Abstract
Ovarian cancers often highly express inflammatory cytokines and form implants throughout the peritoneal cavity. However, the mechanisms that drive inflammatory signaling and peritoneal metastasis of ovarian cancer are poorly understood. We previously identified that high expression of DLX4, a transcription factor encoded by a homeobox gene, is associated with reduced survival of ovarian cancer patients. In this study, we identified that DLX4 stimulates attachment of ovarian tumor cells to peritoneal mesothelial cells in vitro and increases the numbers of peritoneal implants in xenograft models. DLX4 induced expression of the cell surface molecule CD44 in ovarian tumor cells, and inhibition of CD44 abrogated the ability of DLX4 to stimulate tumor–mesothelial cell interactions. The induction of CD44 by DLX4 was attributed to increased activity of NF-κB that was stimulated by the inflammatory cytokine IL-1β, a transcriptional target of DLX4. The stimulatory effects of DLX4 on CD44 levels and tumor–mesothelial cell interactions were abrogated when IL-1β or NF-κB was inhibited in tumor cells. Furthermore, DLX4 expression levels strongly correlated with NF-κB activation and disease stage in clinical specimens of ovarian cancer. Collectively, these findings indicate that DLX4 induces CD44 by stimulating IL-1β–mediated NF-κB activity, thereby promoting peritoneal metastasis of ovarian cancer.
More than 60% of women with a diagnosis of ovarian cancer present with advanced-stage disease that has spread throughout the peritoneal cavity.1 Most patients with advanced-stage ovarian cancer relapse within 18 months after platinum-taxane chemotherapy, and the 5-year survival rate of these women is less than 30%.2 I.P. seeding is a pattern of spread that is unique to ovarian cancer and markedly differs from the hematogenous or lymphatic metastasis of many other types of solid tumors. Ovarian cancer cells often spread by shedding into the peritoneal fluid that transports tumor cells throughout the peritoneal cavity.3–5 Disseminated tumor cells frequently form implants on the omentum and other peritoneal surfaces that are lined by a protective monolayer of mesothelial cells.3–5 Seeding of the peritoneal cavity with tumor cells is often associated with ascites that contains inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor-α.6 Interactions of ovarian tumor cells with peritoneal mesothelial cells are mediated by several cell surface molecules. CD44 promotes attachment of ovarian tumor cells to mesothelial cells by binding hyaluronic acid, a glycosaminoglycan that is synthesized by mesothelial cells.7,8 Ovarian tumor–mesothelial cell interactions are also mediated by P-cadherin molecules that are expressed on the surfaces of tumor cells and mesothelial cells.9 Other cell surface molecules, such as α5β1 integrin, facilitate access of ovarian tumor cells to the submesothelial matrix.10 However, the mechanisms that induce expression of these cell adhesion molecules in ovarian cancer are poorly understood.
Homeobox genes encode transcription factors, often termed homeoproteins, which play essential roles in controlling cell lineage specification and tissue morphogenesis.11 Aberrant expression of many homeobox genes has been observed in a variety of malignancies, including ovarian cancer.12–15 The mechanisms of homeoproteins in tumor progression are poorly understood because only few transcriptional target genes have been identified. DLX4 is a homeobox gene that is not expressed in most normal adult tissues.16 We previously identified that high expression of DLX4 is strongly associated with reduced survival of ovarian cancer patients.17 A study using i.p. xenograft models revealed that DLX4 promotes ovarian tumor progression in part by inducing expression of vascular endothelial growth factor-A that stimulated ascites formation and tumor angiogenesis.17 Because DLX4 primarily functions as a transcription factor, we investigated the possibility that DLX4 also promotes tumor progression by stimulating other processes. In this study, we identified that DLX4 stimulates attachment of ovarian tumor cells to peritoneal mesothelial cells by inducing expression of CD44. The induction of CD44 by DLX4 was dependent on NF-κB activation and was attributed to the ability of DLX4 to induce expression of IL-1β directly. DLX4 might, therefore, contribute to poor outcomes in ovarian cancer in part by promoting peritoneal implantation of tumor cells via stimulation of inflammatory signaling.
Materials and Methods
Antibodies
DLX4 antibodies (Abs) for flow cytometry and tissue staining were purchased from Abcam (Cambridge, UK) and for chromatin immunoprecipitation were purchased from Abnova (Taipei City, Taiwan). CD44 Abs for flow cytometry were purchased from BD Biosciences (San Jose, CA) and for neutralization were purchased from Abcam. Phosphorylated NF-κB p65 (Ser536) Ab was purchased from Cell Signaling Technology (Danvers, MA). Secondary Abs were purchased from BD Biosciences.
Plasmids
The pIRES-EGFP2 FLAG-tagged DLX4 plasmid has been previously described.18 DLX4 cDNA was subcloned into the pRetroQ-AcGFP vector (Clontech, Mountain View, CA). Sources of other plasmids were as follows: IL1B cDNA and pGFP-V-RS nontargeting and DLX4 shRNA plasmids (OriGene Technologies, Rockville, MD); pGIPZ nontargeting and IL1B shRNA plasmids (MD Anderson Cancer Center shRNA and ORFeome Core Facility, Houston, TX); and pBabe-GFP-ΙκΒα-dominant-negative (ΙκΒα-dn)19 (plasmid 15264; Addgene, Cambridge, MA; construct originally deposited to Addgene by Dr. William Hahn, Dana-Farber Cancer Institute, Boston, MA).
Cell Culture and Transfection
Culture media were purchased from Invitrogen (Carlsbad, CA) and were supplemented with penicillin-streptomycin and fetal bovine serum (10% for tumor cells, 20% for mesothelial cells). Stable vector-control and DLX4-overexpressing ES2 cell lines have been previously described and were cultured in McCoy's 5A medium.17 Parental A2780, OVCAR8, and OVCA429 lines were provided by Dr. Gordon Mills (MD Anderson Cancer Center, Houston, TX) and cultured in RPMI 1640 medium (A2780, OVCAR8) and Dulbecco's minimal essential medium (OVCA429). The 2008 cell line was provided by Dr. Zahid Siddik (MD Anderson Cancer Center, Houston, TX) and cultured in RPMI 1640 medium. Ampho293 packaging cells were provided by Dr. Douglas Boyd (MD Anderson Cancer Center, Houston, TX) and cultured in Dulbecco's modified Eagle's medium. Transfections were performed using Lipofectamine 2000 reagent (Invitrogen). Viral supernatants were harvested from pRetroQ-AcGFP–transfected Ampho293 cells and were used to infect A2780 cells. Cells were selected with 0.5 μg/mL puromycin. Primary cultures of normal human omental mesothelial cells have been previously described20 and were provided by Dr. Ernst Lengyel (University of Chicago, Chicago, IL). Mesothelial cells were cultured in RPMI 1640 medium.
Xenograft Studies
Four-week-old female nude mice were purchased from the National Cancer Institute (Frederick, MD) and were inoculated i.p. with 3 × 106 cells of A2780 lines and with 1 × 106 cells of ES2 lines (n = 5 mice per group). Mice were euthanized by carbon dioxide asphyxiation at 4 weeks (A2780 groups) and at 3 weeks (ES2 groups). Formalin-fixed, paraffin-embedded tissue sections were stained with hematoxylin and eosin and viewed by light microscopy.
Cell Attachment Assays
Green fluorescent protein–expressing ovarian tumor cells were seeded in 96-well plates (1.5 × 104 per well) containing confluent monolayers of normal omental mesothelial cells as previously described.21 Where indicated, tumor cells were pre-incubated for 1 hour with neutralizing CD44 Ab or control IgG at a final concentration of 10 μg/mL. Tumor cells were also seeded into plates coated with collagen I, fibronectin, and laminin (Sigma-Aldrich, St. Louis, MO) as previously described.21 At 1 hour after seeding of tumor cells, wells were washed with culture medium to remove unattached tumor cells. Attached tumor cells were viewed by fluorescence microscopy using a Nikon TS100 microscope with fluorescein filter (Nikon, Melville, NY). Three independent experiments were performed in which attached cells were counted in five random ×200 microscopic fields in each experiment.
Flow Cytometry and Enzyme-Linked Immunosorbent Assay
Abs were diluted in phosphate-buffered saline containing 1% bovine serum albumin. For cell surface staining, tumor cells were incubated with CD44 Ab (1:10) for 30 minutes at 4°C, washed, and incubated with peridinin-chlorophyll-protein complex–conjugated anti-mouse IgG (1:5). For intracellular staining, cells were fixed in 1% paraformaldehyde (20 minutes at 4°C) and permeabilized in 0.1% saponin (15 minutes at room temperature). Cells were incubated with Abs to DLX4 (1:20) and phosphorylated NF-κB p65 (1:500) for 30 minutes at 4°C, washed, and incubated with peridinin-chlorophyll-protein complex– or phycoerythrin-conjugated secondary Abs. Thereafter, cells were washed and fixed in 4% paraformaldehyde. Staining was detected by flow cytometry (FACSCalibur, BD Biosciences). IL-1β levels in cell lysates were assayed using the IL-1β enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN).
