats did not differ from the treated injury groups with peripheral zone TA.
3.3.3 Carryover Trials
There was a main effect of group (p=0.001) and trial day (p<0.001) with average percent TA spent in the peripheral zone (Figure 2D). There were no differences among CCI groups or Shams. Shams performed better than all CCI groups in pair-wise comparison (p<0.05) with the exception of CCI/BE compared to Sham/BE and Sham/NT rats, which performed similarly.
Target Zone Time Allocation
3.3.4 Acquisition Trials
There was a main effect of group (p<0.001), trial day (p<0.001), and a group*day interaction (p<0.05) on target zone TA (Figure 2E). CCI/NT rats had lower target zone TAs than all other groups, including CCI/BE and CCI/CT (p<0.001, all comparisons) (Figure 2E). There was no difference in target zone TA between CCI/BE and CCI/CT. There were also no performance differences between CCI/BE and Sham/NT or CCI/CT and Sham/NT, indicating that both treatments may have the ability to partially compensate for injury-related deficits in localizing the general platform area during acquisition trials. However, each CCI group performed worse compared to their respective Shams.
3.3.5 Visible Platform Trials
There was a main effect of group with VP target zone TA [p<0.05 (Figure 2E)]. While CCI/NT had lower target zone TA during acquisition trials, this group did not perform differently from CCI/BE or CCI/CT on VP trials. Sham/BE also did not outperform Sham/CT or Sham/NT. Also, the training paradigms did not enhance navigation to the VP as an egocentric cue when presented in the context of extra-maze cues.
3.3.6 Carryover Trials
A main effect of group (p<0.001) and trial day (p<0.001) was observed for target zone TA during CO trials. All Shams spent more time in the target zone than their CCI counterparts (p<0.05, all comparisons). There were no differences between treatment groups within injured rats or between sham rats. Overall, Sham groups appear to spend increasingly more time in the target zone throughout CO days while CCI group performance plateaued (Figure 2F).
3.3.7 Short-term Memory Retention
There was a main effect of group on target zone TA during the D24 probe following CO trials (p<0.05). There was a detrimental injury effect for CCI/CT and CCI/NT on short-term memory retention (p<0.05). However, CCI/BE compared to Sham/BE did not show a significant injury effect (p<0.10).
3.4 Swim Strategy Selection
All CCI groups showed some thigmotaxis on D14 (CCI/BE=1.7%, CCI/CT=3.6%, and CCI/NT=41.7%), which was largely absent by D18; 0%, 0%, 2.1%, respectively). Thus, both treatments effectively reduced thigmotaxis over testing days among injured animals. All injured groups increased spatial strategy utilization over the acquisition trial period (Figure 4). Sham/BE, Sham/CT, and Sham/NT groups displayed 50.1%, 34.4%, and 18.4% spatial swim strategy selection on D14 and 75%, 87.5%, and 55% on D18, respectively. However, CCI/BE rats performed comparably to Sham/NT rats on both D14 and 18.
Figure 4.
