Abstract
Vitamin D has known importance to bone health including calcium and phosphate homeostasis and appears to have a role in skeletal muscle health as well. Cases of vitamin D deficiency and insufficiency have been associated with poor muscle health. While the exact effects and mechanism of action remains controversial, current data lean towards insufficient vitamin D playing a role in musculoskeletal pain, sarcopenia, myopathy, falls and indirectly via cerebellar and cognitive dysfunction. Sophisticated experimental techniques have allowed detection of the vitamin D receptor (VDR) on skeletal muscle and cerebellar tissue, which if validated in further large studies, could confirm the mechanism of vitamin D in these associations. While further study is required, vitamin D repletion can have a substantial impact on muscle as well as bone health.
Keywords: bone, calcium, muscle, musculoskeletal pain, myopathy, vitamin D receptors, calcitriol, cholecalciferol, ergocalciferol, sarcopenia, vitamin D, vitamin D deficiency, falls
Introduction
The importance of vitamin D on bone mineralization is well established. Often considered more of a steroid hormone than a vitamin, vitamin D has been recognized to exert wide-ranging effects, including a potentially important role in muscle health. Vitamin D status is usually defined by the concentration of calcidiol or 25-hydroxy vitamin D [25(OH)D] because this form reflects total body storage and is the precursor to the activated metabolite, 1,25-dihydroxyvitamin D [Schwartz et al. 2014]. Vitamin D insufficiency, as measured by serum 25(OH)D levels, is considered endemic according to the US National Health and Nutrition Examination Survey (NHANES) for 2005 and 2006 when the mean 25(OH)D level among several age groups was 24 ng per milliliter (60 nmol/l). Furthermore, supplementation with vitamin D to achieve a serum 25(OH)D level of between 75 and 110 nmol/l is thought to provide optimal benefits without increasing health risks [Bischoff-Ferrari et al. 2010]. Thus it is important to identify potential health consequences of insufficient vitamin D levels [Moshfegh et al. 2009].
Vitamin D deficiency has been shown to be a contributor to diffuse and nonspecific musculoskeletal pain [Heidari et al. 2010]. Its deficiency has also been linked to muscle weakness in the elderly [Girgis, 2014] as well as sarcopenia [Wagatsuma and Sakuma,. 2014]. Vitamin D exerts its effects on extra skeletal tissues through the vitamin D receptor (VDR), a transcription factor that is activated by 1,25-dihydroxyvitamin D to regulate gene transcription [Pike et al. 2014]. For this reason, localization of this receptor has been felt to be a prerequisite for determining how vitamin D works in these extra skeletal tissues [Pike, 2014]. Unfortunately, the VDR is not easy to detect. In this review, we examine the literature regarding vitamin D and muscle health and its potential mechanism of action.
Vitamin D and diffuse musculoskeletal pain
In clinical practice, it is commonplace to check a 25(OH)D level in patients with diffuse soft tissue pain with the notion that repleting low levels might improve pain. Studies examining this association between insufficient vitamin D and muscle pain have typically been small and observational. The rational is based largely on the work of Plotnikoff and Quigley, who published an uncontrolled study that examined 150 patients with chronic, nonspecific musculoskeletal pain of uncertain etiology and found that as many as 96% had vitamin D deficiency, with a mean 25(OH)D level of 10.49 ng/ml (winter values) [Plotnikoff and Quigley, 2003]. This was questioned by Block in a letter to the editor stating that he found vitamin D deficiency at a similar latitude to be similar among those with chronic pain and other populations, and furthermore that in his experience, repleting vitamin D did not improve pain [Block, 2004]. A few randomized trials have been published regarding the pain issue, with conflicting results. One trial randomized 50 patients with 25(OH)D levels <20 ng/ml to 50,000 international units (IU) ergocalciferol weekly for 3 months versus placebo [Warner and Arnspriger, 2008]. The study was adequately powered to detect changes in pain scores and no effect was observed.
