Abstract
Chronic lymphocytic leukemia (CLL) is the most common lymphoproliferative disorder in the Western world and predominantly affects older people. Until recently, most studies in CLL focused on younger patients in whom intensive therapy with the addition of rituximab to fludarabine and cyclophosphamide was shown to improve survival. Obinutuzumab is a novel type II anti-CD20 monoclonal antibody (mAb) that recently demonstrated an overall survival advantage when combined with chemotherapy in previously untreated older patients with CLL and comorbidities. Obinutuzumab was superior to rituximab in this same study in terms of response rates and progression-free survival. Several preclinical and early phase clinical studies also support the efficacy of obinutuzumab. The most frequent adverse event noted with obinutuzumab is infusion-related reactions, which occur more frequently than with rituximab and are typically restricted to the first cycle of therapy. Based on these results, obinutuzumab should be considered the gold standard mAb for combination with chemotherapy in previously untreated patients with CLL and comorbidities. The marked efficacy of obinutuzumab with a weak chemotherapy backbone implies significant potency of this mAb, making it the ideal partner for combination studies with other agents in CLL.
Keywords: CD20, chronic lymphocytic leukemia, monoclonal antibody, obinutuzumab
Introduction
The development of the anti-CD20 monoclonal antibody (mAb) rituximab has greatly optimized the treatment of CD20+ lymphoproliferative disorders, including that of chronic lymphocytic leukemia (CLL). CD20 is an ideal target for directed therapy, being highly expressed on most B cells [Glennie et al. 2007] but not expressed on stem cells, precursor cells, or the majority of plasma cells. As such, B-cell development and mature antibody production are not impaired by anti-CD20 therapy [Czuczman and Gregory, 2010].
Despite low-level expression of CD20 on CLL cells, rituximab added to intensive chemotherapy including fludarabine and cyclophosphamide (FCR) in previously untreated, young, fit patients with CLL led to an overall survival (OS) advantage, the first such demonstration of an OS advantage in any phase III clinical trial in CLL [Hallek et al. 2010]. However, despite this important impact of rituximab in CLL, its single agent efficacy is only modest [Hainsworth et al. 2003], and most patients with CLL eventually either fail to respond or relapse after rituximab-containing therapies. Because CD20 mAbs are of such importance in the treatment of B-cell malignancies, great efforts have been underway to develop novel mAbs that can provide greater efficacy than rituximab. Several such mAbs have been developed and are currently being investigated with the majority (including rituximab) being type I antibodies. These antibodies function via stabilization of CD20 on lipid rafts, resulting in strong complement (C1q) binding in vitro and potent induction of complement-dependent cytotoxicity (CDC) and significant antibody-dependent cellular cytotoxicity (ADCC) [Bannerji et al. 2003; Cragg et al. 2003; Di Gaetano et al. 2003; Cragg and Glennie, 2004; Kennedy et al. 2004; Uchida et al. 2004; Bologna et al. 2011; Dalle et al. 2011]. A second class of mAbs, the type II antibodies, do not require lipid rafts and thus leave CD20 distributed across the surface of the B cell (Figure 1). They have much lower in vitro complement binding and CDC, but result in significantly greater ADCC and direct cell death (DCD) compared with type I mAbs [Bologna et al. 2011; Dalle et al. 2011; Niederfellner et al. 2011]. Obinutuzumab (GA101, RO5072759) is the first type II mAb investigated in CLL and has shown efficacy in in vitro studies, animal models and clinical trials, and is the focus of this review.
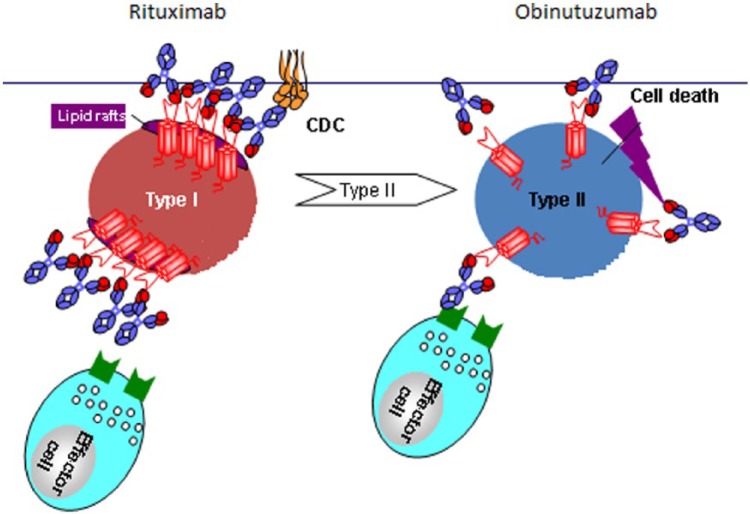
Figure 1.
