Abstract
Erythrodermic psoriasis (EP) is a very severe variant of psoriasis whose management poses a challenge to physicians, as currently available therapies often provide unsatisfactory results. Many biologics have been used to treat chronic plaque psoriasis, the most common form of psoriasis; however, their effectiveness for EP is poorly understood. A recently developed biologic, golimumab, has been extensively studied for the treatment of moderate-to-severe active rheumatoid arthritis, psoriatic arthritis, active ankylosing spondylitis, and chronic plaque psoriasis. However, no clinical trials have been performed for EP. Here, we report the case of a 32-year-old man who presented with severe psoriasis that previously failed to respond satisfactorily to methotrexate, cyclosporine, retinoid, narrow-band ultraviolet B phototherapy, and topical agents (i.e., steroids and calcipotriol). Skin lesions worsened progressively and developed into erythroderma. Psoriatic arthritis was also detected. Conventional therapies lacked efficacy. Therefore, we administered golimumab 50 mg. The skin lesions improved significantly according to the Psoriasis Area and Severity Index score after the first administration; lesions improved further throughout the treatment course. Although additional studies are required to fully evaluate the efficacy and safety of golimumab, this agent may be an alternative treatment strategy for some patients with recalcitrant EP.
Keywords: Biological products, Erythrodermic psoriasis, Golimumab, Psoriasis, Psoriatic arthritis
INTRODUCTION
Erythrodermic psoriasis (EP) is a severe and disabling form of psoriasis that is often challenging to treat; it incurs serious morbidity and can even lead to mortality. EP can result from aggravation of a previous case of psoriasis or can appear as the initial presentation of psoriasis. Drugs such as methotrexate, cyclosporine, and oral retinoid are effective for treating EP; however, numerous factors limit their use, including organ-specific toxicity, inconvenience, and the risk of opportunistic infections. Targeted biological therapies have the advantages of high selectivity for targeting specific pathways involved in the inflammation cascade and have improved the safety profiles of several inflammatory arthropathies and psoriasis1.
Golimumab (GLM) (Simponi; Janssen Korea Ltd.), a human γ-1 immunoglobulin-κ anti-tumor necrosis factor-α (TNF-α) monoclonal antibody, was approved by the US Food and Drug Administration and the European Agency for the Evaluation of Medicinal Products for adults for the treatment of active rheumatoid arthritis, active psoriatic arthritis, and active ankylosing spondylitis. GLM 50 mg is administered as a monthly subcutaneous injection2.
Here, we report the first case of the treatment of EP with GLM without any increase in dosage, demonstrating its efficacy for controlling and preventing the occurrence of erythrodermic flares with satisfactory compliance.
CASE REPORT
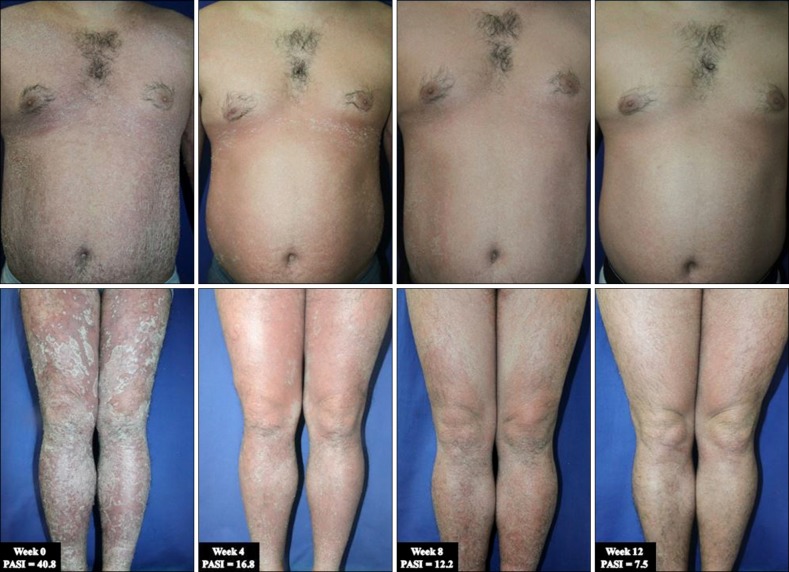
A 32-year-old man with a 17-year history of moderateto-severe plaque psoriasis was referred to our clinic in April 2013 for a flare-up of a skin lesion. On physical examination, the involved body surface area was approximately 90% with a Psoriasis Area and Severity Index (PASI) score of 40.8 (Fig. 1). The patient had joint tenderness and swelling on both hips, the right thumb, and the left fourth toe; his Classification Criteria for Psoriatic Arthritis score was 5. Therefore, he was diagnosed with EP and active psoriatic arthritis. The skin lesion responded poorly to previous conventional treatments including methotrexate, cyclosporine, acitretin, narrow-band ultraviolet B phototherapy, and topical agents (i.e., steroids and calcipotriol). Because of the severity of the clinical features and intolerance to conventional systemic therapies, he was considered to have a high need for biologics. Accordingly, the patient was administered GLM 50 mg subcutaneously once per month. Dramatic improvements were observed: just 4 weeks after the first injection of GLM, his PASI score decreased to 16.8 (Fig. 1). Gradual improvements were observed thereafter: his PASI scores were 12.2 and 7.5 after the second (week 8) and third (week 12) injections, respectively. Subjective symptoms including itching and burning sensations also disappeared. Moreover, dramatic improvements were observed in arthritis: the numbers of tender and swollen joints decreased from 7 to 2 and 5 to 0, respectively. The patient has been undergoing monthly GLM treatments for 11 months and has maintained a reduced PASI score. No significant adverse events have been observed throughout the course of treatment.