Quantitative RT-PCR
CD44, CDH3, and IL1B transcripts were detected by using SYBR Green qPCR Master Mix (SABiosciences, Valencia, CA) and the following primers: CD44, 5′-GGCTTTCAATAGCACCTTGC-3′ (forward) and 5′-ACACCCCTGTGTTGTTTGCT-3′ (reverse); CDH3, 5′-CAGGTGCTGAACATCACGGACA-3′ (forward) and 5′-CTTCAGGGACAAGACCACTGTG-3′ (reverse); IL1B, 5′-CCACAGACCTTCCAGGAGAATG-3′ (forward) and 5′-GTGCAGTTCAGTGATCGTACAGG-3′ (reverse). RPL32 transcript levels were used as controls for normalization and were detected by using the following primers: 5′-ACAAAGCACATGCTGCCCAGTG-3′ (forward) and 5′-TTCCACGATGGCTTTGCGGTTC-3′ (reverse). The expression of NF-κB target genes and genes associated with NF-κB signal transduction were assayed by using the Human NF-κB Signaling Pathway RT2 Profiler PCR Array (SABiosciences) according to the manufacturer's instructions.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation assays were performed by using the EZ-ChIP Assay Kit (Millipore, Temecula, CA). Sheared chromatin was incubated overnight with 1 μg of DLX4 Ab. DNA was purified from precipitated complexes. The following primers were used to amplify a 316-bp fragment of the IL1B promoter: 5′-GGTAGAGACCCACACCCTCA-3′ (forward) and 5′-CATGGAAGGGCAAGGAGTAG-3′ (reverse). As a negative control, a 166-bp fragment of the GAPDH gene was amplified by using the following primers: 5′-TACTAGCGGTTTTACGGGCG-3′ (forward) and 5′-TCGAACAGGAGGAGCAGAGAGCGA-3′ (reverse).
Reporter Assays
A firefly luciferase reporter construct driven by tandem NF-κB binding sites was used to assay NF-κB transcriptional activity (Cignal NF-κB Reporter Kit; SABiosciences). Tumor cells were cotransfected with firefly luciferase reporter plasmid, cDNA, or shRNA plasmids and Renilla luciferase reporter plasmid to normalize transfection efficiency as previously described.18 Luciferase activities were assayed using the Dual-Luciferase Reporter Assay Kit (Promega, Madison, WI). Three independent experiments were performed for each assay.
Tissue Microarray Analysis
Tissue cores were taken from formalin-fixed, paraffin-embedded specimens of 135 cases of ovarian cancer and used to construct tissue microarrays (Beecher Instruments, Sun Prairie, WI). Clinicopathologic features of cases are described in Table 1. Replicate microarray slides were stained with Abs to DLX4 (1:100) and phosphorylated NF-κB p65 (1:500), and staining was detected by streptavidin-biotin-peroxidase and 3,3′-diaminobenzidine. The percentage of tumor cells with positive staining was scored in two random 200× microscopic fields per core. An average staining score was determined for each case.
Table 1.
Clinicopathologic Features of Ovarian Cancer Cases Represented on Tissue Microarrays
| Histological subtype | Tumor | Disease stage |
Total | |||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| Serous | Low grade | 1 | 10 | 11 | ||
| High grade | 1 | 4 | 66 | 18 | 89 | |
| Clear cell | 4 | 2 | 4 | 10 | ||
| Endometrioid | Low grade | 5 | 5 | |||
| High grade | 3 | 1 | 4 | |||
| Mucinous | 1 | 3 | 4 | |||
| Mixed | 2 | 8 | 2 | 12 | ||
| Total | 13 | 10 | 91 | 21 | 135 | |
Statistical Analysis
Statistical analysis was performed by using STATISTICA 6 software (StatSoft, Tulsa, OK). Significance of differences in staining scores between groups of patients was assessed by Mann-Whitney U-test. Correlation coefficients were determined by Spearman test. Statistical significance of other data was assessed by unpaired two-tailed Student's t-test. Data represent means ± SD. P < 0.05 was considered significant.
Results
DLX4 Increases the Number of Tumor Implants in I.P. Xenograft Models of Ovarian Cancer
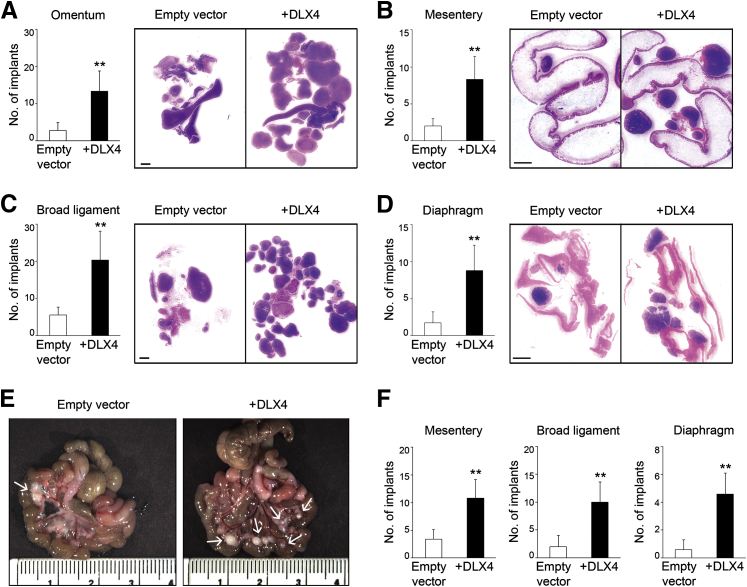
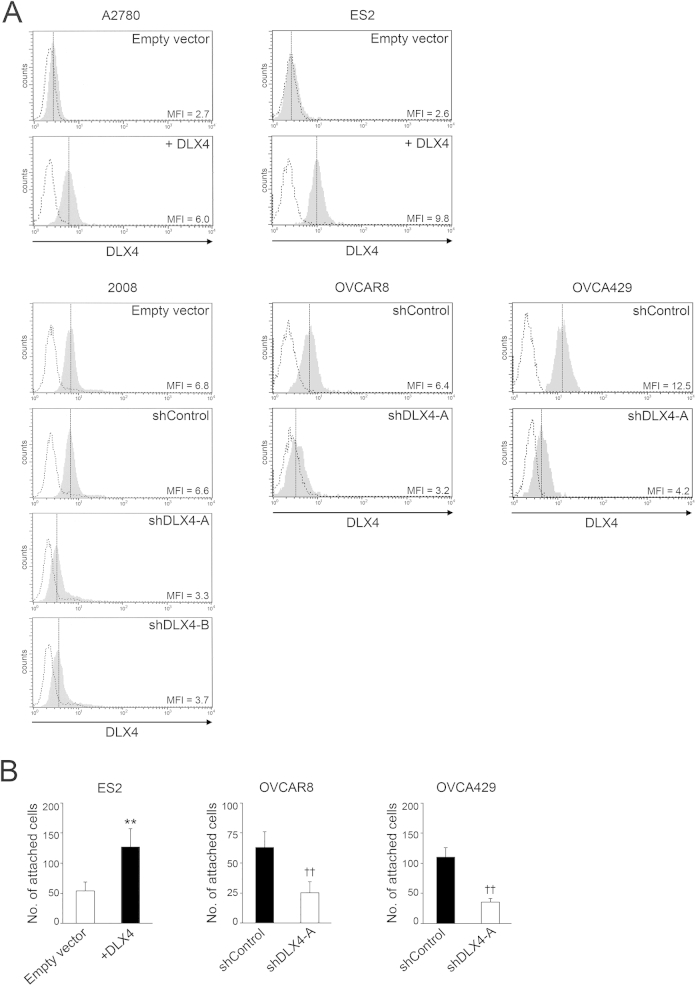
We previously identified that the A2780 and ES2 ovarian cancer cell lines express almost no DLX4.17 Initially, to investigate the effect of DLX4 on tumor implantation, we inoculated mice i.p. with equivalent numbers of cells of stable vector-control and DLX4-overexpressing (+DLX4) A2780 lines. Levels of DLX4 in these lines are shown in Supplemental Figure S1A. Mice that were inoculated with +DLX4 A2780 cells developed significantly higher numbers of omental implants than did mice that were inoculated with vector-control A2780 cells (P < 0.01) (Figure 1A). The +DLX4 group also developed significantly higher numbers of implants on the mesentery, broad ligament, and diaphragm than did the vector-control group (P < 0.01) (Figure 1, B–D). To confirm our findings, we generated i.p. xenograft models from stable vector-control and +DLX4 ES2 lines (Supplemental Figure S1A). Similar to our findings in the A2780 models, the +DLX4 ES2 group developed significantly higher numbers of peritoneal implants than did the vector-control ES2 group (P < 0.01) (Figure 1, E and F).
Figure 1.
DLX4 increases the number of tumor implants in i.p. xenograft models of ovarian cancer. A–D: Female nude mice were inoculated i.p. with equivalent numbers of cells (3 × 106) of vector-control (empty vector) and +DLX4 A2780 lines. Mice were sacrificed at 4 weeks thereafter. Numbers of implants on the omentum (A), mesentery (B), broad ligament (C), and diaphragm (D) were counted in tissue sections of each mouse. The average numbers of tumor implants are shown at each site in each group of mice and representative examples of tissue sections stained with hematoxylin and eosin. E and F: Female nude mice were inoculated i.p. with 1 × 106 cells of vector-control and +DLX4 ES2 lines and were sacrificed at 3 weeks thereafter. E: Representative examples of resected intestines show mesenteric implants (arrows). F: Bar graphs show average numbers of tumor implants on the mesentery, broad ligament, and diaphragm in each group of mice. Numbers of individual nodules on the omentum could not be calculated because ES2 cells formed a large, solid omental mass. n = 5 per group of female nude mide. ∗∗P < 0.01 versus empty vector. Scale bar = 2 mm (A–D); 10 mm (E).
