Abstract
The optimal treatment of follicular lymphoma (FL) is not established. Rituximab is an important component of FL treatment making the impact of chemotherapy on FL less clear. We reviewed 649 FL at the University of Iowa/Mayo Clinic from 2002 to 2009 looking for the association of total dose delivery (TDD), delivered dose intensity (DDI) of cyclophosphamide and doxorubicin on survival. 337 of 649 FL received R-monotherapy/R-chemotherapy as frontline systemic therapy. After a median follow-up duration of 52.7 months, EFS and OS were similar between the two groups with a trend toward better EFS in R-chemotherapy cohort (HR=1.24, p=0.28). In the R-chemotherapy group, increased TDD and DDI of doxorubicin were associated with improved EFS only in patients who did not receive R-maintenance (HR=0.81; P=0.02 and HR=0.94; P=0.04) but not in patients who underwent R-maintenance. Cyclophosphamide was not associated with EFS. Thus, in the immunochemotherapy era, chemotherapy dose delivery tradition requires further evaluation in FL
Keywords: Follicular Lymphoma, Immunochemotherapy, Treatment delivery, dose intensity
Introduction
Follicular Lymphoma (FL) is the most common indolent lymphoma in the Americas and Europe. There is no consensus on optimal initial management. Most patients respond to treatment but in many cases the disease leads to death from progressive disease or transformation to aggressive lymphoma [1].
First line management can be varied and depends upon several factors including disease burdens and host specific factors [2]. Historically, systemic chemotherapy was the backbone of non-Hodgkin’s lymphoma (NHL) when patients with FL needed treatment [3, 4]. Rituximab monotherapy (R-monotherapy) has improved PFS versus observation in a randomized trial [5]. In a trial of patients with low-risk disease of initial treatment with R-monotherapy weekly times 4 followed by observation versus maintenance rituximab every 3 months, there was no difference in outcomes between the two arms in time to treatment failure or quality of life [6]. The addition of rituximab (R) to chemotherapy (“immunochemotherapy” or “R-chemotherapy”) has improved event-free survival (EFS) and overall survival (OS) when compared to chemotherapy [7–9]. Prolonged treatment with rituximab (R-maintenance) in high risk FL initially treated with immunochemotherapy further extends EFS but not OS allowing for variability in current management strategies [10].
Systemic immunochemotherapy for NHL is commonly administered in 6–8 cycles [11]. Most studies support the goal of maintaining dose intensity and total dose for optimal outcomes in diffuse large B cell lymphoma (DLBCL) [12–16]. However, there is a paucity of data assessing the role of chemotherapy dose intensity in FL [17]. In the era of immunochemotherapy with a frequently implemented strategy of R-maintenance, the importance of chemotherapy dosing on outcomes particularly EFS deserves re-examination.
The University of Iowa (UI) and Mayo Clinic (MC) have collaboratively developed a unique resource through the Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) [18]. Herein, we analyze FL patients from the MER to report relationships between choice of frontline systemic therapy (R-monotherapy VS R-chemotherapy), chemotherapy dosing (total dose and dose intensity) and EFS in FL. We identify potential clinicopathologic factors associated with variation in treatment delivery.
Patients and Methods
This study was reviewed and approved by the human subjects institutional review board at the UI and MC, and written informed consent was obtained from all participants. All subjects in this analysis were from the MER of the UI/MC Lymphoma SPORE, which has been previously reported [18]. Briefly, since September 2002, we offered enrollment to consecutive, newly diagnosed NHL patients (within 9 months) ≥ 18 years old who were evaluated at the UI and MC. All diagnoses were confirmed by study hematopathologists. Baseline clinical, laboratory, and treatment data were abstracted from medical records. All patients were systematically queried for events every 6 months for the first 3 years, and annually thereafter. Disease progression, retreatment, transformation, and death were verified through review of pathology and medical records. Treatment follow-up and surveillance depended upon primary oncologists’ discretion. Inclusion criteria for this analysis were patients with initial diagnosis of grade 1, 2, 3a FL enrolled through 12/31/2009 with systemic therapy as initial management which included all patients who received at least one dose of R-monotherapy or R-chemotherapy as given by primary oncologists. Rituximab monotherapy without maintenance was defined as up to 8 weekly doses of rituximab prior to response assessment. Medical records were further abstracted for all dates and doses of delivered first course therapy.
