Abstract
Acute generalized exanthematous pustulosis (AGEP) is a cutaneous reaction principally induced by drugs. Spontaneous resolution is observed in most patients. However, severe cases required systemic corticosteroid administration. Hydroxychloroquine, which is used to treat some dermatologic and rheumatologic diseases because of its anti-inflammatory and immunosuppressive effects, is an uncommon cause of AGEP. A 67-year-old female patient presented with severe AGEP due to hydroxychloroquine treatment. She was recalcitrant to supportive care and systemic corticosteroid treatment butwas successfully treated with cyclosporine. Hydroxychloroquine-induced AGEP occurs in women with underlying rheumatologic diseases, has a longer latent period, and has a severe course usually requiring systemic treatment.
Keywords: Acute generalized exanthematous pustulosis, Cyclosporine, Hydroxychloroquine
INTRODUCTION
Acute generalized exanthematous pustulosis (AGEP) is a rare cutaneous reaction principally induced by drugs. Antibiotics, mainly β-lactams and macrolides, are the most common causative agents, followed by calcium channel blockers and antimalarials1. AGEP is classically characterized by a sudden onset of widespread non-follicular sterile pustules arising within large areas of edematous erythema, high fever (>38℃), and neutrophilia (>5,000/mL). Together with the abovementioned typical cutaneous eruption, targetoid lesions and purpura are also observed in some patients. Although mucosal involvement is uncommon, if present, it is mild and usually accompanied by oral involvement. Although disturbing, AGEP has a favorable prognosis. Spontaneous resolution within 15 days after the cessation of the responsible drug is usually observed in most patients2.
Here, we report the case of a female patient with hydroxychloroquine (HCQ)-induced severe and recalcitrant AGEP who was successfully treated with cyclosporine. Furthermore, we reviewed similar cases in the literature identified through an English-language PubMed search using the term "acute generalized exanthematous pustulosis" AND "hydroxychloroquine."
CASE REPORT
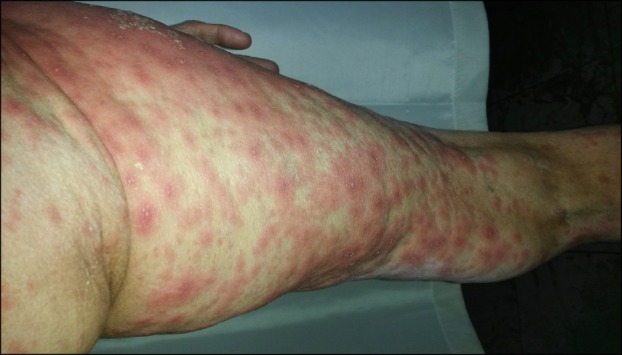
A 67-year-old woman was admitted to our clinic with an acute widespread pruritic pustular rash and high fever for the last 3 days. She reported that this started 15 days after the beginning of HCQ100 mg twice daily prescribed by the Rheumatology Department for a diagnosis of seronegative polyarthritis. She denied psoriasis in her personal and family history. On dermatologic examination, she had facial edema with erythema; widespread millimetric pustules on large areas of edematous and erythematous plaques located on her back, chest, and upper extremities mainly accentuated on intertriginous areas such as the axilla and neck; and slightly painful multiple erythematous targetoid papular lesions on her thighs (Fig. 1, 2). Oral and genital mucosae were normal. Her body temperature was 39℃. Laboratory examination showed white blood cell count was 34,000/ml with neutrophilia (90%) and an erythrocyte sedimentation rate of 100 mm/h. Other laboratory parameters were normal, and all bacterial cultures including pustule, throat, urine, and blood were negative. Skin biopsy from the shoulder of the patient was performed, and histopathologic examination revealed necrotic keratinocytes, spongiform subcorneal and intradermal pustules, papillary edema, and perivascular neutrophil infiltrate with some eosinophils. HCQ was immediately withdrawn on admission, and supportive treatment including oral antihistamine, wet dressing, and paracetamol were administered. As the skin lesions worsened and body temperature failed to be regulated with these measures, oral prednisolone 0.8 mg·kg-1·day-1 was started on the 5th day of hospitalization. Body temperature returned to normal. However, the dose was increased to 1 mg·kg-1·day-1 on the 9th day of hospitalization because of exacerbation of the skin lesions. In the first few days of the treatment with prednisolone 1 mg·kg-1·day-1, pustules began to desquamate, erythema and edema subsided, and targetoid lesions on the legs partially improved. However, 1 week later, the disease suddenly exacerbated without any known triggering factor. As the patient was hospitalized throughout treatment, no other triggering factor such as mercury or plant exposure, which can very rarely cause AGEP, was identified. The ineffectiveness of the treatment was considered, and cyclosporine 4 mg·kg-1·day-1 was added to prednisolone 1 mg·kg-1·day-1 on the 19th day of treatment. Within 5 days, the skin lesions gradually improved and recovered completely thereafter. Oral prednisolone was tapered and stopped rapidly. The cyclosporine dosage was tapered gradually to 3 mg·kg-1·day-1 and then to 1.5 mg·kg-1·day-1 and discontinued within 2 months.
