Abstract
The current standard medical therapy for atopic dermatitis (AD) mainly focuses on symptomatic relief by controlling skin inflammation with topical corticosteroids and/or topical calcineurin inhibitors. However, the clinical efficacy of pharmacological therapy is often disappointing to both patients and physicians. The terminology of AD contains a historical meaning of eczematous dermatitis caused by hypersensitivity reaction to environmental inhalant or food allergen. Complex interrelationships among genetic abnormalities, environmental triggers, skin barrier defects, and immune dysfunction resulting in a vicious domino-circle seem to be involved in the development and maintenance of AD. In the viewpoint of AD as an allergic disease, complete avoidance of clinically relevant allergen or induction of specific immune tolerance through administrations of allergen (allergen immunotherapy) can provide clinical remission by breaking the vicious domino-circle maintaining a chronic disease state. In recent clinical studies, monoclonal antibodies including the anti-interleukin-4 receptor antibody and anti-B cell antibody induced significant clinical improvements in patients with AD. The detailed characteristics of immune dysfunction are heterogeneous among patients with AD. Therefore, a personalized combination of immunomodulatory therapies to reduce hypersensitivity (allergen immunotherapy) and correct immune dysfunction (monoclonal antibody therapy) could be a reasonable therapeutic approach for patients with AD. Future immunomodulatory therapies for AD should be developed to achieve long-term treatment-free clinical remission by induction of immune tolerance.
Keywords: Atopic dermatitis, Hypersensitivity, Immunomodulation, Allergens, Therapeutics
INTRODUCTION
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin disease characterized by itching, dry skin, inflammation, and exudation and is frequently associated with a personal or familial history of allergic diseases1. Hypersensitivity reaction to environmental agent has been suggested to be the pathogenetic mechanism responsible for the development and maintenance of chronic skin inflammation in AD patients2. However, the pathogenetic mechanism of AD seems to be more complexly associated with genetic abnormalities, environmental triggering factors, skin barrier defects, and immune dysfunction. In addition, the precise pathogenetic mechanism of AD is not yet completely understood2,3.
The current standard medical therapies for AD, including the use of topical corticosteroids and/or topical calcineurin inhibitors, are focused mainly on symptomatic relief, and their clinical efficacies are often disappointing to both patients and physicians1. Although the condition of a considerable number of AD patients can be improved by systemic treatment with corticosteroid, cyclosporine, or mycophenolate mofetil, there is a possibility of toxicity from long-term treatment with these compounds1. Various approaches to modulate immune system using monoclonal antibodies have been attempted in patients with severe AD4,5,6,7. Recent clinical trials with monoclonal antibodies showed conflicting results in terms of clinical efficacies4,5,6,7. Positive clinical efficacy results have been reported in clinical trials with anti-interleukin (IL)-4 receptor antibody and anti-B cell antibody in AD patients4,5. Negative clinical efficacy results have been reported in clinical trials with anti-IgE antibody and anti-activated T cell antibody6,7. Further studies on the long-term clinical efficacy and safety of monoclonal antibody-based immunomodulatory therapies for AD are needed. Additionally, development of a new therapeutic modality for AD patients is required.
In this review, the rationale for a personalized immunomodulatory therapy as a therapeutic approach for AD will be discussed.
HISTORY OF THE TERMINOLOGY OF "ATOPIC DERMATITIS"
The term "atopy" was first coined by Coca and Cooke8 in 1923 to describe a genetic predisposition toward the development of immediate-type hypersensitivity reaction (allergic reaction) against common environmental antigen, frequently manifested as hay fever (allergic rhinitis), bronchial asthma, eczematoid dermatitis, or food allergy. In 1933, Wise and Sulzberger proposed the name "atopic dermatitis" in place of the older traditional terms "neurodermatitis," "prurigo Besnier," and "allergic eczema" on the basis of their belief that hypersensitivity to food and airborne antigens was important in the development of eczematous skin lesions in a certain group of patients9,10. They also proposed the following 9 diagnostic criteria for AD: (1) atopic family history; (2) antecedent infantile eczema; (3) flexural localization; (4) gray-brown discoloration of the skin; (5) absence of vesicles; (6) vasomotor instability; (7) negative patch test reactions to contact irritants; (8) positive skin test reactions to various environmental and food antigens; and (9) the presence of reagins in the serum (presence of specific IgE antibodies to common allergens in the serum)10. Wise and Sulzberger stated that the logical therapy for AD was the avoidance of all foods and inhalants giving positive skin reactions, and they also advocated desensitization therapy with the most suspected substance10,11. Therefore, the term of AD originally referred to eczematous dermatitis caused by allergic reaction to inhalant or food allergens. In contrast to the belief of the earlier researchers who coined the term of AD, the pathogenetic significance of hypersensitivity reaction (allergic reaction) in the development of AD seems be currently underestimated, and therapy for AD tends to be focused on skin inflammation and skin barrier defect11,12,13.