Total dose delivery (TDD) was defined as a summation of chemotherapy dose delivered to the patient from the first to last cycle of treatment expressed as mg/m2. Delivered dose intensity (DDI) was defined as a measure of the amount of drug delivered per unit of time expressed as mg/m2/day [19, 20]. All patients who received at least one cycle of chemotherapy were included.
Rituximab maintenance was defined as either a) infrequent monotherapy dosing (no briefer than 8 weeks) for any number of time intervals, or b) predetermined repeated courses of 4 weekly doses at six-month intervals beginning after an identified induction regimen confirmed by intent stated in medical record by treating physicians.
Statistical Analysis
Demographics, baseline disease characteristics, treatment strategy and chemotherapy delivery pattern were summarized with descriptive statistics. The primary endpoint was EFS, which was defined as the time from diagnosis to disease progression, transformation, re-treatment (including rituximab retreatment when it was not stated as pre-planned treatment and chemotherapy), or death due to any cause. The secondary endpoint was OS, which was defined as the time from diagnosis to death due to any cause. Subjects for whom an endpoint of interest was not observed were censored at the date of their last follow-up for the analyses.
Bivariate associations with categorical clinicopathologic characteristics were assessed with Pearson’s chi-squared tests (or Fisher’s exact tests if warranted by sample size) for rituximab therapy (monotherapy vs. chemotherapy), and with analysis of variance for chemotherapy amount (TDD and DDI) delivered. Survival probabilities were estimated with the method of Kaplan-Meier [21]. Univariate and multivariate Cox survival regression was used to assess the continuous effects of TDD, DDI, and age, and the categorical effects of clinicopathologic characteristics. Regression estimates are reported as hazard ratios (HRs) and 95% confidence intervals (CIs). To address the primary goals of the study, two multivariate models were employed – one for TDD and another for DDI. Both models included effects for rituximab maintenance, cyclophosphamide dose, doxorubicin dose, and interactions between maintenance and each of the chemotherapeutic doses. Additionally, Follicular Lymphoma International Prognostic Index (FLIPI) [22] was included in the models because of its standard use as a prognostic indicator of survival for lymphoma patients. The number of events in the study (EFS: 58) limited the number of predictors in the multivariate models to the aforementioned set. All statistical testing was two-sided and assessed for significance at the 5% level using the SAS v9.3 (SAS Institute, Cary, NC) statistical software.
Results
Patient Characteristics
649 patients with newly diagnosed FL grade 1–3a were enrolled between 2002 and 2009. The median age at diagnosis was 60 years (range 25–94) and 54% were male. At the time of analysis, the median follow up duration was 52.7 months (range 0.8–108). 337 FL patients underwent frontline management with R containing regimens including 94 R-monotherapy and 243 R-chemotherapy.
Of 243 FL patients who were treated with R-chemotherapy, 94 received RCVP (C-Cyclophosphamide, V-Vincristine, P-Prednisone) and 139 received RCHOP (H-Adriamycin, O-Vincristine). Other chemotherapy regimens included 5 R-Bendamustine, 4 R-Fludarabine based regimens and 1 R-Chlorambucil.
The median time from diagnosis to systemic therapy (R-monotherapy or R-chemotherapy) was 23 days (range 7–653). Patients treated with R-chemotherapy had comparable age and equal stage distribution but had higher proportion of high-risk FLIPI (30.9 % VS 19.1%, p=0.01) and grade 3a disease (30.9% VS 8.5%, p<0.001) (Table 1). Patients with missing detailed chemotherapy data had similar baseline characteristics including median age, stage, grade and FLIPI distribution to patients with detailed chemotherapy data (Supplementary Table S1).
Table 1.
