Abstract
OBJECTIVE:
To quantify microbial contamination of human milk purchased via the Internet as an indicator of disease risk to recipient infants.
METHODS:
Cross-sectional sample of human milk purchased via a popular US milk-sharing Web site (2012). Individuals advertising milk were contacted to arrange purchase, and milk was shipped to a rented mailbox in Ohio. The Internet milk samples (n = 101) were compared with unpasteurized samples of milk donated to a milk bank (n = 20).
RESULTS:
Most (74%) Internet milk samples were colonized with Gram-negative bacteria or had >104 colony-forming units/mL total aerobic count. They exhibited higher mean total aerobic, total Gram-negative, coliform, and Staphylococcus sp counts than milk bank samples. Growth of most species was positively associated with days in transit (total aerobic count [log10 colony-forming units/mL] β = 0.71 [95% confidence interval: 0.38–1.05]), and negatively associated with number of months since the milk was expressed (β = −0.36 [95% confidence interval: −0.55 to −0.16]), per simple linear regression. No samples were HIV type 1 RNA-positive; 21% of Internet samples were cytomegalovirus DNA-positive.
CONCLUSIONS:
Human milk purchased via the Internet exhibited high overall bacterial growth and frequent contamination with pathogenic bacteria, reflecting poor collection, storage, or shipping practices. Infants consuming this milk are at risk for negative outcomes, particularly if born preterm or are medically compromised. Increased use of lactation support services may begin to address the milk supply gap for women who want to feed their child human milk but cannot meet his or her needs.
Keywords: human milk, aerobic bacteria, Internet, infant, breastfeeding
What’s Known on This Subject:
Sharing human milk between those with an abundant supply and those seeking milk for their child may be growing in popularity, facilitated by Web sites recently established to link providers and recipients.
What This Study Adds:
This study documents the potential for human milk shared via the Internet to cause infectious disease by estimating the extent of microbial contamination among samples purchased via a leading Internet Web site.
Human milk is the optimal nutrition for infants worldwide.1 Approximately 77% of US infants are fed their mother’s milk at least once.2 Many infants are bottle-fed their mothers’ expressed milk in addition to feeding directly at the breast. In fact, 85% of breastfeeding mothers in the US Infant Feeding Practices Study II expressed milk within the first 5 months postpartum.3 Sharing human milk between those with an abundant supply and those seeking milk for their child may be growing in popularity, facilitated by Web sites recently established to link providers and recipients.4–7 The US Food and Drug Administration (FDA) recommends against feeding milk obtained in this way, and the American Academy of Pediatrics discourages feeding preterm infants fresh milk from unscreened donors.8,9 Concerns about the informal sharing of unpasteurized milk include the potential for infectious disease and exposure to chemicals, pharmaceuticals, and drugs.8
Breastfeeding has been shown to confer some protection to infants against infectious disease, and commensal bacteria and other bioactive compounds including antibodies in human milk appear to be important for healthy gut colonization and immune system modulation.10–16 However, exposure to pathogenic bacteria like group B streptococci (GBS), Staphylococcus aureus, Salmonella sp, Klebsiella pneumoniae, and certain strains of Escherichia coli remains a concern for neonatal sepsis, meningitis, necrotizing enterocolitis, and diarrheal disease, particularly for preterm and immunocompromised infants. Cases linked to contaminated breast milk have been documented.11,17–23 Human milk collected and distributed by Human Milk Banking Association of North America (HMBANA) milk banks undergoes pasteurization, which is largely effective in limiting the risk of bacterial and viral illness to recipients.24 However, human milk that is informally shared among individuals may come from an unfamiliar source, and the milk may be collected, stored, and transported under varying conditions, presenting opportunities for contamination with pathogenic bacteria and bacterial overgrowth, which are unchecked without pasteurization. Additionally, milk from unfamiliar sources carries the potential for transmission of viral diseases including HIV type 1 and cytomegalovirus (CMV).
Our objective was to document the potential for human milk shared via the Internet to cause infectious disease by estimating the extent of microbial contamination among samples purchased via a leading Internet Web site, compared with unpasteurized milk donated to a milk bank. We hypothesized that milk bank samples would be similar to Internet samples in that both were expressed outside of clinical settings and handled and stored in a home environment until provided. However, we expected them to differ in that milk bank donors are instructed on hygienic milk collection and storage techniques and optimal shipping procedures and are screened for some viral diseases like HIV.24
Methods
Purchase of Human Milk Via the Internet
Two of the 4 major Internet sites that exist in the United States to facilitate human milk sharing use a classified advertising format. The primary role of the sites is to connect donors and recipients. They are not involved in communications beyond the initial contact, in the transaction, or shipping. Although milk sharing Web sites post guidance on how to minimize health and safety risk, the onus is on individuals to protect themselves and their children. During 3 months in 2012, individuals who posted a public advertisement on these 2 sites searching for recipients for their milk were sent a standard e-mail inquiry expressing interest in buying a small amount of milk. Communications were confined to carrying out the transaction and were terminated if sellers inquired about a recipient infant or insisted on telephone or in-person communication. Sellers were offered the advertised price and encouraged to choose whichever commercial shipper and service, ice, and packing materials they believed to be appropriate. Payment was sent via PayPal payment system.25 To approximate real-life transactions, the e-mail address, PayPal account information, and delivery address were anonymous and tied to a rented mailbox, not the investigators’ or institutional information.