A second randomized trial randomized 30 women with fibromyalgia and 25(OH)D levels<32 ng/ml to 1 of 2 doses of cholecalciferol (1200–2400 daily IU cholecalciferol), depending on their baseline 25(OH)D levels and placebo [Wepner et al. 2014]. This study showed a marked reduction in perception of pain with optimization of vitamin D status compared with controls. Interestingly, this study monitored 25(OH)D levels throughout the trial and withheld treatment if the level reached more than 48 ng/ml for ‘safety reasons’ including one case of mild hypercalcemia with a 25(OH)D level of 63.6 ng/ml and serum calcium level of 2.71 mmol/l. Besides the limitation of the study due to the small sample size, the authors also noted that other parameters of fibromyalgia such as health-related quality of life, disease impairment scores and somatization scales did not show a significant treatment effect and furthermore there were no statistically significant correlations between changes in serum 25(OH)D levels and pain scales within the treatment group. The selective pain response is intriguing in this small study and invites a better understanding of the role of vitamin D in specific fibromyalgia generated pain as opposed to other generalized musculoskeletal pain.
Vitamin D and falls
Sarcopenia, or low muscle mass, occurs with aging and is associated with increased morbidity and mortality [Bunout et al. 2011]. Insufficient vitamin D may be related to the development of sarcopenia, which contributes to poor muscle function. Elderly people have many reasons to be insufficient in vitamin D including frequency of institutionalization, decreased capacity for the skin to endogenously synthesize vitamin D, and decreased VDR expression in the muscle tissue [Bischoff-Ferrari et al. 2004]. In this section, we examine the evidence regarding low vitamin D and decreased muscle function.
Several studies suggest an association between vitamin D insufficiency and falls in the elderly. One large prospective cohort study out of the Netherlands followed 1231 men and women over the age of 65 years [Snijder et al. 2006]. In this study, baseline vitamin D status was measured followed by a year of recording of falls. They found that low serum 25(OH)D levels (<10 ng/ml) were significantly associated with 2 or more falls in those aged between 65 and 75, even when adjusted for cofounders including age, sex, region, season, education level, lifestyle variables, weight, body mass index (BMI), number of chronic diseases, serum creatinine level and physical performance. They noted that physical performance mediated the effect of vitamin D status on falls, suggesting that poor muscle function in those with low vitamin D is an important factor in the risk of falling.
A 3-year randomized controlled trial studied the effects of 3 years of cholecalciferol plus calcium supplementation on the risk of falling [Bischoff-Ferrari et al. 2006]. Patients were randomized to receive either 700 IU of cholecalciferol plus 500 mg calcium daily versus placebo. They found that supplementation reduced the odds of falling in older women by 46%, and in less active women by 65%, though did not show any difference in men. This study was in the setting of what the US Institute of Medicine declares to be a normal baseline serum 25(OH)D level (>20 ng/ml) [Ross et al. 2011]. Other randomized studies have shown similar results [Bischoff et al. 2003, Flicker et al. 2005].
There are also randomized trials that show a lack of effect on vitamin D and muscle strength. For example, a 2005 randomized trial that looked at a large cohort of patients (5292) greater than age 70 who had suffered a low-trauma osteoporotic fracture in the previous 10 years and were randomized to cholecalciferol plus calcium, cholecalciferol alone, calcium alone, and placebo [Grant et al. 2005]. They found that the incidence of new low-trauma fractures in this population was not different among the various treatment arms. This was a secondary prevention trial in those who had already suffered a low-trauma fracture, which differentiates it from the positive trials. In addition, the population was older, a population which has been shown in prospective trials to be less sensitive to the effects of vitamin D supplementation, perhaps because this population has other risk factors for falls that are more important [Snijder et al. 2006]. A subsequent large meta-analysis analyzing 26 trials and 45,782 participants came to the conclusion that vitamin D supplementation combined with calcium reduces the risk of falls, specifically in those who are deficient at baseline [Murad et al. 2011].
One randomized trial from 2010 actually showed an increased incidence of falls in those in the treatment group [Sanders et al. 2010]. A total of 2258 women over the age of 70 years and at increased risk of fracture were randomized to receive either one dose of 500,000 IU of oral cholecalciferol versus placebo. They found a statistically increased risk of first fall in those in the treatment group, in addition to a trend towards first fracture in the same group. Interestingly, these trends were most pronounced in the first 3 months after therapy. For this reason, it has been speculated that the high level of serum 25(OH)D that results from mega-doses causes an increase in 24-hydroxylase levels, which converts 25(OH)D to its inactive metabolite, leading to rapid degradation of the dose and subsequent deficiency [Girgis et al. 2014b].