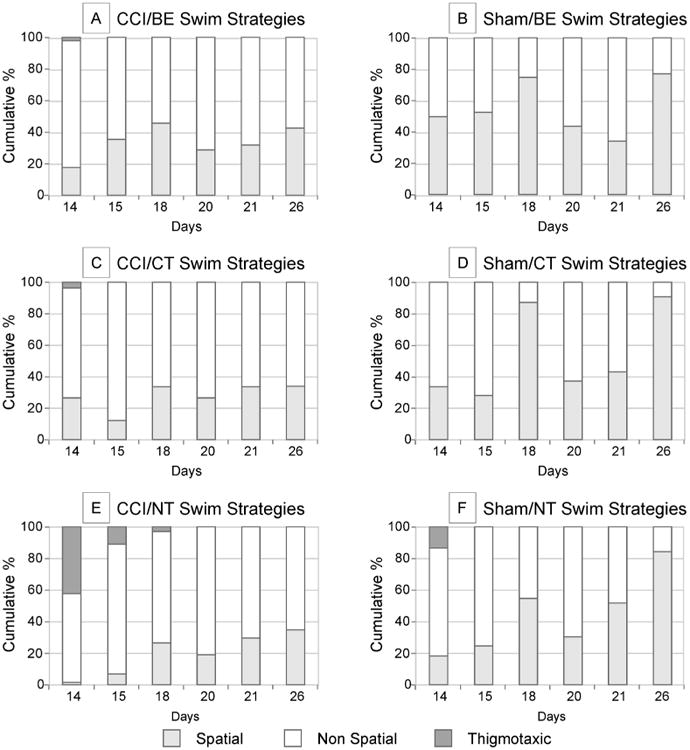
Swim strategy selection distributions by group on specific acquisition and carryover trial days. By the last day of acquisition (D18), CCI/BE and Sham/NT rats had comparable spatial swim strategy selection. At the end of CO (D26), Sham/NT displayed more spatial strategy selection then CCI/BE (p<0.05). On D14, CCI/BE did not perform significantly better than CCI/CT, but on D15 the two treatments diverged in relation to strategy selection as spatial strategy increased for the BE group over CT (p<0.05). (CCI=controlled cortical impact; NT=no training; CT=cognitive training; BE=brief exposure; TA=time allocation; CO=carryover)
CCI/BE rats had the greatest increase in spatial strategy selection (+28.6%) and the highest percentage of spatial strategy use among injured groups on D18 (46.3%). A similar pattern for CCI/BE animals emerged across CO testing days, where CCI/BE animals returned to comparable spatial strategy utilization with CO vs. acquisition trials (D26=42.7%). However, shams had greater spatial strategy selection on CO day 26 relative to CCI rats (Figure 4). When inspecting the acquisition data for day 18, CCI/BE rats performed similarly to Sham/NT rats on strategy selection; on CO day 26, spatial strategy utilization was lower for CCI/BE versus Sham/NT (CCI/BE=42.7%, Sham/NT=85%).
Swim strategy selection between groups was compared quantitatively using GEE. Among injured animals, both BE and CT (p<0.05, OR: 5.50 and 3.98, respectively) had a significant effect on strategy selection compared to NT during acquisition. When comparing CCI/BE to CCI/CT, there were no overall treatment effects on swim strategy.
To examine more specific relationships in strategy adoption between groups, GEE was conducted on single test days. These showed treatment effects (BE versus CT) for injured animals on D15 (p<0.05, OR: 3.81), demonstrating that priming increases early initial adoption of spatial navigation among injured animals. Similarly on D15, sham/BE rats were more likely to choose a spatial strategy compared to sham/CT rats (p<0.05, OR: 2.90). Swim strategy selection was not different between CCI/BE and Sham/NT on D18. However, the same comparison on D26 demonstrates the odds of CCI/BE animals using a more spatial strategy are significantly decreased compared to Sham/NT animals (p<0.05, OR: 0.13), suggesting shams continue to incorporate more spatial strategies over testing days while CCI/BE animal performance plateaus. Similarly, CCI/CT rats had comparable spatial strategy use to Sham/NT on D18, but the odds of CCI/CT rats selecting a more spatial strategy were lower by D26 compared to Sham/NT (p<0.001, OR: 0.09), illustrating CCI/CT animals also plateau with spatial strategy utilization during CO trials.
4. Discussion
The current study validates previously reported findings15 and advances the CT paradigm by adding visual cue priming (BE) to improve extra-maze cue utilization and MWM performance for injured rats. Results indicate that visual priming during implicit cognitive training improves MWM performance in injured rats over CT alone, specifically with initial acquisition platform latencies and early spatial swim strategy adoption. CT allowed rats to learn the implicit components of the task, including appropriate behavioral search strategies and thigmotaxis suppression while precluding spatial mapping. BE was designed to increase salience of these cues when presented during testing trials. BE improved platform latencies in both injured and sham.14,15,20 CCI/BE rats displayed shorter latencies than CCI/CT rats, primarily on the first two days of acquisition, suggesting that BE reduces the initial place learning burden, such that CCI/BE rats did not differ from Sham/NT rats. Additionally, thigmotaxis suppression was similar among CT and BE groups for CCI and Sham rats. BE and CT rats exhibited increased target zone TA, and did not differ on this measure during acquisition, VP, or CO trials, indicating that both treatments conferred benefit in locating the general platform location. Similar target zone TA for BE and CT animals during the VP trial may be due to their inability to recognize the VP as an egocentric cue.14 Target zone TA was similar between BE and CT groups with CO trials. However, D24 BE probe trial target zone TA data showed improved short-term memory retention with CCI/BE versus Sham/BE relative to both CCI/CT and CCI/NT rats versus their shams (Figure 3). These findings suggest CCI/BE functions as an intermediate group with probe trial performance, such that it neither differed from other injury or sham groups. The data also suggest that while target zone TA acquisition was not measurably different, BE may support better memory consolidation compared to other CCI groups on this probe trial.