The schematic represents the differing mechanisms of action of type I (rituximab) and type II (obinutuzumab) antibodies. Type I antibodies function via stabilization of CD20 on lipid rafts, resulting in vitro in strong complement-dependent cytotoxicity (CDC) while type II antibodies leave CD20 distributed across the surface of the B cell and have much lower in vitro CDC, but greater ADCC and direct cell death (DCD). Obinutuzumab has a glycoengineered Fc portion, selected to increase its affinity for FcγRIIIa receptors on immune effector cells, and a modified elbow hinge region to provide superior antigen binding.
Source: GA101 overview presentation at B021005 and B021223 study investigator meetings, July 2011, San Francisco, CA. Permission to use and modify from Michael Wenger, Global Clinical Lead GA101, Hoffman-La Roche.
Mechanism of action
Obinutuzumab is a humanized anti-CD20 mAb that has a glycoengineered Fc portion, selected to increase its affinity for FcγRIIIa receptors on immune effector cells. This increased affinity for immune effector cells such as neutrophils and macrophages is intended to elicit enhanced ADCC. Obinutuzumab also contains a modified elbow hinge region to provide superior antigen binding [Mossner et al. 2010; Niederfellner et al. 2011]. The elbow hinge modification is reported to increase DCD but at the expense of reduced CDC activity. Both antibody modifications were designed to induce much greater cell killing by obinutuzumab compared with rituximab [Alduaij et al. 2011].
Many of the mechanisms of action of obinutuzumab appear different from those of rituximab. Obinutuzumab activates neutrophils and mediates phagocytosis through CD16B on neutrophils more potently than rituximab. Additionally, the glycoengineered obinutuzumab elicits neutrophil-induced phagocytosis much more effectively than the parental nonglycoengineered antibody. Because of these differences, in whole blood, efficient induction of phagocytosis was elicited by obinutuzumab whereas no significant phagocytosis was observed with rituximab [Golay et al. 2013]. Other studies have determined that the DCD induced by obinutuzumab occurs by a nonapoptotic process involving actin reorganization and lysosomes, with this leading to more DCD with obinutuzumab compared with rituximab [Ivanov et al. 2009; Alduaij et al. 2011; Jak et al. 2011]. The importance of lysosomes in the induction of cell death is very important to the mechanism of action of obinutuzumab because it is a novel mechanism that appears unique to type II antibodies [Ivanov et al. 2009]. This mechanism of cell killing is independent of classic apoptosis pathways so it has been postulated that using lysosomal-induced cell death, obinutuzumab may be able to overcome resistance to chemotherapy-induced apoptosis [Ivanov et al. 2009; Alduaij et al. 2011]. Obinutuzumab has also been shown to induce apoptosis in a caspase-dependent manner, with mitochondria important to early caspase activity [Reslan et al. 2014]. Another study showed that reactive oxygen species were critical for programmed cell death induced by several mAbs, including obinutuzumab, observations made in human B-cell lymphoma cell lines and primary CLL cells [Honeychurch et al. 2012]. Thus, the exact mechanism of cell killing by obinutuzumab is clearly multifaceted.
The distinct actions of obinutuzumab compared with rituximab are hoped to overcome the different mechanisms of resistance that have been described with rituximab. The mechanisms of resistance to rituximab are complex and multiple with several studies clarifying different mechanisms, including CD20 ‘shaving’ in which rituximab/CD20 complexes are removed from the B-cell surface by monocytes through an endocytic reaction called trogocytosis [Beum et al. 2008; Pedersen et al. 2011], aberrant lipid raft composition of some malignant B cells [Boyd et al. 2009], complement depletion [Klepfish et al. 2009], polymorphisms in the FcγRIIIa receptor reducing the affinity of the Fc receptor for rituximab [Cartron et al. 2002], downregulation of proapoptotic proteins [Olejniczak et al. 2008] and reduction in CD20 antigen expression levels after treatment with rituximab [Hiraga et al. 2009]. However, to date, studies have not determined how many of these mechanisms might also apply to type II mAbs like obinutuzumab or whether obinutuzumab will be able to overcome these resistance mechanisms.
Preclinical data
In vitro studies
Several in vitro studies of obinutuzumab have been performed and demonstrate superior efficacy over rituximab. The first studies attempted to mimic in vivo conditions by using whole blood assays that incorporated immune effector cells as well as complement so that ADCC, CDC and DCD could all be measured [Mossner et al. 2010]. Effective B-cell depletion by obinutuzumab was demonstrated in whole blood from 10 health donors with significantly greater B-cell depletion than noted with rituximab. Similar findings were reported when examining malignant B cells in the whole blood of a patient with CLL. Other assays have included binding to non-Hodgkin lymphoma (NHL) cells lines, assays of cell death, ADCC and CDC, and B-cell depletion measures in peripheral blood from healthy donors. In these studies, obinutuzumab exhibited increased DCD compared with type I mAbs in a panel of NHL cells lines, and exhibited up to 100 times higher ADCC activity but significantly less CDC compared with the type I anti-bodies [Mossner et al. 2010; Herter et al. 2013].