Several lines of evidence suggest that the risk of falls in those with low 25(OH)D levels may be due to a more indirect pathway via cognitive and cerebellar dysfunction [Marcelli et al. 2015]. People with cognitive dysfunction have been shown to fall more than those without. One study showed that 62% of elderly adults living in residential care facilities who carry a diagnosis of dementia fell, compared with 41% of those living in the same facilities without a diagnosis of dementia [Eriksson et al. 2008]. Studies have shown that deficiencies in cognitive domains such as dual tasking, executive function and reaction time all contribute to fall risk [Segev-Jacubovski et al. 2011].
It is not surprising that vitamin D is now being shown to be a factor in cognitive decline. The VDR has been identified in cerebellar tissue and its expression is reduced in those patients with Alzheimer’s disease [Sutherland et al. 1992]. Many studies have looked at the significance of vitamin D supplementation on cognition. One systematic review found that of 25 cross-sectional studies, 18 (72%) showed that lower 25(OH)D levels or decreased vitamin D intake was associated with worse outcomes on 1 or more cognitive function tests and/or a higher frequency of dementia [van der Schaft et al. 2013]. The same study also noted that of 6 prospective studies, patients with a lower baseline 25(OH)D level at baseline had a higher risk of cognitive decline after a follow-up period of 4–7 years. While randomized trials are needed that address vitamin D, falls and cognition, the literature supports that vitamin D has numerous potential mechanisms that contribute to falls and subsequent fracture.
The effect of vitamin D on falls is felt to be significant enough that consensus societies have made recommendations regarding the topic. The US Preventative Services Task Force has made a grade B recommendation (high certainty) that vitamin D supplementation reduces falls. They recommend 600 IU daily for adults aged 51–70 and 800 IU for adults >70 [Moyer et al. 2012]. The American Society of Geriatrics gives a grade A recommendation (strong) that all older adults with proven vitamin D deficiency get at least 800 IU of vitamin D supplementation daily to prevent falls, and a grade B recommendation that at least 1000 IU be given to those with suspected vitamin D deficiency or at otherwise increased risk of falls. They go so far as to say that 25(OH)D levels do not need to be checked unless the patient has risk factors for hypercalcemia (malignancy, renal disease, sarcoidosis) [American Geriatrics Society Workgroup on Vitamin D Supplementation in Older Adults, 2014]. These recommendations highlight that the potential benefits of vitamin D supplementation in fall prevention outweigh the potential risks.
Vitamin D and myopathy
Vitamin D insufficiency has also been linked to low muscle mass and muscle weakness. Patients with idiopathic inflammatory myopathies have been shown to have lower serum 25(OH)D levels [Azali et al. 2013]. There are conflicting reports that those with statin-related adverse muscle events are at higher risk if they are deficient in vitamin D [Mergenhagen et al. 2014]. Low 25(OH)D levels may also be a cofounder in alcoholic skeletal muscle myopathy [Wijnia et al. 2013]. Osteomalacia is documented to cause proximal muscle myopathy [Russell, 1994; Suresh and Wimalaratna, 2013]. In Saudi Arabia, women who are veiled and heavily covered while outdoors have been shown to develop a proximal muscle weakness that is reversible with vitamin D supplementation [Al-Said et al. 2009]. This body of evidence points to vitamin D’s essential role not only in falls and fall prevention, but also in muscle strength and function.
