Abstract
Categorizing ‘deficit schizophrenia’ (DS) as distinct from ‘non-deficit’ schizophrenia (NDS) may help reduce heterogeneity within schizophrenia. However, it is unknown if DS has a discrete white matter signature. Here we used MRI to compare white matter volume (voxel-based morphometry) and microstructural integrity (fractional anisotropy, FA) in first-episode treatment-naïve patients with DS and NDS and their unaffected relatives to control groups of similar age. We found that white matter disruption was prominent in DS compared to controls; the DS group had lower volumes in the cerebellum, bilateral extra-nuclear and bilateral frontoparietal regions, and lower FA in the body of corpus callosum, posterior superior longitudinal fasciculus and uncinate fasciculus. The DS group also had lower volume in bilateral extra-nuclear regions compared to NDS, and the volume of these clusters was negatively correlated with deficit symptom ratings. NDS patients however, had no significant volume alterations and limited disruption of microstructural integrity compared to controls. Finally, first-degree relatives of those with DS shared volume abnormalities in right extra-nuclear white matter. Thus, white matter pathology in schizophrenia is most evident in the deficit condition, and lower extra-nuclear white matter volumes in both DS patients and their relatives may represent a brain structural ‘endophenotype’ for DS.
Clinical and genetic heterogeneity within schizophrenia has contributed to a relative lack of progress in understanding the illness1,2. The concept of deficit schizophrenia (DS) is regarded as one of the most promising attempts to reduce the heterogeneity within the spectrum3. It is proposed that those with DS represent a clinically homogeneous subgroup of patients with primary and enduring negative symptoms4. Consistent with this, the diagnosis of DS, separate from non-deficit schizophrenia (NDS), has been demonstrated as stable and enduring5,6. Compared to NDS, DS is characterized by more impaired social, global cognition and executive functions, and worse long-term prognosis. The neurobiological underpinnings of DS and NDS have also been suggested to be at least partly distinct7.
Clinically homogeneous subgroups within spectrum conditions such as schizophrenia may have a recognizable ‘endophenotype’, a biological or behavioral marker thought to reflect an inherited vulnerability for the disorder8. Endophenotypes are state-independent, co-segregate within families, and are found in some unaffected relatives of individuals with the disorder (though to a milder extent than in patients)9. Identifying endophenotypes may be a beneficial strategy in investigations of complex and multi-factorial, inherited disorders such as schizophrenia10. The rationale being that the number of genes involved in a phenotype is directly related to the complexity of the phenotype11.
In support of this, there is evidence that, compared to the relatives of those with NDS, the family members of individuals with DS tend to have a higher prevalence of subclinical deficit symptoms, such as social withdrawal; they also have an increased risk of schizophrenia12,13. However, no study has extended the investigation of brain biology in DS and NDS to include family members.
Cerebral white matter subserves all cognitive and neurological functions through fiber pathways which link geographically distant cortical and subcortical regions14. White matter abnormalities have therefore been associated with a number of psychiatric and affective manifestations15,16. For example, lower white volumes have been reported across whole brain17, corpus callosum18, bilateral frontal lobe and internal capsule19 in schizophrenia and white matter pathology has been linked to both negative and positive symptoms16. In addition, DTI studies of schizophrenia indicate that lower FA measures in fronto-limbic-striatal circuits20,21.
Such white matter anomalies are particularly prominent in studies of individuals with chronic DS22,23,24,25,26,27,28. For example, DS is associated with greater density of interstitial cells of the white matter in frontal and parietal lobe26,27 and disrupted fiber integrity in superior longitudinal fasciculus and uncinate fasciculus22,24,25. Thus the pattern of white matter pathology in DS may constitute an ‘endophenotypic’ marker. However, in imaging studies, the range of techniques used, small sample sizes, heterogeneous treatment conditions, or duration of illness, may confound interpretation of DS studies3; and to examine potential white matter ‘endophenotypic’ markers of DS, a study of untreated first-episode patients is needed, alongside a study of their relatives.
Therefore, we conducted a bimodal imaging analysis to compare both white matter volume (voxel-based morphometry, VBM) and microstructural integrity (fractional anisotropy, FA) in first-episode and treatment-naïve patients with schizophrenia, with either DS or NDS, their unaffected first-degree relatives and matched healthy controls. Symptom severity of patients was evaluated using the Positive and Negative Syndrome Scale (PANSS)29. We addressed the following questions: (1) Does DS have distinct patterns of white matter alterations at first clinical presentation relative to controls and those with NDS? (2) If so, are these linked to negative symptom severity? Finally, (3) are similar white matter structural differences also present in their non-psychotic relatives?
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of the participants are shown in Table 1. Compared to respective control groups, patients and relatives showed lower IQ, there were no statistically significant differences in whole brain volume, total white matter volume, age, gender, and educational years in patients or relative groups. However, as expected DS patients had longer duration of untreated psychosis (DUP), higher PANSS total scores and PANSS negative subscale scores than NDS patients. There was no significant difference between two patient groups at age of illness onset, PANSS positive subscale scores and PANSS general psychopathological subscale scores.