Upon delivery, shipments were transported to the laboratory and processed. Shipment time-in-transit, packaging, and shipping method were recorded. On average, 3.23 containers arrived per shipment (SD = 1.10); 1 was designated for bacterial analysis, and 1 for viral analysis. Container surface temperature and physical condition were documented immediately upon opening the box. Incidentally, 93% of sellers wrote a date on the sample container, which we considered the date of milk expression and recorded. These “Internet samples” were stored at −20°C until analyzed within 2 months.
In addition to data on samples themselves, an abstraction form was used to record information conveyed in each advertisement, including whether the seller mentioned adopting any of the following health behaviors that may affect milk safety: the use of a hygienic milk handling or storage practice (ie, pump sterilized or cleaned, milk frozen immediately, containers cleaned, or other), screening for viral disease(s) transmissible via milk (ie, stated she has been screened, offered to provide test results or medical records, stated she has been milk bank donor certified, or offered to provide a health care provider recommendation letter), limiting or abstaining from legal or illegal drugs or pharmaceuticals (ie, consumed limited or no alcohol; nonsmoking; stated she is “drug free” or took no illicit drugs; took no medications). Information about a healthy diet or exercise habit(s) (ie, took vitamins or supplements; stated having a healthy, well-balanced, vegetarian or organic diet; limited or excluded particular allergic or sensitive foods; or regularly exercised) was also recorded because it was often mentioned even if not directly related to milk safety. Any statements the seller made to advertise the quality of the milk (eg, “great quality,” “organic”) were also documented.
Identifiers used to track payments and shipments were purged from study records before laboratory results became available. This study was exempt from review by the Institutional Review Board at Nationwide Children’s Hospital.
Acquisition of Human Milk Donated to a Milk Bank
Twenty donated, anonymized, unpasteurized samples (“milk bank samples”) were obtained from an HMBANA-member milk bank for comparison. Samples were sent to the milk bank according to their guidelines for frozen, well-insulated, and overnight shipment. The samples available for this research would have been discarded because they came from donors temporarily disqualified per HMBANA guidelines (eg, contraindicated medication use) or exceeded guidelines for how long ago the milk had been expressed (>6 months). The reason for each sample was unavailable. These samples were transported via car to the laboratory, maintained at –20°C, and underwent no pasteurization. No additional information about the donors or these samples was available. Donors provided written informed consent for the research use of their milk.
Bacterial Analysis
All samples were analyzed in a single batch by milk bacteriology technicians blind to the source of the samples. Bacterial populations were quantified by serial dilutions of milk surface plated on Plate Count agar (total aerobic count), MacConkey agar (total Gram-negative and coliform counts), Modified Edwards Medium (streptococcal counts), Staph 110 medium (staphylococcal counts), and Salmonella agar (Salmonella counts). Positive cultures were expressed as colony-forming units (CFUs)/mL. Samples were considered to have no growth if the total count was ≤24 CFU/mL, the lower limit of detection. Confirmatory identification was per a standard reference handbook.26
Viral Analysis
Samples were analyzed for HIV type 1 and CMV by a technician blind to the source of the samples. To prevent cross-contamination, DNA/RNA extractions were performed in a laminar-flow hood dedicated to DNA/RNA extraction from clinical isolates, and polymerase chain reaction (PCR) reactions were prepared in a dead-air box.27 RNA was extracted from 140 µL unfractionated milk with the QIAamp viral mini kit (Qiagen). Reverse-transcription PCR was performed with the Invitrogen High-Fidelity Reverse-Transcription PCR kit and HIVgag-specific primers: SK38 (HXB22 coordinates: 1544–1571) and SK39 (HXB2 coordinates: 1658–1631).28 NL4-3 (AIDS Reagent Program) was used as a positive control.29 DNA was extracted from unfractionated milk by using the Plasma-Serum Circulating DNA Purification Mini kit (Norgen Biotek, Ontario, Canada). PCR was performed by using the 2× PCR master mix (Norgen Biotek) and CMV-specific primers (CMV PCR primer set and controls, Norgen Biotek). A CMV-specific positive control (Norgen Biotek) was used in an assay with a lower limit of detection of 700 CMV-particles/µL. PCR amplicons were separated on an agarose gel, stained with ethidium bromide, and visualized under UV light.
Statistical Analysis
The prevalence of selected bacteria species was calculated (defined as >24 CFU/mL), and univariate statistics for each were estimated. Geometric mean bacteria counts and prevalence for each bacteria type among the Internet samples were compared with the milk bank samples (Satterthwaite t test, Fisher’s exact test). Univariate statistics for shipment and sample characteristics were estimated. The log of bacterial counts were analyzed as continuous variables in relation to milk sample characteristics (time-in-transit, shipment contents, temperature, and the time since the milk was expressed) and topics communicated in advertisements using simple linear regression models. Binary variables indicating viral status were analyzed in relation to the same variables by using logistic regression. All analyses used SAS software (version 9.3, SAS Institute, Inc, Cary, NC).