Figure 3.

Target zone TA data from probe conducted after D24 of CO trials. Error bars designate standard error of the mean. CCI/CT vs. Sham/CT (p<0.001) and CCI/NT vs. Sham/NT (p<0.05) revealed a detrimental effect of injury on short-term memory retention. CCI/BE compared to Sham/BE did not show an injury effect (p<0.10). (CCI=controlled cortical impact; NT=no training; CT=cognitive training; BE=brief exposure; TA=time allocation; CO=carryover)
Brief exposure to non-contextualized extra-maze cues increased spatial strategy selection among injured animals (Figure 4). We hypothesized CT and BE would suppress thigmotaxis and allow animals to adopt a search strategy. CCI/BE rats exhibited the largest increase and highest overall spatial strategy selection across all injured groups during acquisition, and highest percentage on D26 CO trials, suggesting an additional benefit of increased generalizability of extra-maze cue utilization to novel testing conditions. Still, CCI rats plateau with cue utilization relative to shams. One possibility for this finding is that BE treatment decreases pliancy deficits among injured rats, an area in which hippocampal damage can have marked deficits.21,22 We specifically explored spatial strategy selection on D14-D15 and found that CCI/BE rats adopt spatial strategies earlier in the acquisition phase than do CCI/CT rats. The frequency of spatial selection on D26, and lower (but not significant) platform latencies, also suggest a longer-term benefit of BE over CT for spatial navigation. These findings may carry some translational relevance to clinical studies assessing strategy training effects on elements like “far transfer,” or evidence that learning from training can be applied in new contexts.23
While not always significantly different from CCI/CT, CCI/BE rats behaved similarly to untrained sham controls in several measures. D18 acquisition trial performance shows that CCI/BE rats did not differ from Sham/NT with spatial strategy percentages. In contrast, differences in multiple endpoints were noted for CCI/BE vs. Sham/NT for D26, the last day of CO trials, suggesting that CCI animals reached a performance limit during CO trials that did not occur for uninjured animals. CCI/NT rats also performed similarly to CCI/CT rats during CO trials, suggesting some training benefits for NT rats during acquisition trials. CCI/BE rats, though, performed better than CCI/NT rats on carryover trials, suggesting that the combination of visual priming, followed by acquisition trial training associated with BE provided an advantage over CT on CO trials. Future work designed to extend priming effects over CO trials is an important future consideration to identify treatment constructs that have a lasting and generalizable effect on cognitive recovery.24,25
Although CCI/NT rats performed worse than all other groups in acquisition latencies, during VP trials they performed similarly to CCI/BE and better than CCI/CT rats. This finding may be attributable to reduced pliancy among trained CCI rats 22, where CT and BE training paradigms may reduce an injured animal's cognitive flexibility to utilize the VP as an egocentric cue, due to prior training involving exposure to extra-maze (allocentric) cues and/or preference toward the trained use of non-spatial strategies. The CCI/NT group's lack of implicit training may have improved set-shifting ability for NT rats during this VP task, where the cue type is novel and all animals must alter their adopted strategies. Pliancy, or cognitive flexibility, needed to solve the VP task remained intact for trained sham rats.
In summary, our findings provide strong evidence that post-injury visual priming augments implicit cognitive training to improve learning tasks that require spatial navigation after experimental TBI. Implications for clinical rehabilitation include the clinical use/integration of therapeutic implicit learning approaches where procedural, patterning, or strategy focused training is primed with non-contextualized learning content, with the goal of improving adaptation and/or carryover of strategies, procedures, and/or patterns to new learning and to performing a range of specific functional tasks. Future studies will evaluate how variations on timing and the priming strategy itself, including increased duration of training and incorporation of contextual interference, further improve MWM performance, pliancy with strategy selection, and strategy generalization across testing conditions in our injury model.
