Abstract
Tooth organogenesis depends on genetically programmed sequential and reciprocal inductive interactions between the dental epithelium and neural crest–derived mesenchyme. Previous studies showed that the Msx1 and Runx2 transcription factors are required for activation of odontogenic signals, including Bmp4 and Fgf3, in the early tooth mesenchyme to drive tooth morphogenesis through the bud-to-cap transition and that Runx2 acts downstream of Msx1 to activate Fgf3 expression. Recent studies identified Osr2 as a repressor of tooth development and showed that inactivation of Osr2 rescued molar tooth morphogenesis in the Msx1-/- mutant mice as well as in mice with neural crest–specific inactivation of Bmp4. Here we show that Runx2 expression is expanded in the tooth bud mesenchyme in Osr2-/- mutant mouse embryos and is partially restored in the tooth mesenchyme in Msx1-/-Osr2-/- mutants in comparison with Msx1-/- and wild-type embryos. Whereas mandibular molar development arrested at the bud stage and maxillary molar development arrested at the bud-to-cap transition in Runx2-/- mutant mice, both mandibular and maxillary molar tooth germs progressed to the early bell stage, with rescued expression of Msx1 and Bmp4 in the dental papilla as well as expression of Bmp4, p21, and Shh in the primary enamel knot in the Osr2-/-Runx2-/- compound mutants. In contrast to the Msx1-/-Osr2-/- compound mutants, which exhibit nearly normal first molar morphogenesis, the Osr2-/-Runx2-/- compound mutant embryos failed to activate the expression of Fgf3 and Fgf10 in the dental papilla and exhibited significant deficit in cell proliferation in both the dental epithelium and mesenchyme in comparison with the control embryos. These data indicate that Runx2 synergizes with Msx1 to drive tooth morphogenesis through the bud-to-cap transition and that Runx2 controls continued tooth growth and morphogenesis beyond the cap stage through activation of Fgf3 and Fgf10 expression in the dental papilla.
Keywords: Bmp4, Fgf3, Fgf10, Msx1, odontogenesis, genetic interaction
Introduction
Tooth development involves morphogenesis of the tooth germ through bud, cap, and bell stages regulated by sequential and reciprocal interactions between the adjacent dental epithelium and mesenchyme (Thesleff and Sharpe 1997; Pispa and Thesleff 2003). The bud-to-cap transition is a critical step and is regulated by multiple transcription factors and signaling pathways (Tucker and Sharpe 2004; Jussila and Thesleff 2012). Expression of the Msx1 transcription factor is induced in the developing tooth mesenchyme by Bmp and Fgf signals from the dental epithelium (Vainio et al. 1993; Chen et al. 1996; Bei and Maas 1998; Tucker et al. 1998). Msx1-/- mutant mice exhibit developmental arrest of all tooth germs at the bud stage, accompanied by significantly reduced expression of Bmp4 in the dental mesenchyme (Satokata and Maas 1994; Chen et al. 1996). Mice lacking the Pax9 transcription factor also exhibit tooth developmental arrest at the bud stage (Peters et al. 1998). Pax9 expression is induced in the prospective tooth mesenchyme at the onset of tooth development by Fgf signaling, and Pax9 function is required for maintenance of both Msx1 and Bmp4 expression in the tooth mesenchyme (Neubüser et al. 1997; Peters et al. 1998; Mandler and Neubüser 2001; Zhou et al. 2011). Remarkably, addition of recombinant Bmp4 protein rescued Msx1-/- mutant mandibular first molar tooth germs to late bell stage in explant cultures (Bei et al. 2000; Chen et al. 1996), which suggested that Bmp4 is a major mesenchymal odontogenic signal downstream of Msx1 to drive tooth morphogenesis through the bud-to-cap transition (Maas and Bei 1997). However, mice with tissue-specific inactivation of the Bmp4 gene in the early cranial neural crest cells, which showed absence of functional Bmp4 mRNA expression in the tooth bud mesenchyme, exhibited bud-stage developmental arrest of mandibular molar tooth germs but developed maxillary molars and incisors to mineralized teeth, suggesting that other Msx1-dependent mesenchymal factors also play critical roles in the bud-to-cap transition (Jia et al. 2013).
