Abstract
Apart from their role in humoral immunity, B cells can exhibit IL-10-dependent regulatory activity (Bregs). These regulatory subpopulations have been shown to inhibit inflammation and allograft rejection. However, our understanding of Bregs has been hampered by their rarity, lack of a specific marker, and poor insight into their induction and maintenance. We previously demonstrated that TIM-1 identifies over 70% of IL-10-producing B cells, irrespective of other markers. We now show that TIM-1 is the primary receptor responsible for Breg induction by apoptotic cells (AC). However, B cells that express a mutant form of TIM-1 lacking the mucin domain (TIM1Δmucin) exhibit decreased phosphatidylserine binding and are unable to produce IL-10 in response to ACs or by specific ligation with anti-TIM-1. TIM1Δmucin mice also exhibit accelerated allograft rejection, which appears to be due in part to their defect in both baseline and induced IL-10+ Bregs, since a single transfer of wt TIM-1+ B cells can restore long-term graft survival. These data suggest that TIM-1 signaling plays a direct role in Breg maintenance and induction both under physiological conditions (in response to apoptotic cells) and in response to TIM-1 ligation. Moreover, they directly demonstrate that the mucin domain regulates TIM-1 signaling.
Introduction
In addition to humoral immunity, B cells play an important role regulating immune responses(1,2). B cell deficiency or depletion can worsen autoimmunity and prevent allograft tolerance, while transfer of certain B cell subpopulations can inhibit inflammation and allograft rejection in an IL-10 dependent fashion(1-5). However, our understanding of such regulatory B cells (Bregs) is hampered because Bregs are rare and lack a specific marker. Various B cell subsets can exhibit regulatory activity (including Marginal Zone, Transitional, CD1dHiCD5+ B cells, and possibly plasma cells(1-3,6-8)). However, these appear to contain the highest proportion of IL-10+ B cells, rather than representing a true “Breg phenotype”. In contrast, TIM-1+ B cells are 8-20 fold enriched for IL-10 across all other B cell subsets, and comprise over 70% of all IL-10+ B cells(5). Thus, TIM-1 is an inclusive marker for IL-10+ Bregs, and TIM-1+ but not TIM-1- B cells can transfer allograft tolerance(5). However, the physiological triggers that lead to Breg generation and IL-10 production are largely unknown(1).
Apoptotic cells can induce IL-10+ B cells that can inhibit EAE and collagen–induced arthritis(6,7). Increased B cell IL-10 expression induced by ACs involves both BCR and TLR9 ligation. However, the expression of TIM-1, a known phosphatidylserine (PS) receptor(9-11), by Bregs raises the question as to whether TIM-1 is involved in AC binding and IL-10 production by B cells.
TIM-1 (T cell immunoglobulin mucin domain-1) is a type I cell-surface glycoprotein, with an immunoglobulin V-like domain, a mucin domain, a single transmembrane region, and a cytoplasmic tail containing a tyrosine phosphorylation motif(12,13). TIM-1 plays a costimulatory role on activated CD4 cells. Ligation with a high affinity anti-TIM-1 mAb, 3B3, promotes Th1 and Th17 responses, blocks allograft tolerance and exacerbates EAE(14,15). In contrast, a lower affinity anti-TIM-1 mAb (RMT1-10) induces IL-10+ Bregs which promote Th2 and Treg responses, and inhibit EAE and allograft rejection(5,15,16).
While the TIM-1 IgV domain contains binding sites for its ligands, TIM-4 and PS, polymorphisms in the mucin domain are linked to susceptibility to asthma and allergy(12,13). Moreover, differences in the TIM-1 mucin domain in B6 and BALB/c mice are thought to contribute to differences in Th2 responsiveness(17). Based on this putative immunoregulatory role, we generated TIM-1Δmucin knock-in mice bearing TIM-1 lacking the mucin domain(18). While young mice were healthy, they developed age-related T cell hyper-responsiveness, with a large increase in Teffector/memory Th1 cells and autoantibodies. When crossed onto a Fas-deficient background, TIM-1Δmucin mice exhibit markedly accelerated lupus(18). Interestingly, a mild impairment in B cell IL-10 expression seen at 4-6 months became more pronounced by 10 months of age. This suggests that TIM-1, and the TIM-1 mucin domain in particular, could play a role in Breg maintenance required for immune homeostasis. However, causality was not established and it remains uncertain whether the observed age-related deficiency in Bregs reflects impaired cellular survival, or stems from a baseline defect in Bregs. Additionally, while polymorphisms in the mucin domain alter immune function, the role of this region in TIM-1 signaling has never been directly tested. B cells from TIM1Δmucin mice express normal levels of TIM-1 protein consisting of an intact IgV domain bound directly to the transmembrane region(18), allowing us to specifically evaluate the role of the mucin domain in TIM-1 signaling.
We now identify TIM-1 as the receptor primarily responsible for Breg induction by apoptotic cells (AC). TIM1Δmucin B cells exhibit decreased TIM-1-mediated PS binding, and are unable to produce IL-10 in response to ACs. Moreover, TIM-1 signaling mediated by ligation with tolerogenic anti-TIM-1 (RMT1-10) is also defective in TIM1Δmucin B cells which are unable to induce TIM-1 or IL-10 expression, or prolong allograft survival in a Breg dependent setting. Importantly, accelerated allograft rejection by TIM1Δmucin recipients can be ameliorated by a single transfer of wt TIM-1+ B cells at the time of transplantation. Together, these data demonstrate that the mucin domain regulates TIM-1 signaling, and that TIM-1 plays a direct role in Breg induction and maintenance, possibly through apoptotic cell binding to TIM-1.
Materials and Methods
Mice
6–10 week old B6 (H-2b), BALB/c (H-2d), μMT, B6.C-H2bm12/KhEg mice (JAX) and B6.TIM-1Δmucin(18) were used.
Flow cytometry
Fluorochrome-conjugated mAbs were from BD Biosciences, eBioscience or Biolegend. α-TIM-1-PE (RMT1-4; Biolegend) whose binding does not overlap with RMT1-10, was used to determine TIM-1 expression(5). All staining was performed in the presence of Fc block. Data was acquired on LSRII analyzers (BD), and analyzed using FlowJo software (TreeStar). Background staining was determined with isotype-matched controls. For detection of intracellular cytokines, B cells were cultured for 5 hours with LPS, PMA, ionomycin, and monensin(5). Leukocytes from IL-4-/-, IL-10−/− mice served as negative control to demonstrate specificity and background-staining.