Results
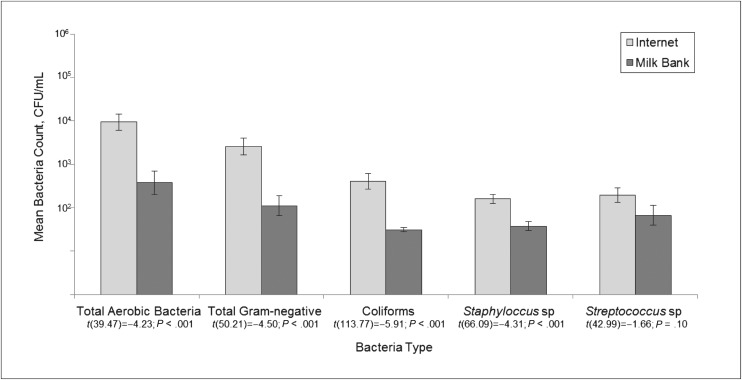
Of 495 inquiries sent, 191 individuals never replied, whereas 41 stopped corresponding after at least 1 reply. Communications were ceased with 57 who wanted to communicate verbally or inquired about an infant. Some (79) agreed to send milk but never followed through with the transaction, 17 transactions were unresolved, 8 sellers accepted payment but did not send milk (5 refunded payment), and 102 milk shipments were received (1 not analyzed due to late receipt). The prevalence of selected bacteria types in the Internet samples (n = 101) and milk bank samples (n = 20) is displayed in Table 1. Gram-negative bacteria and Staphylococcus sp were detected in a majority of Internet samples, and coliforms and Streptococcus sp were fairly common as well. Three Internet samples were contaminated with Salmonella sp; due to low prevalence, Salmonella sp was not included in subsequent analyses. Compared with the unpasteurized milk bank samples, the Internet samples more frequently presented with Gram-negative and Staphylococcus sp growth (Table 1) and exhibited higher mean total aerobic, total Gram-negative, coliform, and Staphylococcus sp counts (all P < .01, Fig 1). No samples from either source were HIV-RNA positive. Twenty-one percent of the Internet samples were CMV DNA positive, whereas 5% of the milk bank samples were CMV DNA positive (Fisher’s exact test, P = .12).
TABLE 1.
Proportions of Human Milk Samples With Each Bacteria Type Isolated
| Bacteria Types | Prevalence, n (%)a | ||
|---|---|---|---|
| Internet Purchased Samples, n = 101 | Milk Bank Samples, n = 20 | P | |
| Gram-negative bacteria | 73 (72) | 7 (35) | .003 |
| Coliforms (lactose-fermenting Gram-negative bacteria) | 44 (44) | 5 (25) | .14 |
| Salmonella sp | 3 (3) | 0 (0) | .58b |
| Staphylococcus sp | 64 (63) | 5 (25) | .002 |
| Streptococcus sp | 36 (36) | 4 (20) | .20 |
| No detectable growth | 9 (9) | 5 (25) | .07 |
>24 CFU/mL.
Calculated with Fisher’s exact test.
FIGURE 1.
Geometric mean bacteria counts (SEs) for human milk samples purchased via the Internet (n = 101) and acquired from a milk bank (n = 20). Results of the Satterthwaite t test for unequal variances reported. Error bars represent SEM.
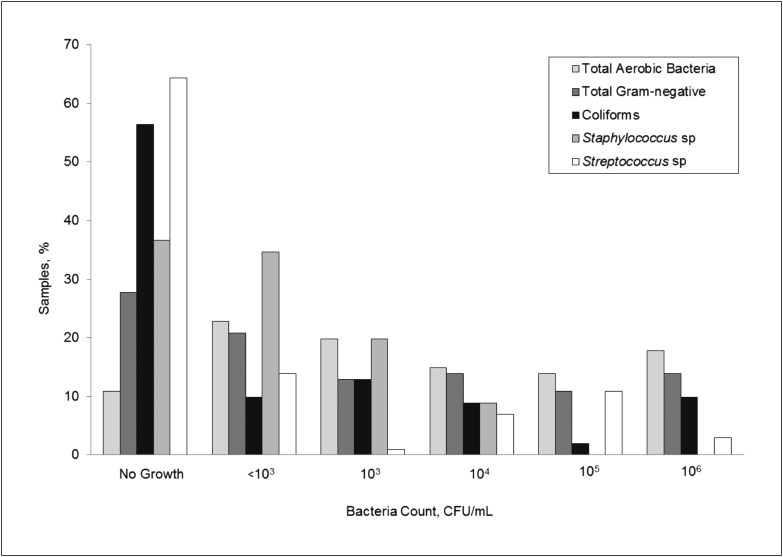
Counts for most bacteria types exhibited a wide distribution among the Internet samples (Fig 2), up to 107 CFU/mL for total aerobic, total Gram-negative, coliforms, and Streptococcus sp; 104 CFU/mL for Staphylococcus sp; and 103 CFU/mL for Salmonella sp. More than 17% of samples had counts >106 CFU/mL for at least 1 bacteria type or in total.
FIGURE 2.
Distribution of bacteria counts by bacteria type for human milk samples purchased via the Internet (n = 101). Salmonella sp was detected in 3 samples (counts not shown).