DLX4 Stimulates Attachment of Tumor Cells to Mesothelial Cells
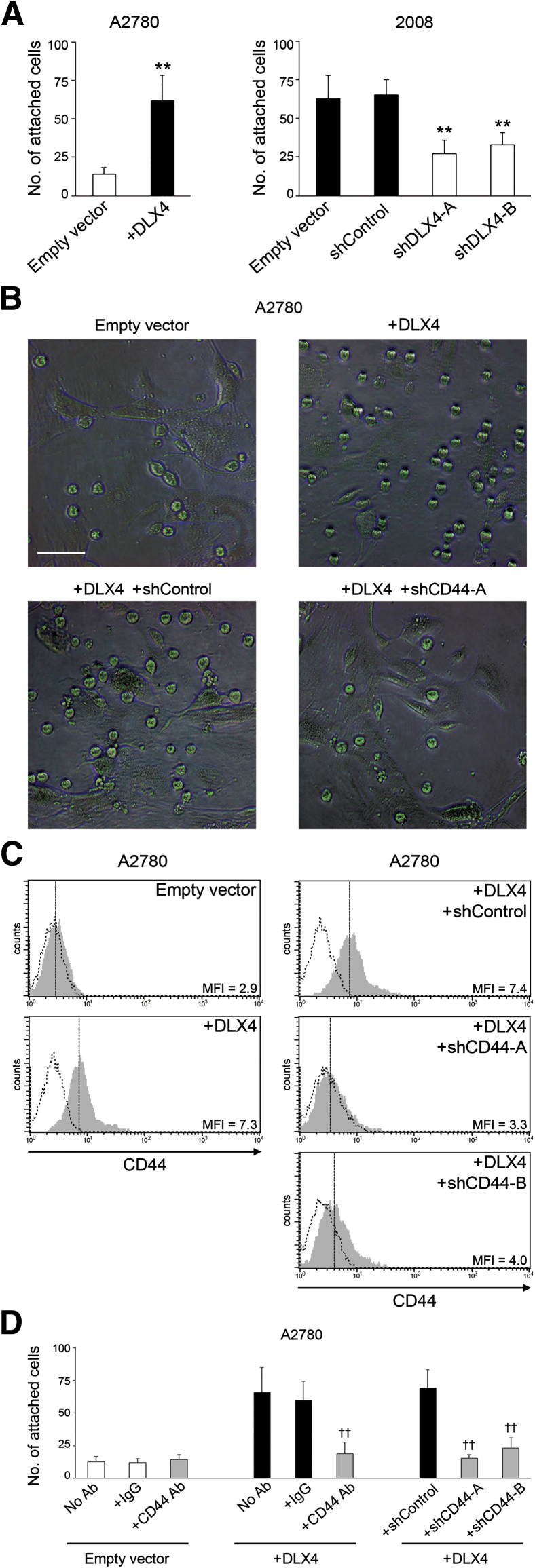
The peritoneal surfaces on to which ovarian tumor cells implant are lined by a monolayer of mesothelial cells.4 To evaluate the effect of DLX4 on interactions between tumor cells and mesothelial cells independently of growth-stimulatory effects, we performed short-term in vitro attachment assays. Equivalent numbers of vector-control and +DLX4 A2780 cells were seeded onto confluent monolayers of normal human omental mesothelial cells. Attachment of A2780 cells to mesothelial cells was assayed at 1 hour after seeding during which time no change in tumor cell survival occurred. The number of +DLX4 A2780 cells that attached to mesothelial cells was significantly higher than the number of vector-control A2780 cells that attached (P < 0.01) (Figure 2, A and B). Similarly, the number of +DLX4 ES2 cells that attached to mesothelial cells was significantly higher than the number of vector-control ES2 cells that attached (P < 0.01) (Supplemental Figure S1B). Conversely, knockdown of endogenous DLX4 using two different shRNAs (shDLX4-A, shDLX4-B) significantly reduced the ability of 2008 cells to attach to mesothelial cells (P < 0.01) (Supplemental Figure S1A and Figure 2A). Because the ovarian origin of 2008 cells has been recently disputed,22 we evaluated two additional ovarian cancer cell lines, OVCAR8 and OVCA429. Knockdown of DLX4 in both OVCAR8 and OVCA429 cells significantly reduced the mesothelial attachment ability of these cells (P < 0.01) (Supplemental Figure S1B).
Figure 2.
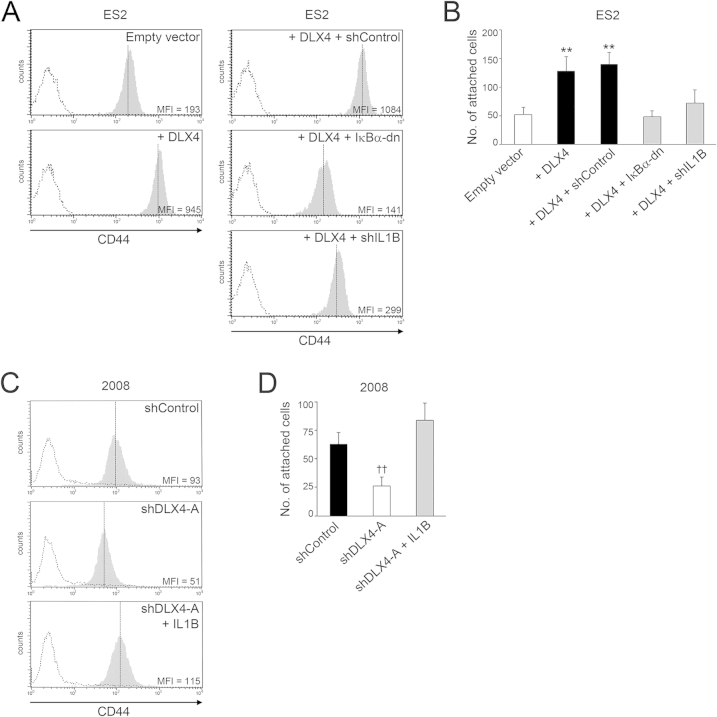
DLX4 stimulates attachment of tumor cells to mesothelial cells by inducing CD44 expression. A: Equivalent numbers of vector-control (empty vector) and +DLX4 A2780 cells that stably expressed green fluorescent protein were seeded onto confluent monolayers of normal human omental mesothelial cells cultured in 96-well plates. At 1 hour thereafter, tumor cells that were attached to mesothelial cells were viewed by fluorescence microscopy and counted in five random ×200 microscopic fields per assay. Green fluorescent protein–expressing 2008 cells transfected with empty vector, nontargeting shRNA (shControl), and DLX4 shRNAs (shDLX4-A, shDLX4-B) were assayed for attachment as described for A2780 cells. B: Representative examples of mesothelial attachment of vector-control and +DLX4 A2780 cells and of +DLX4 A2780 cells transfected with nontargeting shRNA and CD44 shRNA (shCD44-A). C: Flow cytometric analysis of cell surface staining of CD44 in vector-control and +DLX4 A2780 cells and in +DLX4 A2780 cells transfected with nontargeting shRNA and CD44 shRNAs (shCD44-A, shCD44-B). Representative examples are shown of staining and mean fluorescence intensities (MFIs). Solid gray histograms represent staining with antibody (Ab) to CD44. Dotted histograms represent staining with isotype control. D: Attachment of A2780 cells to mesothelial cells was assayed as described in A. Where indicated, A2780 cells were pre-incubated for 1 hour with neutralizing Ab to CD44 or control IgG or with no Ab before seeding onto mesothelial monolayers. Data are expressed as means ± SD for values of three independent attachment assays (A and D). ∗∗P < 0.01 versus empty vector; ††P < 0.01 versus +DLX4 with no Ab. Scale bar = 50 μm (B).
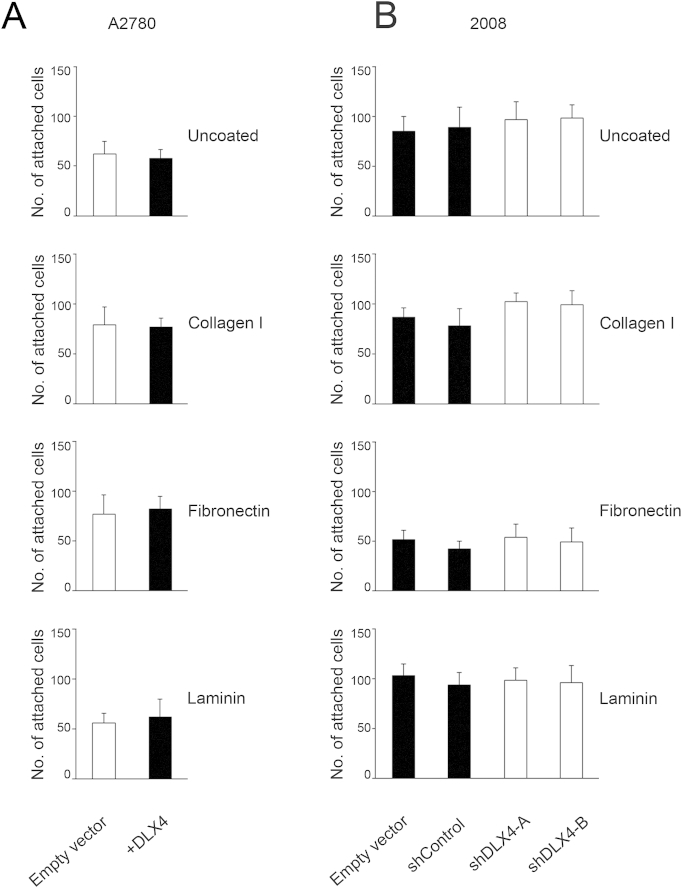
Collagen I, fibronectin, and laminin are major components of the matrix that underlies the mesothelial monolayer.4 We assayed attachment of ovarian tumor cells to wells coated with each of these extracellular matrix proteins to determine whether DLX4 stimulates interactions of tumor cells with the submesothelial matrix. The numbers of +DLX4 A2780 cells that attached to collagen I, fibronectin, or laminin or to uncoated plates were not significantly different from the numbers of attached vector-control A2780 cells (Supplemental Figure S2A). Knockdown of DLX4 in 2008 cells also did not significantly affect attachment to the extracellular matrix proteins (Supplemental Figure S2B). These findings indicate that DLX4 promotes attachment of ovarian tumor cells to mesothelial cells rather than to the submesothelial matrix.