Comparison of Baseline Characteristics of FL between R-monotherapy and R-chemotherapy group
| Treatment
|
P-Value | ||
|---|---|---|---|
| R-monotherapy (N=94) | R-chemotherapy (N=243) | ||
|
| |||
| Mean age at diagnosis (year, range) | 60.0 (27–91) | 56.8 (24–87) | 0.06 |
|
| |||
| Median time from diagnosis to treatment (days, range) | 37.5 (0–175) | 21.0 (0–653) | <0.001 |
|
| |||
| Gender | 0.48 | ||
| ▪ Male | 49 (52.1%) | 137 (56.4%) | |
| ▪ Female | 45 (47.9%) | 106 (42.6%) | |
|
| |||
| FLIPI | 0.01 | ||
| ▪ Low | 33 (35.1%) | 81 (33.3%) | |
| ▪ Intermediate | 42 (44.7%) | 73 (30.0%) | |
| ▪ High | 18 (19.1%) | 75 (30.9%) | |
| ▪ Missing data | 1 (1.1%) | 14 (5.8%) | |
|
| |||
| Stage | 0.16 | ||
| ▪ I–III | 44 (46.8%) | 114 (46.9%) | |
| ▪ IV | 49 (52.1%) | 115 (47.3%) | |
| ▪ Missing data | 1 (1.1%) | 14 (5.8%) | |
|
| |||
| Grade | <0.001 | ||
| ▪ I–II | 85 (90.4%) | 154 (63.3%) | |
| ▪ IIIa | 8 (8.5%) | 75 (30.9%) | |
| ▪ Missing data | 1 (1.1%) | 14 (5.8%) | |
|
| |||
| Elevated LDH > normal | 0.02 | ||
| ▪ <=Normal | 66 (70.2%) | 130 (53.5%) | |
| ▪ >Normal | 14 (14.9%) | 61 (25.1%) | |
| ▪ Missing Data | 14 (14.9%) | 52 (21.4%) | |
|
| |||
| Hemoglobin > 12 g/dL | 0.07 | ||
| ▪ <12 g/dL | 10 (10.6%) | 34 (14.0%) | |
| ▪ >=12 g/dL | 77 (81.9%) | 171 (70.4%) | |
| ▪ Missing Data | 7 (7.5%) | 38 (15.6%) | |
|
| |||
| Number of nodal involvement | 0.10 | ||
