Since the 1960s, different clinical, laboratory and radiological recording systems have been used to measure the prevalence, extent and severity of periodontal diseases at individual and population levels. Some of these recording systems are known as ‘indices’ (i.e. numeric scales with upper and lower limits), requiring validity, reliability, clarity, simplicity, objectivity, quantifiability, sensitivity and acceptability by both the examiner and the subject (7, 14). This paper reviews different recording systems used to measure periodontal diseases at the population level and their integration into surveillance systems (Table 1). We do not review indices designed to measure plaque or residual accumulation around the tooth, indices focussed exclusively on gingival inflammation, or radiographic approaches with limited applicability in surveillance systems.
Table 1.
Indices of periodontal diseases: advantages and disadvantages
| Index | Developed by | Type | Time | Premise | Use | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Recession Index | Stahl & Morris (80) | Periodontal | Mid-1950s | Number of teeth with exposed cementum | Epidemiological | Directly related to scores for supragingival calculus and debris Correlation with Russell’s periodontal index and the Oral Hygiene Index |
Inflammation is not considered in the score for recession |
| Periodontal Index | Russell (71) | Periodontal composite | Late 1950s | Continuity between gingival and periodontal involvement | Epidemiological | Empirically derived First index to assess periodontal disease at the epidemiological level |
Assumes continuity between gingival and periodontal inflammation Underestimates disease levels |
| Periodontal Disease Index | Ramfjord (66) | Periodontal | Late 1950s | Full mouth or selected teeth | Epidemiological, clinical trials | The first use of a calibrated periodontal probe Explicit need to train examiners Flexible in number of teeth and sites to be examined |
Partial assessment of teeth and sites could lead to underestimation of disease Coding based on ‘zones’ in the probe is less sensitive than measuring at a precision of 1 mm in loss of periodontal attachment |
| Periodontal screening examination | O’Leary (58) | Composite | Early 1960s | Little agreement among general practitioners without training, and impractical to examine all teeth | Screening individuals who need periodontal treatment | Introduces the concept of ‘sextants‘ | Not considered a ‘diagnostic tool‘ Not widely used |
| Periodontal Status Index | World Health Organization (87) | Composite | 1970s | Measurements of oral hygiene and periodontal status by sextants | Epidemiological (in first two editions of Oral Health Surveys Basic Methods) | Combines Russell‘s periodontal index with Greene and Vermillion‘s Oral Hygiene Index (34) To assess prevalence and provide a simple estimate of treatment needs in communities |
Complex Replaced by Community Periodontal Index of Treatment Needs |
| Community Periodontal Index of Treatment Needs | World Health Organization (89) | Composite | Late 1970s–1980s | Uses World Health Organization probe sextants and index teeth | Epidemiological surveys | Simplicity, speed and international uniformity Provides an overview of the magnitude of periodontal care services Widely used |
Assesses only pocket depth Questionable extension into treatment needs Underestimation of disease levels |
| Periodontal Screening and Recording Index | American Dental Association / American Academy of Periodontology (47) | Composite | Early 1990s | Same as Community Periodontal Index of Treatment Needs | Clinical screening | An adaptation of the Community Periodontal Index of Treatment Needs with six sites measured around each index tooth | Same as Community Periodontal Index of Treatment Needs |
| Community Periodontal Index | World Health Organization (90) | Composite | Late 1990s | Elimination of the treatment needs component of the Community Periodontal Index of Treatment Needs Included in the 4th Edition of Oral Health Surveys Basic Methods |
Epidemiological | Same as the Community Periodontal Index of Treatment Needs A measure of loss of periodontal attachment was included in the Oral Health Surveys Basic Methods to compensate for lack of loss of attachment Modified in 5th edition to include all teeth in each sextant |
Same as the Community Periodontal Index of Treatment Needs |
| Loss of periodontal attachment | Evolved from the Periodontal Disease Index | Periodontal | Measuring all or a set of teeth or sites around each tooth | Clinical and epidemiological | Validity depends on number of teeth and sites Current de-facto standard |
Measures disease sequelae Difficult to train and implement in epidemiological studies |
Early measurement tools
The first attempts to measure periodontal diseases at the population level were made in the late 1950s, using Russell’s Periodontal Index, an innovative recording system that scored the presence and severity of both gingival bleeding and pocket depth (68–71), also known as ‘composite’ indices (15). Russell, from the National Institute of Dental and Craniofacial Research (formerly known as National Institute of Dental Research), participated in a series of nutritional studies sponsored by the Interdepartmental Committee on Nutrition for National Defense (8) and collected information on dental caries and periodontal diseases from multiple countries (69, 70). Russell designed and tested the Periodontal Index as an epidemiological tool in which all teeth were examined and scored using five well-distinguished categories (0, 1, 2, 6 and 8) representing incremental degrees of disease severity. In the USA, Russell’s Periodontal Index was used in national surveys that reported mean periodontal index scores as population estimates, the effect of age on periodontal diseases, comparisons between racial /ethnic groups and epidemiological trends (40, 43–46, 72).
Russell’s Periodontal Index was simple, had clear detection criteria for each score level, was developed and tested empirically and was able to detect differences between population groups. Furthermore, Russell recognized the need to train examiners to minimize between-examiner differences in scoring. By the early 1980s, owing to a better understanding of periodontal diseases, Russell’s Periodontal Index had become seldom used because of concerns about its validity (interval scale between scores) and underlying assumptions (continuity between gingivitis and periodontal diseases) (14).
Simultaneously, Sigurd Ramfjord introduced the Periodontal Disease Index, a tooth- and site-specific measurement tool. Contrary to Russell’s Periodontal Index, the Periodontal Disease Index was essentially a clinical tool that used a periodontal probe (66), with marks at 3, 6 and 8 mm, to measure the distance from the cemento–enamel junction to the bottom of the pocket (i.e. attachment loss) (64). Ramfjord preferred these cut-off point marks because of improved consistency in the measurements (66). In addition, Ramfjord recommended the assessment of six ‘index teeth’ that soon became known as the ‘Ramfjord teeth’ These teeth (with the notation of the Fédération Dentaire Internationale) were: maxillary right first molar (tooth 16), maxillary left central incisor (tooth 11), maxillary left first bicuspid (tooth 24), mandibular left first molar (tooth 36), mandibular right central incisor (tooth 41) and mandibular right first bicuspid (tooth 44). Originally, the Periodontal Disease Index required measurements to be made on the mesial, buccal, distal and lingual sides, but later it was modified to include just two sites, mesial and mid-buccal (66). Validation research on the ‘Ramfjord teeth’ as representing the entire mouth showed mixed results (32). The Periodontal Disease Index was used by Ramfjord in one epidemiological study among youths and young adults aged 11 to 30 from India (65). He presented these findings side-by-side with results from another five surveys, conducted using Russell’s Periodontal Index between 1957 and 1963 in Sri Lanka, Iran, Nigeria and Sudan (67). Later, both indices were described as reporting lower disease levels when compared with radiographic methods (76).
In the 1960s, O’Leary et al. developed the Periodontal Screening Examination, an index to screen military recruits (58, 59). The Periodontal Screening Examination is important in our discussion of periodontal disease measurement tools because it introduced the idea of examination by ‘sextants’, that is, teeth grouped into six anatomical regions, which are, in the notation of the Fédération Dentaire Internationale: teeth 17 to 14, 13 to 23, 24 to 27, 37 to 34, 33 to 43 and 44 to 47.
