Abstract
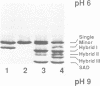
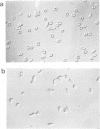
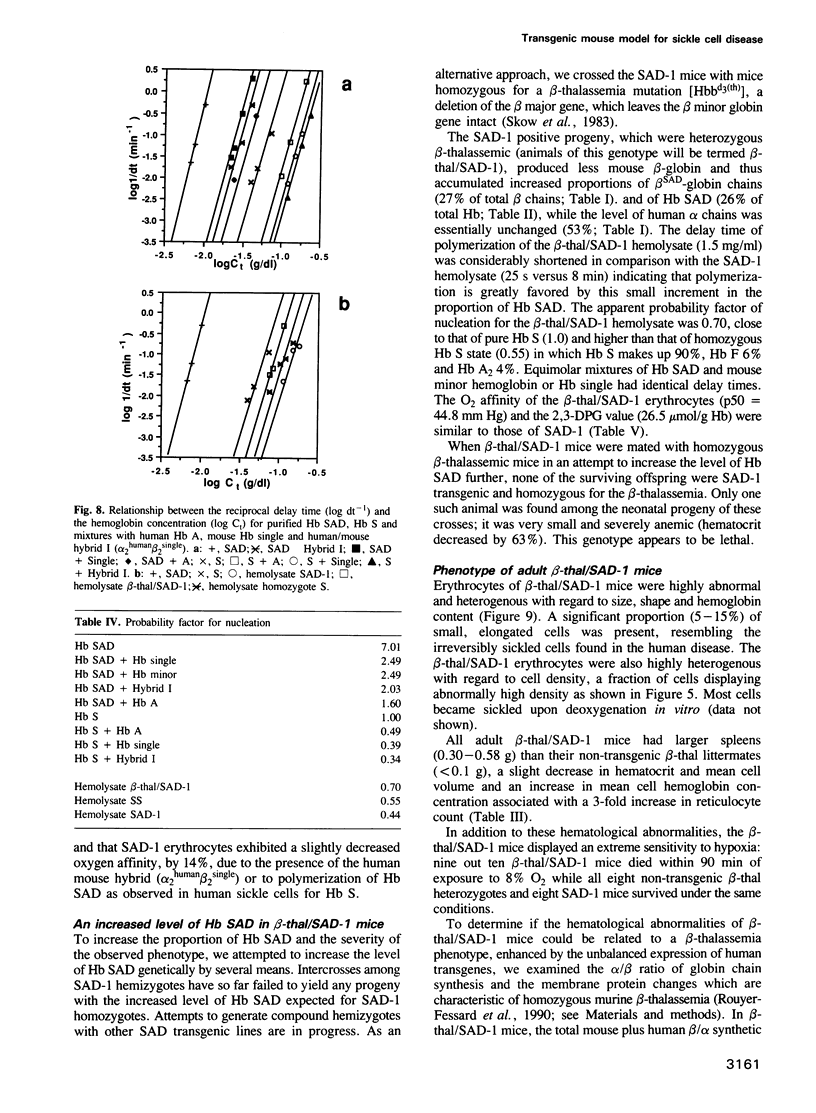
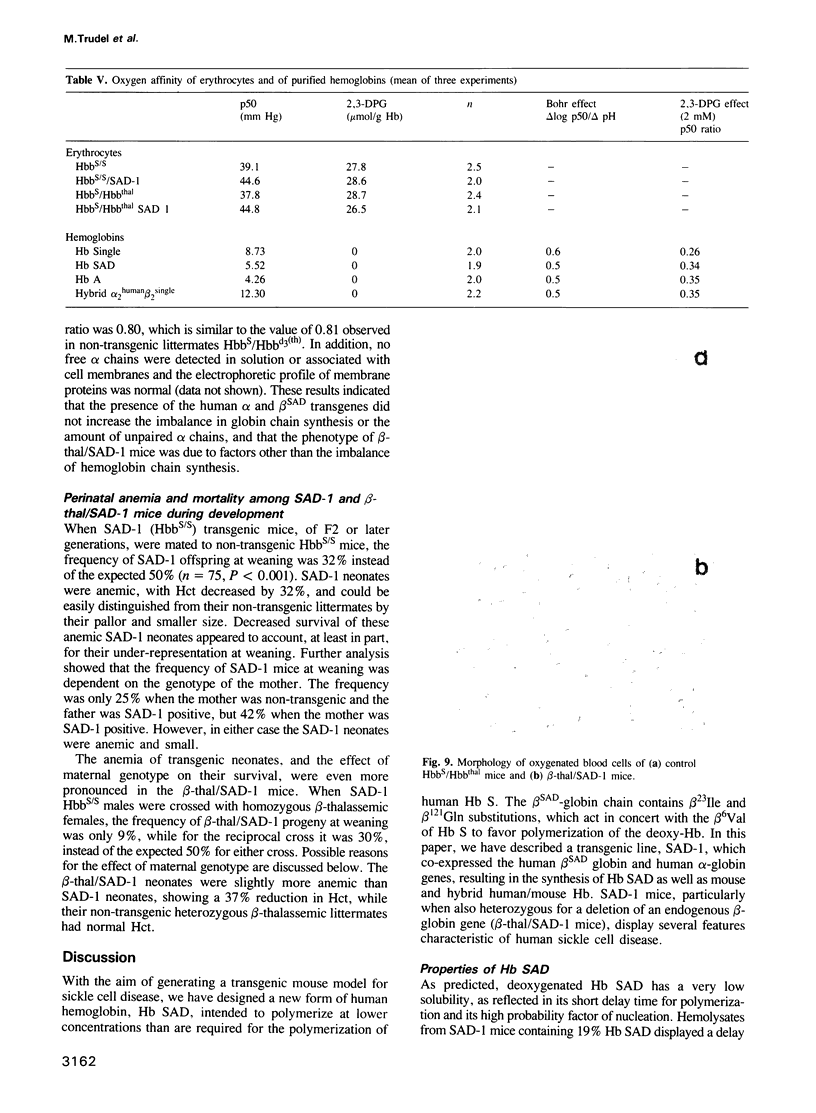
In order to obtain a transgenic mouse model of sickle cell disease, we have synthesized a novel human beta-globin gene, beta SAD, designed to increase the polymerization of the transgenic human hemoglobin S (Hb S) in vivo. beta SAD (beta S-Antilles-D Punjab) includes the beta 6Val substitution of the beta S chain, as well as two other mutations, Antilles (beta 23Ile) and D Punjab (beta 121Gln) each of which promotes the polymerization of Hb S in human. The beta SAD gene and the human alpha 2-globin gene, each linked to the beta-globin locus control region (LCR) were co-introduced into the mouse germ line. In one of the five transgenic lines obtained, SAD-1, red blood cells contained 19% human Hb SAD (alpha 2 human 1 beta 2SAD) and mouse-human hybrids in addition to mouse hemoglobin. Adult SAD-1 transgenic mice were not anemic but had some abnormal features of erythrocytes and slightly enlarged spleens. Their erythrocytes displayed sickling upon deoxygenation in vitro. SAD-1 neonates were anemic and many did not survive. In order to generate adult mice with a more severe sickle cell syndrome, crosses between the SAD progeny and homozygous for beta-thalassemic mice were performed. Hemoglobin SAD was increased to 26% in beta-thal/SAD-1 mice which exhibited: (i) abnormal erythrocytes with regard to shape and density; (ii) an enlarged spleen and a high reticulocyte count indicating an increased erythropoiesis; (iii) mortality upon hypoxia; (iv) polymerization of hemolysate similar to that obtained in human homozygous sickle cell disease; and (v) anemia and mortality during development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. C., Reese A., Stallings M., Huisman T. H. Separation of human hemoglobins by DEAE-cellulose chromatography using glycine-KCN-NaC1 developers. Hemoglobin. 1976;1(1):27–44. doi: 10.3109/03630267609031020. [DOI] [PubMed] [Google Scholar]
- Adachi K., Kim J., Ballas S., Surrey S., Asakura T. Facilitation of Hb S polymerization by the substitution of Glu for Gln at beta 121. J Biol Chem. 1988 Apr 25;263(12):5607–5610. [PubMed] [Google Scholar]
- Adachi K., Kim J., Kinney T. R., Asakura T. Effect of the beta 73 amino acid on the hydrophobicity, solubility, and the kinetics of polymerization of deoxyhemoglobin S. J Biol Chem. 1987 Aug 5;262(22):10470–10474. [PubMed] [Google Scholar]