INCOMPLETENESS OF CURRENT PHARMACOLOGICAL THERAPIES FOR AD AND COMPLEMENTATION BY SYSTEMIC IMMUNOMODULATORY THERAPY TARGETING HYPERSENSITIVITY REACTION AND IMMUNE DYSFUNCTION
The majority of AD patients want a cure or long-term treatment-free clinical remission of AD. However, currently, AD patients and their families are generally informed that there is no curative treatment for AD and that this disease should be controlled by continuous medical management14. This mismatch between demand and supply in AD treatment seems to be the main reason for the current abundance of unconventional or alternative treatment approaches for AD by patients and their families seeking a cure for AD. This "AD problem" is resulting in substantial medicosocial problems and economical burdens in many developed countries15.
The present author suggests that the incompleteness or inefficacy of the current standard medical therapies for AD and the overwhelming "AD problem" might partly be a result of the under-diagnosis and under-treatment of AD in the viewpoint of AD as an allergic disease (the absence of the "allergy concept" in the current treatment approaches). Besides pharmacological therapy, allergen-specific therapies including the avoidance of sensitized allergen and allergen immunotherapy are clinically effective in AD patients16,17. This review proposes that the active clinical application of the concept of AD as an allergic disease and the introduction of allergen-specific therapies could produce additional clinical improvements in AD patients and contribute to the resolution of the current "AD problem." Clinically relevant allergens should be screened by an allergy skin test or a serum allergen-specific IgE assay, and the clinical relevance of the sensitized allergen should be confirmed either by a careful history on the relationship between exposure to the allergen and triggering of clinical symptoms or by objective provocation tests (including the atopy patch test) in AD patients. Moreover, AD patients should be advised and educated about the methods for avoiding clinically relevant sensitized allergens (e.g., house dust mites, skin-colonizing fungi, and food allergens). However, avoidance of environmental triggering factors including allergens and irritants is often technically difficult and insufficient in AD patients. In these cases, allergen immunotherapy could be helpful17. Allergen immunotherapy is a treatment in which small amounts of sensitized allergens are repeatedly administered either subcutaneously or sublingually to induce allergen-specific immune tolerance in patients with allergic diseases18. Allergen immunotherapy has been shown to be clinically beneficial in AD patients sensitized to inhalant allergens such as house dust mites in a meta-analysis of multiple randomized clinical trials19.
ONE-WAY VERTICAL FLOW PATHOGENESIS MODEL OF AD
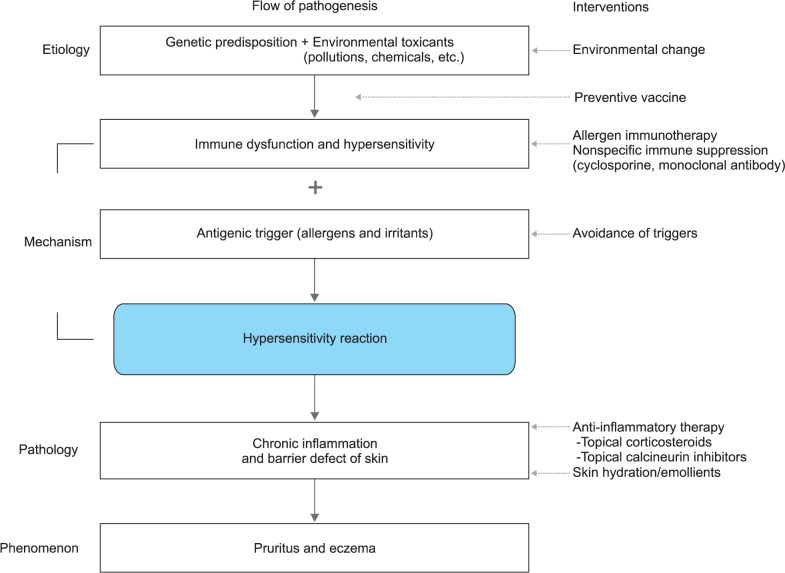
AD is currently regarded as a multifactorial disorder caused by multiple pathogenetic elements including genetic predisposition, environmental trigger, immune dysfunction including hypersensitivity reaction, chronic skin inflammation, and skin barrier defect2,3. However, the precise inter-relationships between multiple pathogenetic elements involved in the development and maintenance of AD are not yet completely understood. Many complex models have described the interactions between the multiple pathogenetic elements in the development of AD11,12,13,20. The main controversy is regarding which event between skin barrier defect and immune dysfunction occurs first and is more important in AD development13. The present author proposes a one-way vertical flow pathogenesis model of AD showing the associations among multiple pathogenetic elements (Fig. 1). In this model, (1) environmental toxicants (e.g., volatile inorganic chemicals, air pollution, and food additives) absorbed through the respiratory mucosa, gastrointestinal mucosa, or skin induce immune dysfunction in genetically susceptible human subjects; (2) immune dysfunction induced by the toxicants produces hypersensitivity to environmental allergens and irritants by decreasing the threshold for developing hypersensitivity reaction; and (3) exposure to the allergens and/or irritants induces hypersensitivity reaction, chronic inflammation, skin barrier defect, and the clinical manifestations of AD (pruritus and eczema), as suggested by an environmental scientist ("toxicant-induced loss of tolerance" theory)21,22. The present author proposes that multiple modalities could be introduced to stop the functioning of the pathogenetic pathway to prevent or treat AD in this one-way vertical flow pathogenesis model of AD (Fig. 1). In addition, blocking the upper stream of the pathogenetic pathway of this model might be a more effective and fundamental therapeutic approach than blocking the lower stream of the pathogenetic pathway (Fig. 1).