Most of these earlier indices were widely discussed at a conference on clinical methods in periodontal diseases, held during May 1967 at the University of Pennsylvania (18), and are included in Table 1, together with other indices. We have included the original references to guide the interested reader. In addition, a detailed description of Russell’s Periodontal Index, Ramfjords’s Periodontal Disease Index, as well as other indices, can be obtained from the Data Resource Center at the National Institute of Dental and Craniofacial Research / Centers for Disease Control and Prevention (http://drc.hhs.gov/catalog.htm).
Current epidemiologic recording systems for periodontal diseases
Quantifying loss of periodontal attachment
The method proposed by Ramfjord in 1959 (64), of measuring the distance from the bottom of the periodontal pocket to the cemento–enamel junction using a periodontal probe and applied to multiple sites, the entire dentition or a subset of teeth, was the basis of what is currently known as loss of periodontal attachment, loss of attachment or clinical attachment loss. This distance represents the amount of periodontal support lost beyond the cemento– enamel junction. It is measured in millimeters by subtracting the distance between the gingival crest to the cemento–enamel junction from the distance between the gingival crest to the base of the pocket. When the cemento–enamel junction is exposed, the measurement from the gingival crest to the cemento–enamel junction is negative, adding to the total number of millimeters of attachment loss. This measuring process is sometimes described as ‘probing’ (38).
Loss of periodontal attachment measures primarily diseases sequelae rather than active disease because probing cannot detect inflammatory activity in the periodontal pocket. Probing requires the tactile identification of the cemento–enamel junction, the standardized insertion of the periodontal probe in terms of angulation and pressure and the reading of the number of millimeters marked on the probe (38, 64). With a precision of 1 mm, probing may be subject to measurement errors influenced by the prevalence of periodontal disease among participants and the physical environment where the examination takes place (49). Therefore, probing requires trained examiners (66), time and participant cooperation. With practice, clinicians develop the skills necessary to perform these examinations (up to 224 measurements at four sites on each of the 28 permanent teeth). Despite these limitations, loss of periodontal attachment is the de-facto tool to measure periodontal diseases and has been used to test the validity of other measurement tools.
Loss of periodontal attachment has been used as a tool in epidemiological studies despite its complexity and limitations. In the USA, efforts in translating loss of periodontal attachment into an epidemiological tool have focussed on reducing the number of measurements at both tooth and site levels, for example, by the random selection of two quadrants (one maxillary and one mandibular), as used in large national surveys in the USA (11, 25, 84). Random selection in these partial recording protocols provides estimates with some level of underestimating prevalence (4, 81) while reducing the examination time (29, 37). The more critical issue may not be the random selection of quadrants, but the number and location of measurements around the tooth (50).
One important issue in using loss of periodontal attachment in epidemiological studies is the training of examiners, especially in multiyear surveys requiring multiple examiners. Organizers of national surveys in the USA have included quality assurance protocols reporting on examiner reliability and bias (21–23).
Data from national surveys in the USA have been questioned when unexpected disease levels are detected. For example, Slade & Beck (78) reported lower estimates of periodontal diseases in NHANES III Phase 2 (1991–1994) than in Phase 1 (1988–1991). Survey organizers attributed these differences to sampling variations (85). Another example is the ‘decline’ in periodontal diseases reported when both NHANES 1988–1994 and NHANES 1999–2004 are compared (24).
A major challenge in using loss of periodontal attachment at the population level is that tooth and site-specific information need to be summarized into values representing the individual and the population. For example, the most recent data from the USA (24) reported up to eight different estimates – mean gingival recession, periodontal pocket depth, loss of periodontal attachment, prevalence of the population at millimeter cut-off points for each of these three measures, prevalence of periodontal diseases (expressed as having one periodontal site with ≥3 mm of periodontal attachment loss and a pocket depth of more than 4 mm), and the prevalence of moderate or severe periodontitis using the case definition by the Centers for Disease Control and Prevention-American Academy of Periodontology (61). The public, decision making people and even some clinical practitioners may get confused by what these figures represent in terms of disease prevalence and severity.
The World Health Organization approach: Community Periodontal Index system
The third edition of the World Health Organization Oral Health Basic Methods proposed a new measure of periodontal diseases that could be translated into planning for resources – a needs-assessment tool (Table 1) (89). The process of developing this new measure started in 1977 with the deliberations of the World Health Organization Scientific Group in charge of reviewing the epidemiology, etiology and prevention of periodontal diseases (3, 86). The objective of this group was ‘to seek and consider a more realistic system of measurement than those available’ (3). In addition, the new measurement should allow comparisons at national and global levels (3). The group concluded that the most useful information on periodontal diseases would be obtained by including gingival bleeding, the presence of calculus and periodontal pocket depth measurements, and proposed a prototype index – the TRS 621 method (86). This index was later renamed as the Community Periodontal Index of Treatment Needs (1).
In order to simplify the clinical data-collection process, the group recommended assessment of a selected group of teeth, a proposal that combined Ramfjord’s index teeth with the assessment by segments (sextants) (86). Measurements were obtained on the buccal and mesio-buccal aspects of the index teeth using a special probe with a 0.5-mm ball tip, a black band between 3.5 and 5.5 mm, and marks at 8.5 and 11.5 mm. Measurements were made within these levels of precision.
The Community Periodontal Index of Treatment Needs is a hierarchical measurement (36). The score identifying the sextant is based on the most severe finding among its index teeth, and the score identifying the person is based on the most severe finding among sextants. The score for each person contributes to the sample proportion of participants within each Community Periodontal Index of Treatment Needs category (89). In addition, the person’s scores in each sextant contribute to sample proportion of sextants within each of the hierarchical scores and the mean number of such sextants is reported (89), which, as originally designed, was translated into treatment needs (1).
The Community Periodontal Index of Treatment Needs was extensively tested (3, 36). The interested reader may review a series of papers published by the World Health Organization (91) and the contributing papers of a meeting in Manila (19). Overall, these papers provided empirical evidence for the validity of using index teeth and the proposed partial recording system. Because the Community Periodontal Index of Treatment Needs is a partial examination recording system, some under-estimation of population estimates may be expected (2), but some underestimation of population estimates is acceptable in surveillance as long as the method is used consistently (7). Indeed the Community Periodontal Index of Treatment Needs has been widely used around the world and comparable data exist to assess disease distribution and trends (63). From these data, it has been estimated that around 10–15% of the population has severe periodontal diseases (equivalent to having one or more periodontal pockets of ≥6 mm). Within countries, Community Periodontal Index of Treatment Needs data have been used in planning community oral health interventions and programs (63).
As with other periodontal disease measurement tools, the Community Periodontal Index of Treatment Needs has its advantages and limitations. Besides criticism about the underestimation of disease levels (33), others have focussed on the translation of the information into treatment needs. In the 4th edition of the World Health Organization Oral Health Surveys Basic Methods, the index was labeled Community Periodontal Index, with no reference to treatment needs (89). Another criticism has been its reliance on just periodontal pockets. In response, the 4th edition of the Oral Health Surveys Basic Methods also includes a measure of loss of periodontal attachment using the same probe and the same index teeth (90). In this later approach, loss of periodontal attachment is categorized in multiples of millimeters (i.e. 0–3, 4–5, 6–8, 9–11 and 12+) using the marks on the probe, but the examiner needs to calculate loss of periodontal attachment, not just register the values for the depth of the pocket and the position of the gingival crest with respect to the cemento–enamel junction.