VDR
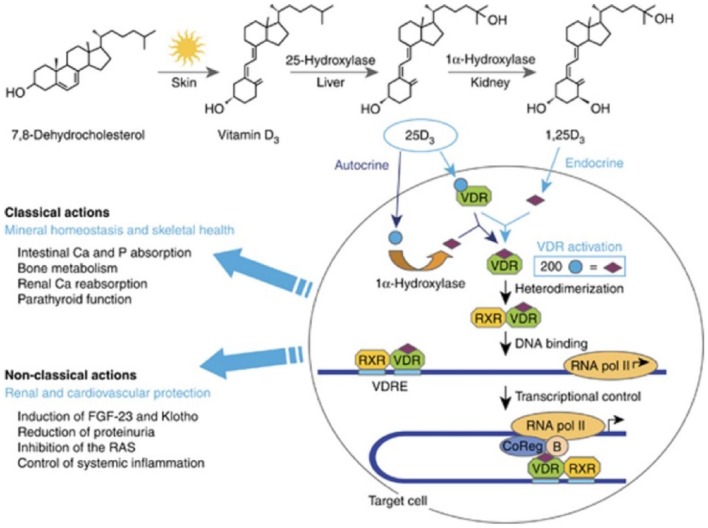
The biologic effects of vitamin D (namely regulating calcium and phosphate metabolism) are mediated by the VDR. The VDR belongs to a subfamily of receptors that form heterodimers with retinoid X receptor (RXR). Once dimerized, the complex binds to the vitamin D response element (VDRE) in the promoter regions of target genes or at distant sites to positively or negatively regulate their expression [Feldman et al. 2013]. Classical actions involve mineral homeostasis and skeletal health and nonclassical actions are being further elucidated (Figure 1) as the VDR has been found in virtually all cell types [Bouillon et al. 2008].
Figure 1.
Vitamin D activation and target cell effects. Reprinted with permission from [Dusso, 2011].
RAS, renin–angiotensin system; RXR, retinoid X receptor ; VDR, vitamin D receptor; VDRE, vitamin D response element.
As many associations have been shown relating vitamin D status to muscle health, in order to further define the pathophysiology numerous studies have focused on identifying VDR in skeletal muscle and to prove that it is responsive to vitamin D levels. The results thus far have been contradictory, with several studies showing the presence of VDR in skeletal muscle [Simpson et al. 1985; Garcia et al. 2011, 2013; Girgis et al. 2014a] and others calling the presence of VDR in skeletal muscle into question [Bischoff et al. 2001; Ceglia et al. 2010; Wang and Deluca, 2011; Srikuea et al. 2012]. Recently, Girgis and colleagues conducted a mouse study to add to the body of literature regarding VDR presence in skeletal muscle [Girgis et al. 2014c]. In this study, mouse skeletal muscle was harvested and propagated. Using polymerase chain reaction (PCR), Western blotting and immunohistochemistry, they were able to show the presence of VDR in murine quadriceps muscle. The amount of VDR was much less than that found in the duodenum and also diminished with age. In in vitro models, both expression of VDR and CYP24A1 (target gene of VDR which encodes 24-hydroxylase) increased after treatment with 1,25-dihydroxyvitamin D. This effect was absent in VDR null controls. Though this study was set up to settle experimentally the controversy of whether VDR is present in skeletal muscle, it was still questioned due to a number of technical factors, including protein extraction methods, variability in different muscle models, past problematic antibodies, and the low level of VDR that is present in mature muscle at baseline [Pike, 2014].
More recently, a study was released that not only looked for the presence of VDR in human myoblasts, but also studied the relationship of vitamin D to the expression of VDR [Pojednic et al. 2015]. This study had 3 parts, the first of which harvested and cloned human myoblasts from healthy volunteers then treated the myoblasts for 18 hours with 1 of 3 doses of 1,25-dihydroxyvitamin D. Using PCR they measured VDR and CYP24A1 before and after treatment, and found that after treatment, expression of both went up in a dose-dependent manner. In the second part, they obtained muscle biopsy data from a previous clinical study that included 20 women over age 65 with moderately low baseline 25(OH)D levels and mobility limitations. These women were participating in a randomized controlled intervention study examining the effects of vitamin D3 versus placebo on skeletal muscle morphology and VDR protein concentration [Ceglia et al. 2013]. Biopsies of the vastus lateralis were taken at baseline and after 16 weeks of treatment with 4000 IU of daily vitamin D3. PCR, immunoblotting and immunofluorescent marker staining showed more VDR detection in the vitamin D treated group. In the third part, muscle biopsy data was obtained from a second clinical study that included 20 older adults who were also mobility limited. These subjects had been selected for a larger randomized study for which inclusion and exclusion criteria are presented elsewhere [Chale et al. 2013]. In this study, fasting serum 25(OH)D levels and muscle biopsies were obtained at baseline for the purposes of the study. They found that at baseline there was no correlation between VDR mRNA expression and 25(OH)D levels; however, there was a positive correlation between VDR protein expression by immunoblotting. Furthermore, when the patients were divided into vitamin D sufficient, insufficient and deficient [25(OH)D levels of ⩾20 ng/ml, 12–19 ng/ml and <12 ng/ml, respectively], there was greater VDR concentration in those with sufficient 25(OH)D compared with those with insufficient and deficient levels combined. This study contributes to the body of literature supporting the presence of VDR on skeletal muscle and that the VDR has biological activity in that location. Larger human studies are needed to further clarify their findings.