Approximately one-half of the samples arrived within 2 days; 12% required 3 to 6 days. Dry ice was included in 62% of shipments; 20% used freezer ice or gel packs to attempt to maintain low temperature (Table 2), and 19% included no cooling agent. Milk temperature ranged −49 to +27°C (mean = 2°C, SD = 18). The time elapsed between milk expression and bacterial analysis varied widely (median = 46, interquartile range = 21–103, maximum = 272); only 3 samples exceeded 6 months.
TABLE 2.
Bacterial Counts (log10 CFU/mL) in Relation to Milk Shipment and Sample Characteristics and Advertised Health Behaviors and Quality Indicators, Milk Purchased Via the Internet (n = 101), Results From Simple Linear Regression Models
| No. (%) | Total Aerobic Bacteria | Total Gram-negative | Coliforms | Staphylococcus sp | Streptococcus sp | |
|---|---|---|---|---|---|---|
| Time-in-transit, d | 0.71 (0.38 to 1.05) | 0.76 (0.41 to 1.11) | 0.83 (0.51 to 1.14) | −0.09 (−0.29 to 0.12) | 0.33 (0.02 to 0.66) | |
| Shipping container contentsa | ||||||
| Dry ice | 63 (62) | −0.53 (−1.50 to 0.43) | −0.75 (−1.74 to 0.23) | −0.79 (−1.71 to 0.12) | 0.01 (−0.55 to 0.57) | 0.11 (−0.79 to 1.01) |
| Freezer ice or gel packs | 20 (20) | −0.67 (−1.84 to 0.51) | −0.84 (−2.08 to 0.41) | −0.58 (−1.81 to 0.65) | −0.28 (−0.88 to 0.33) | −0.41 (−1.33 to 0.50) |
| Styrofoam | 80 (79) | 0.09 (−0.81 to 0.99) | 0.05 (−0.89 to 0.98) | −0.24 (−1.11 to 0.63) | 0.05 (−0.45 to 0.55) | 0.19 (−0.61 to 0.99) |
| Milk container damaged or leaking | 12 (12) | −0.31 (−1.44 to 0.81) | −0.79 (−1.96 to 0.37) | −0.57 (−1.66 to 0.52) | 0.06 (−0.57 to 0.68) | −0.13 (−1.13 to 0.87) |
| Temperature > 0°C | 65 (64) | 0.06 (−0.71 to 0.82) | 0.19 (−0.60 to 0.98) | 0.26 (−0.48 to 0.99) | −0.51 (−0.92 to −0.10) | −0.06 (−0.74 to 0.62) |
| Time from milk expression to analysis, mob | −0.36 (−0.55 to −0.16) | −0.29 (−0.49 to −0.08) | −0.19 (−0.39 to −0.00) | −0.22 (−0.33 to −0.10) | −0.20 (−0.38 to −0.02) | |
| Hygienic milk handling or storagec | 26 (26) | 0.29 (−0.54 to 1.12) | 0.21 (−0.65 to 1.08) | 0.21 (−0.60 to 1.02) | −0.45 (−0.91 to 0.00) | 0.31 (−0.43 to 1.05) |
| Screened for infectious diseaseb,d | 22 (22) | 0.09 (−0.80 to 0.97) | 0.03 (−0.89 to 0.95) | 0.01 (−0.85 to 0.87) | −0.36 (−0.85 to 0.12) | −0.19 (−0.97 to 0.60) |
| Healthy diet or exercise habit(s)b,e | 62 (62) | −0.12 (−0.87 to 0.64) | −0.19 (−0.97 to 0.59) | −0.19 (−0.92 to 0.54) | 0.09 (−0.33 to 0.51) | 0.01 (−0.66 to 0.68) |
| Limit or abstain from legal or illegal drugs or pharmaceuticalsf | 72 (71) | −0.15 (−0.96 to 0.65) | −0.41 (−1.25 to 0.42) | −0.15 (−0.93 to 0.63) | −0.07 (−0.52 to 0.38) | 0.15 (−0.57 to 0.86) |
| Makes claims about quality of milkg | 27 (27) | −0.11 (−0.93 to 0.72) | −0.60 (−1.45 to 0.25) | −0.57 (−1.36 to 0.22) | 0.22 (−0.24 to 0.67) | 0.11 (−0.63 to 0.84) |
Unless noted otherwise, data are presented as β (95% CI).
One shipment contained both dry ice and freezer ice or gel packs.
Seven samples did not indicate the date of milk expression.
Pump sterilized or cleaned, milk frozen immediately, or containers cleaned or other hygiene or milk handling practice mentioned.
Stated has been screened for infectious disease(s), offered to provide screening test results or medical records, milk bank donor certified, or offered to provide letter of recommendation from health care provider.
Took vitamins or supplements; stated healthy, well-balanced, vegetarian or organic diet; limited or excluded particular allergic or sensitive foods; or regularly exercised.
Consumed limited or no alcohol; nonsmoking; stated she is drug free or took no illicit drugs, or took no prescription or over the counter medications.
Described milk as safest, great quality, organic, “tasty,” “sweet,” or similar.