Table 1. Demographic and clinical features.
| NDS (n = 42) | DS (n = 33) | HC1 (n = 41) | NDS_R (n = 37) | DS_R (n = 21) | HC2 (n = 37) | |
|---|---|---|---|---|---|---|
| Female/Male | 17/25 | 11/22 | 17/24 | 21/16 | 10/11 | 19/18 |
| Age | 23.38 ± 7.08 | 22.33 ± 6.90 | 23.49 ± 7.33 | 42.76 ± 8.13 | 43.00 ± 8.23 | 43.13 ± 9.50 |
| tWMV | 0.431 ± 0.05 | 0.420 ± 0.06 | 0.435 ± 0.04 | 0.432 ± 0.04 | 0.428 ± 0.05 | 0.441 ± 0.06 |
| WBV | 1.173 ± 0.12 | 1.175 ± 0.13 | 1.173 ± 0.09 | 1.112 ± 0.09 | 1.130 ± 0.11 | 1.144 ± 0.12 |
| Education years | 12.31 ± 2.46 | 11.45 ± 2.71 | 12.66 ± 2.42 | 9.89 ± 3.79 | 9.24 ± 3.71 | 10.35 ± 4.80 |
| IQ | 98.32 ± 13.49 | 95.95 ± 18.18 | 119.25 ± 12.89* | 95.48 ± 15.25 | 98.43 ± 16.76 | 105.34 ± 15.52* |
| DUP (months) | 5.71 ± 6.93 | 12.22 ± 12.04* | ||||
| Age of Onset | 22.73 ± 7.14 | 20.41 ± 6.74 | ||||
| PANSS-T | 87.83 ± 16.40 | 97.64 ± 17.55* | ||||
| PANSS-P | 25.36 ± 6.21 | 22.48 ± 7.62 | ||||
| PANSS-N | 16.52 ± 5.87 | 27.15 ± 7.82* | ||||
| PANSS-G | 45.95 ± 9.22 | 48.00 ± 9.33 |
Note: Demographic data are shown as mean ± standard deviation; *p < 0.05; DS, deficit schizophrenia; NDS, non-deficit schizophrenia; HC1, healthy controls age-and sex-matched with DS and NDS; DS_R, first degree relatives of DS patients; NDS_R first degree relatives of NDS patients; HC2, healthy controls age- and sex-matched with DS_R and NDS_R; tWMV, total white matter volume; WBV, whole brain volume = total gray matter volume + total white matter volume; DUP, duration of untreated psychosis; PANSS-T, PANSS total scores; PANSS-P, PANSS positive symptoms subscale scores; PANSS-N, PANSS negative symptoms subscale scores; PANSS-G, PANSS general psychopathological symptoms subscale scores.
Voxel-wise comparisons of white matter volume
Results of voxel-wise comparisons are shown in Table 2.
Table 2. White matter volume differences between groups.
| Regions | Cluster P value (FWE corrected) | Voxels | Peak Z value | MNI Coordinates (x, y, z) | ||
|---|---|---|---|---|---|---|
| Analyses of patient groups | ||||||
| DS > HC1 | ||||||
| Precentral Gyrus | 0.013 | 774 | 5.88 | 20 | −19 | 64 |
| Precentral Gyrus | 0.036 | 501 | 5.69 | −26 | −17 | 60 |
| HC1 > DS | ||||||
| Cerebellum Posterior Lobe | 0.020 | 659 | 5.76 | −1 | −56 | −17 |
| Extra-Nuclear | 0.000 | 4053 | 5.24 | −14 | −16 | 15 |
| Extra-Nuclear | 0.000 | 5980 | 5.09 | 34 | −17 | 12 |
| NDS > DS | ||||||
| Extra-Nuclear | 0.021 | 640 | 4.50 | −22 | −4 | 2 |
| Extra-Nuclear | 0.015 | 730 | 4.37 | 16 | −1 | −0 |
| Analyses of relatives | ||||||
| HC2 > DS_R | ||||||
| Insula | 0.662 | 105 | 3.57 | 34 | −18 | 13 |
| NDS_R > DS_R | ||||||
| Extra-Nuclear | 0.453 | 515 | 4.00 | 15 | −14 | 8 |
Note: Analyses of patient groups were thresholded at voxel level p < 0.0001 & cluster level corrected (FWE < 0.05); Analyses of relatives were thresholded at voxel level p < 0.001 & cluster size >100 voxles.
Patients
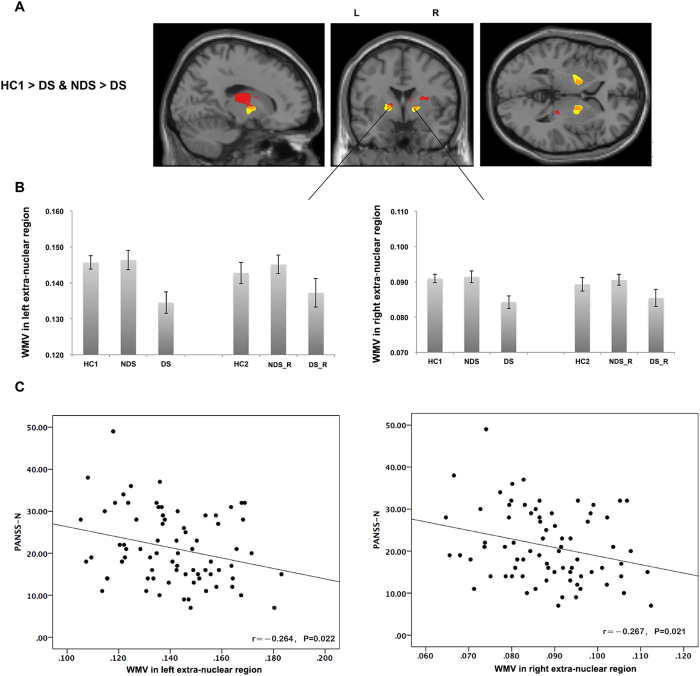
Compared with matched healthy controls (HC1), DS patients had significantly lower white matter volume in the posterior lobe of the cerebellum and bilateral extra-nuclear regions (around the basal ganglia, extending to insula); and greater white matter volume in bilateral sub-lobar frontoparietal areas. In contrast, NDS patients had no significant white matter volume alterations. Direct comparison of DS and NDS patients revealed that the volume of extra-nuclear white matter was smaller in DS patients (Fig. 1A,B).