- Adachi K., Ozguc M., Asakura T. Nucleation-controlled aggregation of deoxyhemoglobin S. Participation of hemoglobin A in the aggregation of deoxyhemoglobin S in concentrated phosphate buffer. J Biol Chem. 1980 Apr 10;255(7):3092–3099. [PubMed] [Google Scholar]
- Alter B. P., Goff S. C., Efremov G. D., Gravely M. E., Huisman T. H. Globin chain electrophoresis: a new approach to the determination of the G gamma/A gamma ratio in fetal haemoglobin and to studies of globin synthesis. Br J Haematol. 1980 Apr;44(4):527–534. doi: 10.1111/j.1365-2141.1980.tb08706.x. [DOI] [PubMed] [Google Scholar]
- Bardakdjian-Michau J., Galactéros F., Craescu C. T. Functional and NMR studies of Hb Sassari (Asp-126 alpha----His); role of the inter-subunit contacts in the affinity control of human hemoglobin. Biochim Biophys Acta. 1990 Dec 5;1041(3):250–253. doi: 10.1016/0167-4838(90)90279-o. [DOI] [PubMed] [Google Scholar]
- Basset P., Beuzard Y., Garel M. C., Rosa J. Isoelectric focusing of human hemoglobin: its application to screening, to the characterization of 70 variants, and to the study of modified fractions of normal hemoglobins. Blood. 1978 May;51(5):971–982. [PubMed] [Google Scholar]
- Blouquit Y., Bardakdjian J., Lena-Russo D., Arous N., Perrimond H., Orsini A., Rosa J., Galacteros F. Hb Bruxelles: alpha 2A beta (2)41 or 42(C7 or CD1)Phe deleted. Hemoglobin. 1989;13(5):465–474. doi: 10.3109/03630268908998085. [DOI] [PubMed] [Google Scholar]
- Brugnara C., Bunn H. F., Tosteson D. C. Ion content and transport and the regulation of volume in sickle cells. Ann N Y Acad Sci. 1989;565:96–103. doi: 10.1111/j.1749-6632.1989.tb24155.x. [DOI] [PubMed] [Google Scholar]
- Costantini F., Chada K., Magram J. Correction of murine beta-thalassemia by gene transfer into the germ line. Science. 1986 Sep 12;233(4769):1192–1194. doi: 10.1126/science.3461564. [DOI] [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves D. R., Fraser P., Vidal M. A., Hedges M. J., Ropers D., Luzzatto L., Grosveld F. A transgenic mouse model of sickle cell disorder. Nature. 1990 Jan 11;343(6254):183–185. doi: 10.1038/343183a0. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991 Jan 15;77(2):214–237. [PubMed] [Google Scholar]
- Milner P. F., Miller C., Grey R., Seakins M., DeJong W. W., Went L. N. Hemoglobin O arab in four negro families and its interaction with hemoglobin S and hemoglobin C. N Engl J Med. 1970 Dec 24;283(26):1417–1425. doi: 10.1056/NEJM197012242832601. [DOI] [PubMed] [Google Scholar]