In vivo treatment protocols
Anti–TIM-1 mAb RMT1-10 (rat IgG2a;BioXCell) or control rat IgG2a were administered i.p. on days –1 (0.5 mg), 0, and 5 (0.3 mg) relative to transplantation.
Cell preparation and adoptive transfer
CD19+, CD19+TIM-1+ and CD19+TIM-1– B cells were isolated by FACS (>95% purity). For adoptive transfer studies, 5-7×106 purified B cell subsets from syngeneic mice were injected i.v. into otherwise untreated μMT, wt, or TIM-1Δmucin allograft recipients. In some experiments, B cells/subsets were obtained from B6 mice 14 days after alloantigen exposure (2×107 mitomycin C–treated BM12 splenocytes i.p.).
Cardiac transplantation
Vascularized intra-abdominal heterotopic transplantation of cardiac allografts was performed as described(5,15,16). Allograft survival (shown as MST in days) was assessed by daily palpation. Rejection was defined as complete cessation of cardiac contractility.
Islet isolation and transplantation
Islets from BALB/c donors were isolated and placed under the renal capsule of B6 or μMT recipients with streptozocin-induced diabetes (400 islets/recipient) (5). All recipients had glycemia <150 mg/dl within 2 days after transplant. Blood glucose >250 mg/dl for two consecutive days after engraftment was defined as rejection.
Generation of apoptotic cells
AC were induced by culturing thymocytes overnight with 1 μM dexamethasone (>80% AnnexinV+). 107 AC were adoptively transferred (I.V.).
In vitro culture
106 isolated splenic B cells, flow-sorted TIM1+CD19+ or TIM1-CD19+ B cells from B6.WT and B6.TIM1Δmucin mice were co-incubated with AC (5×105) for 48hrs. After which, intracellular cytokine staining for IL-10 was performed on CD19+B cells. In some experiments, B cells were also co-cultured with naïve OTII T cells (5×105/ml) plus OVA (1mg/ml) plus AC(6,7).
Generation of PS-coated liposomes
Phospahtidylserine (PS)/ phosphatidylcholine (PC) coated latex beads were generated by mixing PS and PC at a 1:1 ratio (Avanti). Lipid mixtures were dessicated until dry and then resuspended in HBSS. For fluorescent labeling, liposomes were either generated with 1-oleoyl-2-{12-[7-nitro-2,1,3-benzoxadiazol-4-yl)amino]dodecanoyl-sn-glycero-3-PC (Avanti), or pHRodo succinimidyl ester (LifeTechnologies), a pH-sensitive red fluorophore, whose emission increases dramatically at pH<6.0 (i.e. upon entry into phagolysosomes). The liposome mixture was then diluted 1:10 in serum-free media and incubated with splenic B cells from wt or TIM-1Δmucin B6 mice. In some experiments, apoptotic cells labeled with CFSE/pHRodo were used.
Microscopy
Co-localization images (5-7 random fields/condition) were captured on a Nikon C1 confocal microscope. Quantification of co-localization between green fluorescence-labeled liposomes and DAPI stained B cells was determined using Volocity software (PerkinElmer). Live cell imaging utilized a Nikon Eclipse Ti inverted microscope in a humidified chamber with 5% CO2.
Statistics
Statistical analyses used unpaired 2-tailed Student's t test and log-rank (Mantel-Cox) test. Differences were considered to be significant at P values <0.05.
Study approval
Animal studies were approved by the Institutional Animal Care and Use Committees at the University of Pittsburgh and Harvard Medical School.
Results
TIM-1-mediated PS-binding requires an intact mucin domain
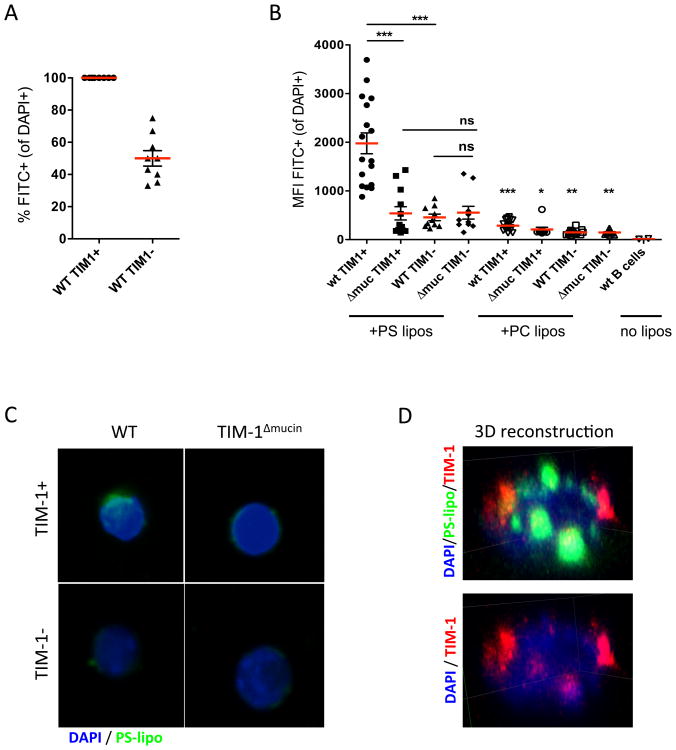
Exposure to apoptotic cells (ACs) has been shown to promote B cell IL-10 expression(6,7). Since TIM-1 identifies >70% of IL-10+ B cells (Bregs) and is a known PS receptor, we hypothesized that it may be involved in AC binding and IL-10 production by B cells. To determine this, we examined binding of green fluorescence-labeled PS-coated liposomes to WT versus TIM-1Δmucin B cells by confocal microscopy. Using a cut-off of ∼350 MFI (similar to the MFI of phosphatidylcholine (PC)-coated “control” liposomes (Fig1B)), 100% of wt TIM-1+ B cells bound PS-coated liposomes compared to ∼50% of WT TIM-1- B cells (Fig1A). While essentially all B cells exhibited some degree of liposomal binding, TIM-1- B cells bind significantly less than TIM-1+ B cells, resulting in a 4-5-fold reduction of mean fluorescence intensity (Fig1B). For these reasons, MFI provides a more accurate measure of liposome binding than percent of positive cells. Not only do wt TIM-1+ B cells bind substantially more PS-liposomes than wt TIM-1- B cells, but TIM-1 co-localizes with regions of high PS-liposomal binding on wt B cells (Fig1D, Supplemental Movie1). TIM-1 is also localized in areas where there are no liposomes, suggesting that there may be different pools of TIM-1. Indeed, some of the areas of most intense TIM-1 staining appear to be intracellular, consistent with large intracellular pools of TIM-1 seen in CD4+ T cells(19) and in renal cancer 769p cells(20).