Animal models
The efficacy of obinutuzumab has also been demonstrated in animal models, showing effective B-cell depletion by the drug in Cynomolgus monkeys with particular improvement in B-cell depletion in the spleen and lymph nodes of the monkeys compared with rituximab [Mossner et al. 2010]. Xenograft models using severe combined immune deficiency (SCID) beige mice and NHL cell lines also demonstrated improved tumor killing by obinutuzumab compared with rituximab [Dalle et al. 2011; Herter et al. 2013]. Xenograft models were also used to examine chemoimmunotherapy by combining obinutuzumab or rituximab with the chemotherapeutic agents fludarabine, bendamustine or cyclophosphamide. Obinutuzumab in combination with fludarabine or bendamustine in a mantle cell lymphoma model exhibited significantly better tumor inhibition than the same chemotherapy with rituximab. In these studies, obinutuzumab as a single agent was as effective as the combination of rituximab with bendamustine or fludarabine [Herting et al. 2014]. Similarly, obinutuzumab induced stronger inhibition of tumor growth than rituximab in a model using a follicular lymphoma cell line, both as a single agent and in combination with cyclophosphamide [Dalle et al. 2011].
Finally, a study using a diffuse large B-cell lymphoma cell line in xenografted mice attempted to investigate the efficacy of obinutuzumab in the setting of rituximab resistance. The mice were treated with weekly rituximab until tumors developed at an advanced stage. The mice were then randomized to receive more rituximab, a placebo injection or obinutuzumab. The tumors continued to grow rapidly in the rituximab and placebo arms but tumor growth was successfully arrested in the obinutuzumab-treated animals, suggesting efficacy of obinutuzumab in this model of rituximab resistance [Mossner et al. 2010].
Pharmacokinetic studies
Pharmacokinetic data were analyzed in two phase I/II studies of obinutuzumab monotherapy with results used to determine the dose for all subsequent phase III studies. Plasma concentrations of obinutuzumab were obtained pre and post infusion in the GAUGUIN study (in which obinutuzumab was dosed every 3 weeks for eight cycles) and the GAUSS study (in which obinutuzumab was provided weekly for 4 weeks) [Sehn et al. 2012; Cartron et al. 2014]. Higher levels of obinutuzumab were obtained more quickly with the weekly infusion schedule and higher plasma concentrations were also noted in the higher dose (1600 mg/800 mg) than the lower dose (400 mg/400 mg) groups in the GAUGUIN study [Cartron et al. 2014].
The elimination of obinutuzumab appears to be complex and involves both a linear clearance and time-dependent (nonlinear) clearance pathway. Based on population pharmacokinetic studies, the steady-state mean volume of distribution is approximately 3.8 L with an elimination half life of 28 days and terminal clearance of 0.09 L/day. The elimination of obinutuzumab is likely target-mediated drug disposition [Shah, 2014]. The relationship between pharmacokinetics and clinical response or tumor burden could not be concluded based on phase I/II trials due to small population numbers; however, the phase II study in patients with CLL reported disappointing response rates in patients with higher tumor burden, suggesting that dosing schedules may be very important for obinutuzumab’s efficacy [Cartron et al. 2014]. Insufficient data exist on the effect of severe renal or hepatic impairment, however no modifications are expected since monoclonal antibodies are metabolized via ubiquitous proteolytic enzymes [Shah, 2014]. Further pharmacokinetic data are anticipated with the ongoing GAGE study, which compares the efficacy and safety of obinutuzumab dosed intravenously at 1000 versus 2000 mg [Flynn et al. 2014]. The preliminary results suggest higher drug levels with the higher dose with a resultant higher overall response rate (ORR) at 67% compared with 49% (p = 0.08) and no new safety signals noted with the higher dose.
Clinical studies
Phase I studies
Small numbers of patients with relapsed/refractory CLL were included in early phase I studies of obinutuzumab as these included mostly patients with NHL [Salles et al. 2012]. The drug was safe with no dose-limiting toxicities and responses were noted at all dose levels. Grade 1–2 infusion-related reactions (IRRs), most frequently with the first dose, were very common, as were minor (grade 1–2) infections. In patients with CLL, grade 3–4 neutropenia was also frequent [Cartron et al. 2014].
The GALTON phase 1b study examined obinutuzumab in combination with FC (fludarabine and cyclophophamide) or B (bendamustine) using investigator choice of chemotherapy backbone in patients with previously untreated CLL. The results revealed high levels of grade 3–4 hematological toxicities as expected with these chemotherapy regimens, as well as high rates of IRRs, which were not dose limiting. The ORR was 62% in the FC-containing arm and 90% in the B arm, though several patients had treatment discontinued due to adverse events (AEs). The conclusion of the authors was that obinutuzumab could safely be administered with intensive chemotherapy to previously untreated patients with CLL [Brown et al. 2013].