For the 5th edition of the Oral Health Surveys Basic Methods (upcoming in 2012) a new, modified Community Periodontal Index system has been suggested that addresses the weaknesses of the original Community Periodontal Index while ensuring simplicity and reproducibility. The new system reflects the effort to give higher public health priority to periodontal diseases as significant contributors to the burden of oral diseases. In the new system, the periodontal status of all teeth will be recorded using the current score system, except for the presence of calculus. The new system has been field tested in several countries of different population sizes and economic development. In addition, it is expected that the full assessment of all teeth would allow the identification of new index teeth for future partial recording systems.
Two modifications of the Community Periodontal Index of Treatment Needs have been proposed for clinical screening of periodontal diseases in private and public settings: The Basic Periodontal Examination, proposed by the British Society of Periodontists (79); and the Periodontal Screening and Recording, proposed by the American Dental Association and the American Academy of Periodontology (47). The Periodontal Screening and Recording uses the sextant and index teeth approach, and the probe has the same characteristics and marks as the World Health Organization periodontal probe. The difference is that at least six areas around the tooth or implant are examined: mesio-buccal, mid-buccal, disto-buccal, disto-lingual, mid-lingual and mesio-lingual.
Use of laboratory tests: markers of periodontal diseases and their use in surveillance
Improved understanding of the microbiological component of periodontal diseases and the host response led to the development of laboratory tests to detect the presence of elements of the bacterial structure (such as surface components and DNA), bacterial metabolic processes or the host inflammatory response (33). These tests are focussed on the detection of ‘high-risk’ individuals utilizing ‘markers’- attributes or exposures associated with an increased probability of disease occurrence, but not necessarily causal (13). Most tests use samples from plaque, saliva or gingival crevicular fluid (33). The interested reader could start the review of this topic with the papers presented at the 1988–1989 symposia organized by the Section of Odontology of the Royal Society of Medicine and edited by Newell Johnson in 1991. Volume 3 deals specifically with markers of periodontal diseases (41). These developments also highlighted the need to understand the validity and reliability of these tests (12, 33, 52).
In our opinion, the use of biomarkers has two main advantages: first, because they are based on physiological events, they may be a more accurate representation of active disease than clinical measurements (such as loss of periodontal attachment or pocket depth); and, second, depending on the validity, reliability, cost and acceptability, they may be used in clinical and epidemiological studies with the added benefit of reducing examiner error because they do not require detection of anatomical landmarks and clinical assessments. The disadvantage, of course, is that no test is 100% accurate and test yield varies when the population used to analyze the test (most of the time this population is high risk) differs from the population on whom the test is applied (6).
Examples of biomarkers that show promise in detecting periodontitis include salivary levels of periodontal pathogens (74) and inflammatory molecules such as interleukin-1beta, interleukin-8, macrophage inflammatory protein and matrix metalloprotease-8 (35, 51, 75). These biomarkers have been proposed for both disease detection and progression (35, 51, 74, 75).
Unfortunately, these approaches have not been translated into effective chair-side tools (17), much less into surveillance tools. In the early 1990s, Bretz et al. (10) used the synthetic substract BANA (n-benzoyl- DL-arginine-β-naph-thylamide [PerioScan™]), to detect proteolytic enzymes produced by Treponema denticola, Porphyromonas gingivalis and Bacteroides forsythus (Tannerella forsythia) associated with adult periodontitis, in subgingival plaque samples in a large sample study. The test results were compared with the immunological presence of periodontal pathogens using ELISA, and clinical measures including bleeding on probing, pocket depth, and a modification of the Russell’s Periodontal Index. The BANA test showed a high degree of sensitivity against ELISA but lower specificity with clinical measures. We are unaware of later studies using these approaches to measure periodontal diseases at the population level.
Characteristics of periodontal measurements for surveillance
Surveillance approaches to measure oral diseases at the population level diverge from clinical approaches in terms of validity and reliability (7). Clinical studies use valid and precise tools to detect teeth or sites where disease is occurring in order to plan an intervention to stop the disease process or to identify risk factors. In contrast, surveillance of oral disease using clinical approaches uses reliable tools that provide consistent results when used by different examiners (7). In addition, a clinical study can include as many diagnostic aids as needed. A surveillance system for oral diseases has inherent limitations in terms of time, number of persons examined and complexity of the measurement tool (7).
With the exception of laboratory tests, epidemiological approaches for periodontal diseases – whether for clinical studies or for surveillance – rely on a clinical assessment, most of which are carried out using a periodontal probe with precision in millimeters. In our opinion, the major concerns of using these tools to measure periodontal diseases are:
The number of teeth to be examined (i.e. full mouth or index teeth only).
The number of sites to be examined around each tooth.
Precision of the measurements.
Pocket depth vs. loss of attachment.
Summarization of site-specific information and case definition(s).
Reliability of periodontal disease measures.
Number of teeth and sites
The question here is which teeth or sites would provide the most valid representation of the presence and level of active disease or disease sequelae when a fullmouth examination is not possible. Based on statistical theory, a probability sample of teeth and sites would be the appropriate choice (4). However, this is technically challenging. An alternative approach used in national surveys in the USA calls for the examination of all teeth in one maxillary and one mandibular randomly selected quadrant (11, 24, 84). Some studies have reported on the bias and underestimation of these partial recordings, including randomly selected quadrants and Ramfjord’s teeth for different fixed or randomly selected sites around the tooth (50, 81). The study by Susin et al. (81) reported that bias depended not only on the type of partial recording, but also on the number and type of sites assessed and the severity of the disease in the sample. A paper by Eke et al. (29) reported underestimation of disease prevalence when using partial recording and fixed sites in a convenience sample of adults. The current NHANES protocol (2009–2012) includes a full periodontal examination of all teeth and six sites to assess the effects of this underestimation at the population level. As a corollary, these data will allow the identification partial examination approaches with less bias.
Precision of the measurements
Dexterity in performing accurate periodontal measurements at the 1-mm level of precision depends on training and repetition. Ramfjord understood this issue and proposed using less-precise measurements (marks on the probe were at 3, 6 and 8 mm). This has also been the approach for the World Health Organization probe used in the Community Periodontal Index of Treatment Needs and the Community Periodontal Index (89, 90). The issue here is a trade-off between more precise and probably less reliable measurements vs. less precise but more reliable measurements. This is not unique to periodontal diseases and the choice for clinicians may be different from that for epidemiologists (6).
Periodontal pocket depth vs. loss of attachment
Both periodontal pocket depth and loss of attachment measure periodontal disease sequelae. Based on the pathology of the disease, pocket depth reflects directly the damage to the supporting structure, while loss of attachment reflects the cumulative effects of the disease. Pocket depth is one of the two components in loss of attachment measurements, but while measuring periodontal pocket is done by direct visual inspection, measuring the distance from the gingival crest to the cemento– enamel junction requires the tactile detection of this anatomical landmark when it is covered by gingiva. In addition, loss of attachment is affected by recession of the gingival margin, especially in the buccal surfaces, which may not be associated with periodontal diseases (60). Most current ‘case definitions’ of periodontal diseases (discussed later) use a combination of both pocket depth and loss of attachment.