Conclusion
In addition to the widely understood mechanisms of vitamin D in relation to skeletal health and calcium and phosphate homeostasis, we are learning of new and important physiologic roles for this vitamin in other cell types. The importance of vitamin D in skeletal muscle health has been known for more than a century as demonstrated in severe cases of vitamin D deficiency, but more recently associations have been made showing more subtle effects in those with insufficient values. While the exact effects and mechanism of action remains controversial, current data lean towards insufficient vitamin D playing a role in specific fibromyalgia musculoskeletal pain, sarcopenia, myopathy, and falls related to cerebellar and cognitive dysfunction.
Furthermore, experimental techniques are becoming more sophisticated and have allowed detection of the VDR on skeletal muscle and in cerebellar tissue, which if validated in further large studies, could lead to the discovery of the mechanism of vitamin D in these associations. These data suggest that vitamin D supplementation may contribute to the health and maintenance of muscle function. Vitamin D supplementation is inexpensive and has relatively few side effects and thus, according to the presented data, the benefit of repleting 25(OH)D levels in patients outweighs the risks of such recommendations. The role of vitamin D in muscle health and function remains an exciting and growing area of research with substantial clinical implications.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: S.B.T. is a member of the data safety monitoring board at AMGEN, the advisory board at Mallinckrodt, Celgene, and the speakers’ bureau of Pfizer and Bristol Myers Squibb. He has benefitted from sponsored research from Lilly GSK, Merck, Novartis, Pfizer and Janssen.
Contributor Information
S. Bobo Tanner, Vanderbilt University Medical Center – Rheumatology and Allergy, 2611 West End Ave, Suite 210, Nashville, Tennessee 37203, USA.
Susan A. Harwell, Vanderbilt University Medical Center – Rheumatology, 1161 21st Avenue So., T-3113 MCN, Nashville, Tennessee 37232, USA
References
- Al-Said Y., Al-Rached H., Al-Qahtani H., Jan M. (2009) Severe proximal myopathy with remarkable recovery after vitamin D treatment. Can J Neurol Sci 36: 336–339. [DOI] [PubMed] [Google Scholar]
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults (2014) Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc 62: 147–152. [DOI] [PubMed] [Google Scholar]
- Azali P., Barbasso Helmers S., Kockum I., Olsson T., Alfredsson L., Charles P., et al. (2013) Low serum levels of vitamin D in idiopathic inflammatory myopathies. Ann Rheum Dis 72: 512–516. [DOI] [PubMed] [Google Scholar]
- Bischoff H., Borchers M., Gudat F., Duermueller U., Theiler R., Stahelin H., et al. (2001) In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J 33: 19–24. [DOI] [PubMed] [Google Scholar]
- Bischoff H., Stahelin H., Dick W., Akos R., Knecht M., Salis C., et al. (2003) Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res 18: 343–351. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H., Borchers M., Gudat F., Durmuller U., Stahelin H., Dick W. (2004) Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res 19: 265–269. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H., Orav E., Dawson-Hughes B. (2006) Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med 166: 424–430. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H., Shao A., Dawson-Hughes B., Hathcock J., Giovannucci E., Willett W. (2010) Benefit-risk assessment of vitamin D supplementation. Osteoporos Int 21: 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block S. (2004) Vitamin D deficiency is not associated with nonspecific musculoskeletal pain syndromes including fibromyalgia. Mayo Clin Proc 79: 1585–1586; author reply 1586–1587. [DOI] [PubMed] [Google Scholar]
- Bouillon R., Carmeliet G., Verlinden L., Van Etten E., Verstuyf A., Luderer H., et al. (2008) Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29: 726–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunout D., De La Maza M., Barrera G., Leiva L., Hirsch S. (2011) Association between sarcopenia and mortality in healthy older people. Australas J Ageing 30: 89–92. [DOI] [PubMed] [Google Scholar]