DLX4 Promotes Tumor–Mesothelial Cell Interactions by Inducing CD44 Expression
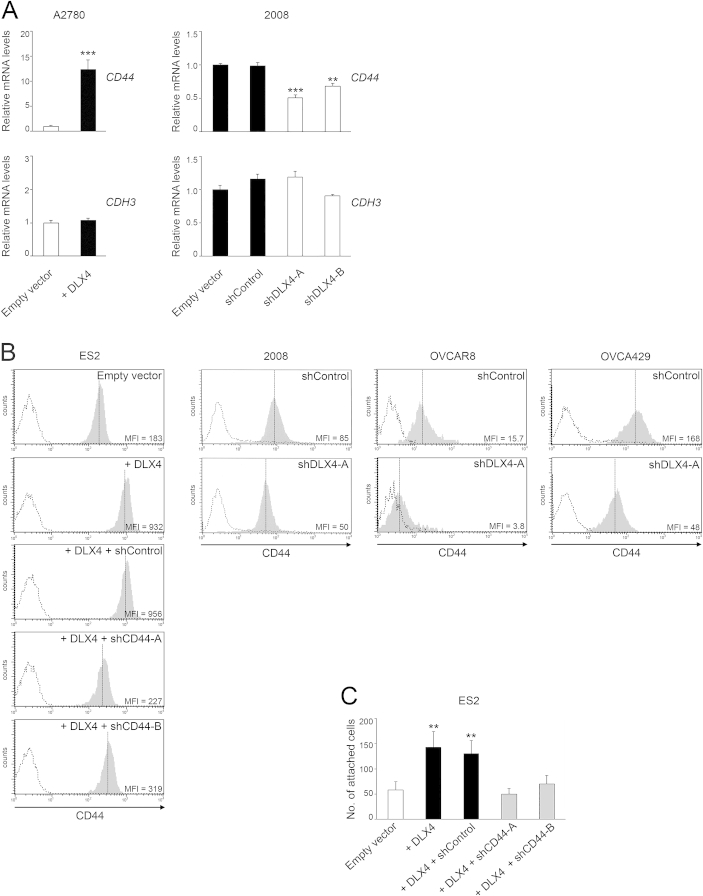
Because DLX4 functions as a transcription factor, we investigated the possibility that DLX4 induces expression of cell surface molecules such as CD44 and P-cadherin that mediate attachment of ovarian tumor cells to mesothelial cells.7–9 Quantitative RT-PCR analysis revealed that DLX4 induces CD44 mRNA levels but not mRNA levels of CDH3 (encoding P-cadherin) (Supplemental Figure S3A). To confirm our findings, flow cytometric analysis of cell surface staining of CD44 was performed. CD44 levels were induced when DLX4 was overexpressed in A2780 cells (Figure 2C) and in ES2 cells (Supplemental Figure S3B). Conversely, CD44 levels were reduced when DLX4 was knocked down in 2008, OVCAR8, and OVCA429 cells (Supplemental Figure S3B).
To determine whether DLX4 promotes tumor–mesothelial cell interactions by inducing CD44, we knocked down CD44 in +DLX4 A2780 cells by using two different CD44 shRNAs (shCD44-A, shCD44-B) (Figure 2C). The number of attached +DLX4 A2780 cells in which CD44 was knocked down was almost equivalent to the number of attached vector-control A2780 cells (Figure 2, B and D). Similarly, knockdown of CD44 in +DLX4 ES2 cells reduced the attachment ability of these cells to a level equivalent to that of vector-control ES2 cells (Supplemental Figure S3, B and C). To confirm our findings, we pre-incubated tumor cells with neutralizing Ab to CD44 and then assayed attachment to mesothelial cells. Attachment of vector-control A2780 cells, which express almost no CD44, was not significantly affected by CD44 Ab (Figure 2D). In contrast, treatment with CD44 Ab significantly inhibited attachment of +DLX4 A2780 cells, as compared to treatment with control IgG or no Ab (P < 0.01) (Figure 2D). These findings indicate that DLX4 primarily promotes attachment of tumor cells to mesothelial cells by inducing CD44 expression.
DLX4 Induces CD44 Expression by Stimulating IL-1β Expression
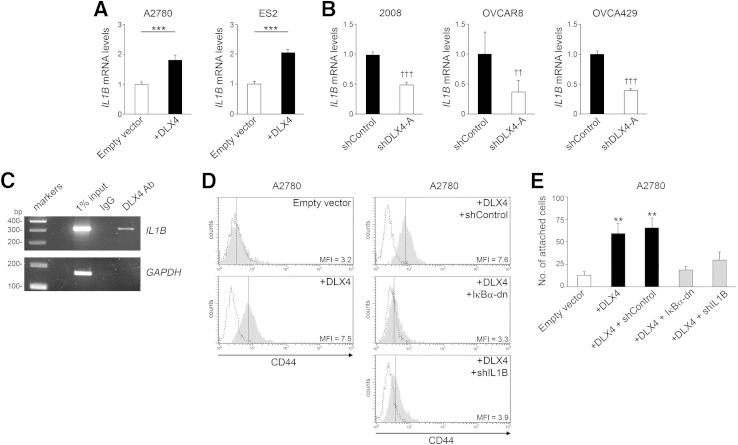
Because no potential DLX4 binding sites were identified in the CD44 promoter, it was likely that DLX4 induces CD44 expression by an indirect mechanism. CD44 expression is induced by IL-1β in inflammatory diseases such as arteriosclerosis.23 Because IL-1β is expressed by ovarian cancers,6,24 we investigated the possibility that DLX4 induces CD44 by stimulating IL-1β expression. IL1B mRNA levels were induced when DLX4 was overexpressed in A2780 and ES2 cells (P < 0.001) (Figure 3A) and were down-regulated when DLX4 was knocked down in 2008, OVCAR8, and OVCA429 cells (P < 0.001 for 2008 and OVCA429; P < 0.01 for OVCAR8) (Figure 3B). Induction of IL-1β protein levels by DLX4 was confirmed by enzyme-linked immunosorbent assay (Supplemental Figure S4). A putative DLX4 binding site was identified at positions -359 to -353 in the IL1B promoter. Binding of endogenous DLX4 to this region was detected in 2008 cells (Figure 3C). These findings indicate that IL1B is a direct transcriptional target of DLX4.
Figure 3.
Stimulatory effects of DLX4 on CD44 expression and tumor–mesothelial cell interactions are dependent on NF-κB and are mediated by its induction of IL-1β. A: Quantitative RT-PCR analysis of relative IL1B mRNA levels in A2780 and ES2 cells. B: Relative IL1B mRNA levels in 2008, OVCAR8, and OVCA429 cells. C: Detection of binding of endogenous DLX4 in parental 2008 cells to the IL1B promoter by chromatin immunoprecipitation. GAPDH was amplified as control. D: Flow cytometric analyses of CD44 levels in vector-control (empty vector) and +DLX4 A2780 cells and in +DLX4 A2780 cells transfected with nontargeting shRNA (shControl), pBabe-GFP-ΙκΒα-dominant-negative (ΙκΒα-dn), and IL1B shRNA (shIL1B). Solid gray histograms represent staining with antibody (Ab) to CD44. Dotted histograms represent staining with isotype control. E: Equivalent numbers of vector-control and +DLX4 A2780 cells were seeded onto confluent monolayers of normal human omental mesothelial cells cultured in 96-well plates. At 1 hour thereafter, tumor cells that were attached to mesothelial cells were counted in five random ×200 microscopic fields per assay. Data are expressed as means ± SD for values of three independent assays (A, B, and E). ∗∗P < 0.01, ∗∗∗P < 0.001 versus empty vector; ††P < 0.01, †††P < 0.001 versus shControl. MFI, mean fluorescence intensity; shDLX4-A, DLX4 shRNA.
To determine whether DLX4 induces CD44 by stimulating IL-1β levels, we knocked down IL-1β in +DLX4 A2780 cells to a level comparable to that of vector-control A2780 cells (Supplemental Figure S4A). Knockdown of IL-1β in +DLX4 A2780 cells reduced CD44 expression almost to the level seen in vector-control cells (Figure 3D). Knockdown of IL-1β also significantly reduced the ability of +DLX4 A2780 cells to attach to mesothelial cells (P < 0.01) (Figure 3E). We also knocked down IL-1β in +DLX4 ES2 cells to a level comparable to that of vector-control ES2 cells (Supplemental Figure S4B) and observed that knockdown of IL-1β reduced CD44 expression and mesothelial attachment ability of +DLX4 ES2 cells (Supplemental Figure S5, A and B). Conversely, we expressed an IL1B cDNA in DLX4-knockdown 2008 cells to reconstitute IL-1β to a level comparable to that of control 2008 cells (Supplemental Figure S4C). Reconstitution of IL-1β in DLX4-knockdown 2008 cells restored CD44 expression and mesothelial attachment ability to levels comparable to those of control 2008 cells (Supplemental Figure S5, C and D). These findings indicate that the stimulatory effects of DLX4 on CD44 expression and tumor–mesothelial cell interactions are primarily mediated by its induction of IL-1β.