Phase II studies
The GAUGIN phase II study in patients with CLL included 20 patients with relapsed/refractory CLL who received obinutuzumab at 1000 mg on day 1, 8, 15, 22 and then every 3 weeks for a total of 10 infusions. Like the phase I study, there were several (six) grade 3–4 IRRs and four grade 3–4 neutropenias. The end of treatment response was 20% with four patients with partial remission (PR) and five with stable disease (SD). A relationship was noted between the level of tumor burden and the response rate as the four patients with PR had lower tumor burdens than the nonresponding patients. The conclusion from this study was that obinutuzumab was safe in patients with advanced CLL but that the single-agent activity was modest and combination chemoimmunotherapy was likely to be necessary for most patients with CLL, especially those with higher tumor burdens [Cartron et al. 2014].
Phase III
The German CLL Study Group (GCLLSG) CLL11 study was a multicenter, open-label, randomized, three-arm phase III study investigating the efficacy and safety of obinutuzumab plus chlorambucil (CLB) versus rituximab plus CLB versus CLB monotherapy in previously untreated patients with CLL of advanced age with comorbidities [Goede et al. 2014b]. The CLL11 study focused on a patient population that had previously been neglected in clinical trials, with most CLL studies having focused on young, fit patients who are better able to tolerate novel or intensive therapies. The study design included two stages of analysis, the first examining the comparison of both antibody arms against CLB monotherapy to determine if anti-CD20 mAbs were valuable in this older, unfit population and the second stage investigating a comparison between the obinutuzumab–CLB and rituximab–CLB arms. A safety run-in study in six patients first ensured that the combination of obinutuzumab and CLB was safe and feasible in this older, frailer CLL population [Goede et al. 2010]. In the safety run-in, AEs were noted but were not dose limiting and included a high incidence of IRRs (five of six patients) that were generally limited to the first infusion and grade 3–4 neutropenias that were not associated with fever, infection or requirement for antibiotics. Some treatment delays occurred but all patients completed the planned therapy.
The final results of the study demonstrate an advantage in terms of overall response rate (ORR) with more complete remissions (CRs) and improved progression-free survival (PFS) with the addition of an anti-CD20 mAb (obinutuzumab or rituximab) to CLB monotherapy. At the time of the original publication, an OS advantage was noted in the obinutuzumab–CLB arm compared with the CLB monotherapy arm (p = 0.002) but no such difference was observed in the rituximab–CLB group (p = 0.11). Recently, an update of the study was presented with nearly another year of follow up. The updated results demonstrate an OS advantage also for the rituximab–CLB group compared with CLB monotherapy (p = 0.0242), confirming that anti-CD20 mAb therapy is valuable for the treatment of all patients with CLL with first-line therapy [Goede et al. 2015].
Obinutuzumab was also superior to rituximab with a statistically significant and clinically important improvement in PFS (26.7 months versus 11.1 months) and a trend to an OS advantage (p = 0.08) [Goede et al. 2014b]. The advantage of obinutuzumab was noted in all analyzed subgroups with the exception of patients with del(17p), who did poorly in both groups. After the lengthier follow up, the PFS was still much longer for the obinutuzumab-treated patients (29.2 months) than for the rituximab-treated patients (15.4 months) (p < 0.001) and the time to next treatment was longer in the obinutuzumab–CLB group (42.7 months versus 32.7 months, p < 0.001). However, there was still no difference in OS between the obinutuzumab–CLB and rituximab–CLB groups (p = 0.0632) [Goede et al. 2015].
Minimal residual disease (MRD) analysis was also performed as a secondary outcome in the study, and surprisingly, demonstrated high rates of MRD negativity in the obinutuzumab–CLB group, with more than a 10-fold higher incidence in the peripheral blood than that observed with rituximab. No MRD eradication was noted in the CLB monotherapy arm. MRD eradication has been correlated with improvements in OS in younger, fit patients with CLL [Böttcher et al. 2012]; but until now, such deep responses were not thought possible in frailer or older patients. The ability to elicit high CR and MRD negativity rates in combination with a weak chemotherapy agent like CLB was very surprising and supports a marked potency of obinutuzumab in CLL. The significant improvement in MRD eradication in the obinutuzumab versus rituximab arms also suggests that an OS advantage may develop with longer follow up in the obinutuzumab versus rituximab comparison.
The improved outcomes in the obinutuzumab–CLB arm were not without some challenges. Toxicity profiles were notable in the study with a high incidence of IRRs with the first obinutuzumab infusion, leading to treatment discontinuation in 7% of patients. However, IRRs were extremely uncommon after cycle 1 and the majority of IRRs were grade 1–2, suggesting that treatment discontinuation should not be necessary. More importantly, the incidence of severe infections and/or treatment-related deaths was not increased in the obinutuzumab group compared with the rituximab or CLB monotherapy groups. The only other difference in toxicity was a small increase in severe thrombocytopenia in the obinutuzumab group.