| - < 4 nodal areas | 60 (63.8%) | 129 (53.1%) | |
| - > 4 nodal areas | 31 (33.0%) | 94 (38.7%) | |
| - Missing data | 3 (3.2%) | 20 (8.2%) | |
Pattern of chemotherapy delivery and R-maintenance
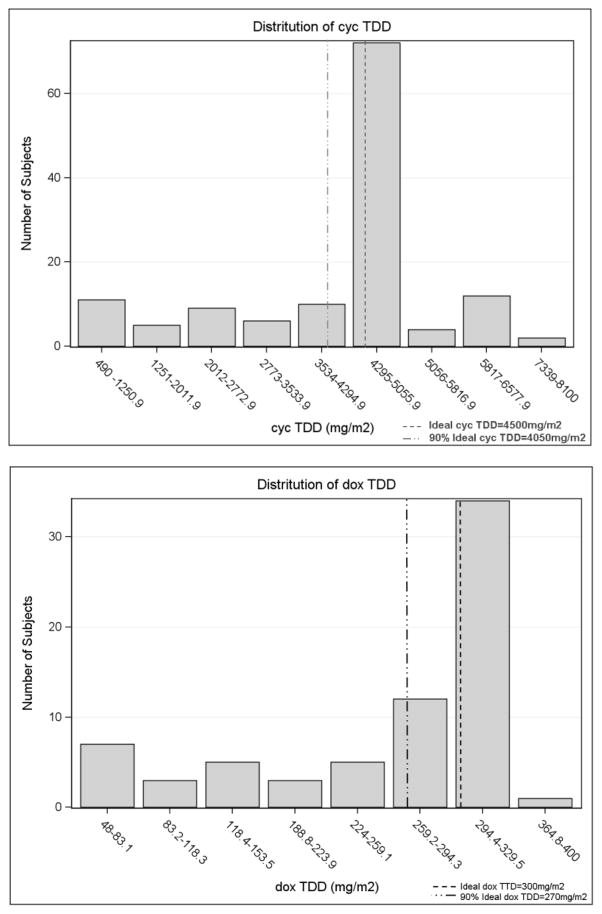
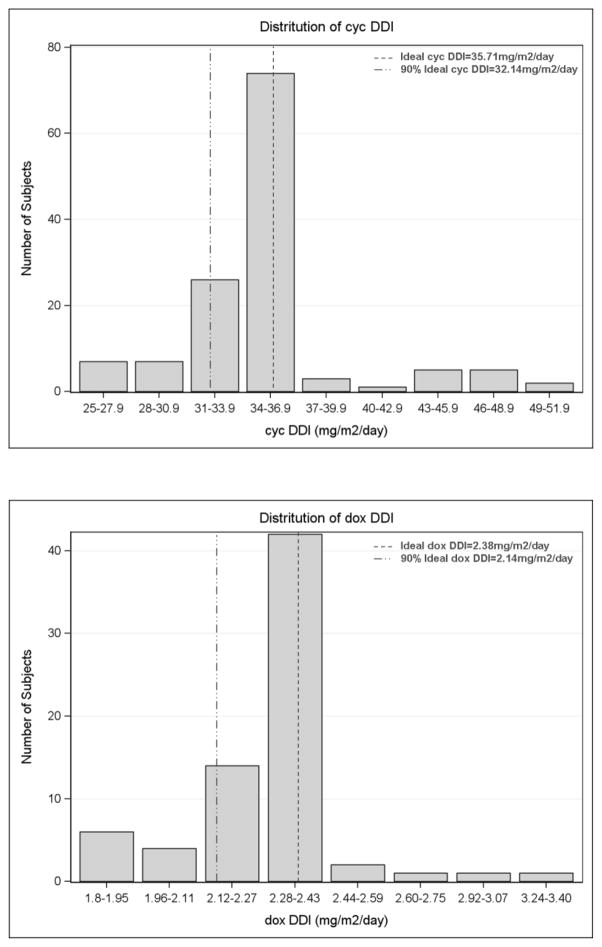
TDD and DDI of cyclophosphamide and doxorubicin (cycTDD, doxTDD, cycDDI, and doxDDI) were evaluated. Complete data on chemotherapy dose and schedule were available for TDD and DDI calculation in 131 patients (71 RCHOP and 60 RCVP), for a total of 131 cyclophosphamide-treated and 71 doxorubicin-treated patients. The median numbers of cycle were 6 cycles (range 1–8 cycles). The overall distribution of TDD and DDI for cyclophosphamide and doxorubicin are shown in figure 1. The median cycTDD and doxTDD were 4506.6 mg/m2 and 295.2 mg/m2 respectively. The median was 35.4 mg/m2/day for cycDDI and 2.3 mg/m2/day for doxDDI. Supplementary Table S2 shows the TDD and DDI distribution stratified by clinical characteristics. Age, grade and FLIPI were not associated with differences of TDD or DDI in R-chemotherapy treated FL. Of 131 patients treated with cyclophosphamide, 85% received more than 4000 mg/m2. Eighty-five percent of doxorubicin treated patients received more than 90% of the standard doxorubicin regimen. Of 131 patients who had complete data on R-chemotherapy treatment, 71 underwent R-maintenance.
Figure I.