Summarization of site-specific data and case definitions
In epidemiological studies and surveillance, all clinical information needs to be aggregated and summarized into measures of the disease occurrence at the individual level – who has the disease and who does not – and later reported as measures of population prevalence or severity. Various case definitions have been proposed for periodontal diseases that combine loss of periodontal attachment and / or pocket depths in a specific number of sites in the same or different teeth (5, 54). The following are three examples of case definitions used in literature.
The first example is the three case definitions for ‘serious’ periodontitis as reviewed by Burt & Eklund (16).
Four or more sites with ≥5 mm of periodontal attachment loss, with probing depths of ≥4 mmat one or more of those sites.
Two or more sites with ≥6 mm of periodontal attachment loss, plus one or more sites with probing depths of ≥5 mm.
Mean periodontal attachment loss in the 20th percentile of the distribution.
Burt & Eklund added that there is some moderate consensus in the literature for using 6 mm as the cut-off point between moderate and severe periodontitis.
The second example includes a two level case definition adopted by consensus at the 5th European Workshop on Periodontology in 2005 (83).
Presence of proximal loss of periodontal attachment of ≥3 mm in two or more nonadjacent teeth.
Presence of proximal loss of periodontal attachment of ≥5 mm in ≥30% of teeth present.
The consensus group also indicated that the first case definition should be considered ‘sensitive’ because it included lower levels of cumulative disease, while the second should be considered as more ‘specific’ because it included individuals with substantial extent and severity of periodontal disease. These definitions, however, were proposed to identify risk factors for the initiation and progression of periodontal diseases, and not for assessing periodontal diseases in populations.
The third example is the case definitions proposed by the Centers for Disease Control and Prevention and the American Academy of Periodontology and used in the validation of self-reported measures of periodontal diseases (28, 61).
Severe: two or more interproximal sites with ≥6 mm of loss of attachment not on the same tooth, and one or more interproximal sites with probing depths of ≥5 mm.
Moderate: two or more interproximal sites with ≥4 mm of loss of attachment not on the same tooth or two or more interproximal sites with probing depths of ≥5 mm not on the same tooth.
Mild: two or more interproximal sites with ≥3 mmof loss of attachment and two or more interproximal sites with ≥4 mm of probing depths not on the same tooth, OR one or more interproximal sites with probing depths ≥5 mm.
In Fig. 1, we plotted the prevalence of periodontal disease among dentate adults 20–64 years of age in the USA having moderate or severe periodontal diseases (in red) using the Centers for Disease Control and Prevention – American Academy of Periodontology case definitions, and those having periodontal disease (in blue), defined as having at least one periodontal site with ≥3 mm of periodontal attachment loss and >4 mm of probing depth [This case definition represent ‘any periodontal disease’ as reported by Dye et al. (24)]. The reader should bear in mind that in NHANES 1999–2004 from which these data were derived, only two sites, mesio-buccal and mid-buccal were assessed (24). In addition, the prevalence of moderate or severe periodontal disease in the 20–34 years age group was not included in the figure because it was statistically unreliable (coefficient of variation greater than 30 percent). These data show that males had higher disease prevalence than females, and Non-Hispanic Whites and those who have never smoked had lower prevalence than Non- Hispanic Blacks and Mexican-Americans and current or former smokers.
Fig. 1.
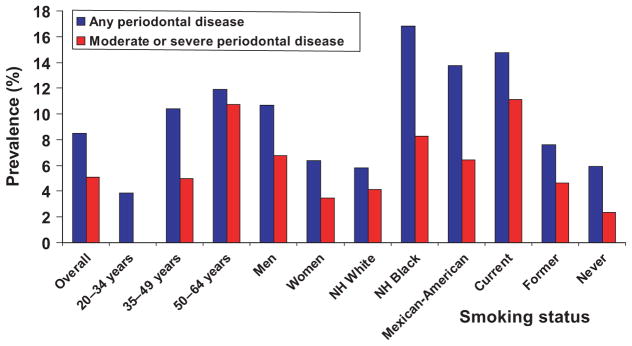
Prevalence of periodontal disease (defined as at least one periodontal site with attachment loss of ≥3 mm and pocket depth of ≥4 mm) and moderate or severe periodontal disease (defined as two or more inter-proximal sites with attachment loss of ≥4 mm in different teeth OR probing depth of ≥5 mm not in the same teeth) among 20- to 64-year-old dentate adults, by age, sex, race/ ethnicity and smoking status. United States, 1999–2004 (24).
Reliability of periodontal disease measures
The reliability of a measurement tool depends on the clarity of the detection criteria and the training of the examiners. There are no studies in the literature with head-to-head comparisons of interexaminer or intra-examiner reliability for different indices of periodontal diseases. However, reliability reported in large national surveys in the USA could provide some guidance. Three published papers have reported on the interexaminer (against the reference examiner) and intra-examiner reliability of measures included in the 1988–1991 (21) and 1999– 2004 NHANES cycles (22, 23). Both Cohen’s kappa statistic and intraclass correlation coefficients were reported. For NHANES 1999–2004 cycles, interexaminer kappa values ranged from 0.58 to 0.77 among five examiners using a cut-off level for loss of periodontal attachment of ≥4 mm. Intraclass correlation coefficients for mean loss of periodontal attachment varied from 0.72 to 0.93 and for mean pocket depth from 0.55 to 0.87. In general, intraexaminer reliability estimates were higher. In 2002, Kingman & Albandar (48) reported on the reliability of three examiners in NHANES 1988–1994 for different cut-off points for both loss of attachment and pocket depth, using kappa and intraclass correlation coefficient statistics. Examiners reported mean values of attachment loss of 0.03–0.20 mm below those of the reference examiner (bias), intraclass correlation coefficients between 0.65 and 0.96 and kappa scores between 0.51 and 0.79. Kingman & Albandar (48) concluded that NHANES examiners had measurement errors similar to those reported in the literature with no significant biases in loss of periodontal attachment or in pocket depth, but there was some variation in their estimates of prevalence. However, they suggested that the latter should be assessed considering that each examiner examined different groups of participants (48).
The World Health Organization Oral Health Program has provided support on methods to calculate measures of examiner reliability for categorical indices such as Community Periodontal Index using percentage agreement and kappa statistics (30, 90). A study by Mojon et al. (57) reported on the reliability of two examiners using the Community Periodontal Index of Treatment Needs in individuals of 65– 80 years of age. Unweighted kappa statistics for intraexaminer reliability were 0.39 and 0.58 for each examiner and 0.48 when both examiners were compared with each other. Agreement was worst in categories 1 (bleeding) and 3 (4–5 mm pocket depths) and best on category 4 (>6 mm pocket depth).
Statistical analysis of periodontal disease data
In contrast to site-specific analysis of data for assessment of reliability (30), data analysis to report estimates of disease occurrence are based on personlevel scores. Teeth and sites within the mouth are clinically correlated (39) and statistical methods based on independent observations cannot be applied (31), otherwise, the statistical power would be artificially increased due to the larger sample size (53). In reporting clinical and surveillance estimates of the prevalence, severity and extent of periodontal diseases, each individual contributes, with one value, to the estimation of population parameters, whether that value is the highest score or the mean among sites / teeth or sextants. The exception is the estimation of percentage of sextants within each category of the Community Periodontal Index of Treatment Needs because of its focus on translating the information into treatment needs, no longer a case in Community Periodontal Index. The interested reader could review the paper by Kingman & Albandar describing different approaches to analyze and report periodontal status data (48).