Conclusion and perspective
Obinutuzumab has now proven to be superior to rituximab in a phase III clinical trial of previously untreated patients with CLL and comorbidities. The results of the GCLLSG CLL11 study are important and practice changing in establishing obinutuzumab–CLB as a gold-standard treatment for patients with CLL and comorbidities, being the first study in such patients to demonstrate an OS advantage. The detection of a survival advantage in the rituximab–CLB group, albeit with a shorter PFS, confirms the importance of anti-CD20 mAb therapy in CLL.
The marked increased incidence of IRRs with the first obinutuzumab infusion in the CLL11 study could cause concern for some physicians and should be anticipated and managed accordingly. As the CLL11 patient population was more frail than that of most clinical trials, there were significantly more treatment discontinuations in the study than was observed in the phase I/II studies. However, as the majority of IRRs are grade 1–2 and are restricted to the first infusion and the patients who experienced grade 3–4 IRRs generally did not experience problems with subsequent infusions, these IRRs should be manageable just like rituximab IRRs are manageable by physicians experienced with its use. The CLL11 study included an amendment late in the accrual period that mandated division of the first dose of the antibody and routine premedications for all patients. Unfortunately, as these measures were implemented so late, it was not possible to confidently conclude if they ameliorated the rate or severity of IRRs. However, the authors have found that the decision to provide a small dose of obinutuzumab on day 1 (100 mg) leads to most patients successfully completing their therapy. While infusion reactions are common, their severity appears to be reduced by the use of premedication with corticosteroids and the majority of patients will have no problems with day 2 of therapy after completing the small dose for day 1. Unfortunately, the study investigators were not able to identify clinical features that predict which patients will experience more profound IRRs making caution necessary for all patients at the time of the first infusion. A recent subanalysis of the study suggested that an increased expression of CD20 on CLL cells predicted for a greater chance of IRRs. The same study was unable to demonstrate that the addition of the glucocorticoid premedication significantly reduced IRRs, however there was no randomization to this treatment, making the analysis difficult [Freeman et al. 2014]. There are other important AEs with obinutuzumab, including an increase in severe thrombocytopenia, also noted only in the first cycle in the CLL11 study; and the risk of reactivation of hepatitis B or the development of progressive multifocal leukoencephalopathy, risks likely shared by all anti-CD20 mAbs. However, the drug is otherwise very well tolerated and none of the reported toxicities should temper the excitement at the introduction of a superior mAb.
Many investigators have criticized the comparison of rituximab at its established dose, with obinutuzumab because of the higher administered dose of obinutuzumab. This is because some early studies of single-agent rituximab suggested improved results in patients with higher serum concentrations of the antibody [Byrd et al. 2001; Cartron et al. 2007] and an advanced modeling study suggested that a higher dose of rituximab might improve results in induction and maintenance therapy in indolent NHL [Ternant et al. 2012]. Also, early studies of rituximab in CLL demonstrated disappointing response rates, much inferior to those noted in most other subtypes of NHL and this was predicted to result from reduced drug levels of rituximab caused by altered pharmacokinetics or to the low density of CD20 expression on CLL cells, both of which were predicted to potentially be improved by increased doses of rituximab [Stolz and Schuler, 2009]. However, a large study of dose-dense rituximab in combination with FC failed to show any improvement in outcomes in young, fit patients with CLL [O’Brien et al. 2005]. The French GOELAMS group is currently conducting a phase II clinical trial of intensified prophase rituximab before FCR with MRD negativity as a primary endpoint [ClinicalTrials.gov identifier: NCT01370772]. While not a phase III study, this trial will hopefully help to answer the question of whether intensifying the dose of rituximab may lead to improved CLL results that may mimic those noted with obinutuzumab.
Future directions
This is a very exciting time in the management of CLL with the recent introduction of several novel agents in this disease, all demonstrating marked efficacy. Targeted therapy of the B-cell receptor via inhibition of Bruton’s tyrosine kinase (BTK) or the phosphoinositide 3-kinase (PI3) kinase pathway and bcl-2 inhibitors are all actively being investigated in phase III studies in previously untreated patients with CLL after early trials demonstrated efficacy and safety [Byrd et al. 2013; Brown et al. 2014; Seymour et al. 2014]. Studies of ibrutinib (the first in class BTK inhibitor) and idelalisib (the first in class PI3k δ inhibitor) in patients with relapsed or refractory CLL have already been published, showing marked efficacy and significant improvements in PFS and OS compared with single-agent type I anti-CD20 mAbs (rituximab or ofatumumab) [Byrd et al. 2014; Furman et al. 2014]. Interestingly, there was initial concern that ibrutinib antagonized the function of rituximab via reduction of immune effector cell function whereas this inhibitory effect is not observed with obinutuzumab in combination with ibrutinib in in vitro and in vivo studies [Herter et al. 2014].