Fig. 1. Dramatic improvement of skin lesions after monthly injections of golimumab 50 mg, with improvement of the Psoriasis Area and Severity Index (PASI) score at 47 weeks of follow-up.
DISCUSSION
EP is a rare subtype of psoriasis characterized by generalized erythema of the entire body with scaling. Furthermore, it is associated with significant mortality3,4,5. In fact, 2 previous studies report mortality rates of 15% and 9% (with 5% and 2% mortality rates attributable to psoriasis) even though the patients had been treated with conventional systemic treatments such as retinoid and methotrexate3,5.
Unfortunately, the management of EP is difficult and has not been well standardized. Traditional systemic therapies include methotrexate, cyclosporine, and oral retinoid. However, treatment failure or intolerance to these therapeutic agents is frequently observed. Therefore, alternative strategies are urgently required4. Biologics including TNF-α inhibitors (e.g., etanercept, infliximab, and adalimumab) and the anti-interleukin-12/23 p40 monoclonal antibody ustekinumab have recently been used to treat patients with EP (Table 1)1,6,7,8,9,10,11,12,13. The results of a multicenter retrospective study of patients in whom conventional systemic treatments failed supports the short-term efficacy of biologics for treating EP, with a high rate of patients reaching a PASI score of 75 or a body surface area improvement of approximately 50% for various TNF-α inhibitors by the 12-week endpoint12.
Table 1. Previous reports on the use of biologics in patients with erythrodermic psoriasis.
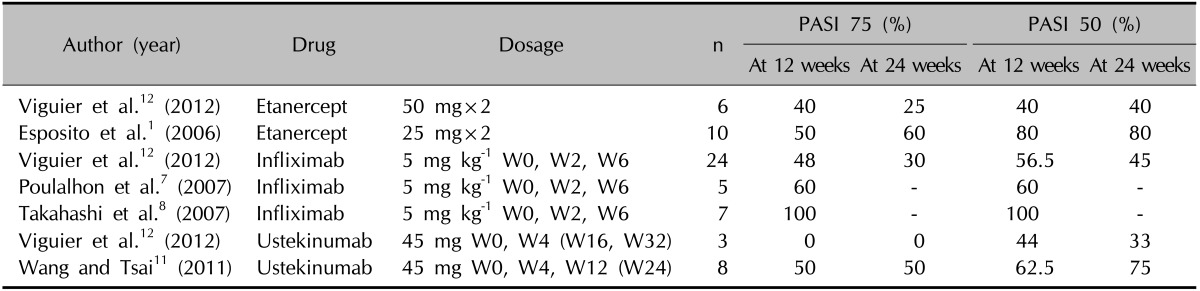
n: number of patients treated, W: week, PASI: Psoriasis Area and Severity Index, PASI 50: percentage of patients obtaining at least a 50% improvement of their baseline, PASI 75: percentage of patients obtaining at least a 75% improvement of their baseline PASI score.
The rationale for administering an anti-TNF-α agent to treat EP is based on the importance of TNF-α in inducing and maintaining skin inflammation during psoriasis. TNF-α is overexpressed in psoriatic skin lesions and is known to promote the proinflammatory cytokine cascade, which leads to the recruitment of leukocytes to the lesional epidermis. Furthermore, TNF-α is distributed throughout the epidermis of lesional psoriatic skin and localized to the upper dermal blood vessels14. Skin inflammation is the predominant feature of EP, and rapid systemic release of TNF-α may be responsible for the disease onset and severity. As GLM was recently demonstrated to exhibit sustained efficacy against multiple facets of psoriatic arthritis including enthesitis and dactylitis, we selected it among 5 biologics (etanercept, adalimumab, infliximab, ustekinumab, and GLM) currently available for the treatment of psoriatic arthritis in Korea15.
GLM is a human γ-1 immunoglobulin-κ anti-TNF-α monoclonal antibody3,9. The amino acid sequence of the heavy and light variable chain regions (Fab) of GLM are virtually identical to those of the human sequence (heavy chain sequence, 98%; light chain sequence, 100%). The constant (Fc) regions of the heavy and light chains of GLM are identical to those of infliximab with respect to amino acid sequence16. GLM is administered as a single-dose prefilled syringe or a single-dose prefilled autoinjector containing 50 mg GLM in 0.5 ml solution for subcutaneous administration.