Examination of shipment and sample characteristics in relation to bacteria counts revealed that each additional transit day was associated with an increase in total bacteria count, Gram-negatives, coliforms, and Streptococcus sp (Table 2). Shipment on any ice or with Styrofoam and container damage was unassociated with bacterial counts. Samples that were thawed upon receipt had lower Staphylococcus sp counts than frozen samples, but were no different in terms of other bacterial counts. The more time that had elapsed between milk expression and analysis, the lower the counts for all bacteria types. These characteristics were not predictive of CMV DNA-positivity.
The majority of sellers promoted their diet or exercise habits or limitations on drug or pharmaceutical use in their advertisements; however, far fewer mentioned adoption of hygiene practices or being screened for infectious disease. Approximately one-quarter of sellers used adjectives like “safest” or great quality to promote the quality of their milk. Whether sellers mentioned adoption of any of these health behaviors or made quality claims about their milk was not predictive of bacteria counts or presence of CMV DNA.
Discussion
This sampling of human milk purchased via the Internet in the United States revealed high levels of overall bacterial growth and frequent contamination with pathogenic bacteria. Most Internet samples (74%) would have failed HMBANA criteria for feeding without pasteurization (>104 CFU/mL total colony count or any detectable pathogens). By almost all measures, the Internet samples exhibited greater contamination than the milk bank samples. The milk bank samples represented an appropriate choice for a comparison group as they were unpasteurized. Nevertheless, this study observed large and clinically meaningful differences between the groups.
Internet sample characteristics that were predictive of bacteria counts were time-in-transit and the age of the milk. Even though many samples arrived at elevated temperature, some shipments included no ice, and some containers were damaged, these factors were unassociated with bacteria growth. Information sellers conveyed in their advertisements about their health and behaviors were poor indicators of milk quality. No variables under study were predictive of CMV DNA.
To our knowledge, no previous studies have evaluated the safety of human milk sold via the Internet. However, studies have documented wide variation in bacteria levels in mother’s own milk fed to hospitalized infants or in milk bank donations. A study of Chinese mothers with hospitalized infants may have had the greatest contamination previously reported, finding 86% of milk samples to have total counts >104 CFU/mL.30 However, the highest total count reported was 1.86 × 106 CFU/mL, and Gram-negative counts did not exceed 103 CFU/mL, significantly lower than here. A study of milk bank donations where women pooled milk over days revealed 61% of pools had Gram-negative rods; however, the degree of contamination was far lower than the current study.31 In general, previous studies have revealed low proportions with coliform contamination (Enterobacter <1%–7%, E coli 0%–2%, Klebsiella sp 0.4%–9%) compared with 44% of Internet samples here.19,32–35
Staphylococcus epidermidis and Viridans streptococci are common skin flora and not usually pathogenic. The prevalence of Staphylococcus sp in the Internet samples was similar to other studies, although Staphylococcus sp counts were inversely associated with temperature. Viridans streptococci have been previously detected in 6% to 30% of milk samples, similar to the 36% prevalence for Streptococcus sp in the current study.34–36 Salmonella sp was not systematically quantified in previous studies. Bacteria counts in the milk bank samples in the current study were within the range of those reported for the largest study of milk bank donations which found 2.5% of samples to have >104 CFU/mL total count and ∼8% to contain Staphylococcus aureus or coliforms before pasteurization.
In most previous studies, women were instructed on hygienic collection and storage and shipping methods to maintain temperature, when relevant. Major milk sharing Web sites display hygiene, storage and shipping, and home pasteurization information. It is unknown what proportion of sellers follow the guidance. The frequency of coliform bacteria contamination in Internet samples points to inadequate hygiene at the point of milk expression, in line with a recent study that revealed 31% of women never sterilize their pump collection kit.37 The large proportion of samples with high bacterial counts indicate storage or transport at improper temperature was also a factor. However, time since milk expression was inversely associated with bacterial counts. This gives the impression that older milk is healthier, but extended storage may reduce bacterial growth-inhibiting properties in milk that prevent overgrowth upon thawing.38
Milk bank donors are instructed on optimal milk collection, storage, and shipping, and their milk is pasteurized before distribution. These measures have been shown to be effective in eradicating bacterial contamination with the possible exception of Bacillus cereus.24,31,39 Even for raw milk expressed by mothers for their hospitalized infants, the benefits of feeding breast milk likely outweigh the risk of bacterial disease.34,40,41 However, for infants receiving raw milk from an unfamiliar source, this benefit-risk calculation may not hold. Milk bank donors are also screened for several viruses including HIV, but not CMV. Pasteurization is an additional safeguard against viral disease transmission.
Quantification of bacterial contamination and presence of CMV is not deterministic of disease risk as much depends on the immune status of the recipient.34,40,42 In fact, compared with formula, many bioactive compounds and immune-modulating factors in human milk protect against bacterial illness and conditions like necrotizing enterocolitis.12,13,43 However, there are numerous reported cases of preterm and immune-compromised infants developing late onset neonatal sepsis attributed to GBS, Gram-negative bacteria, or methicillin-resistant Staphylococcus aureus with some resulting in death, linked to raw human milk.17–19 Several cases of healthy, term infants sickened by Salmonella sp in human milk have also been reported.44,45 Similarly, CMV infection via breast milk may have long-term neurodevelopmental consequences.46,47 Our previous work found that 21% of those seeking milk on Web sites were doing so for a child with a poor birth outcome or other medical condition, which may put them at increased infection risk.48
This study confirms that advertisement information sellers communicate on topics relevant to milk quality were unhelpful signals to buyers. Complicating matters, sellers sometimes communicated virtually unverifiable information, like advertising safe or high-quality milk based on no objective measure, which might distract buyers from considering more relevant factors like screening for viral disease and drug use.