Figure 1. Volume lose in extra-nuclear white matter in DS patients.
DS patients had lower white matter volume in bilateral extra-nuclear regions compared to both healthy controls (red) and NDS patients (yellow) (A,B). The white matter volume of bilateral extra-nuclear regions (the overlapping regions) was inversely correlated with PANSS negative subscale scores in patients (C). Note: L, Left hemisphere; R, right hemisphere; WMV, white matter volume.
Relatives
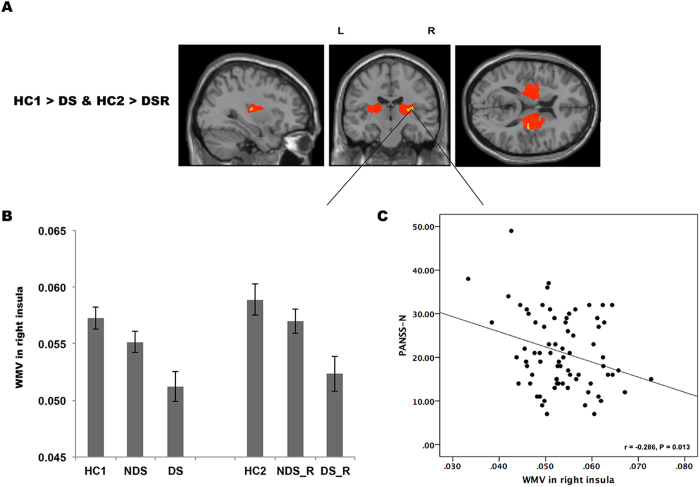
Compared with healthy controls (HC2), relatives of DS patients had significantly less white matter volume in the right insula. This overlapped with the right extra-nuclear cluster where the DS probands had less volume than HC1 (Fig. 2A,B). Relatives of DS patients also had significantly smaller white matter volume in bilateral extra-nuclear regions compared to relatives of NDS patients. No significant volume abnormalities were evident in relatives of NDS patients.
Figure 2. A potential endophenotype of DS.
Both the DS patients (red) and their relatives (yellow) had lower white matter volume in sub-lobar of right insula compared with corresponding controls (A,B). The white matter volume of right insula was inversely correlated with PANSS negative subscale scores in the combined patient groups (C).
Voxel-wise comparisons of FA
Patients
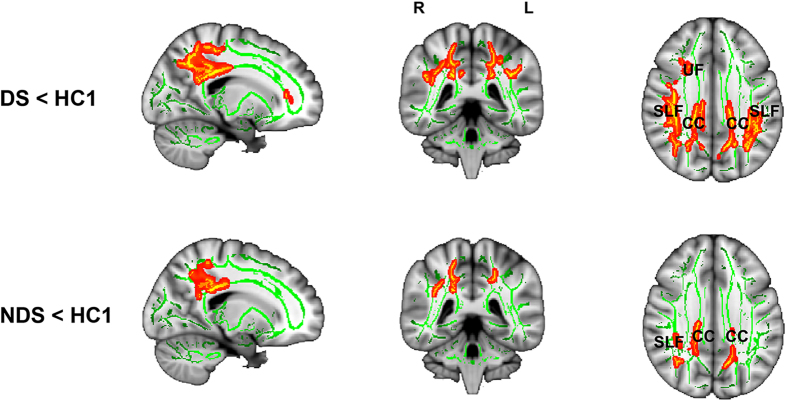
Relative to controls, FA was significantly lower in the body of corpus callosum and the posterior superior longitudinal fasciculus bilaterally in both DS and NDS groups; but regions with lower FA extended to include the right uncinate fasciculus in the DS group (Table 3 and Fig. 3). Direct comparison of whole brain FA maps from DS and NDS groups did not reveal any differences; However, the mean FA extracted from the uncinate fasciculus ROI (which showed an FA difference between patient groups and controls) was significantly lower in the DS group than the NDS group (t73 = 2.852, p = 0.006).
Table 3. Fractional anisotropy differences between groups.
| Regions | P value | Voxels | MNI Coordinates (x, y, z) | ||
|---|---|---|---|---|---|
| Analyses of patient groups | |||||
| DS < HC1 | |||||
| CC extending to SLF | 0.006 | 5984 | 21 | −40 | 43 |
| CC extending to SLF | 0.006 | 4117 | −18 | −44 | 38 |
| Uncinate fasciculus | 0.046 | 140 | 28 | 34 | 4 |
| NDS < HC1 | |||||
| CC extending to SLF | 0.026 | 1131 | 20 | −39 | 41 |
| SLF extending to Posterior Cingulum | 0.035 | 400 | −14 | −60 | 30 |
| CC | 0.031 | 262 | −21 | −39 | 41 |
| SLF | 0.040 | 159 | 35 | −41 | 32 |
| Analyses of relatives | |||||
| NO GROUP DIFFERENCES | |||||
Note: Analyses of patient groups were thresholded at p < 0.05 TFCE corrected; Analyses of relatives were thresholded at p < 0.001 uncorrected; SLF, Superior Longitudinal Fasciculus; CC, corpus callosum.
Figure 3. Fractional anisotropy (FA) differences in DS patients (upper panel) and NDS patients (bottom panel).
The regions showing lower FA in patients compared with healthy controls are shown in red-yellow. Note: SLF, Superior Longitudinal Fasciculus; CC, corpus callosum. UF, uncinate fasciculus.
Relatives
No significant FA differences were found in comparisons including either relative group, even when a threshold of uncorrected p < 0.01 was applied.