Obinutuzumab and CLB has become an accepted comparator for previously untreated patients with CLL in whom intensive therapy with FCR (the only other therapy to demonstrate an OS advantage in frontline therapy of patients with CLL when it was examined in comparison to FC alone in young, fit patients with CLL) is inappropriate. There are now several studies planned or accruing in previously untreated older or frailer patients with CLL, comparing against obinutuzumab and CLB. These studies include the GCLLSG CLL14 study [ClinicalTrials.gov identifier: NCT02242942] comparing GDC-0199 (previously ABT-199) with obinutuzumab versus obinutuzumab–CLB, a study [ClinicalTrials.gov identifier: NCT02264574] comparing ibrutinib with obinutuzumab versus obinutuzumab–CLB, and another study [ClinicalTrials.gov identifier: NCT01980875] comparing idelalisib with obinutuzumab versus obinutuzumab–CLB. Obinutuzumab also proved effective in patients who had relapsing disease or failed to respond to CLB in the CLL11 study, demonstrating that this mAb may have a key role not only in the treatment of therapy-naïve patients [Goede et al. 2014a].
Therefore, the results of the CLL11 phase III study in previously untreated patients with CLL and comorbidities showing an OS advantage with the addition of obinutuzumab to chemotherapy makes obinutuzumab a highly desirable agent in the treatment of patients with CLL. The CLL11 results justify the replacement of rituximab with obinutuzumab in the treatment of CLL, at least in previously untreated patients with comorbidities. The finding of superiority to rituximab in this patient population, which is very representative of the average patient with CLL, suggests that obinutuzumab is likely the best mAb for patients with CLL; though ongoing studies in other CLL patient groups and in the relapsed or refractory setting are required to confirm this.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Owen and Dr Stewart have received honaria and participated in advisory boards with Hoffman La-Roche and have been the local principal investigators for clinical trials sponsored by Hoffman La-Roche.
Contributor Information
Carolyn J. Owen, Departments of Medicine and Oncology, University of Calgary, 603 South Tower, Foothills Medical Centre, Calgary, Alberta T2N 2T9, Canada
Douglas A. Stewart, Departments of Medicine and Oncology, University of Calgary, Alberta, Canada
References
- Alduaij W., Ivanov A., Honeychurch J., Cheadle E., Potluri S., Lim S., et al. (2011) Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 117: 4519–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerji R., Kitada S., Flinn I., Pearson M., Young D., Reed J., et al. (2003) Apoptotic-regulatory and complement-protecting protein expression in chronic lymphocytic leukemia: relationship to in vivo rituximab resistance. J Clin Oncol 21: 1466–1471. [DOI] [PubMed] [Google Scholar]
- Beum P., Lindorfer M., Taylor R. (2008) Within peripheral blood mononuclear cells, antibody-dependent cellular cytotoxicity of rituximab-opsonized Daudi cells is promoted by NK cells and inhibited by monocytes due to shaving. J Immunol 181: 2916–2924. [DOI] [PubMed] [Google Scholar]
- Bologna L., Gotti E., Manganini M., Rambaldi A., Intermesoli T., Introna M., et al. (2011) Mechanism of action of type, I.I., glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol 186: 3762–3769. [DOI] [PubMed] [Google Scholar]
- Böttcher S., Ritgen M., Fischer K., Stilgenbauer S., Busch R., Fingerle-Rowson G., et al. (2012) Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol 30: 980–988. [DOI] [PubMed] [Google Scholar]
- Boyd R., Jukes-Jones R., Walewska R., Brown D., Dyer M., Cain K. (2009) Protein profiling of plasma membranes defines aberrant signaling pathways in mantle cell lymphoma. Mol Cell Proteomics 8: 1501–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Byrd J., Coutre S., Benson D., Flinn I., Wagner-Johnston N., et al. (2014) Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia Blood 123: 3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., O’Brien S., Kingsley C., Eradat H., Pagel J., Lymp J., et al. (2013) Safety and efficacy of obinutuzumab (GA101) with fludarabine/cyclophosphamide (G-FC) or bendamustine (G-B) in the initial therapy of patients with chronic lymphocytic leukemia (CLL): results from the phase 1b galton trial (GAO4779g). Blood (ASH Annual Meeting Abstracts) 122: 523a.23719303 [Google Scholar]