Self-report measures of periodontal diseases
Self-reported measures of periodontal disease have not been widely used. Based on the chronic and subtle clinical process of periodontal diseases with very little symptoms, many people with the disease would remain unaware of their periodontal status. Thus, answers to the question, ‘Do you have periodontal disease?’, may underestimate the level of disease. On the other hand, the question, ‘Does your gums bleed with brushing?’, may overestimate the severity of periodontal disease by including reversible gingival inflammation. Furthermore, the wording of the question and the dental knowledge of the responder are important in the accuracy of the question and answer (9, 42). On the other hand, some conditions (such as diabetes) and some behaviors (such as smoking) which are risk factors of periodontal diseases, can be used to improve the validity of self-report measures (27). Such a tool, with a sufficient level of validity and a high degree of reliability, would allow the monitoring of periodontal diseases at the population level without a clinical examination.
In 2005, the Division of Oral Health convened a group of experts to develop self-reported measures of periodontal disease for surveillance (27). The group conducted a systematic review of potential questions (9, 42), tested the feasibility of these questions in a national survey in Australia (77), submitted the final set of questions for cognitive assessment (56), pilot tested the questions (26) and tested feasibility using a convenience sample in the USA (27). The questions were included in the NHANES 2009–2012 cycle, together with a full periodontal examination of six sites around all teeth (three in the buccal surfaces and three in the lingual surfaces). These data from a nationally representative sample of the US population will provide validation of the self-reported questions for surveillance of periodontal diseases, and, as discussed earlier, identify the sample of teeth and sites providing the most valid representation of the loss of periodontal attachment in the full mouth. At the global level, the upcoming 5th edition of the World Health Organization Oral Health Surveys Basic Methods incorporates the question, ‘How would you describe the state of your gums?’, with potential answers ranging from ‘excellent’ to ‘very poor’.
Surveillance systems for periodontal diseases
In theUSA, public health surveillance has been defined as the ongoing systematic collection, analysis and interpretation of outcome-specific data for use in the planning, implementation and evaluation of public health practice (82).Documenting the levels and trends of disease is important for detecting and addressing disparities and identifying populations at risk. Thus, the importance of valid, reliable and affordable tools to measure periodontal diseases from data vertically integrated from the local into the national level (7).
In the USA, surveillance of periodontal diseases at the national level uses NHANES and site-specific measurements of loss of periodontal attachment and pocket depth (24). These data demand too many resources to be a feasible option at the US state level (7). On the other hand, surveillance of periodontal diseases at the US state level can be done indirectly using three surveillance indicators from the National Oral Health Surveillance System (http://www.cdc.gov/nohss/index.htm) – the percentage of adults aged 18+ years who had their teeth cleaned in the past year, the percentage of adults aged 65 or more years who had lost six or more teeth due to tooth decay or gum disease, and adults aged 65 or more years who had lost all their natural teeth due to tooth decay of gum disease (55). However, these are not focused specifically on periodontal diseases per se. It is expected that self-reported measures will be used in both national and US state level surveillance of periodontal diseases once validation is complete.
At the global level, the rationale, scope and components of surveillance systems for oral diseases (i.e. oral health status and risk factor surveillance) are included within a framework of oral health information (62). One of the fundamental elements of these efforts is using tools that comprise ‘…ease of understanding and application in all countries regardless of the concepts, training and experience of their dental health personnel.’ (87) and facilitating comparisons among countries (73). These have been the goals in all editions of the World Health Organization Oral Health Surveys Basic Methods (20, 87–90). The modifications in measuring periodontal diseases in the upcoming 5th edition of the World Health Organization Oral Health Basic Methods will provide new incentives to measure the occurrence of periodontal diseases at the global level.
Developmental perspectives
The evolution of how wemeasure periodontal diseases shows a continuing struggle to design valid, reliable and feasible measurements of a disease for which a valid case definition (active disease) is still unavailable. This is in contrast to dental caries for which we have had the Klein’s decayed, missing and filled (DMF) in use for more than 80 years despite its limitations. The difference is rather striking considering that both periodontal disease and dental caries are chronic diseases, are caused by microorganisms in a biofilm and can be measured during the lifespan of the person by detecting disease sequelae at the tooth or surface levels. One potential explanation is that Klein’s index has been more resilient, at least until recently, to our evolving understanding of dental caries than the indices of periodontal diseases. Another explanation may be that it is more difficult to train, apply, analyze and report all measures of periodontal diseases than Klein’s DMF index. In addition, at epidemiological and surveillance levels, it was easier to integrate Klein’s DMF index into surveys than to integrate measures of periodontal diseases. Interestingly, the concepts of selecting index teeth and sampling quadrants or sextants used mostly for periodontal diseases has never been fully adapted into measures of dental caries.
It is clear that all current measures of periodontal diseases have limitations as applied into clinical or epidemiological studies and surveillance. Besides issues of validity, all clinical measures are subject to measurement error. Therefore, the choice of a measurement tool should follow an evaluation of level of bias and reliability that could be acceptable in each particular situation. For example, a surveillance system may use the Community Periodontal Index or loss of periodontal attachment in a subset of teeth because the focus is on monitoring disease for which reliability is an issue, while a clinician may opt for a full clinical examination for loss of periodontal attachment supplemented by radiographic measures because the focus is on detection of all periodontal lesions. The latter is also critical when assessing causality or association with other diseases or conditions.
Two nonclinical alternatives have been presented in this paper. The first is self-reported measures that collectively can be used as a proxy for the presence of disease sequelae. These measures are not designed to measure active disease but to gain in reliability and feasibility what may be lost in accuracy. As already noted, these questions are currently being field tested in NHANES against a clinical examination of loss of periodontal attachment. If preliminary results using these questions are confirmed, it will bring a new dimension to monitoring periodontal diseases in populations and will help to standardize the monitoring of periodontal diseases at state and national levels, using a less intrusive, nonclinical, sufficiently valid and highly reliable tool.
The second is the laboratory testing of enzymes from microorganisms considered putative of periodontal diseases. These are probably the closest to a valid representation of disease activity and are relatively easy to carry out, even in large studies. However, to our knowledge, no further development has occurred since the work of Bretz et al. (10). As we rely increasingly on indirect measures and on detecting intermediate aspects of the disease process, it is quite possible that these tests will be reintroduced into surveillance efforts.
Surveillance of periodontal diseases needs to be integrated into surveillance efforts monitoring other dental diseases. Currently, the National Institute of Dental and Craniofacial Research and the Centers for Disease Control and Prevention have initiated the development of a National Surveillance Plan which started with a workshop in 2010. The elements of the proposed plan include details on which health conditions should bemonitored, how frequently they should be measured, and which methods should be used for measurement. In addition, the plan will provide room for future developments on novel measurement tools, as well as provisions for transitional periods when two measures need to be included at the same time to allow for valid assessment of trends. We expect this to be a true surveillance system for oral diseases, including periodontal diseases. New efforts on monitoring periodontal diseases at the global level are expected with the new 5th edition of the World Health Organization Oral Health Surveys Basic Methods.