Region of interest analyses
Correlations with symptoms
The bilateral extra-nuclear and right insula clusters were defined as regions of interest (ROIs) for subsequent correlation analysis. There was a significant inverse correlation between negative symptoms and white matter volume of the left (r = −0.264, p = 0.022) and right (r = −0.267, p = 0.021) extra-nuclear clusters; and significant inverse correlation right insula (r = −0.286, p = 0.013) across combined patient groups (Figs 1C and 2C). There were no significant correlations between IQ (or DUP) and white matter volumes of the ROIs.
The relationship between volume and microstructure (FA) alterations in white matter
No group differences in FA were detected in any of the ROIs with significant group volume differences.
Post hoc analysis for potential confounders
The DUP was longer in DS patients than NDS patients in our sample. To rule out potentially confounding effects of DUP on the group differences in volume observed, two post hoc analyses were carried out in SPM8. First, a regression analyses using DUP as predictor was performed within the white matter volume maps. As the DUP data was log-transformed to conform to assumptions of normality (Kolmogorov-Smirnov Z = 0.998, p = 0.272). Next, a median split of DUP data was used to divide the NDS group into two groups (each n = 21) with shorter and longer DUP and their white matter indices were compared. None of these analyses revealed a significant effect of DUP on white matter volume of extra-nuclear, even at a tolerant threshold of uncorrected p < 0.01(see Supplementary Information). Thus differences in DUP were unlikely to account for white matter volume differences between DS and NDS groups.
Discussion
In present study, we identified a distinct pattern of white matter alterations in DS patients compared to both healthy controls and NDS patients. We found that, relative to healthy controls, patients with DS (but not NDS) had white matter volume alterations in the cerebellum, bilateral extra-nuclear and bilateral frontoparietal regions. Furthermore, patients with DS had smaller white matter volumes in bilateral extra-nuclear regions compared to those with NDS. We also found that the first-degree relatives of patients with DS shared white matter volume abnormalities in the right insula (a part of the extra-nuclear cluster) with their probands. Greater regional volume deficits in bilateral extra-nuclear were associated with more severe negative symptoms across both patient groups. In addition, compared to controls, patients with DS and NDS had lower FA in the body of corpus callosum and posterior superior longitudinal fasciculus; however only patients with DS had lower FA in the uncinate fasciculus.
In this study, we observed white matter volume alterations in a DS group that correspond closely to results of previous studies of more heterogeneous groups of individuals with first-episode schizophrenia. Previous anatomical studies of schizophrenia have reported differences in the frontoparietal30, extra-nuclear31,32 and cerebellar33 regions. Of these, volume alterations in fronto-limbic white matter is one of the most consistent findings [see meta-analyses19,21].
In contrast, differences in FA between DS and NDS groups were less prominent. Compared to controls, both patient groups had lower FA in bilateral corpus callosum and superior longitudinal fasciculus, and left cingulum. This pattern is consistent with previous studies of first-episode patients34,35,36. However, differences between patients with DS and controls extended to the uncinate fasciculus; and this pattern is similar to the abnormalities reported to accompany chronic DS22,24,25. Our ROI comparison of uncinate fasciculus FA measures in the patient groups confirmed that FA was lower in this region in DS. Taken together, these results suggest that there are some common microstructural white matter alterations in fronto-limbic circuitry in DS and NDS in the early stages of the disorder, but pathology in DS is more extensive than NDS.
Our study reveals that a relatively selective volume reduction in bilateral extra-nuclear pathways characterizes DS; and that there are similar abnormalities in first-degree relatives of those with DS. These results fit with previous reports of volume deficits in extra-nuclear white matter and in the superior longitudinal fasciculus in patients with primary negative symptoms23, or severe negative symptoms37. Disrupted white matter connections between the prefrontal cortex and other cortical regions such as the temporal, insular and occipital cortices; and to sub-cortical structures such as the striatum have been consistently linked with the severity of negative symptoms25,38. Nevertheless, the exact role extra-nuclear white matter in DS is not clear. This extra-nuclear region contains major fibers linking the striatum and frontoparietal cortex, and structural alterations in this pathway could potentially affect the cortico-striatal mechanisms of reward39. In addition, functional abnormalities within the cortico-striatal system are associated with two major negative symptoms, amotivation and anticipatory anhedonia40,41. Thus, structural alterations in the pathway observed here could provide a basis for this functional – and subsequently clinical – phenotype.
Lower extra-nuclear white matter volume is also thought to contribute to the ventricular enlargement31 associated with DS and with features such as poor response to treatment, poor outcome and greater negative symptoms42,43. We captured a similar ‘pathology’ in relatives of patients with DS, suggesting that this volume deficit may be genetically determined. That is, these findings may reflect neurodevelopmental abnormalities which disrupt neuronal migration and organization44 in those at increased genetic risk45,46. This fits with the proposal that DS might represent an early onset non-progressive developmental process which interferes with the acquisition of basic cognitive and social skills from childhood3. It is also consistent with evidence that patients with DS have poor premorbid adjustment across all stages of development; impairment in those with NDS tends to appear in late adolescence or early adulthood47.
White matter volume and FA abnormalities in DS were observed in different brain regions. This may indicate that discrete mechanisms disrupt macrostructural volume and microstructural fibre indices early in the course of schizophrenia. This explanation fits with results from prior studies reporting a similar ‘dissociation’ between white matter volume and FA in patients with schizophrenia48,49. The underlying reason for this dissociation is not clear and may be multi-factorial. For example, lower FA with normal volume measures could arise in regions where there is fiber crossing, myelin loss, a change in the chemical composition of the white matter or differences in the proportion of axonal, oligodendrocyte and glial cellular components20,50. Further research will however be needed to better understand the cellular underpinnings of white matter abnormalities in schizophrenia.