Figure 1. TIM-1-mediated PS-binding requires an intact mucin domain.
A) Flow-sorted CD19+TIM1+ or CD19+TIM1- B cells from WT mice were incubated with green fluorescence-labeled phosphatidylserine-coated liposomes (PS-liposomes). After 12h, cells were fixed and stained with DAPI. Random fields (n=7-10) were imaged by confocal microscopy (20×), and co-localization between liposomes and B cells determined using Volocity software. The frequency of FITC+ B cells in each field is shown. Cells were considered positive using a cut-off of >350 MFI (similar to the MFI of phosphatidylcholine (PC)-coated “control” liposomes). Data shown is from a single experiment, representative of 3 experiments, in duplicate wells.
B) Flow-sorted CD19+TIM-1+ or CD19+TIM-1- B cells from wt and TIM-1Δmucin mice were co-incubated with green fluorescence-labeled (PS-liposomes), or control phosphatidylcholine (PC)-liposomes, as above. Co-localization between liposomes and B cells, as defined by the mean fluorescence intensity (MFI) of FITC+ liposomes on each interacting CD19+ B cell, was determined using Volocity software. Data expressed as mean±SEM (*p<0.05; **p<0.01; ***p<0.001) from images of 12-20 random fields. Representative of 3 independent experiments, in triplicate wells.
C) Representative images (100× magnification). Green=PS-liposomes, Blue=DAPI.
C) 3D reconstruction of confocal z-stack images of wt CD19+TIM1+ B cells incubated with PS-liposomes as in A. Green=PS-liposomes, Red=TIM-1, Blue=DAPI.
Importantly, binding of PS-liposomes to TIM-1- wt B cells was comparable to binding by either TIM-1+ or TIM-1- TIM-1Δmucin B cells (Fig1B,C). All B cell populations bound more PS-coated liposomes than control PC-liposomes, indicating that other PS receptors are present on B cells and contribute to low-level binding. However, TIM-1 is the dominant PS receptor and importantly, loss of the TIM-1 mucin domain (TIM-1Δmucin B cells) precludes TIM-1-mediated binding of PS.
Induction of IL-10 in Bregs by apoptotic cells is defective in TIM-1Δmucin mice
Since TIM-1Δmucin B cells do not bind PS through TIM-1, these B cells can be used to evaluate the role of TIM-1 in IL-10 induction by ACs. To address this, wt and TIM-1Δmucin mice received 107 syngeneic apoptotic thymocytes (ACs), as described(6). After 7d, IL-10 expression in total (CD19+) and TIM-1+ B cells was determined. In wt mice, AC administration led to a 1.8-fold increase in the percent of IL-10+ B cells, and this increase was much more pronounced in TIM-1+ B cells (Fig2A,B). However, in TIM-1Δmucin mice, AC administration failed to increase IL-10 expression. Even at baseline, 6-week old TIM-1Δmucin mice exhibited a ∼40-50% decrease in basal B cell IL-10 expression in comparison to WT (Fig2A,B).
Figure 2. Induction of IL-10 in B cells by apoptotic cells (ACs) is defective in TIM-1Δmucin mice.

A) Representative flow plots showing IL-10 expression on CD19+ and TIM-1+CD19+ B cells from wt vs. TIM-1Δmucin mice 7d after administering 107 ACs (iv).
B) Cumulative data showing % of IL-10+ B cells derived from flow plots in A.
C) Isolated splenic 106 B cells from wt and TIM-1Δmucin mice were co-incubated with naïve OTII T cells (5×105/ml), Ovalbumin, and increasing numbers of AC for 48h, followed by intracellular staining for IL-10 on CD19+B cells.
D) Flow-sorted CD19+TIM-1+ or CD19+TIM-1- B cells from wt and TIM-1Δmucin mice were incubated with ACs for 48h followed by intracellular staining for IL-10 on CD19+B cells.
Graphed data expressed as mean±SEM (*p<0.05; **p<0.01). Representative of ≥2 independent experiments, in triplicate wells or 3 mice/group.
To more directly examine the effect of ACs on B cell IL-10 expression, we used an established in vitro assay(6). ACs plus Ovalbumin-stimulated OTII cells induced up to a 4-fold increase IL-10 expression in wt, but not TIM-1Δmucin B cells (Fig2C). Moreover, purified TIM-1+ (but not TIM-1- or TIM-1Δmucin) B cells upregulate IL-10 expression when they are directly stimulated with ACs in vitro (Fig2D). These data combined with those in Fig1, indicate that TIM-1 is the major PS receptor on B cells, and TIM-1 is the predominant receptor underlying AC-induced IL-10 by B cells.