- Ceglia L., Da Silva Morais M., Park L., Morris E., Harris S., Bischoff-Ferrari H., et al. (2010) Multi-step immunofluorescent analysis of vitamin D Receptor loci and myosin heavy chain isoforms in human skeletal muscle. J Mol Histol 41: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceglia L., Niramitmahapanya S., Da Silva Morais M., Rivas D., Harris S., Bischoff-Ferrari H., et al. (2013) A randomized study on the effect of vitamin D(3) supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab 98: E1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chale A., Cloutier G., Hau C., Phillips E., Dallal G., Fielding R. (2013) Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J Gerontol A Biol Sci Med Sci 68: 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S., Gustafson Y., Lundin-Olsson L. (2008) Risk Factors for falls in people with and without a diagnose of dementia living in residential care facilities: a prospective study. Arch Gerontol Geriatr 46: 293–306. [DOI] [PubMed] [Google Scholar]
- Feldman D., Krishnan A., Swami S. (2013) Vitamin D: biology,actions, and clinical implications. In: Marcus R., Feldman D., Dempster D., Luckey M., Cauley J. (eds) Osteoporosis, 4th edn. Waltham, MA, Academic Press, Chapter 13. [Google Scholar]
- Flicker L., Macinnis R., Stein M., Scherer S., Mead K., Nowson C., et al. (2005) Should Older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc 53: 1881–1888. [DOI] [PubMed] [Google Scholar]
- Garcia L., Ferrini M., Norris K., Artaza J. (2013) 1,25(OH)2vitamin D3 enhances myogenic differentiation by modulating the expression of key angiogenic growth factors and angiogenic inhibitors in C2C12 skeletal muscle cells. J Steroid Biochem Mol Biol 133: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L., King K., Ferrini M., Norris K., Artaza J. (2011) 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 152: 2976–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis C. (2014) Vitamin D and muscle function in the elderly: the elixir of youth? Curr Opin Clin Nutr Metab Care 17: 546–550. [DOI] [PubMed] [Google Scholar]
- Girgis C., Clifton-Bligh R., Mokbel N., Cheng K., Gunton J. (2014a) Vitamin D Signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 155: 347–357. [DOI] [PubMed] [Google Scholar]
- Girgis C., Clifton-Bligh R., Turner N., Lau S., Gunton J. (2014b) Effects of vitamin D in skeletal muscle: falls, strength, athletic performance and insulin sensitivity. Clin Endocrinol 80: 169–181. [DOI] [PubMed] [Google Scholar]
- Girgis C., Mokbel N., Cha K., Houweling P., Abboud M., Fraser D., et al. (2014c) The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) Uptake in myofibers. Endocrinology 155: 3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A., Avenell A., Campbell M., Mcdonald A., Maclennan G., McPherson G., et al. (2005) Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium or Vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 365: 1621–1628. [DOI] [PubMed] [Google Scholar]
- Heidari B., Shirvani J., Firouzjahi A., Heidari P., Hajian-Tilaki K. (2010) Association between nonspecific skeletal pain and vitamin D deficiency. Int J Rheum Dis 13: 340–346. [DOI] [PubMed] [Google Scholar]
- Marcelli C., Chavoix C., Dargent-Molina P. (2015) Beneficial effects of vitamin D on falls and fractures: is cognition rather than bone or muscle behind these benefits? Osteoporos Int 26: 1–10. [DOI] [PubMed] [Google Scholar]
- Mergenhagen K., Ott M., Heckman K., Rubin L., Kellick K. (2014) Low vitamin D as a risk factor for the development of myalgia in patients taking high-dose simvastatin: a retrospective review. Clin Ther 36: 770–777. [DOI] [PubMed] [Google Scholar]
- Moshfegh A., Goldman J., Jaspreet A., Rhodes D., LaComb R. (2009) What we eat in America, NHANES 2005–2006. Usual nutient intakes from food and water compared to 1997 dietary reference intakes for vitamin D, calcium, phosphorus, and magnesium. Beltsville, MD: US Department of Agriculture, Agricultural Research Service. [Google Scholar]