One limitation is approximately one-quarter of sellers offered to send milk but never sent it for unknown reasons. Second, some potentially useful information was not collected directly from sellers (eg, use of hygienic practices when collecting the milk analyzed, whether the milk was fresh never frozen, objective information about the seller’s health). However, the advertisements and milk included in the study may have been more representative of the milk sold via the Internet because steps were taken to maintain sellers’ and investigators’ anonymity. Nevertheless, the procedures to maintain anonymity may have biased the results if women who were excluded because they inquired about a recipient infant or desired telephone communication were also more careful about hygiene or were less likely to carry viral disease. Several limitations stem from the use of the milk bank comparison group; the number of samples was limited due to a shortage in donations, and this reduced statistical power. The age of the milk bank samples was unknown and may have been related to bacterial counts; however, the association between time since expression and bacterial counts was modest and so would not explain the observed differences between groups. Overall, we felt the milk bank samples were the best comparison group available; although milk bank donors are screened for some infectious diseases and instructed on milk handling, which distinguishes them from some sellers, milk donated to milk banks is similar to purchased milk in that it is self-collected and often undergoes shipment. Another limitation is that we could not make direct comparison for some bacteria species to other studies that distinguished species differently or in more detail. Consequently, the prevalence of pathogenic bacteria was likely underestimated because Staphylococcus aureus or GBS populations were not delineated from total staphylococcal counts. Finally, the Internet samples were exchanged for money, which introduces different incentives for sellers than for individuals who donate their milk. These results may not be generalizable to nonmonetary exchanges, which are predominant on some Web sites or to sharing among relatives or friends.
The FDA does not currently regulate the exchange of human milk, although this was discussed in 2010 by the Pediatric Advisory Committee, which raised concerns about milk sharing via the Internet.49 The results of this and future studies should inform FDA decision-making.
Seller education and standard viral disease screening combined with pasteurization would be largely effective controls against bacterial contamination and many viruses; however, it is clear that many sellers did not adopt proper techniques and appropriate pasteurization is outside the capability of typical households. Unfortunately, buyers cannot rely on information conveyed in seller’s advertisements to help them make better choices about who to buy from, and buyers cannot test the milk they purchase, resulting in contaminated milk and the potential for infectious disease.
Conclusions
Medical and public health organizations have been effective in promoting breastfeeding, resulting in an increase in breastfeeding initiation to 77% of live births.2 However, there are limited options for when the mother does not have enough milk. Lactation difficulties may be addressed with lactation support so that children’s needs may be met with mother’s own milk. Unfortunately, not all women access or are referred for support early. The potential risk of milk sharing to infant health needs to be further examined related to other risks posed (eg, toxins, pharmaceutical, and drug exposure), especially for infants born preterm or who are medically compromised.
Acknowledgments
We are grateful to the OhioHealth Mother's Milk Bank; Janet McCormick, AAS, and Pam Schoenberger, BS, of the Ohio Agricultural Research and Development Center Mastitis Laboratory for laboratory technical assistance; Anne Brown, BS, and Rachel Ronau for research assistance; Tondi Harrison, PhD, RN, CPNP; Kelly Kelleher, MD, MPH; Mark Klebanoff, MD, MPH; Nehal Parikh, DO, MS; Alex Rakowsky, MD; and Jonathan Slaughter, MD, MPH for critical review of the article; and Kamma Smith for administrative support from The Research Institute at Nationwide Children’s Hospital.