AC ligation by TIM-1 is sufficient to induce Breg IL-10 induction
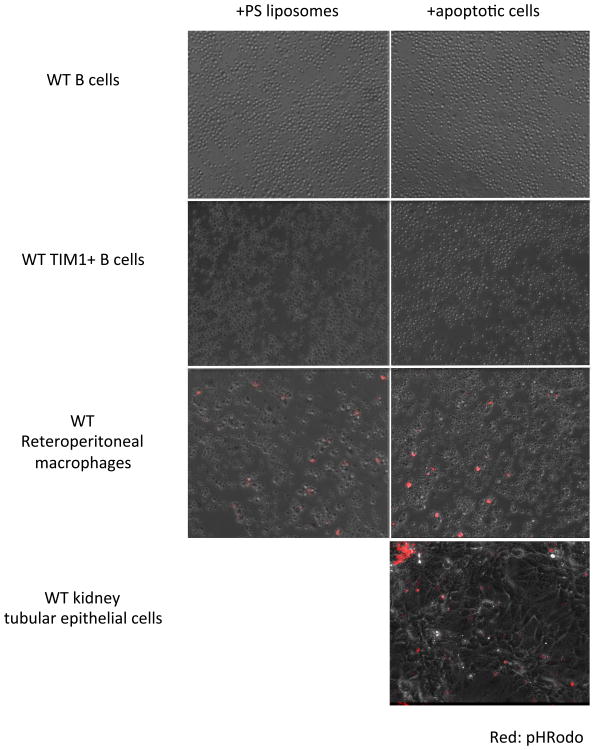
TIM-1 (KIM-1) expressed by injured kidney proximal tubular epithelial cells is the primary receptor responsible for PS-mediated binding of ACs followed by phagocytosis(9). Although ACs induce IL-10 upon interaction with B cells in a TIM-1-dependent fashion, it is unknown whether ligation alone is sufficient, or whether engulfment is necessary. In this regard, TIM-4 appears to play an important role in PS-mediated tethering of ACs by macrophages, but engulfment is actually mediated by other receptors(21,22). To differentiate between binding and internalization, live-cell imaging was performed on B cells incubated with PS-liposomes containing pHRodo succinimidyl ester(22). Despite serum-starvation to promote phagocytosis, no internalization of liposomes by total or purified TIM-1+ B cells was observed over 16h (Fig3 left panels, Supplemental Movie2). Similar results were observed when pHRodo-labeled apoptotic cells were used instead of PS-liposomes (Fig3 right panels, Supplemental Movie3). In comparison, kidney tubular epithelial cells expressing TIM-1 (KIM-1), the only known PS-receptor expressed on these cells, readily bound and engulfed pHRodo-labeled ACs within a few hours (Fig3 bottom, Supplemental Movie4). Furthermore, WT retroperitoneal macrophages, which express multiple PS-receptors, including TIM-1 and TIM-4, demonstrated uptake of both pHRodo liposomes and ACs.
Figure 3. Neither PS-coated liposomes nor apoptotic cells (AC) are internalized by isolated CD19+TIM-1+ splenic B cells.
PS-coated liposomes or ACs were generated as described in methods, and labeled with pHRodo succinimidyl ester, a pH-sensitive red fluorophore whose emission increases dramatically at pH<6.0 (i.e. upon entry into phagolysosomes). Liposomes or ACs were then diluted in serum-free media and incubated with total or sorted TIM-1+ B cells from WT mice. Still images taken at t=16h from time-lapse video of images captured in 15 minute intervals is shown. Time-lapse videos are available in supplemental materials. Left panels: splenic B cells incubated with labeled PS-liposomes; right panels: splenic B cells incubated with ACs. Top row: WT CD19+ B cells; second row: WT CD19+TIM-1+ B cells; third row: WT retroperitoneal macrophages (positive control); bottom row: TIM-1+ (KIM-1+) WT kidney tubular epithelial cells. Red=pHRodo. Images show merged brightfield and TRITC channels.
The TIM-1 mucin domain is also critical for induction of IL-10+ Bregs by anti-TIM-1
The studies above suggest that TIM-1Δmucin B cells do not bind PS on ACs via TIM-1 and are defective in B cell IL-10 induction by ACs. Additionally, these mice exhibit a baseline defect in Bregs suggesting that AC-mediated signals through TIM-1 may contribute to basal Breg levels. We next asked whether induction of Bregs mediated by anti-TIM-1 (RMT1-10) was also dependent on the TIM-1 mucin domain. This is important because unlike ACs, anti-TIM-1 is entirely specific for TIM-1 and anti-TIM-1 binds normally to the intact IgV domain expressed by TIM-1Δmucin B cells, even when titered to non-saturating concentrations (Fig4A, and data not shown). Previously, we showed that anti-TIM-1 treatment prolongs allograft survival in BALB/c mice, by increasing both number and percentage of TIM-1+ B cells expressing IL-10(5). Since TIM-1 expressed by TIM-1Δmucin mice has intact IgV and intracellular domains, we postulated that anti-TIM-1 would still induce Bregs. Treatment of allosensitized wt C57BL/6 mice with anti-TIM-1 led to an ∼1.7-fold increase in both TIM-1 and IL-10 expression by total and TIM-1+ B cells (Fig 4B,C, Supplementary Fig 1A). IL-10 expression was highly enriched (∼16-fold) on TIM-1+ vs. TIM-1- B cells. IL-4 expression, which is important for induction of IL-10+ Bregs(5), was also increased ∼two-fold in total and TIM-1+ B cells, following anti-TIM-1 treatment of allosensitized mice (Fig4C, Supplementary Figure1B). Since neither transplantation nor anti-TIM-1 affect the total number of splenic B cells, these percentages directly reflect differences in cell number(5).
Figure 4. The TIM-1 mucin domain is also critical for induction of IL-10+ Bregs by anti-TIM-1.
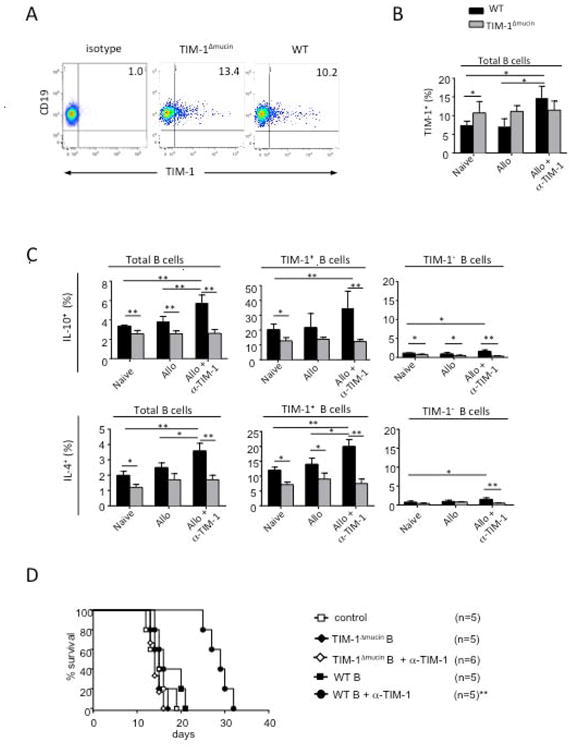
A) Representative flow cytometry plots showing anti-TIM-1 mAb RMT1-10 binding to TIM-1Δmucin vs. WT B cells, as assessed by indirect staining with RMT1-10 followed by PE-conjugated anti-rat Ig secondary mAb.