Fig. 1. Multiple pustular lesions on the erythematous and edematous plaque accentuated in the intertriginous area and axilla.
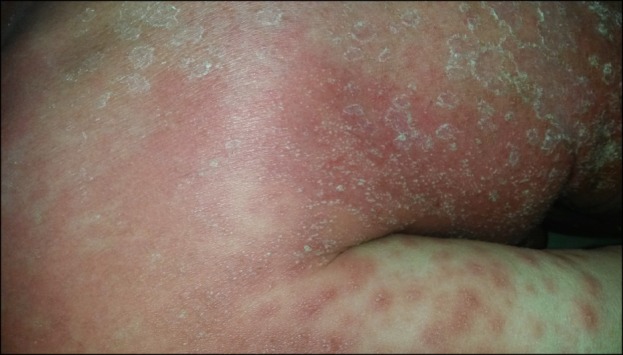
Fig. 2. Targetoid lesions on the thigh.
DISCUSSION
HCQ is an antimalarial drug commonly used to treat several dermatologic and rheumatologic diseases owing to its immunosuppressive and anti-inflammatory effects. However, it is a rare causative agent of AGEP3. In our patient, skin eruption developed following HCQ treatment. HCQ can trigger both pustular psoriasis and AGEP. The absence of personal and family history of psoriasis as well as the presence of targetoid lesions together with pustular eruptions, accentuation of pustules in the intertriginous areas, and the histopathologic presence of eosinophils and necrotic keratinocytes but absence of tortuous or dilated vessels helped us exclude pustular psoriasis in the present case. In the English-language literature, we found 10 cases of rheumatology patients who developed AGEP due to HCQ (Table 1)3,4,5,6,7,8,9,10. Nine patients were women; concordantly, AGEP is known to be more common in women1. However, the fact that there was only 1 male patient is noteworthy; this may be due to the less frequent occurrence of rheumatologic diseases in males. On the other hand, HCQ-induced AGEP occurs mostly in women because of some unknown metabolic or hormonal factor. Nevertheless, the number of patients with HCQ-induced AGEP is too small to draw such conclusions.
Table 1. Features of HCQ-induced acute generalized exanthematous pustulosis patients in the English-language literature.
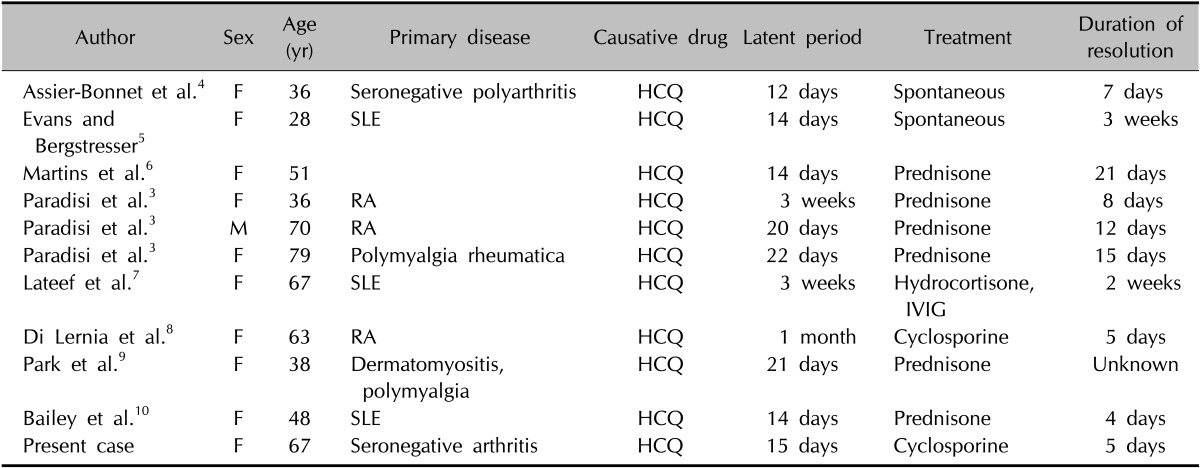
HCQ: hydroxychloroquine, F: female, M: male, SLE: systemic lupus erythematosus, RA: rheumatoid arthritis, IVIG: intravenous immunoglobulin.