The zinc finger transcription factor Osr2 is expressed in a buccolingual gradient in the developing tooth mesenchyme and inhibits Msx1-meditated propagation of mesenchymal odontogenic signals along the buccolingual axis (Zhang et al. 2009). Osr2-/- mutant mice exhibit de novo supernumerary tooth induction lingual to the normal molar tooth germs (Zhang et al. 2009). The induction of supernumerary teeth in Osr2-/- mutant embryos depended on Msx1 function as Msx1-/-Osr2-/- double mutant mice did not form supernumerary tooth germs. Remarkably, however, in contrast to the bud-stage arrest of all tooth germs in Msx1-/- mutant mice, the maxillary and mandibular first molar tooth germs developed to late bell stage in the Msx1-/-Osr2-/- double mutant mice (Zhang et al. 2009). Whereas Bmp4 expression was partially restored in the tooth mesenchyme in Msx1-/-Osr2-/- double mutant embryos compared with the loss of Bmp4 expression in Msx1-/- tooth mesenchyme, we recently found that reducing Osr2 gene dosage by 50% was able to rescue the mandibular first molar morphogenesis in mice with neural crest–specific deletion of Bmp4 (Zhang et al. 2009; Jia et al. 2013). These results suggest that Msx1 and Osr2 antagonistically regulate other critical mesenchymal odontogenic factors, in addition to Bmp4, during early tooth development.
The Runx2 gene encodes a runt-domain containing transcription factor that is essential for bone and tooth development (Otto et al. 1997; D’Souza et al. 1999; Ryoo and Wang 2006). Runx2 is expressed in the dental mesenchyme at the bud and cap stages and mediates Fgf signaling from the dental epithelium to mesenchyme (D’Souza et al. 1999; Aberg, Wang, et al. 2004). Runx2-/- mice exhibit tooth developmental arrest at late bud stages (D’Souza et al. 1999), accompanied by significant reduction or loss of expression of Fgf3 in the dental mesenchyme and of Shh, Edar, and p21 in the enamel knot (Aberg, Wang, et al. 2004). Explant culture assays showed that Msx1 and Runx2 are required for Fgf-induced expression of Fgf3 in the dental mesenchyme (Bei and Maas 1998; Kettunen et al. 2000; Aberg, Wang, et al. 2004). Since Runx2 mRNA expression was significantly reduced in the tooth bud mesenchyme in Msx1-/- mutant embryos whereas Msx1 expression in the tooth mesenchyme was unaltered in Runx2-/- mutant embryos, these data suggest that Runx2 acts downstream of Msx1 to activate Fgf3 expression during early odontogenesis.
In this study, we investigated possible genetic interactions between Osr2 and Runx2 and compared molar tooth morphogenesis in Osr2-/-Runx2-/- mutant mice with that in Msx1-/-Osr2-/- mice. We found that, in contrast to bud-stage developmental arrest in Runx2-/- mutants, both upper and lower first molar tooth germs progressed past the cap stage, with obvious primary enamel knot formation, in Osr2-/-Runx2-/- mutants. However, in contrast to Msx1-/-Osr2-/- mutants, the Osr2-/-Runx2-/- mutant embryos failed to activate the expression of Fgf3 and Fgf10 in the dental papilla and showed significant deficit in cell proliferation in both the upper and lower molar tooth germs. Our data provide new insight into the molecular mechanism through which Runx2 regulates odontogenesis.
Materials and Methods
Mouse Strains
Msx1+/-, Osr2+/-, and Runx2+/- mice, which have been described previously (Satokata and Maas 1994; Otto et al. 1997; Lan et al. 2004), were maintained in the CD1 background. All animal procedures were approved by the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee.