Limitations
There are a number of limitations to our study. 1) The main clinical difference between our DS and NDS groups was a longer DUP in DS group. To address this, we conducted post hoc analyses and the results indicated that the extra-nuclear white matter volume differences in DS were independent of DUP. 2) We used PANSS scores and IQ to explore the clinical and cognitive significance of white matter changes. However, further more specific psychological or cognitive assessment targeted at the deficit syndrome would help us better understand the functional meaning of MRI findings. This will be a focus of future studies. 3) The 15 diffusion gradients DTI data used in this study could also have limited our sensitivity to detected changes in microstructural integrity of white matter fibres. However, at the start of the study we used what was optimal at the time and during the course of this large study we elected not to change the scanning parameters. 4) Finally, as in any study of schizophrenia there are multiple potential confounds such as lifestyle, diet, exercise, relatively low-dosage alcohol and tobacco usage. These are acknowledged as potentially important as they may independently impact on brain structure51,52.
In this study, we identified a distinctive pattern of white matter alterations in first-episode patients with DS. Specifically, we propose that lower volume in extra-nuclear white matter may be a biological marker of DS. To our knowledge, this is the first report of a neuroanatomical endophenotype for a subgroup of schizophrenia. The results should encourage further study of clinically and biologically homogenous samples within the schizophrenia spectrum.
Methods
Participants
Two hundred and eleven subjects, including 75 first-episode patients (33 DS and 42 NDS) with schizophrenia, 58 unaffected first-degree relatives of these patients, and 78 healthy controls participated in this study.
All patients were recruited at the Mental Health Centre of the West China Hospital, Sichuan University. Diagnoses of schizophrenia were confirmed using the Structured Clinical Interview for the DSM-IV Patient Edition (SCID-I/P)53 shortly after presentation to the mental health services in their first episode of psychotic illness. Data collected from patients initially diagnosed with schizophreniform psychosis (n = 20, 6 DS and 14 NDS) were included if a diagnosis of schizophrenia was confirmed after at least 6 months follow-up. The diagnoses of DS and NDS were reached using the Schedule for the Deficit Syndrome (SDS)54, 12 months after admission.
The psychiatric history of each patient was reviewed in order to exclude patients with a history of any other major psychiatric disorder (including schizophrenia, bipolar disorder, depression and schizoaffective disorders), mental retardation, head trauma, current substance abuse and neurological disorders. Information about previous treatment and duration of DUP was collected at diagnosis. Five patients (6.6%; 2 DS and 3 NDS) were taking low dose antipsychotics (risperidone or olanzapine; 25 to 75 mg of chlorpromazine daily dose equivalent) for less than 3 days prior to MRI. All others (93.4%, 23 DS and 39 NDS) were treatment-naïve before scanning. Symptom severity was evaluated using the Positive and Negative Syndrome Scale (PANSS) in the patient groups29.
Current IQ was estimated for all subjects using the seven-subtest version (including Information, Similarities, Arithmetic, Digit Span, Picture Completion, Block Design, and Digit Symbol subtests)55 of the Wechsler Adult Intelligence Scale-revised in China (WAIS-RC)56.
All controls and relatives were screened for a lifetime absence of Axis I illness of DSM-IV psychiatric illnesses with the SCID non-patient version (SCID-I/NP)57. Relatives were assigned to two subgroups; relatives of DS (DS_R) or relatives of NDS (NDS_R). Because relatives were generally older than first-episode patients, two groups of ‘healthy’ controls were age- and sex-matched with patients (HC1) and relatives (HC2) to avoid confound the brain structural differences due to age58. Relatives or controls with any major psychiatric or neurological disorder, serious physical illness, alcohol or drug abuse, pregnancy, head trauma, or mental retardation were excluded from the study. We excluded subjects with a history of alcohol abuse, according to DSM-IV criteria. The number of smokers in HC1, NDS and DS groups was 5, 10 and 8, respectively. The number of smokers in HC2, NDS_R and DS_R was 8, 12 and 8 respectively. The proportion of smokers was not significantly different across patient groups (χ2 = 2.001, p = 0.368), or relative groups (χ2 = 1.353, p = 0.508).
All participants were Han Chinese and right-handed as assessed by the Annett Handedness Scale59. Written informed consent was obtained from all participants individually, and with their guardians as appropriate. This study was carried out in accordance with the Declaration of Helsinki and was approved by were approved by the Institutional Review Board of West China Hospital, Sichuan University.
MRI data acquisition
Structural MRI
High-resolution T1-weighted images were acquired from patients on admission using a 3-Tesla MRI system (EXCITE, General Electric, WI, USA) and an eight-channel phase array head coil with a volumetric 3D Spoiled Gradient Recall (SPGR) sequence (TR = 8.5 ms, TE = 3.4 ms, slice thickness = 1.0 mm with no gap, field of view = 24 × 24 cm2, matrix size = 256 × 256), generating 156 contiguous axial slices with the in-plane resolution of 0.47 mm × 0.47 mm.
DTI
Whole brain diffusion-weighted images were recorded along 15 gradient directions (b = 1000 s/mm2, number of excitations = 2) together with one unweighted (b = 0) image (42 images in total). Each image was acquired using a single-shot spin echo planar imaging (EPI) sequence (TR = 10 000 ms, TE = 70.8 ms, slice thickness = 3.0 mm with no gap, field of view = 24 × 24 cm2, matrix size = 128 × 128, voxel resolution 0.94 × 0.94 × 3 mm3).
An experienced neuroradiologist qualitatively inspected the raw MRI data. No gross abnormalities were observed for any participant.