Fig. 1. One-way vertical flow pathogenesis model of atopic dermatitis.
This vertical model suggests that (1) environmental toxicants (e.g., volatile inorganic chemicals, air pollution, and food additives) induce immune dysfunction and hypersensitivity in genetically susceptible human subjects by decreasing the threshold for developing hypersensitivity reaction to environmental allergens and irritants; and (2) exposure to the allergens and/or irritants induces hypersensitivity reaction, chronic skin inflammation, skin barrier defect, and clinical manifestations of atopic dermatitis (pruritus and eczema). In this model, multiple modalities could be introduced to block multiple elements in the pathogenetic pathway for the treatment of atopic dermatitis. In addition, blocking the upper stream of the pathogenetic pathway of this model might be a more effective and fundamental therapeutic approach than blocking the lower stream of the pathogenetic pathway.
EVIDENCES SUPPORTING THE KEY ROLE OF HYPERSENSITIVITY REACTION (ALLERGIC REACTION) IN THE PATHOGENESIS OF AD
Hypersensitivity reaction (allergic reaction) to environmental allergen play a critical role in the development and maintenance of AD according to the following evidences.
1) Exposure to sensitized allergen (e.g., skin contact, inhalation) aggravates preexisting eczematous skin lesions or induces new eczematous skin lesions in AD patients23,24.
2) Avoidance of sensitized allergen induces clinical improvement in AD patients16,25.
3) Allergen immunotherapy including the repeated administrations of small amounts of sensitized allergen to induce allergen-specific immune tolerance results in clinical improvement in AD patients sensitized to inhalant allergen17,18,19.
4) The majority of AD patients (80%~90%) show allergic sensitization to common inhalants or food allergens26.
5) Serum IgE concentrations are increased in the majority of AD patients27.
6) AD patients frequently have coexisting other allergic diseases (e.g., allergic rhinitis, allergic keratoconjunctivitis, and bronchial asthma)28.
7) Children with AD frequently outgrow AD before puberty and progress to bronchial asthma and/or allergic rhinitis (allergic march)28.
These evidences support that hypersensitivity reaction play a key role in the pathogenesis of AD, and allergen-specific therapies including the avoidance of sensitized allergen and allergen immunotherapy should be introduced for the treatment of AD patients.
EVIDENCES SUPPORTING THE IMMUNE DYSFUNCTION AS A MAJOR THERAPEUTIC TARGET FOR AD
Immune dysfunction could be an ideal therapeutic target to induce long-term treatment-free clinical remission in AD patients according to the following evidences4,5,29,30,31,32,33.
1) Significant clinical improvement of AD can be induced by immunosuppressive drugs (e.g., cyclosporine, mycophenolate) 29,30.
2) Significant clinical improvement of AD can be induced by monoclonal antibodies to specific immune components (anti-IL-4 receptor antibody or anti-B cell antibody) 4,5.
3) Immunoadsorption to remove circulating immunoglobulins (IgG, IgA, IgM, and IgE) from plasma by using an anti-immunoglobulin antibody column induced significant clinical improvements in severe AD patients31.
4) The majority of children with AD experience natural clinical remission before puberty32.
5) Passive transfer of food allergy and subsequent development of AD occurred in a recipient following bone marrow transplantation from a donor with food allergy33.
These evidences suggest that immune dysfunction plays a key role in the pathogenesis of AD and is a useful therapeutic target. Clinical remission might be achieved by the correction of immune dysfunction in AD patients. The present author believes that an increased exposure to environmental toxicants is the most important factor responsible for the recent substantial increase in the prevalence of AD in developed countries as suggested (Fig. 1)21. Current medical therapies focus on controlling skin inflammation by using topical corticosteroids and/or topical calcineurin inhibitors1. However, immune dysfunction and hypersensitivity reaction in the upstream of the pathogenetic pathway might be more useful therapeutic targets than control of skin inflammation for long-term clinical remission in AD patients (Fig. 1).
PREVIOUS REPORTS ON THERAPEUTIC INTERVENTIONS INDUCING LONG-TERM TREATMENT-FREE CLINICAL REMISSION OF AD
Previous reports suggest that long-term treatment-free clinical remission of AD could be achieved by a complete change in the living environment, allergen immunotherapy, or natural remission in children with AD (Table 1).
Table 1. Treatment methods that have been reported to produce long-term clinical remission in patients with atopic dermatitis.