Histology and In Situ Hybridization
Embryos were collected from timed pregnant females, fixed in 4% paraformaldehyde, embedded in paraffin, sectioned at 5- to 7-µm thickness, and stained with hematoxylin and eosin (Zhang et al. 2009). For in situ hybridization, paraffin sections were hybridized with DIG-labeled cRNA probes as described previously (Zhang et al. 2009).
Cell Proliferation and Cell Death Assays
BrdU was injected intraperitoneally into timed pregnant female mice at E14.5 (Sigma-Aldrich, St. Louis, MO, USA; 50 µg/g body weight), and embryos were harvested 1 h after injection. Paraffin sections were prepared as described above. BrdU-incorporated cells were detected by immunofluorescent staining using the Alexa Fluor 594–conjugated anti-BrdU antibody (Life Technologies, Carlsbad, CA, USA; 1:50). The cell proliferation index was defined as the percentage of BrdU-positive nuclei relative to Hoechst-stained nuclei in the dental epithelial and mesenchymal compartments, respectively. Cell proliferation data were statistically analyzed using Student’s t test for pairwise comparison, with a P value less than 0.05 considered significant. Cell death was detected by using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA) following the manufacturer’s instruction.
Results
Runx2 Expression in the Developing Tooth Mesenchyme Is Regulated by Antagonistic Actions of Msx1 and Osr2
To understand possible interactions between Osr2 and Runx2 during tooth development, we first examined Runx2 expression during tooth development in the control and Osr2-/- mutant embryos. Runx2 was expressed in the dental mesenchyme and in osteogenic mesenchyme in the control embryos at the bud (E13.5) and late cap (E15.0) stages (Fig. 1A, C). In the Osr2-/- embryos, Runx2 mRNA expression was up-regulated in the mesenchymal cells lingual to the tooth bud as well as in the palatal mesenchyme (Fig. 1B, D). In contrast, the Osr2 mRNA expression pattern in the developing tooth germs was similar in the control and Runx2-/- embryos (Fig. 1E, F). Since Runx2 mRNA expression in the developing tooth mesenchyme was reduced in the Msx1-/- embryos (Aberg, Wang, et al. 2004; Fig. 1G), we examined Runx2 mRNA expression in the Msx1-/-Osr2-/- mutant embryos and found that it was partially restored in the maxillary tooth mesenchyme in these double mutants (Fig. 1H). These results suggest that Msx1 and Osr2 act antagonistically to regulate Runx2 expression during early tooth development.