Distribution of chemotherapy delivered dose intensity (DDI) and total dose delivery (TDD)
Outcomes
Event Free Survival
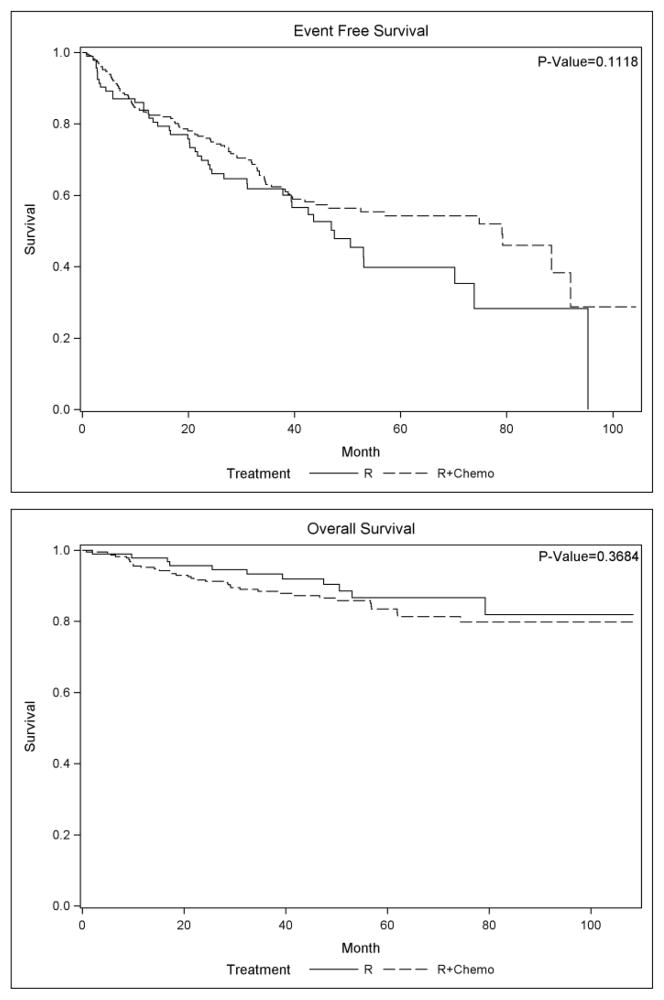
Median EFS for the 337 patients treated with R-monotherapy or R-chemotherapy was 70.2 months, and there were 137 events. Unadjusted EFS for patients treated with R-monotherapy compared to R-chemotherapy was 47.5 versus 79.1 months (p=0.11) (Figure 2). As shown in table 2 higher FLIPI and stage IV disease are significant predictors of lower EFS. After adjusting for FLIPI, stage, and grade in a multivariate Cox regression model, there was no significant difference in EFS between R-monotherapy and R-chemotherapy (HR 1.24, p=0.28).
Figure II.
Kaplan Meier survival curve of R-monotherapy treated patients and R-chemotherapy treated patients
Table 2.
Univariate survival analysis of relevant clinicopathologic prognostic factors
| Event-Free Survival | ||||
|---|---|---|---|---|
| Comparison | HR | 95% CI | P-Value | |
| FLIPI | Intermediate vs Low High vs Low |
3.37 4.00 |
1.49–7.63 1.81–8.80 |
0.002 |
| Stage | IV vs I–III | 2.77 | 1.54–5.00 | <0.001 |
| Grade | IIIa vs I–II | 0.60 | 0.33–1.09 | 0.09 |
| Age at diagnosis (continuous) | 1 Year Increase | 0.98 | 0.96–1.01 | 0.18 |
| R-maintenance | No vs Yes | 1.8 | 0.94–3.51 | 0.08 |
Among patients who were treated with R-chemotherapy, there was no difference in EFS between patients with detailed chemotherapy data and patients with unavailable detailed chemotherapy data (92.0 months and 57.1 months respectively, p=0.26). Of 131 patients treated with R-chemotherapy and who had detailed dosing data on cyclophosphamide and doxorubicin, after adjustment for FLIPI and the other chemotherapy dose, doxorubicin dose (both TDD and DDI) was associated with improved EFS in patients who did not receive R-maintenance (HR 0.81, p=0.02 and HR 0.94, p=0.04). However, doxTDD and DDI were not significant predictors of EFS in patients who received R-maintenance. Neither cycTDD nor cycDDI was a significant predictor of EFS either in R-maintenance or no R-maintenance patients (Table 3).
Table 3.
Multivariate EFS analysis of the effect of chemotherapy dose as a continuous variable by R-Maintenance status, when adjusted for TDD or DDI of the other chemotherapy and FLIPI.
| Chemotherapy Dose | HR | Interaction*** | ||||
|---|---|---|---|---|---|---|
| Relative Increase* | R-Maintenance | Estimate | 95% CI | p-value | p-value | |
| doxTDD1,** | 89 units | No | 0.81 | (0.67, 0.97) | 0.0254 | 0.0524 |
| Yes | 1.28 | (0.84, 1.97) | 0.2560 | |||
| cycTDD2 | 1483 units | No | 0.91 | (0.67, 1.23) | 0.5271 | 0.3320 |
| Yes | 1.33 | (0.66, 2.69) | 0.4331 | |||
| doxDDI3,** | 0.23 units | No | 0.94 | (0.89, 1.00) | 0.0458 | 0.2782 |
| Yes | 1.02 | (0.90, 1.15) | 0.7921 | |||
| cycDDI4 | 5.02 units | No | 0.87 | (0.61, 1.24) | 0.4292 | 0.5854 |
| Yes | 1.02 | (0.66, 1.57) | 0.9480 | |||
Adjusted for flipi and
cycTTD,
doxTDD,
cycDDI, or
doxDDI.