DLX4 Stimulates CD44 Expression and Tumor–Mesothelial Interactions in an NF-κB–Dependent Manner
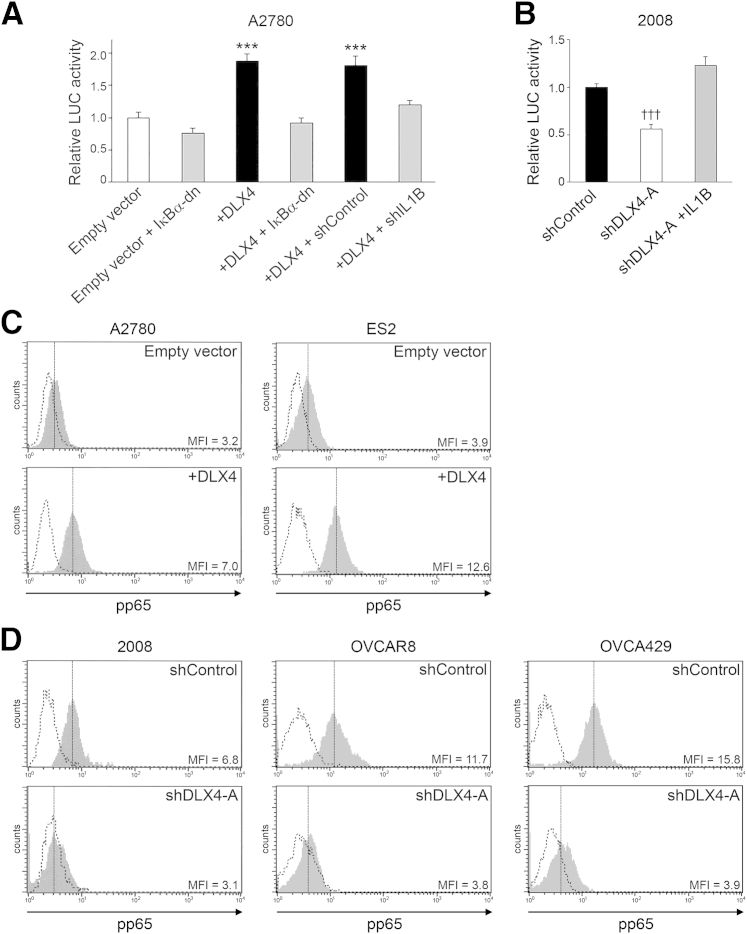
Because IL-1β stimulates NF-κB activation,25 we evaluated whether DLX4 stimulates NF-κB activity via its induction of IL-1β. Activity of a luciferase reporter construct driven by tandem NF-κB–binding elements was induced when DLX4 was expressed in A2780 cells, and this induction was blocked when IL-1β was knocked down (Figure 4A). Conversely, NF-κB–driven promoter activity decreased when DLX4 was knocked down in 2008 cells and was restored when IL-1β was reconstituted (Figure 4B). Transcriptional activity of NF-κB is stimulated upon phosphorylation of its p65 subunit.25 Levels of phosphorylated p65 (pp65) were increased when DLX4 was expressed in A2780 and ES2 cells (Figure 4C) and were decreased when DLX4 was knocked down in 2008, OVCAR8, and OVCA429 cells (Figure 4D). To confirm that DLX4 induces NF-κB activity, we assayed mRNA levels of NF-κB target genes and genes associated with NF-κB signal transduction. Levels of 30 NF-κB–associated genes were found to be 1.7- to 15-fold higher in +DLX4 A2780 cells than in vector-control A2780 cells (Supplemental Figure S6).
Figure 4.
DLX4 stimulates NF-κB activity. A: Activity of a firefly luciferase (LUC) reporter construct driven by tandem NF-κB binding sites was assayed in vector-control (empty vector) and +DLX4 A2780 cells that lacked or expressed pBabe-GFP-ΙκΒα-dominant-negative (ΙκΒα-dn) and in +DLX4 A2780 cells that expressed nontargeting shRNA (shControl) and IL1B shRNA (+shIL1B). Relative LUC activities are shown in three independent experiments. B: NF-κB–driven luciferase reporter activity was assayed in control and DLX4-knockdown 2008 cells and in DLX4-knockdown 2008 cells that expressed IL1B cDNA as described in A. C and D: Flow cytometric analysis of intracellular staining of phosphorylated p65 (pp65) NF-κB in A2780 and ES2 cells (C) and in 2008, OVCAR8, and OVCA429 cells (D). Solid gray histograms represent staining with antibody to pp65. Dotted histograms represent staining with isotype control. Data are expressed as means ± SD for values of three independent reporter assays (A and B). ∗∗∗P < 0.001 versus empty vector; †††P < 0.001 versus shControl. MFI, mean fluorescence intensity; shDLX4-A, DLX4 shRNA.
To determine whether induction of CD44 by DLX4 depends on NF-κB, we blocked NF-κB activity in +DLX4 A2780 cells by expressing a dominant-negative mutant form of ΙκΒα (ΙκΒα-dn) that sequesters NF-κB in the cytoplasm and is resistant to degradation.19 ΙκΒα-dn reduced NF-κB activity in +DLX4 A2780 cells to the level seen in vector-control A2780 cells (Figure 4A). ΙκΒα-dn abrogated the ability of DLX4 to induce CD44 expression in A2780 cells (Figure 3D) and to promote attachment of A2780 cells to mesothelial cells (P < 0.01) (Figure 3E). Similarly, expression of ΙκΒα-dn in +DLX4 ES2 cells abrogated the ability of DLX4 to induce CD44 expression and to promote mesothelial attachment (Supplemental Figure S5, A and B). These observations indicate that DLX4 primarily induces CD44 expression and tumor–mesothelial cell interactions in an NF-κB-dependent manner.
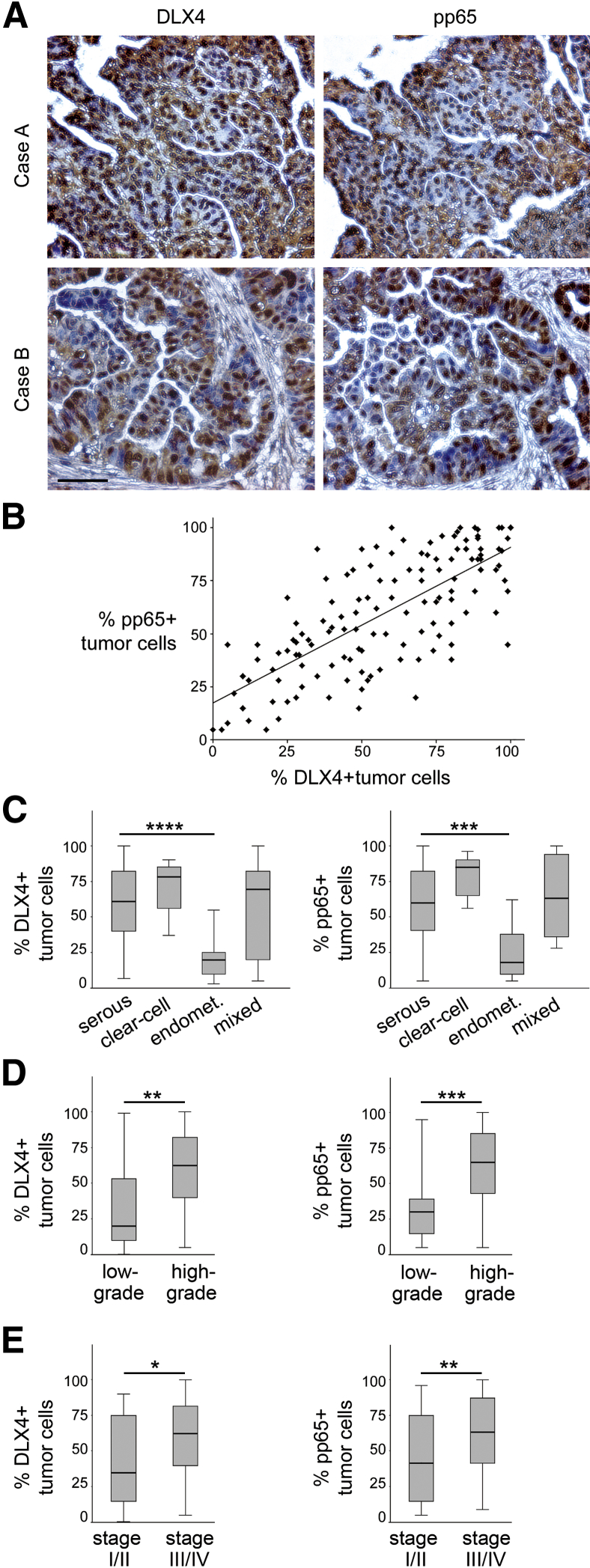
DLX4 Correlates with NF-κB Activation in Clinical Specimens of Ovarian Cancer
We evaluated the relationship between DLX4 and NF-κB activation by analyzing immunohistochemical staining of DLX4 and pp65 in microarrays of tissue cores of 135 ovarian cancer cases. Clinicopathologic features of cases are described in Table 1. Detection of pp65 has been used to assay activation of canonical NF-κB signaling in clinical specimens.26,27 Examples of staining of tissue cores of two cases are shown in Figure 5A. The percentage of DLX4-positive tumor cells strongly correlated with the percentage of pp65-positive tumor cells (R = 0.74, P < 0.001) (Figure 5B). The percentages of DLX4-positive and pp65-positive cells were not significantly different between serous, clear cell, or mixed histology cases but were lower in endometrioid cases (Figure 5C). The reduced DLX4 and pp65 staining in endometrioid cases appeared to be related to these cases being mostly low-grade and/or stage I tumors (Table 1). In contrast, 89 of the 100 serous cases and all of the clear cell and mixed histology cases were high-grade tumors, and most cases that were serous (94/100) or of mixed histology (10/12) were stage III or IV (Table 1). Across all histological subtypes, the percentages of DLX4-positive cells and pp65-positive cells were significantly higher in high-grade cases than in low-grade cases (P < 0.01 for DLX4, P < 0.001 for pp65) (Figure 5D) and were also significantly higher in late-stage cases (stages III and IV) than in early-stage cases (stages I and II) (P < 0.05 for DLX4, P < 0.01 for pp65) (Figure 5E). Together, our findings support a model in which DLX4 promotes aggressive tumor behavior and peritoneal dissemination of ovarian tumor cells by stimulating NF-κB signaling.