Table 1.
Main effects for quantitative MWM data.
| Main effects | Group effect | Time effect | Group*Time interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial Type | DF | F-value | P-value | DF | F-value | P-value | DF | F-value | p-value |
| Acquisition trials | |||||||||
| Latencies | 5, 60 | 26.869 | p<0.001 | 4, 240 | 47.023 | p<0.001 | 20, 240 | 3.969 | p<0.001 |
| Peripheral Zone TA | 5, 60 | 16.726 | p<0.001 | 4, 240 | 52.818 | p<0.001 | 20, 240 | 2.521 | p<0.05 |
| Target Zone TA | 5, 60 | 16.507 | p<0.001 | 3.472, 208.307 | 28.246 | p<0.001 | 17.359, 208.307 | 1.74 | p<0.05 |
| Visible Platform trials | |||||||||
| Latencies | 5, 60 | 5.23 | p<0.001 | ||||||
| Peripheral Zone TA | 5, 60 | 4.985 | p<0.05 | ||||||
| Target Zone TA | 5, 60 | 3.439 | P<0.05 | ||||||
| Carryover trials | |||||||||
| Latencies | 5, 60 | 12.468 | p<0.001 | 4.413, 264.786 | 11.541 | p<0.001 | 22.065, 264.786 | 0.463 | p=0.983 |
| Peripheral Zone TA | 5, 60 | 5.118 | p<0.05 | 6, 360 | 5.607 | p<0.001 | 30, 360 | 1.062 | p=0.381 |
| Target Zone TA | 5, 60 | 7.974 | p<0.001 | 4.557, 273.412 | 22.492 | p<0.001 | 22.784, 273.412 | 0.969 | p=0.505 |
Table 2. Generalized Estimating Equation (GEE) Results Summary.
| Day 14 | Day 15 | |||
| P | OR | P | OR | |
| CCI BE* | 0.082 | 0.68 | <0.05 | 3.81 |
| Sham BE∧ | 0.267 | 1.91 | <0.05 | 2.90 |
| Day 18 | Day 26 | |||
| P | OR | P | OR | |
| CCI BE‡ | 0.545 | 0.71 | <0.05 | 0.13 |
| CCI CT‡ | 0.228 | 0.42 | <0.001 | 0.09 |
Compared to CCI CT;
Compared to Sham CT;
Compared to Sham NT
Acknowledgments
This work was supported by NIH grant NIH R21HD071728.
Abbreviations List
- TBI
traumatic brain injury
- MWM
Morris water maze
- CT
Cognitive training
- CCI
controlled cortical impact
- NT
No training
- BE
Brief Exposure to extra-maze cues during CT trials
- VP
Visible platform trials
- CO
Carryover trials
- BB
Beam Balance
- BW
Beam Walk
- TA
time allocation
- FDR
False Discovery Rate
References
- 1.Bedi SS, Hetz R, Thomas C, et al. Intravenous multipotent adult progenitor cell therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. Stem Cells Tranls Med. 2013;2:953–60. doi: 10.5966/sctm.2013-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monti JM, Voss MW, Pence A, McAuley E, Kramer AF, Cohen NJ. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front Aging Neurosci. 2013;5:1–9. doi: 10.3389/fnagi.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen X, Li A, Zhang Y, et al. The effect of different intensities of treadmill exercise on cognitive function deficit following a severe controlled cortical impact in rats. Int J Mol Sci. 2013;14(11):21598–612. doi: 10.3390/ijms141121598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitter-Edgecombe M. Implications of basic science research for brain injury rehabilitation: A focus on intact learning mechanisms. J Head Trauma Rehab. 2006;21(2):131–41. doi: 10.1097/00001199-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Meth. 1991;39(3):253–62. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 6.Albasser MM, Dumont JR, Amin E, et al. Association rules for rat spatial learning: the importance of the hippocampus for binding item identity with item location. Hippocampus. 2013;23(12):1162–1178. doi: 10.1002/hipo.22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darrah SD, Chuang J, Mohler LM, Chen X, Cummings EE, Burnett T, Wagner AK. Dilantin therapy in an experimental model of traumatic brain injury: effects of limited versus daily treatment on neurological and behavioral recovery. J Neurotraum. 2011;28(1):43–55. doi: 10.1089/neu.2010.1521. [DOI] [PubMed] [Google Scholar]