B) Frequency of TIM-1 expression on CD19+ B cells in wt or TIM-1Δmucin mice that were naive, allosensitized, or allosensitized and treated with anti-TIM-1 (RMT1-10) (*p<0.05, **p<0.01; n=4/group). Error bars indicate SEM. Representative of 3 independent experiments, with 3 mice per condition.
C) Frequency of IL-10 and IL-4 expression on CD19+, CD19+TIM-1+ and CD19+TIM-1- B cells in mice as in B.
D) TIM-1Δmucin B cells fail to promote allograft survival. Naïve B cells from wt or TIM-1Δmucin C57BL/6 mice were transferred into B cell deficient μMT (C57BL/6) recipients of BALB/c islets, and treated with or without anti-TIM-1 (**p<0.01vs. other groups). n=5-6 mice per group.
In comparison to wt B cells, naïve TIM-1Δmucin B cells actually express slightly higher baseline levels of TIM-1 (Fig4A). However, IL-10 expression is reduced on both total and TIM-1+ B cells from naïve TIM-1Δmucin mice, confirming the basal defect in B cell IL-10 expression even in young mice noted above (Fig4C, SupplementaryFig 1A). Moreover, in contrast to wt mice, there was no induction in B cell TIM-1 or IL-10 expression in TIM-1Δmucin mice in response to alloantigen plus anti-TIM-1, exacerbating the relative defect in B cell IL-10 expression seen in untreated mice. Expression of IL-4 by TIM-1Δmucin B cells parallels that of IL-10 (Fig4C, Supplementary Fig1B). It is decreased at baseline and not induced by anti-TIM-1. Thus, both basal and anti-TIM-1-induced Bregs are defective in TIM-1Δmucin mice, consistent with the TIM-1 mucin domain regulating responsiveness to TIM-1 signaling and TIM-1 regulating the induction of Breg.
TIM-1Δmucin B cells fail to promote allograft survival
We previously showed that in the absence of B cells, anti-TIM-1 actually accelerated rejection compared to untreated recipients(5). In contrast, after transfer of wt B cells, anti-TIM-1 treatment significantly prolonged islet allograft survival and this was dependent on induction of IL-10 expression by B cells. Thus, this model is dependent on both IL-10+ Bregs and TIM-1 signaling. Here, we specifically examined the effects of B cell TIM-1 signaling on allograft survival, by transferring B cells from wt versus TIM-1Δmucin mice into B cell deficient μMT recipients of BALB/c islets. After transfer of wt B cells, anti-TIM-1 treatment doubles islet allograft survival, by promoting the induction of IL-10 on B cells (Fig4C, (5)), compared to untreated mice (Fig4D). In contrast, in mice reconstituted with TIM-1Δmucin B cells, where IL-10 induction is impaired, anti-TIM-1 had no effect on allograft survival (Fig4D). Thus, an intact TIM-1 mucin domain on B cells is required for induction of IL-10+ Bregs in vivo and for prolonged graft survival induced by anti-TIM-1.
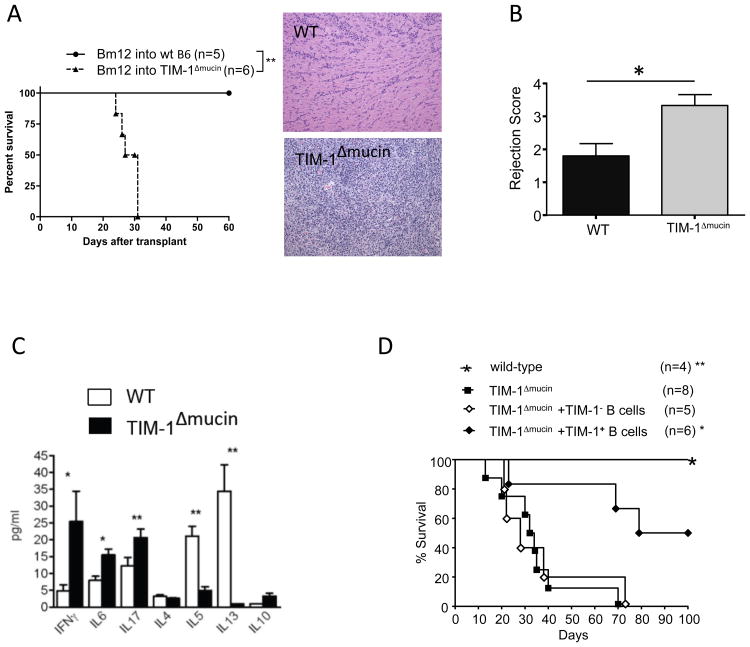
TIM-1Δmucin recipients exhibit accelerated allograft rejection that is ameliorated by transfer of wt TIM-1+ B cells
We previously showed that TIM-1Δmucin mice (B6) develop age-related autoimmunity associated with T cell hyper-responsiveness and decreased B cell IL-10 first observable at 4-6 months of age. However, the degree to which T cell defects were due to an underlying defect in Bregs was unclear. In wt B6 recipients, bm12 cardiac allografts (single MHCII mismatch) exhibit long-term survival, but develop chronic allograft vasculopathy(23). In contrast, 6-8 week-old TIM-1Δmucin recipients exhibit an exaggerated immune response, with allograft rejection by 31d (Fig5A). This is associated with severe mononuclear cell infiltration and vasculopathy at a time when allografts in wt recipients appear minimally inflamed (Fig5A,B). Thus, TIM-1Δmucin mice exhibit immune hyper-responsiveness even at a relatively young age. In general agreement, CD4 cells from young allosensitized TIM-1Δmucin mice exhibit heightened IFNγ, IL-6, and IL-17, and impaired Th2 (IL-4, IL-5, and IL-13), responses (Fig5C), a pattern generally associated with increased allograft injury(24). While IL-10 expression by CD4 cells in TIM-1Δmucin recipients was preserved (Fig6C), B cell IL-10 expression is impaired in these mice (Fig2,4).
Figure 5. TIM-1Δmucin mice exhibit accelerated allograft rejection.
A) bm12 hearts were transplanted into wt or TIM-1Δmucin C57BL/6 mice. Left: Kaplan-Meir plots of graft survival (MST>100d vs. 29d; **p<0.01). Right: Representative H&E staining of allografts 30d post-transplant (100× magnification). n=5-6 mice per group.