Climatotherapy or heliotherapy involving complete changes in the living environment of AD patients has been reported to produce long-term treatment-free clinical remission of AD in the majority of severe AD patients34,35. However, the clinical remission usually disappeared after the patients returned to their previous living environments34,35. These findings suggest that environmental factors are critical in the development and maintenance of AD. However, it is also suggested that the endogenous tendency of AD patients (immune dysfunction and hypersensitivity) prone to develop a chronic skin inflammation is not completely corrected by substantial change in the environmental factor alone.
Another important treatment modality that has been reported to produce long-term treatment-free clinical remission in AD patients is an allergen immunotherapy36. Observational studies reported long-term treatment-free clinical remission in AD patients after allergen immunotherapy or treatment by allergen-antibody complexes36,37,38. An interesting puzzle on the nature of AD is that a significant number of children with AD (up to 70%) experience natural remission of their disease before puberty26. The remission of AD in children seems to be due to a natural induction of immune tolerance. However, the exact mechanism inducing natural remission of AD in children has not yet been determined. If the mechanism is identified, then its application in the treatment of AD might be the most promising therapeutic approach in the future.
EVIDENCES AGAINST THE CRITICAL ROLE OF SKIN BARRIER DEFECT IN THE PATHOGENESIS OF AD
The evidences supporting the importance of immune dysfunction and hypersensitivity in the pathogenesis of AD argue against the hypothesis that defect in the gene for the skin barrier protein (i.e., filaggrin) is a primary cause of AD12,13,39,40. If the mutation of the filaggrin gene is a critical and essential pathogenetic component responsible for the development of AD, how can we explain the marked clinical improvements of AD frequently observed by complete changes in the environment (climatotherapy), allergen immunotherapy, or the interesting finding of natural remission observed in children with AD?
CRITICISM AGAINST THE CRITICAL ROLE OF HYPERSENSITIVITY REACTION TO ENVIRONMENTAL ANTIGEN IN THE PATHOGENESIS OF AD
There has been criticism regarding the importance of hypersensitivity reaction to environmental antigen in the pathogenesis of AD. The rationales supporting this criticism are as follows.
1) A certain number of AD patients have no evidence of allergic sensitization to environmental antigen, known as the "intrinsic type of AD" (about 10%~20% of adult AD patients)41,42,43.
2) Avoidance of the sensitized house dust mite allergen was effective in children with AD but not in adult AD patients in randomized controlled studies25,44.
3) The autoreactivity to self-protein (auto-allergy) seems to play a role in the development of chronicity of AD45.
4) Although a systematic review on allergen immunotherapy for AD showed a positive evidence of clinical efficacy, relatively few well-designed clinical trials have studied the clinical efficacy of allergen immunotherapy, and further clinical trials are needed to obtain a strong evidence19,46.
The relative importance of hypersensitivity reaction to environmental antigen in the pathogenesis of AD could be variable among patients43. In addition, a chemical or metal, rather than a protein, could act as an antigen causing chronic skin inflammation, as suggested in the intrinsic type of AD42. However, the importance of immune dysfunction and hypersensitivity reaction (not confined to IgE-mediated type I hypersensitivity reaction) in the pathogenesis of AD could be supported by the above mentioned evidences.
REASONS FOR THE CLINICAL EFFICACY OF MULTIPLE DIFFERENT THERAPEUTIC APPROACHES FOR AD ("DOMINO THEORY")
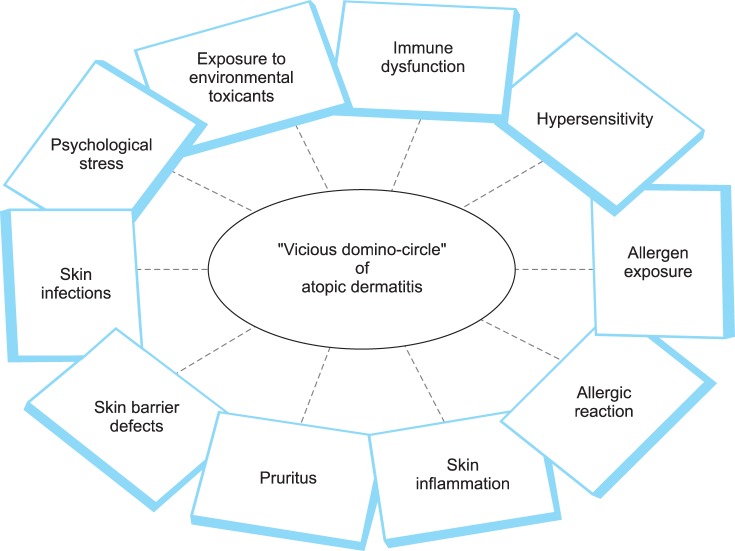
It is unclear how both climatotherapy and allergen immunotherapy can induce long-term clinical remission in AD patients although these 2 approaches target different pathogenetic elements of AD. The present author suggests that a vicious domino-circle formed from the negative interactions among multiple pathogenetic elements is essential for the development and maintenance of AD based on the personal clinical experiences obtained during the treatment of AD patients ("domino theory"). If one specific pathogenetic factor (e.g., environmental trigger, immune dysfunction, skin inflammation, or skin barrier defect) can be sufficiently controlled to break the vicious domino-circle, clinical remission of AD could be possible (Fig. 2). This theory can also explain the long-term clinical remission observed in AD patients induced by various types of therapeutic interventions including climatotherapy, allergen immunotherapy, diet change, nutritional supplementation, and lifestyle change. Therefore, the "domino theory" of AD supports a therapeutic concept that multiple different therapeutic approaches can produce clinical improvements and even induce long-term treatment-free clinical remission in AD patients. In addition, this concept supports that a multidisciplinary therapeutic approach including environmental control, psychological support, and patient education should be attempted to achieve maximal clinical improvements in AD patients.