Figure 1.
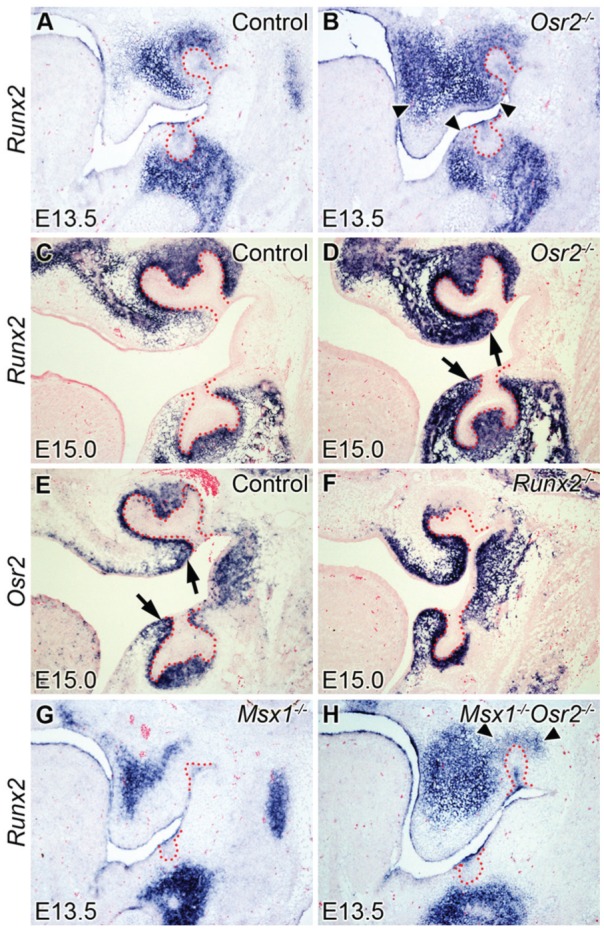
Expression of Runx2 and Osr2 in the tooth germ in control, Osr2-/-, Runx2-/-, Msx1-/-, and Msx1-/-Osr2-/- mutant mouse embryos. (A–H) Frontal sections at E13.5 (A, B, G, H) and E15.5 (C–F). (A, C) Runx2 is expressed in both bone-forming regions and dental mesenchyme in the control group. (B, D) Runx2 is expressed in both bone-forming regions and dental mesenchyme, and it is expanded to the palatal shelf and lingual mesenchyme (arrowheads, arrows) in the Osr2-/- group. (E, F) At E15.0, Osr2 is expressed in the dental mesenchyme and also in the mesenchymal cells surrounding the dental stalk (arrows) in the control group (E); Osr2 expression pattern is similar to the control group in the Runx2-/- group (F). (G, H) Runx2 is expressed only in the bone-forming regions but not in the dental mesenchyme in the Msx1-/- group at E13.5 (G); Runx2 is expressed in both bone-forming regions and the maxillary dental mesenchyme (arrowheads) in the Msx1-/-Osr2-/- group (H).
Deletion of Osr2 Substantially Rescued Tooth Morphogenesis in the Runx2-Deficient Mice
To investigate whether Osr2 and Runx2 interact to regulate tooth development, we generated and analyzed Osr2-/-Runx2-/- double mutant mice. At E18.5, whereas Runx2-/- embryos showed tooth developmental arrest with absence of condensed dental mesenchyme (Fig. 2B) and Osr2-/- embryos had supernumerary tooth germ lingual to the first molar (Fig. 2C), Osr2-/-Runx2-/- double mutant embryos had smaller than normal molar tooth germs resembling a bell shape, with mesenchymal cells condensed inside the bell to form the dental papilla (Fig. 2D).
Figure 2.
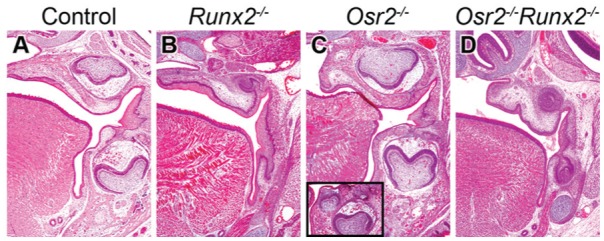
Tooth morphogenesis in the first molar at E18.5. (A–D) Frontal sections of control (A), Runx2-/- (B), Osr2-/- (C), and Osr2-/-Runx2-/- (D) mutant mouse embryos. (A, C) Controls and Osr2-/- mutants exhibit bell stage tooth germs, whereas supernumerary teeth appear in the lingual region in the Osr2-/- mutants (C, box). (B) Tooth germ is arrested at the bud stage in the Runx2-/- mutants. (D) Tooth morphogenesis is substantially rescued, showing a bell shape, in the Osr2-/-Runx2-/- mutants. Supernumerary tooth is not found in the lingual region.