HRs are reported for 1 standard deviation increases in dose.
Values of dox were set to zero for patients in which it was not used.
Interaction p-values are for the interactions between R-Maintenance and chemotherapy dose.
Overall Survival
Of the 337 patients treated with R-monotherapy or R-chemotherapy, 49 died at the time of analysis (42 in R-chemotherapy and 7 in R-monotherapy. There was no difference in OS between R and R-chemotherapy treated patients (HR 0.55, p=0.37) (Figure 2). Impact of chemotherapy delivery on OS was not performed because of inadequate statistical power.
Discussion
To our knowledge, this is the most detailed analysis of chemotherapy dosing intensity and associations with clinical outcomes of FL in the immunochemotherapy era. The addition of R to chemotherapy has changed the landscape of FL treatment resulting in improved response rates, EFS and OS compared to chemotherapy alone [7, 9, 23]. Acknowledging the survival benefits gained from R raises several questions and new issues on the role of chemotherapy in immunochemotherapy era. Overall and complete response rates are doubtless increased by the addition of immunotherapy to chemotherapy, but the details of the role of chemotherapy are uncertain.
A recent report by Nabhan and colleagues using National LymphoCare Study (NLCS) database including advanced stage patients showed superior PFS but not OS for R-chemotherapy compared to R-monotherapy only in patients under age 60 [24]. Our data showed no difference in EFS or OS between R-monotherapy and R-chemotherapy treated patients, although there was a trend toward superior EFS in R-chemotherapy patients. This finding might be because chemotherapy adds little to rituximab induction – this has not been studied in a prospective controlled trial. Other potential explanations include inadequate power to detect modest differences or unmeasured differences in the patient cohorts that could include less bulky or symptomatic disease in the R-monotherapy cohort.
Further evidence undermining the importance of the chemotherapy component of immunochemotherapy in FL in our study comes from the observation that increased doxorubicin dosing (both TDD and DDI) is associated with improved EFS in patients who did not receive R-maintenance but this effect is not seen in patients who received R-maintenance whereas cyclophosphamide dosing does not correlate with difference in EFS irrespective of the status of R-maintenance. Although there was no interaction between R-maintenance and doxorubicin dosing to explain the different effect of doxorubicin dosing on EFS, this might be due to relatively low number of events.
Chemotherapy dose intensity in NHL has long been a topic of interest. There have been several retrospective studies demonstrating inability to maintain standard dose intensity associated with inferior survival outcome in DLBCL [12, 13, 25, 26]. However, most prospective studies in DLBCL show no benefit of increased dose density in DLBCL in the immunochemotherapy era [14, 16]. The issue of dose intensity has not been well studied in FL but is of significant clinical interest.
The evidence on dose intensity in FL has been limited. Recently, Watanabe and colleagues demonstrated similar outcome including response rate, PFS and OS between FL who were treated with RCHOP-14 and R-CHOP-21 [17]. However, there are no studies exploring outcomes associated with substandard chemotherapy delivery in FL. Our study is the first report exploring the relationship between the ability to maintain chemotherapy delivery and outcomes in FL. We used EFS instead of PFS due to the variation of surveillance imaging inherent in observational studies and to prioritize the significance of retreatment, which does not always have a direct relationship to progression in follicular lymphoma.
The issue of using EFS or OS as an endpoint in FL is important to address in the context of goals of therapy. No chemotherapy regimen in the absence of immunotherapy was ever demonstrated to have an impact on OS. Thus upon initiation of therapy, understanding the role of further chemotherapy dosing in achieving EFS is of clinical importance. Therapy for FL may be deferred until there is significant change in the amount of disease or patients are symptomatic with the goal of therapy at time of initiation to reduce the amount of disease or symptoms and maximize time to next treatment. Recently, Martin et al demonstrated that EFS were inferior among FL patients receiving <5 cycles of RCVP compared to those receiving 5–6 cycles. No difference in EFS was noted among cycles received for patients treated with RCHOP and impact of delivered cycles controlled for R-maintenance was not specifically reported [27]. The findings from both studies should be interpreted within the accepted limitations of observational studies – namely that the observed associations between dosing and outcome may fail to fully account for important unmeasured variables and causality cannot be concluded with certainty. Future analyses in subsequent cohorts could be designed to measure any hypothesized variables that are deemed a threat to such conclusions.