B) Grafts, from A, were scored for rejection using ISHLT guidelines: Grade 1, mild (interstitial or perivascular infiltrate with < 1 focus of myocyte damage; Grade 2, moderate (>2 foci of myocyte damage); Grade 3 severe (diffuse infiltrate with multiple foci of myocyte damage, vasculitis, edema) in a blinded fashion. *p<0.05; n=5 in each group. Error bars indicate SEM.
C) Splenic CD4 cells from recipients 21d post-transplantation were restimulated in vitro with irradiated bm12 splenocytes for 48h. Cytokine concentration in culture supernatants was determined by Luminex (*p<0.05, **p<0.01). Error bars indicate SEM. Representative of 2 independent experiments, each with 3 mice per group.
D) Kaplan-Meier plots of graft survival of TIM-1Δmucin recipients of bm12 cardiac allografts after receiving no treatment vs. 5×106 TIM-1+CD19+ or TIM-1-CD19+ B cells from (d14) alloimmunized wt C57BL/6 mice (*p=0.01, **p=0.0025 vs. all other groups). n= 4-8 mice per group.
We previously showed that transfer of TIM-1+ Bregs prolongs allograft survival by reducing Th1 and augmenting Th2 responses in a B cell-IL-10 dependent fashion(5, 16). To determine whether the defect in TIM-1+ Bregs contributes to the observed hyper-responsiveness of TIM-1Δmucin mice, we transferred TIM-1+ versus TIM-1- B cells from allosensitized syngeneic wt mice into TIM-1Δmucin recipients of bm12 cardiac allografts. TIM-1+ B cell transfer significantly prolonged graft survival, with 50% of TIM-1Δmucin recipients achieving long-term engraftment, whereas TIM-1- B cells had no effect (Fig5D). Thus, young TIM-1Δmucin mice exhibit a baseline deficit in Bregs and their immunological hyper-responsiveness can be ameliorated by a single transfer of wt Bregs at the time of transplantation.
Discussion
We previously showed that TIM-1 is an inclusive marker for IL-10-producing B cells with regulatory activity (5). Moreover, TIM-1 ligation induces IL-10+ Bregs. Using mice expressing a mutant version of TIM-1 has now allowed us to directly examine the role of the mucin domain in TIM-1-mediated signals and to delineate the role of TIM-1 in Breg homeostasis. Indeed, we find that TIM-1 is more than a phenotypic marker and is intimately involved in maintaining both basal and inducible Breg levels.
Tolerance is maintained despite continuous exposure to self-antigens through apoptotic cells (ACs). It is estimated that over the course of a single day, >109 cells turnover/die in our body(25). These include cells that are constantly generated in the course of tissue regeneration, used/aged cells, and damaged cells. In the context of transplantation, additional dying cells likely arise from the allograft itself, as well as from the constant immune response that it generates. Rapid removal of ACs inhibits DC and macrophage maturation, and promotes their expression of anti-inflammatory cytokines(25-27). Conversely, if AC clearance is defective, secondary necrosis causes release of auto-antigens and DAMPs that promote autoimmunity(26).
AC engulfment is mediated by “eat-me” signals including PS, which is expressed on cell membranes early in apoptosis(25). PS is recognized directly by PS-receptors that include TIM-family members, or indirectly, by bridging proteins that link PS to Mer tyrosine kinase or β3/5 integrins on phagocytes(25-27). TIM-4 expressed by macrophages and TIM-1 expressed by kidney epithelial cells are directly involved in AC engulfment(10). Additionally, natural IgM binds PS, and possibly self-antigens, enhancing AC uptake in concert with FcR and complement proteins(28). While deficiency in IgM, C1q, and Mer, are associated with defective AC clearance and lupus, deficiency in other engulfment receptors, leads to AC accumulation without autoimmunity(25,26,28). This suggests that ACs may also promote tolerance through other pathways(25).
Another mechanism by which ACs promote tolerance is by inducing IL-10 expression by B cells (MZ and B1 B cells in particular)(6,7). MZ and B1 B cells highly express natural IgM, which may bind ACs through PS and self-antigens (as noted above). BCR and TLR9 engagement are also involved in Breg induction by ACs(7). Our data now indicate that TIM-1 ligation on B cells is essential for AC-mediated induction of IL-10. In this regard, TIM-1 is expressed at two-fold higher frequency on MZ and B1 B cells than on other B cell subsets(5).
Unlike macrophages or kidney epithelial cells, we showed that B cells do not engulf ACs, and engulfment is not required for IL-10 expression. Our data also suggest that TIM-1 is not merely required for tethering, but also for signaling upon AC binding. While significantly reduced, ACs do still bind to TIM-1- and to TIM-1Δmucin B cells, presumably through interactions with other PS receptors. However, this does not induce IL-10. Thus, TIM-1 mediates the majority of PS binding to B cells, and is essential for IL-10 expression. While the TIM-1 crystal structure shows that PS binds to the IgV domain and does not directly interact with the mucin domain(11), the mucin domain may contribute sterically to PS-mediated binding of ACs. Alternatively, loss of the mucin domain could alter IgV domain conformation in a manner that decreases AC binding (although both anti-TIM-1 and TIM-4-Ig binding to the TIM-1 IgV domain remain intact(18) and (Fig4A)). Regardless, the decrease in Bregs observed in TIM-1Δmucin mice, and the loss of age-related increase in Bregs (discussed below), suggests that AC interactions with TIM-1 may promote peripheral tolerance by maintaining baseline levels of Bregs.
In the absence of the TIM-1 mucin domain, anti-TIM-1 binding to the IgV domain is intact, but is incapable of inducing IL-10 expression by B cells. Thus, anti-TIM-1 prolongs graft survival in B-deficient mice after the transfer of WT, but not TIM-1Δmucin B cells. This supports our previous findings that TIM-1 ligation in B cells induces IL-10+ Bregs which are required to prolong graft survival. Moreover, our study provides the most concrete evidence, to date, that the mucin domain actually regulates TIM-1 signaling. Previous studies have only inferred such a role. For example, differences in mucin domain length in different mouse strains correlate with differences in TIM-1 responsiveness; BALB/c mice exhibit increased Th2 responses compared to C57BL/6 mice which have a shorter TIM-1 mucin domain(17). Whereas we previously showed that in BALB/c mice, alloantigen plus anti-TIM-1 increased B cell TIM-1, IL-10 and IL-4 expression, four-fold(5), we now show that in C57BL/6 mice, antigen plus anti-TIM-1 leads to only a two-fold induction (Fig4). Thus, B cells from wt BALB/c mice (longer mucin domain) are more responsive to antibody-mediated TIM-1 ligation than those from wt C57BL/6 mice (short mucin domain), while B cells from TIM-1Δmucin mice lacking the TIM-1 mucin domain altogether, fail to induce B cell IL-4 or IL-10. Additionally, deletional polymorphisms of the human TIM-1 mucin domain correlate with susceptibility to asthma and allergy(12,13). Combined with our current findings, these data strongly suggest that regions within the mucin domain affect TIM-1 signaling and immunological reactivity.