MRI data analysis
Structural MRI
Structural data were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) running on Matlab7.6 (MathWorks, Inc., Natick, MA, USA). The preprocessing included the following steps: (1) T1-weighted images were realigned and then manually reoriented so that the anterior commissure was positioned at coordinate [0, 0, 0]; (2) MINC non-parametric, non-uniformity intensity normalization software (http://wiki.bic.mni.mcgill.ca /index.php/MINC) was used to rectify the uniformity of high-magnetic field signals in reoriented images; (3) the images were then segmented into gray matter, white matter, and cerebrospinal fluid probability maps using the unified segmentation option; (4) The white matter images were spatially normalized to MNI space and Jacobian modulated into volume images using the Dartel (Diffeomorphic Anatomical Registration using the Exponentiated Lie algebra) toolbox60 implemented in SPM8, and finally (5) smoothed with a 6 mm FWHM Gaussian kernel.
DTI
DTI data were preprocessed using the FMRIB’s Diffusion Toolbox (FDT) within FSL (http://www.fmrib.ox.ac.uk/fsl). The preprocessing included the following steps: (1) the diffusion-weighted volumes were aligned to their corresponding b0 image with an affine transformation to minimize image distortion from eddy currents and head motion. (2) The non-brain tissue and background noise were removed from b0 image using BET. (3) The diffusion tensor for each voxel was estimated by the multivariate linear fitting algorithm. Finally (4) voxel-wise values of FA (fractional anisotropy) were calculated.
Whole brain analysis of FA images was performed using the TBSS toolbox61. In brief, FA maps of all subjects were first realigned to a common target and then normalized to a 1 × 1 × 1 mm3 Montreal Neurological Institute standard space (MNI152) via the FMRIB58_FA template. Thereafter, the registered FA images were averaged to generate a cross-subject mean FA image, and the mean FA image applied to create a mean FA skeleton representing the center of all fiber tracts common to the group. The mean FA skeleton was further thresholded by a FA value of 0.2 to exclude peripheral tracts with significant inter-subject variability and/or partial volume effects due to grey matter. Each participant’s aligned FA dataset was then projected onto this skeleton.
Statistical analysis
Demographic data
Statistical analysis of demographic data was conducted using SPSS16.0 for Windows (SPSS Inc., Chicago, IL, USA). Where appropriate parametric (analysis of variance, ANOVA: or Student’s t-test) and non-parametric (Mann-Whitney’s U test) procedures were used to compare the distribution and differences of categorical and continuous data between groups. For categorical data, χ2 tests were applied.
VBM
Voxel-wise comparisons of white matter volume in the patient and control groups were performed using an analysis of covariance (ANCOVA) followed by post hoc, two-sample t-tests (between DS and HC1, NDS and HC1, and DS and NDS, respectively) in SPM8. The threshold for ANCOVA test was set at p < 0.001, this initial screening threshold was adopted to optimize sensitivity for small structural changes in patients. However, in order to control more stringently for multiple comparisons, a combined voxel-level p < 0.0001 and cluster-level p < 0.05 (FWE corrected) was adopted for post hoc, two-sample t-tests.
The analyses of data from the relatives groups (contrasts between DS_R and HC2, NDS_R and HC2, and DS_R and NDS_R) were also completed using ANCOVA and post hoc two-sample t-tests. However, anticipating less volume alteration in otherwise unaffected relatives, we adopted a somewhat less conservative statistical threshold of uncorrected p < 0.001 and cluster extent of 100 voxels for both ANCOVA and post hoc two-sample t-tests.
For all the voxel-wise comparisons of white matter volume, an explicit white matter mask constructed from all subjects (n = 211) was applied to ensure that only voxels within the white matter were analyzed. Finally, whole brain volume (total gray and white matter volume; WBV) was used as covariate in every analysis. To further evaluate the potential impact of age we also specified a model with both WBV and age as nuisance variables. The pattern of results remained unchanged - not shown.
FA
The voxel-wise comparisons of skeletonised FA maps were performed using the randomise62 toolkit implemented in FSL, which carries out permutation-based, nonparametric inference. Comparisons of patients and relatives with their respective control groups were performed separately using ANCOVA and post hoc two-sample t-tests, with WBV as covariate. As in the analysis of volume, the threshold of the initial ANCOVA was set at an uncorrected P < 0.001. For comparisons involving the patient groups, we reported results at P < 0.05 corrected for multiple comparisons using the threshold-free cluster enhancement (TFCE) method63 in post hoc two-sample t-tests. For comparisons involving the relative groups we adopted a less stringent statistical threshold of uncorrected p < 0.001.
ROI analysis
To further characterize the functional significance of potential biological markers, clusters that showed selective volume or FA alterations in DS (or NDS) were defined as ROIs. The volume and FA values within each ROI were extracted from each patient’s dataset and correlations with PANSS scores (including total score, positive, negative and general psychopathological subscale scores, respectively), IQ and DUP were explored.
We also planned to examine any relationship between the volume and FA of ROI clusters showing volume or FA differences in patients.
The alpha value was accepted as p < 0.05 for ROI analyses.
Additional Information
How to cite this article: Lei, W. et al. White matter alterations in first episode treatment-naïve patients with deficit schizophrenia: a combined VBM and DTI study. Sci. Rep. 5, 12994; doi: 10.1038/srep12994 (2015).
Supplementary Material
Acknowledgments
This work was partly funded by National Nature Science Foundation of China (81130024 and 81461168029 to TL, 81271479 to QW), National Key Technology R & D Program of the Ministry of Science and Technology of China during the 12th Five-Year Plan (2012BAI01B06, TL), the Ph.D. Programs Foundation of Ministry of Education of China (20110181110014, TL). GMcA receives salary support from the Sackler Institute for Translational Neurodevelopment at King’s College London. She also receives support from the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Author Contributions W.L. and N.L. conducted the data analyses and wrote the first version of the manuscript with intellectual input from G.M. and T.L. W.D. and W.L. provided the analysis tools. M.L., Z.H., Y.H., C.H., X.M., Q.W., W.G., Y.L., L.J., Y.Z. and X.H. contributed in the data collection. All authors contributed to and have approved the final manuscript.