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Ainamo J, Barmes D, Beagrie G, Cutress T, Martin J, Sardo-Infirri J. Development of the World Health Organization (WHO) Community Periodontal Index of Treatment Needs (CPITN) Int Dent J. 1982;32:281–291. [PubMed] [Google Scholar]
- 2.Baelum V, Fejerskov O, Manji F, Wanzala P. Influence of CPITN partial recordings on estimates of prevalence and severity of various periodontal conditions in adults. Community Dent Oral Epidemiol. 1993;21:354–359. doi: 10.1111/j.1600-0528.1993.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 3.Barmes D. CPITN – a WHO initiative. Int Dent J. 1994;44:523–525. [PubMed] [Google Scholar]
- 4.Beck JD, Caplan DJ, Preisser JS, Moss K. Reducing the bias of probing depth and attachment level estimates using random partial-mouth recording. Community Dent Oral Epidemiol. 2006;34:1–10. doi: 10.1111/j.1600-0528.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 5.Beck JD, Koch GG, Rozier RG, Tudor GE. Prevalence and risk indicators for periodontal attachment loss in a population of older community-dwelling blacks and whites. J Periodontol. 1990;61:521–528. doi: 10.1902/jop.1990.61.8.521. [DOI] [PubMed] [Google Scholar]
- 6.Beltrán ED, Malvitz DM, Eklund SA. Validity of two methods for assessing oral health status in populations. J Public Health Dent. 1997;57:206–214. doi: 10.1111/j.1752-7325.1997.tb02977.x. [DOI] [PubMed] [Google Scholar]
- 7.Beltrán-Aguilar ED, Malvitz DM, Lockwood SA, Rozier RG, Tomar SL. Oral health surveillance: past, present and future challenges. J Public Health Dent. 2003;63:141–149. doi: 10.1111/j.1752-7325.2003.tb03492.x. [DOI] [PubMed] [Google Scholar]
- 8.Berry FB, Schaefer AE. Program of the Interdepartmental Committee on Nutrition for National Defense. JAMA. 1958;166:775–777. doi: 10.1001/jama.1958.62990070017014. [DOI] [PubMed] [Google Scholar]
- 9.Blicher B, Joshipura K, Eke P. Validation of self-reported periodontal disease: a systematic review. J Dent Res. 2005;84:881–890. doi: 10.1177/154405910508401003. [DOI] [PubMed] [Google Scholar]
- 10.Bretz WA, Eklund SA, Radicchi R, Schork MA, Schork N, Schottenfeld D, Lopatin DE, Loesche WJ. The use of rapid enzymatic assay in the field for the detection of infections associated with adults periodontitis. J Public Health Dent. 1993;53:235–240. doi: 10.1111/j.1752-7325.1993.tb02710.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown LJ, Brunelle JA, Kingman A. Periodontal status in the United States, 1988–91: prevalence, extent, and demographic variation. J Dent Res. 1996;75(Spec Iss):672–683. doi: 10.1177/002203459607502S07. [DOI] [PubMed] [Google Scholar]
- 12.Brunette DM. Critical thinking. Understanding and evaluating dental research. Chicago, IL: Quintessence Publishing Co, Inc; 1996. Reliability, sensitivity, and specificity of diagnostic tests and measurements; pp. 99–111. [Google Scholar]
- 13.Burt BA. The distribution of periodontal destruction in the populations of industrialized countries. In: Johnson NW, editor. Periodontal diseases. Markers of disease susceptibility and activity. Cambridge: Cambridge University Press; 1991. pp. 9–26. [Google Scholar]
- 14.Burt BA, Eklund SA. The methods of oral epidemiology. In: Burt BA, Eklund SA, editors. Dentistry, dental practice, and the community. 4. Philadelphia, PA: W.B. Saunders Company; 1992. pp. 64–68. [Google Scholar]
- 15.Burt BA, Eklund SA. Measuring periodontal diseases. In: Burt BA, Eklund SA, editors. Dentistry, dental practice, and the community. 6. St. Louis, MO: Elsevier Saunders; 2005. pp. 203–209. [Google Scholar]
- 16.Burt BA, Eklund SA. Periodontal diseases. In: Burt BA, Eklund SA, editors. Dentistry, dental practice and the community. 6. St. Louis, MO: Elsevier Saunders; 2005. pp. 259–286. [Google Scholar]
- 17.Chapple ILC. Periodontal diagnosis and treatment – where does the future lie? Periodontol 2000. 2009;51:9–24. doi: 10.1111/j.1600-0757.2009.00319.x. [DOI] [PubMed] [Google Scholar]
- 18.Cohen DW, Ship II. Clinical methods in periodontal diseases. Based on a conference held on May 20–23, 1967. J Periodontol. 1967;38:576–795. doi: 10.1902/jop.1967.38.6_part2.3. [DOI] [PubMed] [Google Scholar]
- 19.Cutress T, Pilot T, Purdell-Lewis D. Introduction. Int Dent J. 1994;44:521. [Google Scholar]
- 20.Davies GN, Horowitz HS, Wada W. The assessment of periodontal disease for public health purposes. J Periodontal Res. 1974;9:62–70. doi: 10.1111/j.1600-0765.1974.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 21.Drury TF, Winn DM, Snowden CB, Kingman A, Kleinman DV, Lewis B. An overview of the oral health component of the 1988–1991 National Health and Nutrition Examination Survey (NHANES III-Phase 1) J Dent Res. 1996;75(Spec Iss):620–630. doi: 10.1177/002203459607502S02. [DOI] [PubMed] [Google Scholar]
- 22.Dye BA, Barker LK, Selwitz RH, Lewis BG, Wu T, Fryar CD, Ostchega Y, Beltran ED, Ley E. Overview and quality assurance for the National Health and Nutrition Examination Survey (NHANES) oral health component, 1999–2002. Community Dent Oral Epidemiol. 2007;35:140–151. doi: 10.1111/j.1600-0528.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 23.Dye BA, Nowjack-Raymer R, Barker LK, Nunn JH, Steele JG, Tan S, Lewis BG, Beltran-Aguilar ED. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey 1999–2002. J Public Health Dent. 2008;68:218–226. doi: 10.1111/j.1752-7325.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 24.Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltrán-Aguilar ED, Horowitz AM, Li C-H. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat. 2007;11:1–92. [PubMed] [Google Scholar]
- 25.Dye BA, Thornton-Evans G. A brief history of national surveillance efforts for periodontal disease in the United States. J Periodontol. 2007;78(Suppl):1373–1379. doi: 10.1902/jop.2007.060210. [DOI] [PubMed] [Google Scholar]
- 26.Eke PI, Dye BA. Assessment of self-reported measures for predicting population prevalence of periodontitis. J Periodontol. 2009;80:1371–1379. doi: 10.1902/jop.2009.080607. [DOI] [PubMed] [Google Scholar]
- 27.Eke PI, Genco RJ. CDC Periodontal Disease Surveillance Project: background, objectives, and progress report. J Periodontol. 2007;78(Suppl):1366–1371. doi: 10.1902/jop.2007.070134. [DOI] [PubMed] [Google Scholar]
- 28.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83 doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eke PI, Thornton-Evans GO, Wei L, Bognakke WS, Dye BA. Accuracy of NHANES periodontal examination protocols. J Dent Res. 2010;89:1208–1213. doi: 10.1177/0022034510377793. [DOI] [PubMed] [Google Scholar]