To our knowledge, there are no published reports of EP treated with GLM. Thus, the present case is the first to demonstrate GLM is highly effective for EP and induces a rapid and significant clinical response associated with an excellent safety profile. Our patient's global assessment and compliance were consistently good. Although further studies are required to clarify the long-term profile of GLM for this indication, the rapidity of clearance and excellent safety profile of GLM suggest it can play a role in the management of EP.
References
- 1.Esposito M, Mazzotta A, de Felice C, Papoutsaki M, Chimenti S. Treatment of erythrodermic psoriasis with etanercept. Br J Dermatol. 2006;155:156–159. doi: 10.1111/j.1365-2133.2006.07217.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyce EG, Halilovic J, Stan-Ugbene O. Golimumab: review of the efficacy and tolerability of a recently approved tumor necrosis factor-α inhibitor. Clin Ther. 2010;32:1681–1703. doi: 10.1016/j.clinthera.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Boyd AS, Menter A. Erythrodermic psoriasis. Precipitating factors, course, and prognosis in 50 patients. J Am Acad Dermatol. 1989;21:985–991. [PubMed] [Google Scholar]
- 4.Jalal O, Houass S, Laissaoui K, Hocar O, Charioui S, Amal S. Severe psoriasis: 160 cases. Ann Dermatol Venereol. 2005;132:126–128. doi: 10.1016/s0151-9638(05)79222-x. [DOI] [PubMed] [Google Scholar]
- 5.Marks J. Erythrodermas and uric acid aberrations in psoriasis. In: Farber EM, Cox AJ, editors. Psoriasis: proceedings of the International Symposium Stanford University. Stanford, CA: Stanford University Press; 1971. pp. 89–98. [Google Scholar]
- 6.Heikkilä H, Ranki A, Cajanus S, Karvonen SL. Infliximab combined with methotrexate as long-term treatment for erythrodermic psoriasis. Arch Dermatol. 2005;141:1607–1610. doi: 10.1001/archderm.141.12.1607. [DOI] [PubMed] [Google Scholar]
- 7.Poulalhon N, Begon E, Lebbé C, Lioté F, Lahfa M, Bengoufa D, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329–336. doi: 10.1111/j.1365-2133.2006.07639.x. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi MD, Castro LG, Romiti R. Infliximab, as sole or combined therapy, induces rapid clearing of erythrodermic psoriasis. Br J Dermatol. 2007;157:828–831. doi: 10.1111/j.1365-2133.2007.08111.x. [DOI] [PubMed] [Google Scholar]
- 9.Richetta AG, Maiani E, Carlomagno V, Carboni V, Mattozzi C, Giancristoforo S, et al. Treatment of erythrodermic psoriasis in HCV+ patient with adalimumab. Dermatol Ther. 2009;22(Suppl 1):S16–S18. doi: 10.1111/j.1529-8019.2009.01266.x. [DOI] [PubMed] [Google Scholar]
- 10.Santos-Juanes J, Coto-Segura P, Mas-Vidal A, Galache Osuna C. Ustekinumab induces rapid clearing of erythrodermic psoriasis after failure of antitumour necrosis factor therapies. Br J Dermatol. 2010;162:1144–1146. doi: 10.1111/j.1365-2133.2010.09669.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang TS, Tsai TF. Clinical experience of ustekinumab in the treatment of erythrodermic psoriasis: a case series. J Dermatol. 2011;38:1096–1099. doi: 10.1111/j.1346-8138.2011.01224.x. [DOI] [PubMed] [Google Scholar]
- 12.Viguier M, Pagès C, Aubin F, Delaporte E, Descamps V, Lok C, et al. Groupe Français de Recherche sur le Psoriasis. Efficacy and safety of biologics in erythrodermic psoriasis: a multicentre, retrospective study. Br J Dermatol. 2012;167:417–423. doi: 10.1111/j.1365-2133.2012.10940.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee EJ, Shin MK, Kim NI. A clinical trial of combination therapy with etanercept and low dose cyclosporine for the treatment of refractory psoriasis. Ann Dermatol. 2010;22:138–142. doi: 10.5021/ad.2010.22.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lizzul PF, Aphale A, Malaviya R, Sun Y, Masud S, Dombrovskiy V, et al. Differential expression of phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and downregulation of NF-kappaB in response to treatment with etanercept. J Invest Dermatol. 2005;124:1275–1283. doi: 10.1111/j.0022-202X.2005.23735.x. [DOI] [PubMed] [Google Scholar]
- 15.Kavanaugh A, Mease P. Treatment of psoriatic arthritis with tumor necrosis factor inhibitors: longer-term outcomes including enthesitis and dactylitis with golimumab treatment in the Longterm Extension of a Randomized, Placebo-controlled Study (GO-REVEAL) J Rheumatol Suppl. 2012;89:90–93. doi: 10.3899/jrheum.120254. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Jang H, Fleischmann RM, Bouman-Thio E, Xu Z, Marini JC, et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol. 2007;47:383–396. doi: 10.1177/0091270006298188. [DOI] [PubMed] [Google Scholar]