Fig. 2. "Domino theory".
A vicious domino-circle formed from negative interactions among multiple pathogenetic elements is essential for the development and maintenance of atopic dermatitis, and if one of the pathogenetic elements (e.g., environmental triggers, immune dysfunction, skin inflammation, or skin barrier defects) can be sufficiently controlled to break the vicious domino-circle, then clinical remission of atopic dermatitis could be possible.
REGULATORY T CELL AS A CRITICAL TARGET FOR THE TREATMENT OF AD
Regulatory T cell plays a critical role in maintaining immune tolerance including the suppression of autoimmunity (immune tolerance to self antigens) and hypersensitivity (immune tolerance to non-self antigens)47. Deficiency of regulatory T cell function has been suggested to be a key immune dysfunction responsible for the development of autoimmune and allergic diseases, as observed in animal experiments48. Allergen immunotherapy decreases allergic inflammation (T helper type2 cell activation and IgE-mediated reaction) and induces clinical improvement in patients with allergic diseases possibly through an activation of regulatory T cell49. Lactobacillus or vitamin D supplementation has also been shown to activate regulatory T cell in animal models50,51. There have been reports on the positive clinical efficacy of lactobacillus or vitamin D supplementation in AD patients52,53,54,55. Therefore, combinations of various approaches to activate regulatory T cell, including allergen immunotherapy, vitamin D supplementation, and lactobacillus supplementation, might provide maximal clinical improvements in AD patients by the induction of immune tolerance51.
RATIONALE FOR A PERSONALIZED COMBINATION OF MULTIPLE THERAPIES TARGETING DIFFERENT STEPS IN THE PATHOGENETIC PATHWAY OF AD
The pathogenetic mechanisms involved in the development of AD seem to be variable among AD patients. Because of this heterogeneity in the pathogenetic mechanism, the clinical efficacy of a single type of therapy can be insufficient and unpredictable in AD patients. In real clinical practice, many physicians apply various combinations of pharmacological therapies targeting skin inflammation (use of topical corticosteroid and/or topical calcineurin) and immune dysfunction (use of immunosuppressant drugs including cyclosporine) and provide advices regarding the avoidance of environmental triggers (allergens and irritants) in the treatment of AD patients. For the achievement of maximal clinical improvement, a personalized combination of immunomodulatory therapies including allergen immunotherapy and monoclonal antibody therapy (e.g., anti-IgE or anti-IL4 receptor antibody) should be tried and personalized according to the characteristics of immune dysfunction and hypersensitivity in individual AD patients in order to achieve a maximal clinical improvement.
PRESENT AND FUTURE OF IMMUNOMODULATORY THERAPY FOR AD
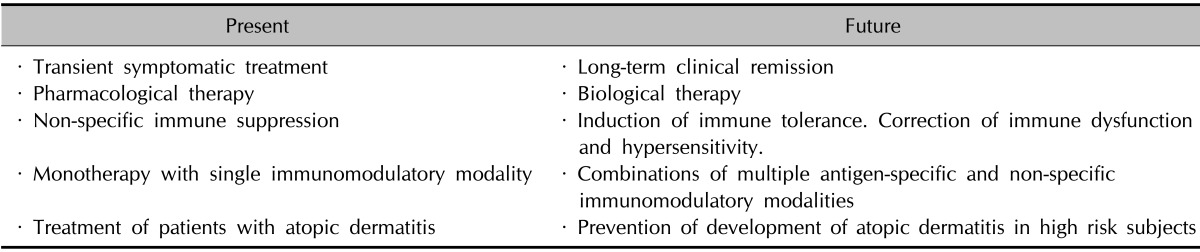
Current immunomodulatory therapies for AD using immunosuppressive drugs and monoclonal antibodies are directed to a nonspecific suppression of immune function, and this approach could be harmful in AD patients because of the possible increase in the risk of infection and/or malignancy. Therefore, future development of immunomodulatory therapy for AD should be directed to the normalization of immune dysfunction without immune suppression. The duration of the clinical efficacy of the current immunomodulatory therapies for AD is relatively short, and regular maintenance therapy is essential for long-term clinical improvement. Future immunomodulatory therapy for AD should aim for long-term clinical remission without the need for maintenance therapy (Table 2). Currently, none of the available immunomodulatory therapies has proven to modify the disease course of AD. Future immunomodulatory therapies should aim for the modification of the long-term disease course and ultimately the long-term treatment-free clinical remission and cure of AD. Unfortunately, no definite tool is currently available for the primary prevention of AD. Future studies should also focus on the development of preventive vaccines for AD.