Figure 5.
DLX4 correlates with p65 phosphorylation in clinical specimens of ovarian cancer. Replicate slides of tissue microarrays representing a total of 135 cases of ovarian cancer were stained with antibodies to DLX4 and to phosphorylated p65 (pp65). For each case, an average staining score for each protein was calculated from the percentage of stained tumor cells for which two independent ×200 microscopic fields were evaluated in each core. A: Examples of staining of DLX4 and pp65 in cores of two different cases of serous ovarian carcinoma. B: Correlation between percentages of DLX4-positive and pp65-positive tumor cells as evaluated by Spearman test, where each symbol represents an individual case (R = 0.74, P < 0.001). C–E: Percentages of DLX4-positive and pp65-positive tumor cells in cases grouped by histological subtype (C), tumor grade (D), and disease stage (E). Differences between groups were evaluated by Mann-Whitney U-test. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. Scale bar = 50 μm. Endomet., endometrioid.
Discussion
The ability of ovarian cancer to seed the peritoneal cavity with nests of tumor cells is a hallmark of this disease. Increasing evidence indicates that interactions of ovarian tumor cells with mesothelial cells and the submesothelial matrix are dynamically regulated by several cell surface molecules, including CD44, P-cadherin, and various integrins.7–10 However, the mechanisms that control expression of these cell adhesion molecules in tumor cells are poorly understood. In this study, we identified that the homeoprotein DLX4 stimulates the attachment of ovarian tumor cells to mesothelial cells by inducing expression of CD44. Although DLX4 did not induce expression of P-cadherin, we cannot exclude the possibility that DLX4 also induces expression of other adhesion molecules. However, knockdown or neutralization of CD44 substantially abrogated the stimulatory effect of DLX4 on tumor–mesothelial cell interactions, indicating that DLX4 primarily stimulates these interactions by inducing CD44.
The present study indicates that DLX4 induces CD44 levels and tumor–mesothelial cell interactions in an NF-κB–dependent manner and that this occurs via the ability of DLX4 to activate IL1B, a transcriptional target of DLX4. Knockdown of IL-1β or blockade of NF-κB signaling abrogated the stimulatory effect of DLX4 on CD44 levels. These observations are consistent with the ability of IL-1β to activate NF-κB25 and a report in which CD44 was identified as an NF-κB target gene.28 Although our findings indicate that the stimulatory effect of DLX4 on NF-κB activity substantially occurs via its induction of IL-1β, we cannot exclude the possibility that DLX4 also directly regulates transcription of genes encoding other ligands or components of the NF-κB pathway. Several genes that encode activators and other components of the pathway, including IL1B, are transcriptional targets of NF-κB29 (Supplemental Figure S6). By inducing expression of IL-1β, DLX4 might chronically activate NF-κB autoregulatory loops in tumor cells.
Elevated IL-1β levels have been found to be significantly associated with reduced survival of ovarian cancer patients.24 By inducing IL-1β levels, DLX4 could promote ovarian tumor progression through several other mechanisms in addition to promoting peritoneal attachment. Several lines of evidence indicate that ovarian tumor-derived IL-1β renders the peritoneum more receptive to growth of tumor implants. IL-1β stimulates mesothelial cells to express angiogenic factors such as basic fibroblast growth factor, vascular endothelial growth factor-A, IL-6, and IL-8.30–33 It has also been reported that IL-1β suppresses p53 levels in ovarian tumor-associated fibroblasts and that p53 down-regulation in stromal fibroblasts enhances expression of vascular endothelial growth factor-A, IL-6, IL-8, and growth regulated oncogene-α.24 IL-1β also stimulates ovarian tumor cells to express vascular endothelial growth factor-A.31 We observed that DLX4 induces expression of several NF-κB target genes that encode angiogenic factors such as IL-8 and the chemokine (C-X-C motif) ligands 2 and 3 (Supplemental Figure S6). The reported stimulatory effect of DLX4 on ovarian tumor angiogenesis17 might, therefore, be mediated in part by its induction of IL-1β that in turn stimulates production of angiogenic factors via both autocrine and paracrine mechanisms.
Our finding that p65 phosphorylation is significantly higher in late-stage ovarian cancers than in early-stage tumors is consistent with findings from several studies. Bioluminescence imaging of a mouse model of ovarian cancer that expressed an NF-κB–driven reporter transgene revealed that NF-κB activity is elevated at advanced stages of tumor progression.34 Furthermore, expression of NF-κB subunits is strongly associated with poor outcomes of ovarian cancer patients.35 It has been thought that NF-κB exerts a pro-apoptotic, tumor-suppressive function in early-stage tumors, whereas tumor-promoting functions of NF-κB become unleashed as tumors progress.36 This biphasic role of NF-κB is supported by findings that NF-κB functions as an oncogene in chemoresistant ovarian cancer cell lines but functions as a tumor suppressor in the isogenic parental cell lines.37 Our findings that DLX4 stimulates NF-κB activity and peritoneal implantation and is highly expressed in advanced-stage ovarian cancers support the notion of a predominantly tumor-promoting function of NF-κB in advanced-stage disease.
Our findings that DLX4 promotes the aggressive behavior of ovarian cancers are supported by a previous study in which amplification of the chromosomal region 17q21.3-q22 to which the DLX4 gene maps was found to be associated with poor outcomes of patients with clear cell ovarian carcinoma.38 Our study indicates that high levels of DLX4 are associated with high tumor grade and advanced disease stage but not with the histological subtype. Although DLX4 levels were lower in endometrioid tumors than in other subtypes (Figure 5C), all except one of the endometrioid cases studied were either low-grade or stage I tumors (Table 1). The parental A2780 line, which lacks DLX4, has been recently identified to be an endometrioid carcinoma line.39 It is possible that the high endogenous DLX4 levels in the 2008, OVCAR8, and OVCA429 lines might reflect the clinicopathologic features of the original tumor. It has been recently reported that the 2008 line has clear cell–like features, whereas the OVCAR8 line might be reflective of high-grade serous carcinoma.39,40 The OVCA429 line was established from a late-stage serous carcinoma as described by Bast et al.41 The ES2 line has been described as a clear cell carcinoma line but has been recently reported to be more reflective of high-grade serous carcinoma.40,42 Although parental ES2 cells express very little DLX4, the high endogenous CD44 levels in these cells might explain, in part, the aggressive behavior of this line in xenograft models. At the present time, we can only speculate as to the mechanisms that cause overexpression of DLX4 in ovarian cancers. In addition to gene amplification, overexpression of DLX4 might stem from epigenetic mechanisms. Deregulation of several other homeobox genes in solid tumors has been attributed to aberrant expression of various miRNAs, noncoding RNAs, and chromatin modifiers.43–45
Despite the essential functions of homeoproteins in normal development and their increasingly recognized importance in tumors, relatively few transcriptional targets of homeoproteins have been identified. Several homeoproteins that are aberrantly expressed in tumors control transcription of genes that regulate the cell cycle.14,46 We have identified that DLX4 activates TOP2A promoter activity.47 DLX4 also enables tumor cells to evade the antiproliferative effect of transforming growth factor-β by sequestering Smad4.18 There is also increasing evidence that homeoproteins modulate tumor–stroma interactions. HOXA9, a homeoprotein that is associated with poor outcomes of ovarian cancer patients, stimulates tumor–mesothelial cell interactions by activating transcription of the CDH3 gene that encodes P-cadherin.48 HOXA9 also promotes ovarian tumor growth by activating transcription of gene encoding transforming growth factor-β2, which in turn stimulates peritoneal fibroblasts to acquire features of cancer-associated fibroblasts and stimulates macrophages to acquire immunosuppressive properties.15,49 By controlling expression of distinct sets of ligands and receptors, homeoproteins can, therefore, profoundly perturb signaling pathways in tumor cells and also communications between tumor and stromal cells.
Conclusions
The present study supports increasing evidence of the functional importance of homeobox genes in driving tumor progression. This is the first study to identify a role of a homeobox gene in controlling inflammatory signaling in cancer. Further characterization of the mechanisms of this important class of developmental genes could yield important insights into the control of intra- and intercellular communication in tumors and the identification of more effective focal points for therapeutic intervention.
Acknowledgments
We thank Drs. Gordon Mills, Zahid Siddik, and Douglas Boyd (MD Anderson Cancer Center, Houston, TX) for parental A2780, OVCAR8, and OVCA429 lines; 2008 cells; and Ampho293 packaging cells, respectively. Dr. Ernst Lengyel (University of Chicago, Chicago, IL) provided primary cultures of normal human omental mesothelial cells. pBabe-GFP-ΙκΒα-dominant-negative plasmid was originally generated by Dr. William Hahn (Dana-Farber Cancer Institute, Boston, MA) and deposited to Addgene (plasmid 15264).