- 8.Wagner AK, Kline AE, Sokoloski J, Zafonte RD, Copulong E, Dixon CE. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci Lett. 2002;334(3):165–8. doi: 10.1016/s0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- 9.Wagner AK, Drewencki LL, Chen S, et al. Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J Neurochem. 2009;108(4):986–97. doi: 10.1111/j.1471-4159.2008.05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graf P, Schacter DL. Implicit and explicit memory for new associations in normal and amnesic subjects. J Exp Psychol Learn Mem Cogn. 1985;11:501–18. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- 11.Vakil E. The effect of moderate to sever traumatic brain injury (TBI) on different aspects of memory: a selective review. J Clin Exper Neuropsyc. 2005;27(8):977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- 12.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cain DP, Boon F, Corcoran ME. Thalamic and hippocampal mechanisms in spatial navigation: A dissociation between brain mechanisms for learning how versus learning where to navigate. Behav Brain Res. 2006;170:241–56. doi: 10.1016/j.bbr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Wagner AK, Brayer SW, Hurwitz M, et al. Non-spatial pre-training in the water maze as a clinically relevant model for evaluating learning and memory in experimental traumatic brain injury. Neurobiol Learn Mem. 2013;106:71–86. doi: 10.1016/j.nlm.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brayer S, Ketcham K, Zou H. Developing a clinically relevant model of cognitive rehabilitation training after experimental traumatic brain injury. 2013 doi: 10.1177/1545968314550367. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitter-Edgecombe M. Effects of divided attention on implicit and explicit memory performance following severe closed head injury. Neuropsychology. 1996;10(2):155–67. doi: 10.1017/s1355617700000965. [DOI] [PubMed] [Google Scholar]
- 17.Vakil E, Sigal J. The effect of level of processing on perceptual and conceptual priming; control versus closed-head-injued patients. J Int Neuropsych Soc. 1997;3(4):327–36. [PubMed] [Google Scholar]
- 18.Wagner AK, Postal BA, Darrah SD, Chen X, Khan AS. Deficits in novelty exploration after controlled cortical impact. Journal of Neurotraum. 2007;24(8):1308–20. doi: 10.1089/neu.2007.0274. [DOI] [PubMed] [Google Scholar]
- 19.Graziano A, Petrosini L, Bartoletti A. Automatic recognition of explorative stratedies in the Morris water maze. J Neurosci Meth. 2003;130:33–44. doi: 10.1016/s0165-0270(03)00187-0. [DOI] [PubMed] [Google Scholar]
- 20.Deweer B, Ergis AM, Fossati P, et al. Explicit memory, procedural learning, and lexical priming in Alzheimer's Disease. Cortex. 1994;30(1):113–26. doi: 10.1016/s0010-9452(13)80327-9. [DOI] [PubMed] [Google Scholar]
- 21.Day LB, Weisend M, Sutherland RJ, Schallert T. The hippocampus is not necessary for place response but may be necessary for pliancy. Behav Neurosci. 1999;113(5):914–924. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- 22.Ramos JM. Preserved learning about allocentric cues but impaired flexible memory expression in rats with hippocampal lesions. Neurobiol Learn Mem. 2010;93:506–14. doi: 10.1016/j.nlm.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Dawson DR, Binns MA, Hunt A, Lemsky C, Polatajko HJ. Occupation-based strategy training for adults with traumatic brain injury: a pilot study. Arch Phys Med Rehab. 2013;94:1959–63. doi: 10.1016/j.apmr.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Schweighofer N, Lee J, Goh H, Choi Y, Kim S, Stewart J, Winstein C. Mechanisms of the contextual interference effect in individuals poststroke. J Neurophysiol. 2011;106(5):2632–41. doi: 10.1152/jn.00399.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu W, Young D, Schandler S, Meir G, Judy R, Perez J, Cohen M. Contextual interference and augmented feedback: is there an additive effect for motor learning? Hum Movement Sci. 2011;30(6):1092–101. doi: 10.1016/j.humov.2011.02.004. [DOI] [PubMed] [Google Scholar]