We realize that our report has some limitations. Missing detailed data on chemotherapy dose in some patients limited those available for analysis. However, there is no significant difference between patients with detailed chemotherapy data and patients who did not have detailed chemotherapy data (Supplementary Table S1), which may partly resolve selection biases. Variation of DDI is modest in the studied cohort (Figure 1). In our study over 90% of the patients received at least 70% of standard doxDDI and notably more than 80% received at least 90% of standard doxDDI, which is less variability compared to previous DLBCL series [12, 25]. Variability in accepted definitions of optimal dosing of cyclophosphamide in FL (750 versus 1000 mg/m2 and six versus eight cycles) makes calculation relative to optimal dosing somewhat arbitrary. Data from prospective immunochemotherapy trials in DLBCL showed good adherence to the treatment protocols with lower DDI variability, however, growth factor use in those trials in part led to minimal protocol deviation [14, 16]. The small variation of DDI may explain the lack of survival impact of chemotherapy dose in our study. Exclusion of patients treated with bendamustine-based regimen which has become the backbone to immunochemotherapy programs [11] may limit generalizability of our study to current practice. The relatively short duration of follow up and small number of deaths limited the meaningful analysis of OS. OS remains an important endpoint in FL as there are still several theoretical reasons why OS might be impacted by depth of initial response, which is certainly affected by chemotherapy delivery.
Our study has several unique strengths including a well-vetted prospective observational study cohort with a representative “real world” patient population, central pathology review, excellent long-term follow-up for events and the only reported cohort with available details on actual delivered doses. It is the first report exploring the effect of non-standard chemotherapy dose delivery in FL in the immunochemotherapy era.
The comprehensive use of immunotherapy in FL combined with chemotherapy may lessen the impact of chemotherapy on outcome. Randomized clinical trials are appropriately prioritized for identifying the value of new therapeutic options, and research to determine optimal dosing of older therapies is unlikely to be further studied in randomized trials. The results of our study are not definitive but are hypothesis generating and require further similar analyses in other data sets.
Conclusion
In a retrospective analysis of a prospective observational study of patients with FL treated with frontline immunochemotherapy, the delivered dose of cyclophosphamide was not associated with EFS and greater delivery of doxorubicin was associated with an improved EFS only in patients who did not receive maintenance rituximab. In the immunotherapy era, chemotherapy dose delivery requires re-evaluation in FL.
Supplementary Material
Acknowledgments
We acknowledge the collective contribution of the medical, nursing and administrative staff of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE). Dr. Christopher Flowers and Dr. John Leonard kindly reviewed study design and the manuscript as mentors in the ASH Clinical Research Training Institute (CRTI).
Funding
The study is supported in part by Grant No. P50 CA97274 from the National Institutes of Health, and Dr. Wudhikarn is a scholar of ASH’s CRTI.
Footnotes
Disclosure
BKL receives grant support from Genentech, and serves as a consultant for Genentech. JRC serves on the scientific advisory board of the LymphoCare study funded by Genentech.