Glossary
- CFU
colony-forming unit
- CMV
cytomegalovirus
- FDA
US Food and Drug Administration
- GBS
group B streptococci
- HMBANA
Human Milk Banking Association of North America
- PCR
polymerase chain reaction
Footnotes
Dr Keim conceptualized and designed the study, participated in data analysis, and drafted the initial manuscript; Dr Hogan participated in study design, supervised laboratory analysis, participated in data analysis and interpretation, and critically reviewed the manuscript; Ms McNamara participated in study design, data analysis, and interpretation, supervised data collection, and critically reviewed the manuscript; Mr Gudimetla participated in design of viral contamination assays and manuscript review; Dr Kwiek participated in study design, design and supervision of viral contamination assays, and critically reviewed the manuscript; Ms Dillon participated in study design, carried out data collection, and critically reviewed the manuscript; Dr Geraghty conceptualized and designed the study, participated in results interpretation, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by a grant from The Ohio State University Food Innovation Center and internal support from The Research Institute at Nationwide Children’s Hospital.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.World Health Organization Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization; 2003 [Google Scholar]
- 2.Centers for Disease Control and Prevention. Breastfeeding among US children born 2000--2010, CDC National Immunization Survey. Available at: www.cdc.gov/breastfeeding/data/nis_data/index.htm. Accessed September 9, 2013
- 3.Labiner-Wolfe J, Fein SB, Shealy KR, Wang C. Prevalence of breast milk expression and associated factors. Pediatrics. 2008;122(suppl 2):S63–S68 [DOI] [PubMed] [Google Scholar]
- 4.Human Milk 4 Human Babies. Available at: www.hm4hb.net/. Accessed December 7, 2012
- 5.Walker S. Eats on feets. Available at: www.eatsonfeets.org/. Published 2010. Accessed December 7, 2012
- 6.Only The Breast. Available at: www.onlythebreast.com/. Published 2011. Accessed December 7, 2012
- 7.Faulkner K. Milk share. Available at: http://milkshare.birthingforlife.com/. Published 2002–2005. Accessed December 7, 2012
- 8.US Food and Drug Administration. Use of donor human milk. Available at: www.fda.gov/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/ucm235203.htm. Accessed March 13, 2013
- 9.Gartner LM, Morton J, Lawrence RA, et al. American Academy of Pediatrics Section on Breastfeeding . Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506 [DOI] [PubMed] [Google Scholar]
- 10.Martín R, Langa S, Reviriego C, et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003;143(6):754–758 [DOI] [PubMed] [Google Scholar]
- 11.Wilson C, Nizet V, Maldonado Y, Remington J, Klein J. Infectious Diseases of the Fetus and Newborn. 7th ed. Philadelphia, PA: Saunders; 2010 [Google Scholar]
- 12.Wright AL, Bauer M, Naylor A, Sutcliffe E, Clark L. Increasing breastfeeding rates to reduce infant illness at the community level. Pediatrics. 1998;101(5):837–844 [DOI] [PubMed] [Google Scholar]
- 13.López-Alarcón M, Villalpando S, Fajardo A. Breast-feeding lowers the frequency and duration of acute respiratory infection and diarrhea in infants under six months of age. J Nutr. 1997;127(3):436–443 [DOI] [PubMed] [Google Scholar]
- 14.Chiba Y, Minagawa T, Mito K, et al. Effect of breast feeding on responses of systemic interferon and virus-specific lymphocyte transformation in infants with respiratory syncytial virus infection. J Med Virol. 1987;21(1):7–14 [DOI] [PubMed] [Google Scholar]
- 15.Palma GD, Capilla A, Nova E, et al. Influence of milk-feeding type and genetic risk of developing coeliac disease on intestinal microbiota of infants: the PROFICEL study. PLoS ONE. 2012;7(2):e30791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kainonen E, Rautava S, Isolauri E. Immunological programming by breast milk creates an anti-inflammatory cytokine milieu in breast-fed infants compared to formula-fed infants. Br J Nutr. 2013;109(11):1962–1970 [DOI] [PubMed] [Google Scholar]
- 17.Godambe S, Shah PS, Shah V. Breast milk as a source of late onset neonatal sepsis. Pediatr Infect Dis J. 2005;24(4):381–382 [DOI] [PubMed] [Google Scholar]
- 18.Behari P, Englund J, Alcasid G, Garcia-Houchins S, Weber SG. Transmission of methicillin-resistant Staphylococcus aureus to preterm infants through breast milk. Infect Control Hosp Epidemiol. 2004;25(9):778–780 [DOI] [PubMed] [Google Scholar]
- 19.Botsford KB, Weinstein RA, Boyer KM, Nathan C, Carman M, Paton JB. Gram-negative bacilli in human milk feedings: quantitation and clinical consequences for premature infants. J Pediatr. 1986;109(4):707–710 [DOI] [PubMed] [Google Scholar]
- 20.Qutaishat SS, Stemper ME, Spencer SK, et al. Transmission of Salmonella enterica serotype typhimurium DT104 to infants through mother’s breast milk. Pediatrics. 2003;111(6 pt 1):1442–1446 [DOI] [PubMed] [Google Scholar]
- 21.Ryder RW, Crosby-Ritchie A, McDonough B, Hall WJ, III. Human milk contaminated with Salmonella kottbus. A cause of nosocomial illness in infants. JAMA. 1977;238(14):1533–1534 [PubMed] [Google Scholar]
- 22.Stiver HG, Albritton WL, Clark J, Friesen P, White FM. Nosocomial colonization and infection due to E. coli 0125:K70 epidemiologically linked to expressed breast-milk feedings. Can J Public Health. 1977;68(6):479–482 [PubMed] [Google Scholar]