Footnotes
Supported by NIH grant CA141078 and Cancer Prevention and Research Institute of Texas grant RP120390 (H.N.).
D.H. and B.Q.T. contributed equally to this work as senior authors.
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2015.04.004.
Supplemental Data
Supplemental Figure S1.
Overexpression or knockdown of DLX4 alters the ability of tumor cells to attach to mesothelial cells. A: Flow cytometric analysis of intracellular staining of DLX4. Mean fluorescence intensities (MFIs) are shown for DLX4 staining in A2780 and ES2 cells stably transfected with empty vector and with DLX4 (+DLX4) and in 2008, OVCAR8, and OVCA429 cells transfected with empty vector, nontargeting shRNA (shControl) and DLX4 shRNAs (shDLX4-A, shDLX4-B). Solid gray histograms represent staining with antibody (Ab) to DLX4. Dotted histograms represent staining with isotype control. B: Equivalent numbers of tumor cells were seeded onto confluent monolayers of normal human omental mesothelial cells cultured in 96-well plates. At 1 hour thereafter, attached tumor cells were counted in five random ×200 microscopic fields per assay. Data are expressed as means ± SD for values of three independent attachment assays (B). ∗∗P < 0.01 versus empty vector (ES2); ‡‡P < 0.01 versus shControl (OVCAR8 and OVCA429).
Supplemental Figure S2.
DLX4 does not promote attachment of tumor cells to components of the submesothelial matrix. Equivalent numbers of vector-control (empty vector) and +DLX4 A2780 (A) and control and DLX4-knockdown 2008 (B) cells were seeded into 96-well plates that were coated with collagen I, fibronectin, and laminin or were left uncoated. At 1 hour thereafter, attached tumor cells were counted in five random ×200 microscopic fields per assay. Differences between vector-control and +DLX4 A2780 cells and between vector-control and shRNA-transfected 2008 cells were not significant. Data are expressed as means ± SD for values of three independent attachment assays. shControl, nontargeting shRNA; shDLX4-A and shDLX4-B, DLX4 shRNAs.
Supplemental Figure S3.
DLX4 induces expression of CD44. A: Quantitative RT-PCR analysis of relative mRNA levels of CD44 and CDH3 (encoding P-cadherin) in A2780 and 2008 cells. B: Flow cytometric analysis of cell surface staining of CD44 in vector-control (empty vector) and +DLX4 ES2 cells and in +DLX4 ES2 cells transfected with nontargeting shRNA (shControl) and CD44 shRNAs (shCD44-A, shCD44-B). CD44 levels are also shown in 2008, OVCAR8, and OVCA429 cells transfected with nontargeting and DLX4 shRNAs (shDLX4-A). Solid gray histograms represent staining with antibody to CD44. Dotted histograms represent staining with isotype control. C: Equivalent numbers of tumor cells were seeded onto confluent monolayers of normal human omental mesothelial cells cultured in 96-well plates. At 1 hour thereafter, attached tumor cells were counted in five random ×200 microscopic fields per assay. Data are expressed as means ± SD for values of three independent attachment assays (A and C). ∗∗P < 0.01, ∗∗∗P < 0.001 versus empty vector. MFI, mean fluorescence intensity.
Supplemental Figure S4.
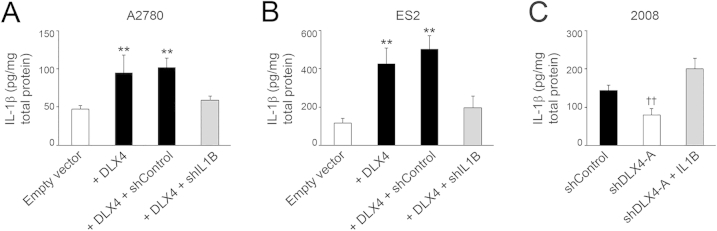
DLX4 induces IL-1β expression. Levels of IL-1β protein were assayed by enzyme-linked immunosorbent assay in lysates of vector-control (empty vector) and +DLX4 A2780 cells and in +DLX4 A2780 cells transfected with nontargeting shRNA (shControl) and IL1B shRNA (shIL1B) (A), vector-control and +DLX4 ES2 cells, and +DLX4 ES2 cells transfected with nontargeting and IL1B shRNAs (B), and control and DLX4-knockdown 2008 cells and DLX4-knockdown 2008 cells transfected with IL1B cDNA (shDLX4-A + IL1B) (C). Data are expressed as means ± SD for values of three independent assays. ∗∗P < 0.01 versus empty vector; ††P < 0.01 versus shControl. shDLX4-A, DLX4 shRNA.
Supplemental Figure S5.
Stimulatory effects of DLX4 on CD44 expression and tumor–mesothelial cell interactions are mediated by its induction of IL-1β. A: Flow cytometric analysis of cell surface staining of CD44 in vector-control (empty vector) and +DLX4 ES2 cells and in +DLX4 ES2 cells transfected with nontargeting shRNA (shControl), pBabe-GFP-ΙκΒα-dominant-negative (ΙκΒα-dn), and IL1B shRNA (shIL1B). B: Attachment of ES2 cells to mesothelial cells. C: CD44 levels in control and DLX4-knockdown 2008 cells and in DLX4-knockdown 2008 cells transfected with IL1B cDNA. D: Attachment of 2008 cells to mesothelial cells. In A and C, solid gray histograms represent staining with antibody to CD44. Dotted histograms represent staining with isotype control. Data are expressed as means ± SD for values of three independent attachment assays (B and D). ∗∗P < 0.01 versus empty vector; ‡‡P < 0.01 versus shControl. MFI, mean fluorescence intensity, shDLX4-A DLX4 shRNA.
Supplemental Figure S6.
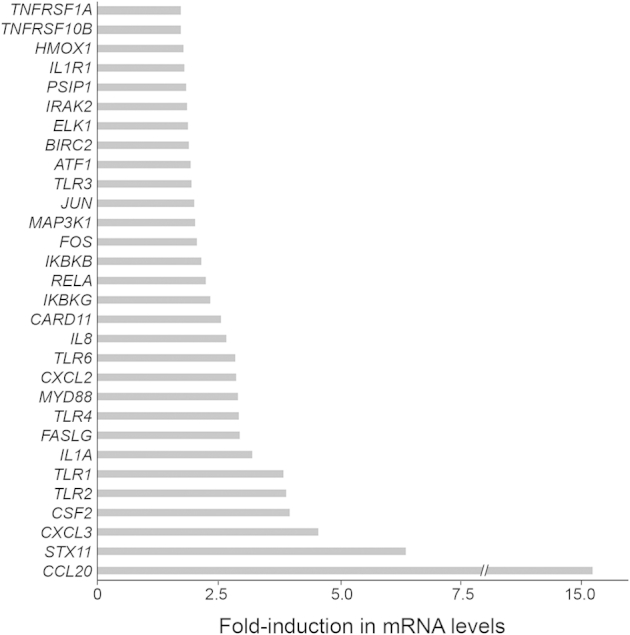
DLX4 induces expression of NF-κB–associated genes. Fold increases are shown in mRNA levels of the indicated NF-κB target genes and genes associated with NF-κB signal transduction in +DLX4 A2780 cells as compared to respective mRNA levels in vector-control A2780 cells mRNA levels were assayed by quantitative RT-PCR.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R., Kaye S.B. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 3.Naora H., Montell D.J. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 4.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodek K.L., Murphy K.J., Brown T.J., Ringuette M.J. Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev. 2012;31:397–414. doi: 10.1007/s10555-012-9351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutteh W.H., Kutteh C.C. Quantitation of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in the effusions of ovarian epithelial neoplasms. Am J Obstet Gynecol. 1992;167:1864–1869. doi: 10.1016/0002-9378(92)91788-c. [DOI] [PubMed] [Google Scholar]
- 7.Cannistra S.A., Kansas G.S., Niloff J., DeFranzo B., Kim Y., Ottensmeier C. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res. 1993;53:3830–3838. [PubMed] [Google Scholar]
- 8.Strobel T., Swanson L., Cannistra S.A. In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: a novel role for CD44 in the process of peritoneal implantation. Cancer Res. 1997;57:1228–1232. [PubMed] [Google Scholar]
- 9.Usui A., Ko S.Y., Barengo N., Naora H. P-cadherin promotes ovarian cancer dissemination through tumor cell aggregation and tumor-peritoneum interactions. Mol Cancer Res. 2014;12:504–513. doi: 10.1158/1541-7786.MCR-13-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwanicki M.P., Davidowitz R.A., Ng M.R., Besser A., Muranen T., Merritt M., Danuser G., Ince T.A., Brugge J.S. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011;1:144–157. doi: 10.1158/2159-8274.CD-11-0010. [Erratum appeared in Cancer Discov 2011, 1:626] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 12.Cheng W., Liu J., Yoshida H., Rosen D., Naora H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11:531–537. doi: 10.1038/nm1230. [DOI] [PubMed] [Google Scholar]
- 13.Behbakht K., Qamar L., Aldridge C.S., Coletta R.D., Davidson S.A., Thorburn A., Ford H.L. Six1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer Res. 2007;67:3036–3042. doi: 10.1158/0008-5472.CAN-06-3755. [DOI] [PubMed] [Google Scholar]