References
- Tsuang M. T., STONE W. S. & Faraone S. V. Genes, environment and schizophrenia. The British Journal of Psychiatry 178, s18–s24 (2001). [DOI] [PubMed] [Google Scholar]
- Tandon R., Nasrallah H. A. & Keshavan M. S. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophrenia research 110, 1–23 (2009). [DOI] [PubMed] [Google Scholar]
- Galderisi S. & Maj M. Deficit schizophrenia: An overview of clinical, biological and treatment aspects. European psychiatry 24, 493–500 (2009). [DOI] [PubMed] [Google Scholar]
- Carpenter W. T., Heinrichs D. W. & Wagman A. M. Deficit and nondeficit forms of schizophrenia: the concept. The American journal of psychiatry 145, 578–583 (1988). [DOI] [PubMed] [Google Scholar]
- Tek C., Kirkpatrick B. & Buchanan R. A five-year followup study of deficit and nondeficit schizophrenia. Schizophrenia research 49, 253–260 (2001). [DOI] [PubMed] [Google Scholar]
- Strauss G. P., Harrow M., Grossman L. S. & Rosen C. Periods of Recovery in Deficit Syndrome Schizophrenia: A 20-Year Multi–follow-up Longitudinal Study. Schizophrenia bulletin 36, 788–799 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B. & Galderisi S. Deficit schizophrenia: an update. World Psychiatry 7, 143–147 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T. P. Role of biomarkers and endophenotypes in prevention and treatment of psychopathological disorders. Biomarkers in Medicine 3, 1–3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman I. I. & Gould T. D. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry 160, 636–645 (2003). [DOI] [PubMed] [Google Scholar]
- Allen A. J., Griss M. E., Folley B. S., Hawkins K. A. & Pearlson G. D. Endophenotypes in schizophrenia: a selective review. Schizophrenia research 109, 24–37 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboyer M. et al. Psychiatric genetics: search for phenotypes. Trends in neurosciences 21, 102–105 (1998). [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B., Buchanan R. W., Ross D. E. & Carpenter W. T. Jr A separate disease within the syndrome of schizophrenia. Archives of General Psychiatry 58, 165–171 (2001). [DOI] [PubMed] [Google Scholar]
- Hong L. E., Avila M. T., Adami H., Elliot A. & Thaker G. K. Components of the smooth pursuit function in deficit and nondeficit schizophrenia. Schizophrenia research 63, 39–48 (2003). [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D., Smith E. E., Eichler F. S. & Filley C. M. Cerebral white matter. Annals of the New York Academy of Sciences 1142, 266–309 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterfang M., Wood S. J., Velakoulis D. & Pantelis C. Neuropathological, neurogenetic and neuroimaging evidence for white matter pathology in schizophrenia. Neuroscience & Biobehavioral Reviews 30, 918–948 (2006). [DOI] [PubMed] [Google Scholar]
- Yao L. et al. Association of white matter deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized VBM study using 3T. Magnetic Resonance Materials in Physics, Biology and Medicine 27, 283–290 (2014). [DOI] [PubMed] [Google Scholar]
- Wright I. C. et al. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry 157, 16–25 (2000). [DOI] [PubMed] [Google Scholar]
- Arnone D., McIntosh A., Tan G. & Ebmeier K. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophrenia research 101, 124–132 (2008). [DOI] [PubMed] [Google Scholar]
- Di X., Chan R. C. & Gong Q. Y. White matter reduction in patients with schizophrenia as revealed by voxel-based morphometry: an activation likelihood estimation meta-analysis. Progress in neuro-psychopharmacology and biological psychiatry 33, 1390–1394 (2009). [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I. & Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia research 108, 3–10 (2009). [DOI] [PubMed] [Google Scholar]
- Bora E. et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophrenia research 127, 46–57 (2011). [DOI] [PubMed] [Google Scholar]
- Kitis O. et al. Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non‐deficit schizophrenia. Psychiatry and Clinical Neurosciences 66, 34–43 (2012). [DOI] [PubMed] [Google Scholar]
- Sigmundsson T. et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. American Journal of Psychiatry 158, 234–243 (2001). [DOI] [PubMed] [Google Scholar]
- Rowland L. M. et al. White matter alterations in deficit schizophrenia. Neuropsychopharmacology 34, 1514–1522 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos A. N. et al. Neuroimaging Evidence for the Deficit Subtype of Schizophrenia. JAMA psychiatry 113, 472–480 (2013). [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B., Conley R. C., Kakoyannis A., Reep R. L. & Roberts R. C. Interstitial cells of the white matter in the inferior parietal cortex in schizophrenia: An unbiased cell‐counting study. Synapse 34, 95–102 (1999). [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B., Messias N. C., Conley R. R. & Roberts R. C. Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. The Journal of nervous and mental disease 191, 563–567 (2003). [DOI] [PubMed] [Google Scholar]
- Buchanan R. W. et al. Structural abnormalities in deficit and nondeficit schizophrenia. American Journal of Psychiatry 150, 59–59 (1993). [DOI] [PubMed] [Google Scholar]
- Kay S. R., Flszbein A. & Opfer L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin 13, 261–276 (1987). [DOI] [PubMed] [Google Scholar]
- Whitford T. et al. Volumetric white matter abnormalities in first-episode schizophrenia: a longitudinal, tensor-based morphometry study. American Journal of Psychiatry 164, 1082–1089 (2007). [DOI] [PubMed] [Google Scholar]
- Chua S. E. et al. Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophrenia research 89, 12–21 (2007). [DOI] [PubMed] [Google Scholar]