- 23.Kayıran PG, Can F, Kayıran SM, Ergonul O, Gürakan B. Transmission of methicillin-sensitive Staphylococcus aureus to a preterm infant through breast milk [published online ahead of print July 24, 2013]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2013.819332 [DOI] [PubMed] [Google Scholar]
- 24.Human Milk Banking Association of North America Guidelines for the Establishment and Operation of a Donor Human Milk Bank. 16th ed. Fort Worth, TX: Human Milk Banking Association of North America; 2011 [Google Scholar]
- 25.PayPal. Available at: https://www.paypal-media.com/about. Accessed March 13, 2013
- 26.Hogan JS, Gonzales RN, Harmon RJ, et al. Laboratory Handbook on Bovine Mastitis. Madison, WI: National Mastitis Council; 1999 [Google Scholar]
- 27.Kumar SB, Handelman SK, Voronkin I, et al. Different regions of HIV-1 subtype C env are associated with placental localization and in utero mother-to-child transmission. J Virol. 2011;85(14):7142–7152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ou CY, Kwok S, Mitchell SW, et al. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988;239(4837):295–297 [DOI] [PubMed] [Google Scholar]
- 29.Adachi A, Gendelman HE, Koenig S, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59(2):284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng DK, Lee SY, Leung LC, Wong SF, Ho JC. Bacteriological screening of expressed breast milk revealed a high rate of bacterial contamination in Chinese women. J Hosp Infect. 2004;58(2):146–150 [DOI] [PubMed] [Google Scholar]
- 31.Landers S, Updegrove K. Bacteriological screening of donor human milk before and after Holder pasteurization. Breastfeed Med. 2010;5(3):117–121 [DOI] [PubMed] [Google Scholar]
- 32.el-Mohandes AE, Schatz V, Keiser JF, Jackson BJ. Bacterial contaminants of collected and frozen human milk used in an intensive care nursery. Am J Infect Control. 1993;21(5):226–230 [DOI] [PubMed] [Google Scholar]
- 33.Carroll L, Osman M, Davies DP, McNeish AS. Bacteriological criteria for feeding raw breast-milk to babies on neonatal units. Lancet. 1979;2(8145):732–733 [DOI] [PubMed] [Google Scholar]
- 34.Eidelman AI, Szilagyi G. Patterns of bacterial colonization of human milk. Obstet Gynecol. 1979;53(5):550–552 [PubMed] [Google Scholar]
- 35.Law BJ, Urias BA, Lertzman J, Robson D, Romance L. Is ingestion of milk-associated bacteria by premature infants fed raw human milk controlled by routine bacteriologic screening? J Clin Microbiol. 1989;27(7):1560–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heikkilä MP, Saris PE. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J Appl Microbiol. 2003;95(3):471–478 [DOI] [PubMed] [Google Scholar]
- 37.Labiner-Wolfe J, Fein SB. How US mothers store and handle their expressed breast milk. J Hum Lact. 2013;29(1):54–58 [DOI] [PubMed] [Google Scholar]
- 38.Hernandez J, Lemons P, Lemons J, Todd J. Effect of storage processes on the bacterial growth-inhibiting activity of human breast milk. Pediatrics. 1979;63(4):597–601 [PubMed] [Google Scholar]
- 39.de Segura AG, Escuder D, Montilla A, et al. Heating-induced bacteriological and biochemical modifications in human donor milk after holder pasteurisation. J Pediatr Gastroenterol Nutr. 2012;54(2):197–203 [DOI] [PubMed] [Google Scholar]
- 40.Schanler RJ, Fraley JK, Lau C, Hurst NM, Horvath L, Rossmann SN. Breastmilk cultures and infection in extremely premature infants. J Perinatol. 2011;31(5):335–338 [DOI] [PubMed] [Google Scholar]
- 41.Hylander MA, Strobino DM, Dhanireddy R. Human milk feedings and infection among very low birth weight infants. Pediatrics. 1998;102(3). Available at: www.pediatrics.org/cgi/content/full/102/3/e38 [DOI] [PubMed] [Google Scholar]
- 42.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357(9255):513–518 [DOI] [PubMed] [Google Scholar]
- 43.Quigley MA, Henderson G, Anthony MY, McGuire W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2007; (4):CD002971. [DOI] [PubMed] [Google Scholar]
- 44.Chen TL, Thien PF, Liaw SC, Fung CP, Siu LK. First report of Salmonella enterica serotype panama meningitis associated with consumption of contaminated breast milk by a neonate. J Clin Microbiol. 2005;43(10):5400–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooke FJ, Ginwalla S, Hampton MD, et al. Report of neonatal meningitis due to Salmonella enterica serotype Agona and review of breast milk-associated neonatal Salmonella infections. J Clin Microbiol. 2009;47(9):3045–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bevot A, Hamprecht K, Krägeloh-Mann I, Brosch S, Goelz R, Vollmer B. Long-term outcome in preterm children with human cytomegalovirus infection transmitted via breast milk. Acta Paediatr. 2012;101(4):e167–e172 [DOI] [PubMed] [Google Scholar]
- 47.Goelz R, Meisner C, Bevot A, Hamprecht K, Kraegeloh-Mann I, Poets CF. Long-term cognitive and neurological outcome of preterm infants with postnatally acquired CMV infection through breast milk. Arch Dis Child Fetal Neonatal Ed. 2013;98(5):F430–F433 [DOI] [PubMed] [Google Scholar]
- 48.Keim SA, McNamara KA, Jayadeva CM, Braun AC, Dillon CE, Geraghty SR. Breast milk sharing via the Internet: the practice and health and safety considerations. Breastfeeding Med. In press [DOI] [PubMed] [Google Scholar]
- 49.US Food and Drug Administration. Pediatric Advisory Committee Meeting, December 6, 2010. Available at: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM251799.pdf. Accessed September 2, 2013