While Breg activity has generally been associated with IL-10, IL-35 was recently shown to be intimately associated with Breg function(8,29). Importantly, IL-35 signaling was required for generation of IL-10+ Bregs, and B cell IL-10 expression was necessary for the prevention of uveitis mediated by IL-35(29). Since IL-35 can upregulate B cell IL-10(8,29), TIM-1 could work at the level of IL-35. Alternatively, IL-35 could bypass the requirement for TIM-1 in terms of B cell IL-10 induction. While addition of IL-35 to LPS for 72h induced IL-10 in WT B cells, it had no effect on IL-10 expression by TIM-1Δmucin B cells (Supplementary Fig2). Thus, TIM-1 signaling has a dominant effect on B cell IL-10 expression, and the defect in IL-10 seen in TIM-1Δmucin B cells cannot be rescued by provision of IL-35.
In addition to an age-related defect in Bregs previously reported(18), we now show that deletion of the TIM-1 mucin domain results in defective Bregs that contribute to disordered immune homeostasis even in young mice. The deficiency of Bregs in TIM-1Δmucin mice appears to be due to both a baseline decrease and a defect in their induction, rather than a survival defect of Bregs over time. Thus, the Bregs start at a lower baseline number in TIM-1Δmucin mice and do not progressively increase with age, as observed in wt mice(18). This may help explain why TIM-1Δmucin mice develop spontaneous autoimmunity with age, and why young mice exhibit accelerated rejection of single MHC mismatched cardiac allografts associated with an exaggerated CD4 cell pro-inflammatory response compared to wt recipients. In this regard, transfer of WT TIM-1+ but not TIM-1- B cells from alloimmunized hosts ameliorated rejection of bm12 cardiac allografts by TIM-1Δmucin mice, with ∼50% surviving long-term.
In summary, our data demonstrate that the TIM-1 mucin domain regulates responsiveness to TIM-1 signaling, and TIM-1 signaling regulates the induction of Breg. Binding of ACs to TIM-1 appears to be an important physiological trigger in Breg maintenance and induction. Moreover, an anti-TIM-1 antibody can mimic the same effects on Bregs and promote allograft tolerance, thus demonstrating the therapeutic potential for targeting TIM-1.
Supplementary Material
Supplementary Figure 1. Representative flow cytometry plots showing the frequency of IL-10 expression (A) or IL-4 expression (B) on CD19+, CD19+TIM-1- or CD19+TIM-1+ B cells in spleen 7 days after AC injection of WT vs. TIM-1Δmucin mice.
Supplementary Figure 2. IL-35 signaling cannot induce IL-10 in TIM-1Δmucin B cells. Representative flow cytometry plots (A) and cumulative data (B) from sort-purified splenic CD19+ B cells from either WT or from TIM-1Δmucin mice that were cultured in the presence of either LPS alone, or LPS plus IL-35 for 72h followed by intracellular staining for IL-10 expression.
Supplemental Movie 1. Areas of major PS-liposomal binding are co-localized with TIM-1. Flow-sorted CD19+TIM-1+ B cells from wt mice were co-incubated with green fluorescence-labeled phosphatidylserine-coated liposomes (PS-liposomes). After 12h, cells were fixed and stained with DAPI, and re-stained for TIM-1. Movie depicts a 3D reconstruction of confocal z-stack images. Green=PS-liposomes, Red=TIM-1, Blue=DAPI.
Supplemental Movie 2. PS-coated liposomes are not internalized by isolated CD19+TIM-1+ splenic B cells. PS-coated liposomes were generated as described in methods section, and labeled using pHRodo succinimidyl ester, a pH-sensitive red fluorophore whose emission increases dramatically at pH<6.0 (i.e. upon entry into phagolysosomes). The liposome mixture was then diluted in serum-free media and incubated with total or sorted TIM-1+ B cells from WT mice. Sixteen-hour time-lapse video of images captured in 15 minute intervals is shown. Left panels: merged brightfield and TRITC channels; right panels: TRITC channel. Top row: WT CD19+ B cells; middle row: WT CD19+TIM-1+ B cells; bottom row: WT retroperitoneal macrophages (positive control). Red=pHRodo.
Supplemental Movie 3. Apoptotic cells are not internalized by isolated CD19+TIM-1+ splenic B cells. Apoptotic cells were generated as described in methods section, and labeled using pHRodo succinimidyl ester, a pH-sensitive red fluorophore whose emission increases dramatically at pH<6.0 (i.e. upon entry into phagolysosomes). ACs were then incubated with total or sorted TIM-1+ B cells at a ratio of 1:10. Sixteen-hour time-lapse video of images captured in 15 minute intervals is shown. Images show merged brightfield and TRITC channels. Top: WT CD19+ B cells; middle: WT CD19+TIM-1+ B cells; bottom: WT retroperitoneal macrophages (positive control). Red=pHRodo.
Supplemental Movie 4. Apoptotic cells labeled with pHRodo succinimidyl ester were incubated with kidney tubular epithelial cells expressing TIM-1 (KIM-1). Sixteen-hour time-lapse video of images captured in 30 minute intervals is shown. Top panel: merged brightfield and TRITC channels; bottom panel: TRITC channel. Red=pHRodo.
Acknowledgments
This work was supported by NIH grants R01AI097361(DR), R01AI091977 (DAV) and R56AI101150(NN), and an American Heart Association fellow-to-faculty transition grant (MYY).
Abbreviations
- AC
apoptotic cell
- BCR
B cell receptor
- Breg
regulatory B cell
- DC
dendritic cell
- EAE
experimental autoimmune encephalitis
- IL-4
interleukin-4
- IL-10
interleukin-10
- IL-35
interleukin-35
- KIM-1
kidney injury molecule-1
- MZ
marginal zone
- PC
phosphatidylcholine
- PS
phosphatidylserine
- TIM-1
T-cell immunoglobulin mucin domain-1
- TLR
Toll-like receptor
- WT
wild-type
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information: Additional Supporting Information may be found in the online version of this article.