Table 2. Present and future of immunomodulatory therapy for atopic dermatitis.
Induction of immune tolerance mimicking the immunological mechanism responsible for the natural remission of AD in children would be an ideal method to achieve clinical remission in AD patients. Induction of an anti-idiotypic immune response (immune response to the antigen-binding site of an antibody) has been suggested as a mechanism responsible for the development of immune tolerance in animal studies56. However, limited clinical data supporting anti-idiotypic immunomodulatory therapy in human subjects with allergic diseases are available. Recently, the clinical efficacy of recombinant idiotype vaccines resulting in the prolongation of disease-free survival was demonstrated in patients with B-cell lymphoma in a randomized placebo-controlled clinical trial57. This result suggests that anti-idiotypic immunomodulatory therapy could be also clinically effective in patients with allergic diseases. The present author hypothesized that repeated intramuscular injections of autologous immunoglobulin could induce clinical improvements in AD patients by stimulating active immune responses to the antigen-binding sites of pathogenic antibodies, thereby correcting immune dysfunction. Repeated intramuscular injections of autologous immunoglobulin (mainly IgG) purified from autologous plasma using Protein A (autologous immunoglobulin therapy) induced significant long-term clinical improvements in 2 of 3 patients with severe recalcitrant AD58. Autologous immunoglobulin therapy also significantly decreased the clinical severity scores and serum IgE concentrations in 17 adult patients with severe AD59. Future immunomodulatory therapies for AD should be personalized to specifically correct the immune dysfunction and hypersensitivity in individual patients.
CONCLUSION
Immune dysfunction and hypersensitivity reaction play key roles in the pathogenesis of AD. Systemic immunomodulatory therapies with allergen immunotherapy or monoclonal antibodies to specific immune components could be effective in AD patients. However, the clinical efficacy of current immunomodulatory therapy is often unpredictable or insufficient in some AD patients due to the heterogeneity of the pathogenetic mechanisms among AD patients. Therefore, a personalized combination of immunomodulatory therapies to reduce hypersensitivity(allergen immunotherapy) and correct immune dysfunction (monoclonal antibody therapy) could be a reasonable therapeutic approach for patients with AD. Future immunomodulatory therapies for AD should be developed to achieve long-term treatment-free clinical remission by induction of immune tolerance.
ACKNOWLEDGMENT
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2014R1A2A1A11049386).
References
- 1.Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, et al. European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol. 2006;118:152–169. doi: 10.1016/j.jaci.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 2.Novak N, Bieber T, Leung DY. Immune mechanisms leading to atopic dermatitis. J Allergy Clin Immunol. 2003;112(6 Suppl):S128–S139. doi: 10.1016/j.jaci.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 5.Simon D, Hösli S, Kostylina G, Yawalkar N, Simon HU. Anti-CD20 (rituximab) treatment improves atopic eczema. J Allergy Clin Immunol. 2008;121:122–128. doi: 10.1016/j.jaci.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Krathen RA, Hsu S. Failure of omalizumab for treatment of severe adult atopic dermatitis. J Am Acad Dermatol. 2005;53:338–340. doi: 10.1016/j.jaad.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Takiguchi R, Tofte S, Simpson B, Harper E, Blauvelt A, Hanifin J, et al. Efalizumab for severe atopic dermatitis: a pilot study in adults. J Am Acad Dermatol. 2007;56:222–227. doi: 10.1016/j.jaad.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Coca AF, Cooke RA. On the classification of the phenomena of hypersensitiveness. J Immunol. 1923;8:163–182. [Google Scholar]
- 9.Sulzberger MB. Historical notes on atopic dermatitis: its names and nature. Semin Dermatol. 1983;2:1–4. [Google Scholar]
- 10.Furue M, Chiba T, Takeuchi S. Current status of atopic dermatitis in Japan. Asia Pac Allergy. 2011;1:64–72. doi: 10.5415/apallergy.2011.1.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halbert AR, Weston WL, Morelli JG. Atopic dermatitis: is it an allergic disease. J Am Acad Dermatol. 1995;33:1008–1018. doi: 10.1016/0190-9622(95)90295-3. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Lee SH. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol Res. 2014;6:276–287. doi: 10.4168/aair.2014.6.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151–161. doi: 10.2332/allergolint.13-RAI-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raimer SS. Managing pediatric atopic dermatitis. . Clin Pediatr (Phila) 2000;39:1–14. doi: 10.1177/000992280003900101. [DOI] [PubMed] [Google Scholar]