- 14.Tan Y., Cheung M., Pei J., Menges C.W., Godwin A.K., Testa J.R. Upregulation of DLX5 promotes ovarian cancer cell proliferation by enhancing IRS-2-AKT signaling. Cancer Res. 2010;70:9197–9206. doi: 10.1158/0008-5472.CAN-10-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko S.Y., Barengo N., Ladanyi A., Lee J.S., Marini F., Lengyel E., Naora H. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122:3603–3617. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chase M.B., Fu S., Haga S.B., Davenport G., Stevenson H., Do K., Morgan D., Mah A.L., Berg P.E. BP1, a homeodomain-containing isoform of DLX4, represses the beta-globin gene. Mol Cell Biol. 2002;22:2505–2514. doi: 10.1128/MCB.22.8.2505-2514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara F., Samuel S., Liu J., Rosen D., Langley R.R., Naora H. A homeobox gene related to Drosophila distal-less promotes ovarian tumorigenicity by inducing expression of vascular endothelial growth factor and fibroblast growth factor-2. Am J Pathol. 2007;170:1594–1606. doi: 10.2353/ajpath.2007.061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinh B.Q., Barengo N., Naora H. Homeodomain protein DLX4 counteracts key transcriptional control mechanisms of the TGF-beta cytostatic program and blocks the antiproliferative effect of TGF-beta. Oncogene. 2011;30:2718–2729. doi: 10.1038/onc.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boehm J.S., Zhao J.J., Yao J., Kim S.Y., Firestein R., Dunn I.F., Sjostrom S.K., Garraway L.A., Weremowicz S., Richardson A.L., Greulich H., Stewart C.J., Mulvey L.A., Shen R.R., Ambrogio L., Hirozane-Kishikawa T., Hill D.E., Vidal M., Meyerson M., Grenier J.K., Hinkle G., Root D.E., Roberts T.M., Lander E.S., Polyak K., Hahn W.C. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Kenny H.A., Krausz T., Yamada S.D., Lengyel E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer. 2007;121:1463–1472. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- 21.Ko S.Y., Lengyel E., Naora H. The Müllerian HOXA10 gene promotes growth of ovarian surface epithelial cells by stimulating epithelial-stromal interactions. Mol Cell Endocrinol. 2010;317:112–119. doi: 10.1016/j.mce.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korch C., Spillman M.A., Jackson T.A., Jacobsen B.M., Murphy S.K., Lessey B.A., Jordan V.C., Bradford A.P. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol Oncol. 2012;127:241–248. doi: 10.1016/j.ygyno.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster L.C., Arkonac B.M., Sibinga N.E., Shi C., Perrella M.A., Haber E. Regulation of CD44 gene expression by the proinflammatory cytokine interleukin-1beta in vascular smooth muscle cells. J Biol Chem. 1998;273:20341–20346. doi: 10.1074/jbc.273.32.20341. [DOI] [PubMed] [Google Scholar]
- 24.Schauer I.G., Zhang J., Xing Z., Guo X., Mercado-Uribe I., Sood A.K., Huang P., Liu J. Interleukin-1beta promotes ovarian tumorigenesis through a p53/NF-kappaΒ-mediated inflammatory response in stromal fibroblasts. Neoplasia. 2013;15:409–420. doi: 10.1593/neo.121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins N.D., Gilmore T.D. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 26.Briones J., Moga E., Espinosa I., Vergara C., Alvarez E., Villa J., Bordes R., Delgado J., Prat J., Sierra J. Bcl-10 protein highly correlates with the expression of phosphorylated p65 NF-kappaB in peripheral T-cell lymphomas and is associated with clinical outcome. Histopathology. 2009;54:478–485. doi: 10.1111/j.1365-2559.2009.03250.x. [DOI] [PubMed] [Google Scholar]
- 27.McCall P., Bennett L., Ahmad I., Mackenzie L.M., Forbes I.W., Leung H.Y., Sansom O.J., Orange C., Seywright M., Underwood M.A., Edwards J. NF-kappaB signaling is upregulated in a subset of castrate-resistant prostate cancer patients and correlates with disease progression. Br J Cancer. 2012;107:1554–1563. doi: 10.1038/bjc.2012.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinz M., Lemke P., Anagnostopoulos I., Hacker C., Krappman D., Mathas S., Dörken B., Zenke M., Stein H., Scheidereit C. Nuclear factor kappaB-dependent gene expression profiling of Hodgkin's disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J Exp Med. 2002;196:605–617. doi: 10.1084/jem.20020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiscott J., Marois J., Garoufalis J., D'Addario M., Roulston A., Kwan I., Pepin N., Lacoste J., Nguyen H., Bensi G. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13:6231–6240. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronauer M.V., Stadlmann S., Klocker H., Abendstein B., Eder I.E., Rogatsch H., Zeimet A.G., Marth C., Offner F.A. Basic fibroblast growth factor synthesis by human peritoneal mesothelial cells: induction by interleukin-1. Am J Pathol. 1999;155:1977–1984. doi: 10.1016/S0002-9440(10)65516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stadlmann S., Amberger A., Pollheimer J., Gastl G., Offner F.A., Margreiter R., Zeimet A.G. Ovarian carcinoma cells and IL-1beta-activated human peritoneal mesothelial cells are possible sources of vascular endothelial growth factor in inflammatory and malignant peritoneal effusions. Gynecol Oncol. 2005;97:784–789. doi: 10.1016/j.ygyno.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Topley N., Jörres A., Luttmann W., Petersen M.M., Lang M.J., Thierauch K.H., Müller C., Coles G.A., Davies M., Williams J.D. Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL-1 beta and TNF alpha. Kidney Int. 1993;43:226–233. doi: 10.1038/ki.1993.36. [DOI] [PubMed] [Google Scholar]
- 33.Topley N., Brown Z., Jörres A., Westwick J., Davies M., Coles G.A., Williams J.D. Human peritoneal mesothelial cells synthesize interleukin-8. Synergistic induction by interleukin-1 beta and tumor necrosis factor-alpha. Am J Pathol. 1993;142:1876–1886. [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson A.J., Barham W., Saskowski J., Tikhomirov O., Chen L., Lee H.J., Yull F., Khabele D. Tracking NF-kappaB activity in tumor cells during ovarian cancer progression in a syngeneic mouse model. J Ovarian Res. 2013;6:63. doi: 10.1186/1757-2215-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Annunziata C.M., Stavnes H.T., Kleinberg L., Berner A., Hernandez L.F., Birrer M.J., Steinberg S.M., Davidson B., Kohn E.C. Nuclear factor kappaB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer. 2010;116:3276–3284. doi: 10.1002/cncr.25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins N.D. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol. 2004;14:64–69. doi: 10.1016/j.tcb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Yang G., Xiao X., Rosen D.G., Cheng X., Wu X., Chang B., Liu G., Xue F., Mercado-Uribe I., Chiao P., Du X., Liu J. The biphasic role of NF-kappaB in progression and chemoresistance of ovarian cancer. Clin Cancer Res. 2011;17:2181–2194. doi: 10.1158/1078-0432.CCR-10-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirasawa A., Saito-Ohara F., Inoue J., Aoki D., Susumu N., Yokoyama T., Nozawa S., Inazawa J., Imoto I. Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas with poor prognosis and identification of PPM1D and APPBP2 as likely amplification targets. Clin Cancer Res. 2003;9:1995–2004. [PubMed] [Google Scholar]
- 39.Anglesio M.S., Wiegand K.C., Melnyk N., Chow C., Salamanca C., Prentice L.M., Senz J., Yang W., Spillman M.A., Cochrane D.R., Shumansky K., Shah S.P., Kalloger S.E., Huntsman D.G. Type-specific cell line models for type-specific ovarian cancer research. PLoS One. 2013;8:e72162. doi: 10.1371/journal.pone.0072162. [Erratum appeared in PLoS One 2013, 8(10). doi:10.1371/annotation/856f0890-9d85-4719-8e54-c27530ac94f4 and PLoS One 2013, 8(9). doi:10.1371/annotation/ffcaf179-872f-470b-8bb6-f06d8ba6d03a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domcke S., Sinha R., Levine D.A., Sander C., Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bast R.C., Jr., Feeney M., Lazarus H., Nadler L.M., Colvin R.B., Knapp R.C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwok A.L., Wong O.G., Wong E.S., Tsun O.K., Chan K.K., Cheung A.N. Caution over use of ES2 as a model of ovarian clear cell carcinoma. J Clin Pathol. 2014;67:921–922. doi: 10.1136/jclinpath-2014-202430. [DOI] [PubMed] [Google Scholar]
- 43.Ma L., Teruya-Feldstein J., Weinberg R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 44.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E., Chang H.Y. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rauch T., Wang Z., Zhang X., Zhong X., Wu X., Lau S.K., Kernstine K.H., Riggs A.D., Pfeifer G.P. Homeobox gene methylation in lung cancer studied by genome-wide analysis with a microarray-based methylated CpG island recovery assay. Proc Natl Acad Sci U S A. 2007;104:5527–5532. doi: 10.1073/pnas.0701059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coletta R.D., Christensen K., Reichenberger K.J., Lamb J., Micomonaco D., Huang L., Wolf D.M., Müller-Tidow C., Golub T.R., Kawakami K., Ford H.L. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci U S A. 2004;101:6478–6483. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trinh B.Q., Ko S.Y., Barengo N., Lin S.Y., Naora H. Dual functions of the homeoprotein DLX4 in modulating responsiveness of tumor cells to topoisomerase II-targeting drugs. Cancer Res. 2013;73:1000–1010. doi: 10.1158/0008-5472.CAN-12-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ko S.Y., Naora H. HOXA9 promotes homotypic and heterotypic cell interactions that facilitate ovarian cancer dissemination via its induction of P-cadherin. Mol Cancer. 2014;13:170. doi: 10.1186/1476-4598-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ko S.Y., Ladanyi A., Lengyel E., Naora H. Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype. Am J Pathol. 2014;184:271–281. doi: 10.1016/j.ajpath.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]