References
- 1.Dilillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 2.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 3.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 5.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121(9):3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(35):14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miles K, Heaney J, Sibinska Z, Salter D, Savill J, Gray D, et al. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(3):887–892. doi: 10.1073/pnas.1109173109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507(7492):366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichimura T, Asseldonk EJPV, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. Journal of Clinical Investigation. 2008;118(5):1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27(6):927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santiago C, Ballesteros A, Tami C, MartÌnez-MuÒoz L, Kaplan GG, Casasnovas JM. Structures of T Cell Immunoglobulin Mucin Receptors 1 and 2 Reveal Mechanisms for Regulation of Immune Responses by the TIM Receptor Family. Immunity. 2007;26(3):299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat Rev Immunol. 2008;8(8):577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- 13.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235(1):172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degauque N, Mariat C, Kenny J, Zhang D, Gao W, Vu MD, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118(2):735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, et al. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204(7):1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueno T, Habicht A, Clarkson MR, Albin MJ, Yamaura K, Boenisch O, et al. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest. 2008;118(2):742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2(12):1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 18.Xiao S, Brooks CR, Zhu C, Wu C, Sweere JM, Petecka S, et al. Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(30):12105–12110. doi: 10.1073/pnas.1120914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angiari S, Donnarumma T, Rossi B, Dusi S, Pietronigro E, Zenaro E, et al. TIM-1 glycoprotein binds the adhesion receptor P-selectin and mediates T cell trafficking during inflammation and autoimmunity. Immunity. 2014;40(4):542–553. doi: 10.1016/j.immuni.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balasubramanian S, Jansen M, Valerius MT, Humphreys BD, Strom TB. Orphan nuclear receptor Nur77 promotes acute kidney injury and renal epithelial apoptosis. J Am Soc Nephrol. 2012;23(4):674–686. doi: 10.1681/ASN.2011070646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park D, Hochreiter-Hufford A, Ravichandran KS. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr Biol. 2009;19(4):346–351. doi: 10.1016/j.cub.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 22.Toda S, Hanayama R, Nagata S. Two-step engulfment of apoptotic cells. Mol Cell Biol. 2012;32(1):118–125. doi: 10.1128/MCB.05993-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayegh MH, Wu Z, Hancock WW, Langmuir PB, Mata M, Sandner S, et al. Allograft rejection in a new allospecific CD4+ TCR transgenic mouse. Am J Transplant. 2003;3(4):381–389. doi: 10.1034/j.1600-6143.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 24.Sho M, Yamada A, Najafian N, Salama AD, Harada H, Sandner SE, et al. Physiological mechanisms of regulating alloimmunity: Cytokines, CTLA-4, CD25+ cells, and the alloreactive T cell clone size. Journal of Immunology. 2002;169(7):3744–3751. doi: 10.4049/jimmunol.169.7.3744. [DOI] [PubMed] [Google Scholar]
- 25.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1):a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140(5):619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nature reviews Immunology. 2007;7(12):964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 28.Notley CA, Brown MA, Wright GP, Ehrenstein MR. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. Journal of immunology. 2011;186(8):4967–4972. doi: 10.4049/jimmunol.1003021. [DOI] [PubMed] [Google Scholar]
- 29.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20(6):633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Representative flow cytometry plots showing the frequency of IL-10 expression (A) or IL-4 expression (B) on CD19+, CD19+TIM-1- or CD19+TIM-1+ B cells in spleen 7 days after AC injection of WT vs. TIM-1Δmucin mice.
Supplementary Figure 2. IL-35 signaling cannot induce IL-10 in TIM-1Δmucin B cells. Representative flow cytometry plots (A) and cumulative data (B) from sort-purified splenic CD19+ B cells from either WT or from TIM-1Δmucin mice that were cultured in the presence of either LPS alone, or LPS plus IL-35 for 72h followed by intracellular staining for IL-10 expression.
Supplemental Movie 1. Areas of major PS-liposomal binding are co-localized with TIM-1. Flow-sorted CD19+TIM-1+ B cells from wt mice were co-incubated with green fluorescence-labeled phosphatidylserine-coated liposomes (PS-liposomes). After 12h, cells were fixed and stained with DAPI, and re-stained for TIM-1. Movie depicts a 3D reconstruction of confocal z-stack images. Green=PS-liposomes, Red=TIM-1, Blue=DAPI.
Supplemental Movie 2. PS-coated liposomes are not internalized by isolated CD19+TIM-1+ splenic B cells. PS-coated liposomes were generated as described in methods section, and labeled using pHRodo succinimidyl ester, a pH-sensitive red fluorophore whose emission increases dramatically at pH<6.0 (i.e. upon entry into phagolysosomes). The liposome mixture was then diluted in serum-free media and incubated with total or sorted TIM-1+ B cells from WT mice. Sixteen-hour time-lapse video of images captured in 15 minute intervals is shown. Left panels: merged brightfield and TRITC channels; right panels: TRITC channel. Top row: WT CD19+ B cells; middle row: WT CD19+TIM-1+ B cells; bottom row: WT retroperitoneal macrophages (positive control). Red=pHRodo.
Supplemental Movie 3. Apoptotic cells are not internalized by isolated CD19+TIM-1+ splenic B cells. Apoptotic cells were generated as described in methods section, and labeled using pHRodo succinimidyl ester, a pH-sensitive red fluorophore whose emission increases dramatically at pH<6.0 (i.e. upon entry into phagolysosomes). ACs were then incubated with total or sorted TIM-1+ B cells at a ratio of 1:10. Sixteen-hour time-lapse video of images captured in 15 minute intervals is shown. Images show merged brightfield and TRITC channels. Top: WT CD19+ B cells; middle: WT CD19+TIM-1+ B cells; bottom: WT retroperitoneal macrophages (positive control). Red=pHRodo.
Supplemental Movie 4. Apoptotic cells labeled with pHRodo succinimidyl ester were incubated with kidney tubular epithelial cells expressing TIM-1 (KIM-1). Sixteen-hour time-lapse video of images captured in 30 minute intervals is shown. Top panel: merged brightfield and TRITC channels; bottom panel: TRITC channel. Red=pHRodo.