- 15.Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192–199. doi: 10.1111/j.1525-1470.2005.22303.x. [DOI] [PubMed] [Google Scholar]
- 16.Sanda T, Yasue T, Oohashi M, Yasue A. Effectiveness of house dust-mite allergen avoidance through clean room therapy in patients with atopic dermatitis. J Allergy Clin Immunol. 1992;89:653–657. doi: 10.1016/0091-6749(92)90370-h. [DOI] [PubMed] [Google Scholar]
- 17.Werfel T, Breuer K, Ruéff F, Przybilla B, Worm M, Grewe M, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy. 2006;61:202–205. doi: 10.1111/j.1398-9995.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 18.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 19.Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;132:110–117. doi: 10.1016/j.jaci.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Novak N, Simon D. Atopic dermatitis-from new pathophysiologic insights to individualized therapy. Allergy. 2011;66:830–839. doi: 10.1111/j.1398-9995.2011.02571.x. [DOI] [PubMed] [Google Scholar]
- 21.Genuis SJ. Sensitivity-related illness: the escalating pandemic of allergy, food intolerance and chemical sensitivity. Sci Total Environ. 2010;408:6047–6061. doi: 10.1016/j.scitotenv.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Lee HS, Park MR, Lee SW, Kim EH, Cho JB, et al. Relationship between indoor air pollutant levels and residential environment in children with atopic dermatitis. Allergy Asthma Immunol Res. 2014;6:517–524. doi: 10.4168/aair.2014.6.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darsow U, Laifaoui J, Kerschenlohr K, Wollenberg A, Przybilla B, Wüthrich B, et al. The prevalence of positive reactions in the atopy patch test with aeroallergens and food allergens in subjects with atopic eczema: a European multicenter study. Allergy. 2004;59:1318–1325. doi: 10.1111/j.1398-9995.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 24.Tupker RA, De Monchy JG, Coenraads PJ, Homan A, van der Meer JB. Induction of atopic dermatitis by inhalation of house dust mite. J Allergy Clin Immunol. 1996;97:1064–1070. doi: 10.1016/s0091-6749(96)70259-2. [DOI] [PubMed] [Google Scholar]
- 25.Tan BB, Weald D, Strickland I, Friedmann PS. Double-blind controlled trial of effect of housedust-mite allergen avoidance on atopic dermatitis. Lancet. 1996;347:15–18. doi: 10.1016/s0140-6736(96)91556-1. [DOI] [PubMed] [Google Scholar]
- 26.Hua TC, Hwang CY, Chen YJ, Chu SY, Chen CC, Lee DD, et al. The natural course of early-onset atopic dermatitis in Taiwan: a population-based cohort study. Br J Dermatol. 2014;170:130–135. doi: 10.1111/bjd.12603. [DOI] [PubMed] [Google Scholar]
- 27.Johnson EE, Irons JS, Patterson R, Roberts M. Serum IgE concentration in atopic dermatitis. Relationship to severity of disease and presence of atopic respiratory disease. J Allergy Clin Immunol. 1974;54:94–99. doi: 10.1016/0091-6749(74)90037-2. [DOI] [PubMed] [Google Scholar]
- 28.Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67–73. doi: 10.4168/aair.2011.3.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitt J, Schmitt N, Meurer M. Cyclosporin in the treatment of patients with atopic eczema-a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2007;21:606–619. doi: 10.1111/j.1468-3083.2006.02023.x. [DOI] [PubMed] [Google Scholar]
- 30.Weatherhead SC, Wahie S, Reynolds NJ, Meggitt SJ. An open-label, dose-ranging study of methotrexate for moderate-to-severe adult atopic eczema. Br J Dermatol. 2007;156:346–351. doi: 10.1111/j.1365-2133.2006.07686.x. [DOI] [PubMed] [Google Scholar]
- 31.Kasperkiewicz M, Schmidt E, Frambach Y, Rose C, Meier M, Nitschke M, et al. Improvement of treatment-refractory atopic dermatitis by immunoadsorption: a pilot study. J Allergy Clin Immunol. 2011;127:267–270. doi: 10.1016/j.jaci.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson D, Sjöberg O, Foucard T. Sensitization to food and airborne allergens in children with atopic dermatitis followed up to 7 years of age. Pediatr Allergy Immunol. 2003;14:448–452. doi: 10.1046/j.0905-6157.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 33.Bellou A, Kanny G, Fremont S, Moneret-Vautrin DA. Transfer of atopy following bone marrow transplantation. Ann Allergy Asthma Immunol. 1997;78:513–516. doi: 10.1016/s1081-1206(10)63240-1. [DOI] [PubMed] [Google Scholar]
- 34.Byremo G, Rød G, Carlsen KH. Effect of climatic change in children with atopic eczema. Allergy. 2006;61:1403–1410. doi: 10.1111/j.1398-9995.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- 35.Vähävihu K, Ylianttila L, Salmelin R, Lamberg-Allardt C, Viljakainen H, Tuohimaa P, et al. Heliotherapy improves vitamin D balance and atopic dermatitis. Br J Dermatol. 2008;158:1323–1328. doi: 10.1111/j.1365-2133.2008.08518.x. [DOI] [PubMed] [Google Scholar]
- 36.Chait I, Allkins V. Remission of life-long atopic dermatitis after hyposensitisation to house dust mite. Practitioner. 1985;229:609, 612. [PubMed] [Google Scholar]