- Byrd J., Brown J., O’Brien S., Barrientos J., Kay N., Reddy N., et al. (2014) Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 371: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J., Furman R., Coutre S., Flinn I., Burger J., Blum K., et al. (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 369: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J., Murphy T., Howard R., Lucas M., Goodrich A., Park K., et al. (2001) Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol 19: 2153–2164. [DOI] [PubMed] [Google Scholar]
- Cartron G., Blasco H., Paintaud G., Watier H., Le Guellec C. (2007) Pharmacokinetics of rituximab and its clinical use: thought for the best use? Crit Rev Oncol Hematol 62: 43–52. [DOI] [PubMed] [Google Scholar]
- Cartron G., Dacheux L., Salles G., Solal-Celigny P., Bardos P., Colombat P., et al. (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 99: 754–758. [DOI] [PubMed] [Google Scholar]
- Cartron G., de Guibert S., Dilhuydy M, Morschhauser F., Leblond V., Dupuis J., et al. (2014) Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood 124: 2196–2202. [DOI] [PubMed] [Google Scholar]
- Cragg M., Glennie M. (2004) Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood 103: 2738–2743. [DOI] [PubMed] [Google Scholar]
- Cragg M., Morgan S., Chan H., Morgan B., Filatov A., Johnson P., et al. (2003) Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood 101: 1045–1052. [DOI] [PubMed] [Google Scholar]
- Czuczman M., Gregory S. (2010) The future of CD20 monoclonal antibody therapy in B-cell malignancies. Leuk Lymphoma 51: 983–994. [DOI] [PubMed] [Google Scholar]
- Furman R., Sharman J., Coutre S., Cheson B., Pagel J., Hillmen P., et al. (2014) Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 370: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle S., Reslan L., Besseyre de Horts T., Herveau S., Herting F., Plesa A., et al. (2011) Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther 10: 178–185. [DOI] [PubMed] [Google Scholar]
- Di Gaetano N., Cittera E., Nota R., Vecchi A., Grieco V., Scanziani E., et al. (2003) Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol 171: 1581–1587. [DOI] [PubMed] [Google Scholar]
- Flynn J., Byrd J., Kipps T., Boxer M., Kolibaba K., Tyson N., et al. (2014) Obinutuzumab (GA101) 1,000 mg versus 2,000 mg in patients with chronic lymphocytic leukemia (CLL): results of the phase II GAGE (GAO4768g) trial. J Clin Oncol 32: abstract 7083. [Google Scholar]
- Freeman C., Morschhauser F., Sehn L., Dixon M., Houghton R., Lamy T., et al. (2014) Pattern of cytokine release in patients with chronic lymphocytic leukemia treated with obinutuzumab and possible relationship with development of infusion related reactions (IRR). Blood (ASH Annual Meeting Abstracts) 122: 4674a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennie M., French R., Cragg M., Taylor R. (2007) Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol 44: 3823–3837. [DOI] [PubMed] [Google Scholar]
- Goede V., Engelke A., Fischer K., Jimenez J., Kuzmin A., Dyer M., et al. (2014a) Salvage therapy with obinutuzumab (GA101) plus chlorambucil (Clb) after treatment failure of Clb alone in patients with chronic lymphocytic leukemia (CLL) and comorbidities: results of the CLL11 study. Blood (ASH Annual Meeting Abstracts) 122: 3327a. [Google Scholar]
- Goede V., Fischer K., Busch R., Engelke A., Eichhorst B., Wendtner C., et al. (2014b) Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370: 1101–1110. [DOI] [PubMed] [Google Scholar]
- Goede V., Fischer K., Engelke A., Schlag R., Lepetre S., Montero L.F., et al. (2015) Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia. [DOI] [PubMed] [Google Scholar]
- Goede V., Fischer K., Raymonde B., Jaeger U., Dilhuydy M., Wickham N., et al. (2010) Chemoimmunotherapy with chlorambucil and the type II CD20 antibody GA101 in patients with chronic lymphocytic leukemia and comorbidity: results of the run-in phase of the CLL11 (B021004) trial. Blood (ASH Annual Meeting Abstracts) 116: 1387a. [Google Scholar]
- Golay J., Da Roit F., Bologna L., Ferrara C., Leusen J., Rambaldi A., et al. (2013) Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 122: 3482–3491. [DOI] [PubMed] [Google Scholar]
- Hainsworth J., Litchy S., Barton J., Houston G., Hermann R., Bradof J., et al. (2003) Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 21: 1746–1751. [DOI] [PubMed] [Google Scholar]
- Hallek M., Fischer K., Fingerle-Rowson G., Fink A., Busch R., Mayer J., et al. (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 376: 1164–1174. [DOI] [PubMed] [Google Scholar]
- Herter S., Herting F., Mundigl O., Waldhauer I., Weinzierl T., Fauti T., et al. (2013) Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther 12: 2031–2042. [DOI] [PubMed] [Google Scholar]