- Price G. et al. Brain pathology in first-episode psychosis: magnetization transfer imaging provides additional information to MRI measurements of volume loss. NeuroImage 49, 185–192 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthaus H. et al. White matter abnormalities in subjects at ultra high-risk for schizophrenia and first-episode schizophrenic patients. Schizophrenia research 102, 141–149 (2008). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. White-matter microstructure in previously drug-naive patients with schizophrenia after 6 weeks of treatment. Psychological medicine 43, 2301–2309 (2013). [DOI] [PubMed] [Google Scholar]
- Cheung V. et al. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychological medicine 38, 877–885 (2008). [DOI] [PubMed] [Google Scholar]
- Lee S.-H. et al. Extensive white matter abnormalities in patients with first-episode schizophrenia: a diffusion tensor imaging (DTI) study. Schizophrenia research 143, 231–238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillere-Martinot M.-L. et al. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophrenia research 50, 19–26 (2001). [DOI] [PubMed] [Google Scholar]
- Filippi M. et al. Patterns of Brain Structural Changes in First-Contact, Antipsychotic Drug-Naïve Patients with Schizophrenia. American Journal of Neuroradiology 35, 30–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring A. M. & Barch D. M. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology 24, 725–736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring A. M., Gur R. E., Blanchard J. J., Horan W. P. & Reise S. P. The clinical assessment interview for negative symptoms (CAINS): Final development and validation. American Journal of Psychiatry 170, 165–172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S. A., Der-Avakian A. & Markou A. Anhedonia, avolition, and anticipatory deficits: assessments in animals with relevance to the negative symptoms of schizophrenia. European Neuropsychopharmacology 24, 744–758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N. C., Olsen S. A., Dennert J. W. & Smith M. R. Ventricular enlargement in schizophrenia: relationship to positive and negative symptoms. American Journal of Psychiatry 139, 297–302 (1982). [DOI] [PubMed] [Google Scholar]
- Davis K. L. et al. Ventricular enlargement in poor-outcome schizophrenia. Biological Psychiatry 43, 783–793 (1998). [DOI] [PubMed] [Google Scholar]
- Rapoport J. L., Addington A. M. & Frangou S. The neurodevelopmental model of schizophrenia: update 2005. Molecular psychiatry 10, 434–449 (2005). [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol H. E. et al. Genetic contributions to human brain morphology and intelligence. The Journal of Neuroscience 26, 10235–10242 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu H. et al. Regional white matter abnormalities in drug-naive, first-episode schizophrenia patients and their healthy unaffected siblings. The Australian and New Zealand journal of psychiatry 49, 246–254 (2015). [DOI] [PubMed] [Google Scholar]
- Galderisi S. et al. Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: a multicenter study. American Journal of Psychiatry 159, 983–990 (2002). [DOI] [PubMed] [Google Scholar]
- Chan W.-Y. et al. White matter abnormalities in first-episode schizophrenia: a combined structural MRI and DTI study. Schizophrenia research 119, 52–60 (2010). [DOI] [PubMed] [Google Scholar]
- Moriya J. et al. Gray and white matter volumetric and diffusion tensor imaging (DTI) analyses in the early stage of first-episode schizophrenia. Schizophrenia research 116, 196–203 (2010). [DOI] [PubMed] [Google Scholar]
- Kubicki M. et al. A review of diffusion tensor imaging studies in schizophrenia. Journal of psychiatric research 41, 15–30 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Schubert F. & Gallinat J. Reduced thickness of medial orbitofrontal cortex in smokers. Biological psychiatry 68, 1061–1065 (2010). [DOI] [PubMed] [Google Scholar]
- Gondoh Y. et al. Effects of aerobic exercise training on brain structure and psychological well-being in young adults. The Journal of sports medicine and physical fitness 49, 129–135 (2009). [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M. & Williams J. B. W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). (New York: State Psychiatric Institute, , 2002) (Website: http://www.scid4.org/index.html). [Google Scholar]
- Kirkpatrick B., Buchanan R. W., McKenny P. D., Alphs L. D. & Carpenter W. T. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry research 30, 119–123 (1989). [DOI] [PubMed] [Google Scholar]
- Schopp L. H., Callahan C. D., Johnstone B. & Schwake C. J. Utility of a seven-subtest version of the WAIS-R among an Alzheimer’s disease sample. Archives of clinical neuropsychology 13, 637–643 (1998). [PubMed] [Google Scholar]
- Gong Y. Wechsler adult intelligence scale-revised in China Version. (Hunan Medical School Press, 1992) (in Chinese). [Google Scholar]
- Spitzer M., Robert L., Gibbon M. & Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP). (New York: State Psychiatric Institute, , 2002) (Website: http://www.scid4.org/index.html). [Google Scholar]
- Nenadić I., Sauer H., Smesny S. & Gaser C. Aging effects on regional brain structural changes in schizophrenia. Schizophrenia bulletin 38, 838–844 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British journal of psychology 61, 303–321 (1970). [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage 38, 95–113 (2007). [DOI] [PubMed] [Google Scholar]
- Smith S. M. et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31, 1487–1505 (2006). [DOI] [PubMed] [Google Scholar]
- Hayasaka S. & Nichols T. E. Validating cluster size inference: random field and permutation methods. Neuroimage 20, 2343–2356 (2003). [DOI] [PubMed] [Google Scholar]
- Smith S. M. & Nichols T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.