- 37.Leroy BP, Lachapelle JM, Somville MM, Jacquemin MG, Saint-Remy JM. Injection of allergen-antibody complexes is an effective treatment of atopic dermatitis. Dermatologica. 1991;182:98–106. doi: 10.1159/000247754. [DOI] [PubMed] [Google Scholar]
- 38.Tuft L. Studies in atopic dermatitis. V. Problems in inhalant hyposensitization and results of treatment. J Allergy. 1960;31:1–11. doi: 10.1016/0021-8707(60)90019-8. [DOI] [PubMed] [Google Scholar]
- 39.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu HS, Kang MJ, Jung YH, Kim HY, Seo JH, Kim YJ, et al. Mutations in the filaggrin are predisposing factor in korean children with atopic dermatitis. Allergy Asthma Immunol Res. 2013;5:211–215. doi: 10.4168/aair.2013.5.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suárez-Fariñas M, Dhingra N, Gittler J, Shemer A, Cardinale I, de Guzman Strong C, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol. 2013;132:361–370. doi: 10.1016/j.jaci.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010;58:1–7. doi: 10.1016/j.jdermsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis? J Allergy Clin Immunol. 2004;114:150–158. doi: 10.1016/j.jaci.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 44.Oosting AJ, de Bruin-Weller MS, Terreehorst I, Tempels-Pavlica Z, Aalberse RC, de Monchy JG, et al. Effect of mattress encasings on atopic dermatitis outcome measures in a double-blind, placebo-controlled study: the Dutch mite avoidance study. J Allergy Clin Immunol. 2002;110:500–506. doi: 10.1067/mai.2002.126791. [DOI] [PubMed] [Google Scholar]
- 45.Tang TS, Bieber T, Williams HC. Does "autoreactivity" play a role in atopic dermatitis. J Allergy Clin Immunol. 2012;129:1209–1215. doi: 10.1016/j.jaci.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Gendelman SR, Lang DM. Specific immunotherapy in the treatment of atopic dermatitis: a systematic review using the GRADE system. Ann Allergy Asthma Immunol. 2013;111:555–561. doi: 10.1016/j.anai.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 47.Nouri-Aria KT. Foxp3 expressing regulatory T-cells in allergic disease. Adv Exp Med Biol. 2009;665:180–194. doi: 10.1007/978-1-4419-1599-3_14. [DOI] [PubMed] [Google Scholar]
- 48.Miyara M, Wing K, Sakaguchi S. Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion. J Allergy Clin Immunol. 2009;123:749–755. doi: 10.1016/j.jaci.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27. doi: 10.1016/j.jaci.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 50.Kim HJ, Kim YJ, Lee SH, Yu J, Jeong SK, Hong SJ. Effects of Lactobacillus rhamnosus on allergic march model by suppressing Th2, Th17, and TSLP responses via CD4(+) CD25(+)Foxp3(+) Tregs. Clin Immunol. 2014;153:178–186. doi: 10.1016/j.clim.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Weiss ST. Bacterial components plus vitamin D: the ultimate solution to the asthma (autoimmune disease) epidemic? J Allergy Clin Immunol. 2011;127:1128–1130. doi: 10.1016/j.jaci.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torii S, Torii A, Itoh K, Urisu A, Terada A, Fujisawa T, et al. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum markers of atopic dermatitis in children. Int Arch Allergy Immunol. 2011;154:236–245. doi: 10.1159/000321110. [DOI] [PubMed] [Google Scholar]
- 53.Yang HJ, Min TK, Lee HW, Pyun BY. Efficacy of probiotic therapy on atopic dermatitis in children: a randomized, double-blind, placebo-controlled trial. Allergy Asthma Immunol Res. 2014;6:208–215. doi: 10.4168/aair.2014.6.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sidbury R, Sullivan AF, Thadhani RI, Camargo CA., Jr Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. Br J Dermatol. 2008;159:245–247. doi: 10.1111/j.1365-2133.2008.08601.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee SA, Hong S, Kim HJ, Lee SH, Yum HY. Correlation between serum vitamin d level and the severity of atopic dermatitis associated with food sensitization. Allergy Asthma Immunol Res. 2013;5:207–210. doi: 10.4168/aair.2013.5.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shoenfeld Y. The idiotypic network in autoimmunity: antibodies that bind antibodies that bind antibodies. Nat Med. 2004;10:17–18. doi: 10.1038/nm0104-17. [DOI] [PubMed] [Google Scholar]
- 57.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves diseasefree survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–2794. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nahm DH, Cho SM, Kim ME, Kim YJ, Jeon SY. Autologous immunoglobulin therapy in patients with severe recalcitrant atopic dermatitis: a preliminary report. Allergy Asthma Immunol Res. 2014;6:89–94. doi: 10.4168/aair.2014.6.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nahm DH, Kim ME, Cho SM. The effects of intramuscular injection of autologous immunoglobulin on clinical severity and serum IgE concentration in patients with atopic dermatitis. Dermatology. 2015;231:145–151. doi: 10.1159/000431173. [DOI] [PubMed] [Google Scholar]