- 30.Eklund S, Moller IJ, Leclercq M-H. Calibration of examiners for oral health epidemiological surveys. Geneva: World Health Organization; 1993. Report No.: ORH / EIS/EPID. 93.1. [Google Scholar]
- 31.Fleiss JL, Park MH, Chilton NW. Within-mouth correlations and reliabilities for probing depth and attachment level. J Periodontol. 1987;58:460–463. doi: 10.1902/jop.1987.58.7.460. [DOI] [PubMed] [Google Scholar]
- 32.Fleiss JL, Park MH, Chilton NW, Alman JE, Feldman RS, Chauncey HH. Representativeness of the “Ramfjord teeth” for epidemiologic studies of gingivitis and periodontitis. Community Dent Oral Epidemiol. 1987;15:221–224. doi: 10.1111/j.1600-0528.1987.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 33.Genco RJ, Jeffcoat M, Caton J, Jr, Papapanou P, Armitage G, Grossi S, Johnson N, Lamster I, Lang N, Robertson P, Sanz M. Consensus Report. Periodontal diseases: epidemiology and diagnosis. Ann Periodontol. 1996;1:216–222. doi: 10.1902/annals.1996.1.1.216. [DOI] [PubMed] [Google Scholar]
- 34.Greene JC, Vermillion JR. Oral hygiene index: a method for classifying oral hygiene status. J Am Dent Assoc. 1960;61:172–179. [Google Scholar]
- 35.Gursoy UK, Könönen E, Pussinen PJ, Tervahartiala T, Hyvärinen K, Suominen AL, Uitto VJ, Paju S, Sorsa T. Use of host- and bacteria-derived salivary markers in detection of periodontitis: a cumulative approach. Dis Markers. 2011;30:299–305. doi: 10.3233/DMA-2011-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmgren CJ. CPITN – Interpretations and limitations. Int Dent J. 1994;44(Suppl 1):533–546. [PubMed] [Google Scholar]
- 37.Hunt RJ. The efficiency of half-mouth examinations in estimating the prevalence of periodontal diseases. J Dent Res. 1987;66:1044–1048. doi: 10.1177/00220345870660051101. [DOI] [PubMed] [Google Scholar]
- 38.Hunter F. Periodontal probes and probing. Int Dent J. 1994;44:577–583. [PubMed] [Google Scholar]
- 39.Imrey PB. Considerations in the statistical analysis of clinical trials in periodontitis. J Clin Periodontol. 1986;13:517–528. doi: 10.1111/j.1600-051x.1986.tb01500.x. [DOI] [PubMed] [Google Scholar]
- 40.Ismail AI, Szpunar SM. XI. The prevalence of total tooth loss, dental caries, and periodontal disease among Mexican Americans, Cuban Americans and Puerto Ricans: findings from HHANES 1982–1984. Am J Public Health. 1990;80:66–70. doi: 10.2105/ajph.80.suppl.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson NW. Periodontal diseases. Markers of disease susceptibility and activity. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- 42.Joshipura KJ, Douglass CW, Garcia RI, Valachovic R, Willett WC. Validity of a self-reported periodontal disease measure. J Public Health Dent. 1996;56:205–212. doi: 10.1111/j.1752-7325.1996.tb02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly JE, EngeL A. Selected examination findings related to periodontal disease among adults. Rockville, MD: National Center for Health Statistics; 1974. DHEW Publication No. (HRA) 75–1293. [PubMed] [Google Scholar]
- 44.Kelly JE, Harvey CR. Basic data on dental examination findings of persons 1–74 years. United States, 1971–1974. Hyattsville, MD: National Center for Health Statistics; 1979. DHEW Publication No. (PHS) 79–1662. [PubMed] [Google Scholar]
- 45.Kelly JE, Sanchez M. Periodontal disease and oral hygiene among children. United States. Rockville, MD: National Center for Health Statistics; 1972. DHEW Publication No. (HSM) 72–1060. [PubMed] [Google Scholar]
- 46.Kelly JE, Van Kirk LE. Periodontal disase in adults. United States – 1960–1962. Rockville, MD: National Center for Health Statistics; 1965. Public Health Service Publication No. 1000. [Google Scholar]
- 47.Khocht A, Zohn H, Deasy M, Chang K-M. Assessment of periodontal status with PSR and traditional clinical periodontal examination. J Am Dent Assoc. 1995;126:1658– 1665. doi: 10.14219/jada.archive.1995.0115. [DOI] [PubMed] [Google Scholar]
- 48.Kingman A, Albandar JM. Methodological aspects of epidemiological studies of periodontal diseases. Periodontol 2000. 2002;29:11–30. doi: 10.1034/j.1600-0757.2002.290102.x. [DOI] [PubMed] [Google Scholar]
- 49.Kingman A, Löe H, Ånerud Å, Boysen H. Errors in measuring parameters associated with periodontal health and disease. J Periodontol. 1991;62:477–486. doi: 10.1902/jop.1991.62.8.477. [DOI] [PubMed] [Google Scholar]
- 50.Kingman A, Susin C, Albandar JM. Effect of partial recording protocols on severity estimates of periodontal disease. J Clin Periodontol. 2008;35:659–667. doi: 10.1111/j.1600-051X.2008.01243.x. [DOI] [PubMed] [Google Scholar]
- 51.Kinney JS, Morelli T, Braun T, Ramseier CA, Herr AE, Sugai JV, Shelburne CE, Rayburn LA, Singh AK, Giannobile WV. Saliva/pathogents biomarker signatures and periodontal disease preogression. J Dent Res. 2011;90:752–758. doi: 10.1177/0022034511399908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lang NP. Clinical markers of active periodontal disease. In: Johnson NW, editor. Periodontal diseases. Markers of disease susceptibility and activity. Cambridge: Cambridge University Press; 1991. pp. 179–202. [Google Scholar]
- 53.Loesche WJ, Hujoel P. Microbiologically based diagnostic tests for periodontitis. Considerations of sensitivity, specificity and accuracy. In: Johnson NW, editor. Periodontal diseases. Markers of disease susceptibility and activity. Cambridge: Cambridge University Press; 1991. pp. 417–440. [Google Scholar]
- 54.Machtei EE, Christersson LA, Grossi SG, Dunford R, Zambon JJ, Genco RJ. Clinical criteria for the definition of “established periodontitis”. J Periodontol. 1992;63:206– 214. doi: 10.1902/jop.1992.63.3.206. [DOI] [PubMed] [Google Scholar]
- 55.Malvitz DM, Barker LK, Phipps KR. Development and status of the National Oral Health Surveillance System. Prev Chronic Dis. 2009;6:A66. [PMC free article] [PubMed] [Google Scholar]
- 56.Miller K, Eke PI, Schoua-Glusberg A. Cognitive evaluation of self-report questions for surveillance of periodontitis. J Periodontol. 2007;78(Suppl):1455–1462. doi: 10.1902/jop.2007.060384. [DOI] [PubMed] [Google Scholar]
- 57.Mojon P, Chung J-P, Favre P, Budtz-Jörgensen E. Examiner agreement on periodontal indices during dental surveys in elders. J Clin Periodontol. 1996;23:56–59. doi: 10.1111/j.1600-051x.1996.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 58.O’Leary T. The periodontal screening examination. J Periodontol. 1967;38:617–624. doi: 10.1902/jop.1967.38.6_part2.617. [DOI] [PubMed] [Google Scholar]