Furthermore, all 10 patients were taking HCQ for rheumatologic but not dermatologic problems. This may be because of the more frequent use of HCQ to treat rheumatologic diseases. However, some immunologic factors in the pathogenesis of rheumatologic diseases may also play roles in the pathogenesis of HCQ-induced AGEP.
The latent period of HCQ-induced AGEP is longer than that of antibiotics-induced AGEP, which are 12~30 days and 1 day, respectively11. The longer latent period of HCQ-induced AGEP may be related to the metabolic characteristics of HCQ or the immunologic dysregulation due to underlying rheumatologic diseases. However, the longer latent period observed in HCQ-induced AGEP is probably due to a lack of previous sensitization with HCQ and the consequent lack of immunologic recall phenomenon11.
Finally, most of the patients were treated with systemic agents3,6,7,8,9,10. Cessation of the culprit drug and supportive care are commonly sufficient for the treatment of mild cases. Thus, the requirement of systemic treatments in those patients suggests most of them had severe or recalcitrant diseases. Accordingly, our patient had recalcitrant disease that did not improve spontaneously or with systemic corticosteroids but did improve only with cyclosporine. Hence, the present case is the second case of HCQ-induced AGEP successfully treated with cyclosporine8. Cyclosporine has many inhibitory effects on T cells12. As drug-specific T cells, which express the neutrophil-attracting chemokine interleukin-8,play an important role in the pathogenesis of AGEP, cyclosporine can be successfully used to treat severe or recalcitrant AGEP13.
In conclusion, HCQ-induced AGEP is a rare disease that can be severe and recalcitrant. It predominantly occurs in women treated with HCQ for their underlying rheumatologic diseases. The latent period of the disease is long. Immediate cessation of HCQ is mandatory for treatment. However, most patients require systemic corticosteroid administration together with supportive care. Moreover, cyclosporine can be a good choice for patients resistant to systemic corticosteroid treatment.
References
- 1.Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87–92. doi: 10.1111/j.1440-0960.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 2.Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol. 2001;28:113–119. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 3.Paradisi A, Bugatti L, Sisto T, Filosa G, Amerio PL, Capizzi R. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: three cases and a review of the literature. Clin Ther. 2008;30:930–940. doi: 10.1016/j.clinthera.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Assier-Bonnet H, Saada V, Bernier M, Clerici T, Saïag P. Acute generalized exanthematous pustulosis induced by hydroxychloroquine. Dermatology. 1996;193:70–71. doi: 10.1159/000246211. [DOI] [PubMed] [Google Scholar]
- 5.Evans CC, Bergstresser PR. Acute generalized exanthematous pustulosis precipitated by hydroxychloroquine. J Am Acad Dermatol. 2004;50:650–651. doi: 10.1016/s0190-9622(03)02733-6. [DOI] [PubMed] [Google Scholar]
- 6.Martins A, Lopes LC, Paiva Lopes MJ, Rodrigues JC. Acute generalized exanthematous pustulosis induced by hydroxychloroquine. Eur J Dermatol. 2006;16:317–318. [PubMed] [Google Scholar]
- 7.Lateef A, Tan KB, Lau TC. Acute generalized exanthematous pustulosis and toxic epidermal necrolysis induced by hydroxychloroquine. Clin Rheumatol. 2009;28:1449–1452. doi: 10.1007/s10067-009-1262-4. [DOI] [PubMed] [Google Scholar]
- 8.Di Lernia V, Grenzi L, Guareschi E, Ricci C. Rapid clearing of acute generalized exanthematous pustulosis after administration of ciclosporin. Clin Exp Dermatol. 2009;34:e757–e759. doi: 10.1111/j.1365-2230.2009.03480.x. [DOI] [PubMed] [Google Scholar]
- 9.Park JJ, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. A case of hydroxychloroquine induced acute generalized exanthematous pustulosis confirmed by accidental oral provocation. Ann Dermatol. 2010;22:102–105. doi: 10.5021/ad.2010.22.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey K, McKee D, Wismer J, Shear N. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: first case report in Canada and review of the literature. J Cutan Med Surg. 2013;17:414–418. doi: 10.2310/7750.2013.12105. [DOI] [PubMed] [Google Scholar]
- 11.Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) Br J Dermatol. 2007;157:989–996. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 12.Dehesa L, Abuchar A, Nuno-Gonzalez A, Vitiello M, Kerdel FA. The use of cyclosporine in dermatology. J Drugs Dermatol. 2012;11:979–987. [PubMed] [Google Scholar]
- 13.Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, et al. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001;107:1433–1441. doi: 10.1172/JCI12118. [DOI] [PMC free article] [PubMed] [Google Scholar]