- Monplaisir N., Merault G., Poyart C., Rhoda M. D., Craescu C., Vidaud M., Galacteros F., Blouquit Y., Rosa J. Hemoglobin S Antilles: a variant with lower solubility than hemoglobin S and producing sickle cell disease in heterozygotes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9363–9367. doi: 10.1073/pnas.83.24.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzarelli A., Hofrichter J., Eaton W. A. Delay time of hemoglobin S polymerization prevents most cells from sickling in vivo. Science. 1987 Jul 31;237(4814):500–506. doi: 10.1126/science.3603036. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Love W. E. Refined crystal structure of deoxyhemoglobin S. II. Molecular interactions in the crystal. J Biol Chem. 1985 Jul 15;260(14):8280–8291. [PubMed] [Google Scholar]
- Popp R. A., Popp D. M., Johnson F. M., Skow L. C., Lewis S. E. Hematology of a murine beta-thalassemia: a longitudinal study. Ann N Y Acad Sci. 1985;445:432–444. doi: 10.1111/j.1749-6632.1985.tb17213.x. [DOI] [PubMed] [Google Scholar]
- Rouyer-Fessard P., Garel M. C., Domenget C., Guetarni D., Bachir D., Colonna P., Beuzard Y. A study of membrane protein defects and alpha hemoglobin chains of red blood cells in human beta thalassemia. J Biol Chem. 1989 Nov 15;264(32):19092–19098. [PubMed] [Google Scholar]
- Rouyer-Fessard P., Leroy-Viard K., Domenget C., Mrad A., Beuzard Y. Mouse beta thalassemia, a model for the membrane defects of erythrocytes in the human disease. J Biol Chem. 1990 Nov 25;265(33):20247–20251. [PubMed] [Google Scholar]
- Rubin E. M., Lu R. H., Cooper S., Mohandas N., Kan Y. W. Introduction and expression of the human Bs-globin gene in transgenic mice. Am J Hum Genet. 1988 Apr;42(4):585–591. [PMC free article] [PubMed] [Google Scholar]
- Rubin E. M., Witkowska H. E., Spangler E., Curtin P., Lubin B. H., Mohandas N., Clift S. M. Hypoxia-induced in vivo sickling of transgenic mouse red cells. J Clin Invest. 1991 Feb;87(2):639–647. doi: 10.1172/JCI115041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. M., Townes T. M., Reilly M. P., Asakura T., Palmiter R. D., Brinster R. L., Behringer R. R. Human sickle hemoglobin in transgenic mice. Science. 1990 Feb 2;247(4942):566–568. doi: 10.1126/science.2154033. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skow L. C., Burkhart B. A., Johnson F. M., Popp R. A., Popp D. M., Goldberg S. Z., Anderson W. F., Barnett L. B., Lewis S. E. A mouse model for beta-thalassemia. Cell. 1983 Oct;34(3):1043–1052. doi: 10.1016/0092-8674(83)90562-7. [DOI] [PubMed] [Google Scholar]