- 59.O’Leary T, Gibson WA, Shannon IL, Schuessler CF, Nabers CL. A screening examination for detection of gingival and periodontal breakdown and local irritants. USAF School of Aerospace Medicine Technical Documentary Report 63-51. 1963 [Google Scholar]
- 60.Oliver RC, Brown LJ, Löe H. Periodontal diseases in the United States population. J Periodontol. 1998;69:269–278. doi: 10.1902/jop.1998.69.2.269. [DOI] [PubMed] [Google Scholar]
- 61.Page RC, Eke PI. Case definitions for use in populationbased surveillance of periodontitis. J Periodontol. 2007;78(Suppl):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 62.Petersen PE, Bourgeois D, Bratthall D, Ogawa H. Oral health information systems – towards measuring progress in oral health promotion and disease prevention. Bull World Health Organ. 2005;83:686–693. [PMC free article] [PubMed] [Google Scholar]
- 63.Petersen PE, Ogawa H. Strengthening the prevention of periodontal disease: the WHO approach. J Periodontol. 2005;76:2187–2193. doi: 10.1902/jop.2005.76.12.2187. [DOI] [PubMed] [Google Scholar]
- 64.Ramfjord SP. Indices for prevalence and incidence of periodontal disease. J Periodontol. 1959;30:51–59. [Google Scholar]
- 65.Ramfjord SP. The periodontal status of boys 11 to 17 years old in Bombay, India. J Periodontol. 1961;32:237–248. [Google Scholar]
- 66.Ramfjord SP. The periodontal disease index (PDI) J Periodontol. 1967;38:602–610. doi: 10.1902/jop.1967.38.6.602. [DOI] [PubMed] [Google Scholar]
- 67.Ramfjord SP, Emslie RD, Greene JC, Held A-J, Waerhaug J. Epidemiological studies of periodontal diseases. Am J Public Health. 1968;58:1713–1722. doi: 10.2105/ajph.58.9.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Russell AL. A system of classification and scoring for prevalence surveys of periodontal disease. J Dent Res. 1956;35:350–359. doi: 10.1177/00220345560350030401. [DOI] [PubMed] [Google Scholar]
- 69.Russell AL. International nutrition surveys: a summary of preliminary dental findings. J Dent Res. 1963;42:233–244. doi: 10.1177/00220345630420012401. [DOI] [PubMed] [Google Scholar]
- 70.Russell AL. World Epidemiology and Oral Health. In: Kreshover SJ, McClure FJ, editors. Environmental variables in oral disease. Washington, DC: American Association for the Advancement of Science; 1966. pp. 21–39. [Google Scholar]
- 71.Russell AL. The Periodontal Index. J Periodontol. 1967;38:585–591. doi: 10.1902/jop.1967.38.6_part2.585. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez M. Periodontal disease among youths 12–17 years. United States. Rockville, MD: National Center for Health Statistics; 1974. DHEW Publication No. (HRA) 74–1623. [PubMed] [Google Scholar]
- 73.Sardo Infirri J, Barmes DE. Epidemiology of oral diseases – differences in national problems. Int Dent J. 1979;29:183– 190. [PubMed] [Google Scholar]
- 74.Saygun I, Nizam N, Keskiner I, Bal V, Kubar A, Açıkel C, Serdar M, Slots J. Salivary infectious agents and periodontal disease status. J Periodontal Res. 2011;46:235–239. doi: 10.1111/j.1600-0765.2010.01335.x. [DOI] [PubMed] [Google Scholar]
- 75.Sexton WM, Lin Y, Kryscio RJ, Dawson DR, 3rd, Ebersole JL, Miller CS. Salivary biomarkers of periodontal disease in response to treatment. J Clin Periodontol. 2011;38:434–441. doi: 10.1111/j.1600-051X.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sheiham A, Striffler DF. A comparison of four epidemiological methods of assessing periodontal diasease. II. Test of periodontal indices. J Periodontal Res. 1970;5:155–161. doi: 10.1111/j.1600-0765.1970.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 77.Slade GD. Interim analysis of validity of periodontitis screening questions in the Australian population. J Periodontol. 2007;78(Suppl):1463–1470. doi: 10.1902/jop.2007.060344. [DOI] [PubMed] [Google Scholar]
- 78.Slade GD, Beck JD. Plausibility of periodontal disease estimates from NHANES III. J Public Health Dent. 1999;59:67–72. doi: 10.1111/j.1752-7325.1999.tb03237.x. [DOI] [PubMed] [Google Scholar]
- 79.Smales FC, Mosedale RF, Floyd PM. Policy for periodontal care. Br Dent J. 1987;163:167–169. doi: 10.1038/sj.bdj.4806229. [DOI] [PubMed] [Google Scholar]
- 80.Stahl SS, Morris AL. Oral health conditions among army personnel at the Army Engineer Center. J Periodontol. 1955;26:180–185. [Google Scholar]
- 81.Susin C, Kingman A, Albandar JM. Effect of partial recording protocols on estimates of prevalence of periodontal diseases. J Periodontol. 2005;76:262–267. doi: 10.1902/jop.2005.76.2.262. [DOI] [PubMed] [Google Scholar]
- 82.Thacker SB. Historical development. In: Teutsch SM, Churchill RE, editors. Principles and practice of public health surveillance. 2. New York: Oxford University Press, Inc; 2000. pp. 1–16. [Google Scholar]
- 83.Tonetti MS, Claffey N. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. J Clin Periodontol. 2005;32(Suppl 6):210–213. doi: 10.1111/j.1600-051X.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 84.U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health. Oral Health of United States Adults. The National Survey of Oral Health in U.S. Employed Adults and Seniors: 1985–1986. National Findings. Bethesda, MD: National Institutes of Health; 1987. NIH Publication No. 87-2868. [Google Scholar]
- 85.Winn DM, Johnson CL, Kingman A. Periodontal disease estimates in NHANES III: clinical measurement and complex sampling design issues. J Public Health Dent. 1999;59:73–78. doi: 10.1111/j.1752-7325.1999.tb03238.x. [DOI] [PubMed] [Google Scholar]
- 86.World Health Organization. Epidemiology, etiology and prevention of periodontal diseases. Geneva: World Health Organization; 1978. Report No.: 621. [PubMed] [Google Scholar]
- 87.World Health Organization. Oral health surveys. Basic methods. Geneva: World Health Organization; 1971. [Google Scholar]
- 88.World Health Organization. Oral health surveys. Basic methods. 2. Geneva: World Health Organization; 1977. [Google Scholar]
- 89.World Health Organization. Oral health surveys. Basic methods. 3. Geneva: World Health Organization; 1987. [Google Scholar]
- 90.World Health Organization. Oral health surveys. Basic methods. 4. Geneva: World Health Organization; 1997. [Google Scholar]
- 91.World Health Organization, Oral Health Unit. Community periodontal index of treatment needs. Development, field testing and statistical evaluation. Geneva: World Health Organization; 1984. Report No.: WHO/ORD/EPID.PD/84.1. [Google Scholar]