- Moyer V. (2012) Prevention of falls in community-dwelling older adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 157: 197–204. [DOI] [PubMed] [Google Scholar]
- Murad M., Elamin K., Elnour N., Elamin M., Alkatib A., Fatourechi M., et al. (2011) The Effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab 96: 2997–3006. [DOI] [PubMed] [Google Scholar]
- Pike J. (2014) Expression of the vitamin D receptor in skeletal muscle: are we there yet? Endocrinology 155: 3214–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike J., Lee S., Meyer M. (2014) Regulation of gene expression by 1,25-dihydroxyvitamin D3 in bone cells: exploiting new approaches and defining new mechanisms. BoneKEy Rep 3: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikoff G., Quigley J. (2003) Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc 78: 1463–1470. [DOI] [PubMed] [Google Scholar]
- Pojednic R., Ceglia L., Olsson K., Gustafsson T., Lichtenstein A., Dawson-Hughes B., et al. (2015) Effects of 1,25-dihydroxyvitamin D and vitamin D on the expression of the vitamin D receptor in human skeletal muscle cells. Calcif Tissue Int 96: 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A., Taylor C., Yaktine A., Del Valle H. (eds) (2011) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Russell J. (1994) Osteomalacic myopathy. Muscle Nerve 17: 578–580. [DOI] [PubMed] [Google Scholar]
- Sanders K., Stuart A., Williamson E., Simpson J., Kotowicz M., Young D., et al. (2010) Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303: 1815–1822. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Lai J., Lizaola B., Kane L., Weyland P., Terrault N., et al. (2014) Variability in free 25(OH) vitamin D levels in clinical populations. J Steroid Biochem Mol Biol 144: 156–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev-Jacubovski O., Herman T., Yogev-Seligmann G., Mirelman A., Giladi N., Hausdorff J. (2011) The interplay between gait, falls and cognition: can cognitive therapy reduce fall risk? Expert Rev Neurother 11: 1057–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R., Thomas G., Arnold A. (1985) Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem 260: 8882–8891. [PubMed] [Google Scholar]
- Snijder M., Van Schoor N., Pluijm S., Van Dam R., Visser M., Lips P. (2006) Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab 91: 2980–2985. [DOI] [PubMed] [Google Scholar]
- Srikuea R., Zhang X., Park-Sarge O., Esser K. (2012) VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol 303: C396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh E., Wimalaratna S. (2013) Proximal myopathy: diagnostic approach and initial management. Postgrad Med J 89: 470–477. [DOI] [PubMed] [Google Scholar]
- Sutherland M., Somerville M., Yoong L., Bergeron C., Haussler M., Mclachlan D. (1992) Reduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus: correlation with calbindin-28K mRNA levels. Brain Res Mol Brain Res 13: 239–250. [DOI] [PubMed] [Google Scholar]
- Van der Schaft J., Koek H., Dijkstra E., Verhaar H., van der Schouw Y., Emmelot-Vonk M. (2013) The association between vitamin D and cognition: a systematic review. Ageing Res Rev 12: 1013–1023. [DOI] [PubMed] [Google Scholar]
- Wagatsuma A., Sakuma K. (2014) Vitamin D signaling in myogenesis: potential for treatment of sarcopenia. Biomed Res Int 2014: 121254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Deluca H. (2011) Is the vitamin D receptor found in muscle? Endocrinology 152: 354–363. [DOI] [PubMed] [Google Scholar]
- Warner A., Arnspiger S. (2008) Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. J Clin Rheumatol 14: 12–16. [DOI] [PubMed] [Google Scholar]
- Wepner F., Scheuer R., Schuetz-Wieser B., Machacek P., Pieler-Bruha E., Cross H., et al. (2014) Effects of vitamin D on patients with fibromyalgia syndrome: a randomized placebo-controlled trial. Pain 155: 261–268. [DOI] [PubMed] [Google Scholar]
- Wijnia J., Wielders J., Lips P., van de Wiel A., Mulder C., Nieuwenhuis K. (2013) Is vitamin D deficiency a confounder in alcoholic skeletal muscle myopathy? Alcohol Clin Exp Res 37(Suppl. 1): E209–E215. [DOI] [PubMed] [Google Scholar]