Presented in part at the American Society of Clinical Oncology in Chicago 2013
References
- 1.Link BK, Maurer MJ, Nowakowski GS, et al. Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: a report from the University of Iowa/MayoClinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol. 2013;31:3272–3278. doi: 10.1200/JCO.2012.48.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedberg JW, Taylor MD, Cerhan JR, et al. Follicular lymphoma in the United States: first report of the national LymphoCare study. J Clin Oncol. 2009;27:1202–1208. doi: 10.1200/JCO.2008.18.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Portlock CS, Rosenberg SA. Combination chemotherapy with cyclophosphamide, vincristine, and prednisone in advanced non-Hodgkin’s lymphomas. Cancer. 1976;37:1275–1282. doi: 10.1002/1097-0142(197603)37:3<1275::aid-cncr2820370307>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Peterson BA, Petroni GR, Frizzera G, et al. Prolonged single-agent versus combination chemotherapy in indolent follicular lymphomas: a study of the cancer and leukemia group B. J Clin Oncol. 2003;21:5–15. doi: 10.1200/jco.2003.05.128. [DOI] [PubMed] [Google Scholar]
- 5.Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol. 2014;15:424–435. doi: 10.1016/S1470-2045(14)70027-0. [DOI] [PubMed] [Google Scholar]
- 6.Kahl BS, Hong F, Williams ME, et al. Results of Eastern Cooperative Oncology Group Protocol E4402 (RESORT): A randomized phase III study comparing two different rituximab dosing strategies for low tumor burden follicular lymphoma. Blood. 2011:118. [Google Scholar]
- 7.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 8.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 9.Herold M, Haas A, Srock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25:1986–1992. doi: 10.1200/JCO.2006.06.4618. [DOI] [PubMed] [Google Scholar]
- 10.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 11.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 12.Hirakawa T, Yamaguchi H, Yokose N, et al. Importance of maintaining the relative dose intensity of CHOP-like regimens combined with rituximab in patients with diffuse large B-cell lymphoma. Ann Hematol. 2010;89:897–904. doi: 10.1007/s00277-010-0956-7. [DOI] [PubMed] [Google Scholar]
- 13.Kwak LW, Halpern J, Olshen RA, Horning SJ. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol. 1990;8:963–977. doi: 10.1200/JCO.1990.8.6.963. [DOI] [PubMed] [Google Scholar]
- 14.Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013;14:525–533. doi: 10.1016/S1470-2045(13)70122-0. [DOI] [PubMed] [Google Scholar]
- 15.Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood. 2004;104:626–633. doi: 10.1182/blood-2003-06-2094. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:1817–1826. doi: 10.1016/S0140-6736(13)60313-X. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T, Tobinai K, Shibata T, et al. Phase II/III study of R-CHOP-21 versus R-CHOP-14 for untreated indolent B-cell non-Hodgkin’s lymphoma: JCOG 0203 trial. J Clin Oncol. 2011;29:3990–3998. doi: 10.1200/JCO.2011.34.8508. [DOI] [PubMed] [Google Scholar]
- 18.Drake MT, Maurer MJ, Link BK, et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28:4191–4198. doi: 10.1200/JCO.2010.28.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyman GH, Dale DC, Friedberg J, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol. 2004;22:4302–4311. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- 20.Gregory SA, Trumper L. Chemotherapy dose intensity in non-Hodgkin’s lymphoma: is dose intensity an emerging paradigm for better outcomes? Ann Oncol. 2005;16:1413–1424. doi: 10.1093/annonc/mdi264. [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method) BMJ. 1998;317:1572. doi: 10.1136/bmj.317.7172.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 23.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 24.Nabhan C, Byrtek M, Latta S, et al. Disease Characteristics, Patterns of Care, and Outcomes of Follicular Lymphoma (FL) in the Oldest Old: Report from the US National Lymphocare Study (NLCS) Hematol Oncol. 2013;31:130. [Google Scholar]
- 25.Yamaguchi H, Hirakawa T, Inokuchi K. Importance of relative dose intensity in chemotherapy for diffuse large B-cell lymphoma. J Clin Exp Hematop. 2011;51:1–5. doi: 10.3960/jslrt.51.1. [DOI] [PubMed] [Google Scholar]
- 26.Lepage E, Gisselbrecht C, Haioun C, et al. Prognostic significance of received relative dose intensity in non-Hodgkin’s lymphoma patients: application to LNH-87 protocol. The GELA (Groupe d’Etude des Lymphomes de l’Adulte) Ann Oncol. 1993;4:651–656. doi: 10.1093/oxfordjournals.annonc.a058619. [DOI] [PubMed] [Google Scholar]
- 27.Martin P, Byrtek M, Dawson K, et al. Patterns of delivery of chemoimmunotherapy to patients with follicular lymphoma in the United States: results of the National LymphoCare Study. Cancer. 2013;119:4129–4136. doi: 10.1002/cncr.28350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.