- Herter S., Sagiv-Barfi I., Chester C., Sadaram M., Hebb J., Czerwinski D., et al. (2014) Obinutuzumab (GA101) is less prone to antagonism of immune effector function by ibrutinib than rituximab in vitro and in vivo. Blood (ASH Annual Meeting Abstracts) 122: 1765a. [Google Scholar]
- Herting F., Friess T., Bader S., Muth G., Hölzlwimmer G., Rieder N., et al. (2014) Enhanced anti-tumor activity of the glycoengineered type II CD20 antibody obinutuzumab (GA101) in combination with chemotherapy in xenograft models of human lymphoma. Leuk Lymphoma 55: 2151–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga J., Tomita A., Sugimoto T., Shimada K., Ito M., Nakamura S., et al. (2009) Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood 113: 4885–4893. [DOI] [PubMed] [Google Scholar]
- Honeychurch J., Alduaij W., Azizyan M., Cheadle E., Pelicano H., Ivanov A., et al. (2012) Antibody-induced nonapoptotic cell death in human lymphoma and leukemia cells is mediated through a novel reactive oxygen species-dependent pathway. Blood 119: 3523–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A., Beers S., Walshe C., Honeychurch J., Alduaij W., Cox K., et al. (2009) Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J Clin Invest 119: 2143–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak M., van Bochove G., Reits E., Kallemeijn W., Tromp J., Umana P., et al. (2011) CD40 stimulation sensitizes CLL cells to lysosomal cell death induction by type II anti-CD20 monoclonal antibody GA101. Blood 118: 5178–5188. [DOI] [PubMed] [Google Scholar]
- Kennedy A., Beum P., Solga M., DiLillo D., Lindorfer M., Hess C., et al. (2004) Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol 172: 3280–3288. [DOI] [PubMed] [Google Scholar]
- Klepfish A., Gilles L., Ioannis K., Rachmilewitz E., Schattner A., et al. (2009) Enhancing the action of rituximab in chronic lymphocytic leukemia by adding fresh frozen plasma: complement/rituximab interactions & clinical results in refractory CLL. Ann NY Acad Sci 1173: 865–873. [DOI] [PubMed] [Google Scholar]
- Mossner E., Brunker P., Moser S., Püntener U., Schmidt C., Herter S., et al. (2010) Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115: 4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederfellner G., Lammens A., Mundigl O., Georges G., Schaefer W., Schwaiger M., et al. (2011) Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood 118: 358–367. [DOI] [PubMed] [Google Scholar]
- O’Brien S., Wierda W., Faderl S., Ferrajoli A., Bueso-Ramos C., Browning M., et al. (2005) FCR-3 as frontline therapy for patients with chronic lymphocytic leukemia (CLL). Blood (ASH Annual Meeting Abstracts) 106: 2117a. [Google Scholar]
- Olejniczak S., Hernandez-Ilizaliturri F., Clements J., Czuczman M. (2008) Acquired resistance to rituximab is associated with chemotherapy resistance resulting from decreased Bax and Bak expression. Clin Cancer Res 14: 1550–1560. [DOI] [PubMed] [Google Scholar]
- Pedersen A., Jungersen M., Pedersen C. (2011) Monocytes mediate shaving of B-cell-bound anti-CD20 antibodies. Immunology 133: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reslan L., Dalle S., Herveau S., Perrial E., Dumontet C. (2014) Apoptotic induction by anti-CD20 antibodies in chronic lymphocytic leukemia: comparison of rituximab and obinutuzumab. Leuk Lymphoma. 55: 188–190. [DOI] [PubMed] [Google Scholar]
- Salles G., Morschhauser F., Lamy T., Milpied N., Thieblemont C., Tilly H., et al. (2012) Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood 119: 5126–5132. [DOI] [PubMed] [Google Scholar]
- Sehn L., Assouline S., Stewart D., Mangel J., Gascoyne R., Fine G., et al. (2012) A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood 119: 5118–5125. [DOI] [PubMed] [Google Scholar]
- Seymour J., Davids M., Pagel J., Kahl B., Wierda W., Puvvada S., et al. (2014) ABT-199 (GDC-0199) in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL): High complete- response rate and durable disease control. J Clin Oncol 32: 7015a. [Google Scholar]
- Shah A. (2014) Obinutuzumab: a novel anti-CD20 monoclonal antibody for previously untreated chronic lymphocytic leukemia. Ann. Pharmacother 48: 1356–1361. [DOI] [PubMed] [Google Scholar]
- Stolz C., Schuler M. (2009) Molecular mechanisms of resistance to rituximab and pharmacologic strategies for its circumvention. Leuk Lymphoma 50: 873–885. [DOI] [PubMed] [Google Scholar]
- Ternant D., Cartron G., Hénin E., Tod M., Girard P., Paintaud G. (2012) Model-based design of rituximab dosage optimization in follicular non-Hodgkin’s lymphoma. Br J Clin Pharmacol 73: 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida J., Hamaguchi Y., Oliver J., Ravetch J., Poe J., Haas K., et al. (2004) The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 199: 1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]