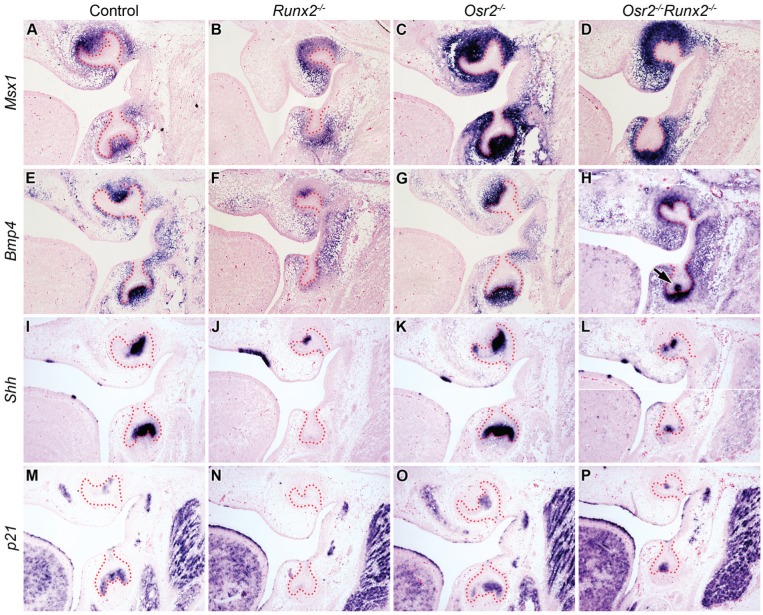
Molecular marker analysis showed that the Runx2-/- mutant tooth germs had reduced expression of Msx1 and Bmp4 in the tooth mesenchyme and lack of Shh expression in the mandibular molar epithelium at E15.5, in comparison with the control littermates (Fig. 3A, B, E, F, I, J). The Osr2-/- littermates showed increased expression of all of these 3 markers (Fig. 3C, G, K). Although the molar tooth germs in the Osr2-/-Runx2-/- mutant embryos were much smaller in size compared with those in the control and Osr2-/- littermates, both Msx1 and Bmp4 were strongly expressed in the molar mesenchyme while Shh was strongly expressed in the distal molar epithelium that corresponds to the primary enamel knot in these double mutant embryos (Fig. 3D, H, L). Furthermore, whereas the primary enamel knot marker p21 was not detected in the molar tooth germs in the E15.5 Runx2-/- mutant embryos, in comparison with the control and Osr2-/- embryos, robust expression of p21 was detected in both the maxillary and mandibular molar tooth germs in the Osr2-/-Runx2-/- double mutant embryos (Fig. 3M–P). These molecular markers confirm that molar tooth morphogenesis in the Runx2-/- mutant embryos was partially rescued by deletion of Osr2.
Figure 3.
Expression of odontogenic markers in control, Runx2-/-, Osr2-/-, and Osr2-/-Runx2-/- mutant mouse embryos at E15.5. (A–H) Down-regulation of Msx1 and Bmp4 in the dental mesenchyme in Runx2-/- mutants (B, F) was rescued in the Osr2-/-Runx2-/- mutants (D, H). Epithelial Bmp4 was also rescued in the enamel knot in Osr2-/-Runx2-/- mutants (H, arrow). (I–P) Down-regulation of Shh and p21 in the enamel knot in Runx2-/- mutants (J, N) was rescued in the Osr2-/-Runx2-/- mutants (L, P).
Runx2 Is Required for Fgf3 and Fgf10 Expression in the Dental Papilla
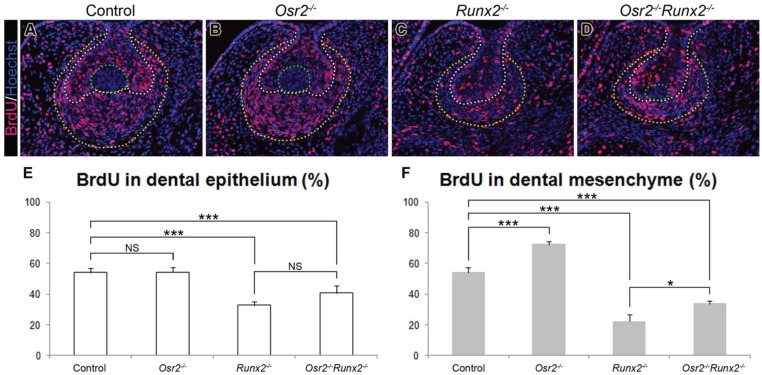
Despite the formation of the primary enamel knot, the Osr2-/-Runx2-/- mutant molar tooth germs appeared significantly smaller at both E18.5 (Fig. 2) and E15.5 (Fig. 3). To investigate whether the reduced tooth germ size was due to aberrant cell death or defective cell proliferation, we performed TUNEL and BrdU-labeling assays at E14.5. No significant differences in the distribution of TUNEL-positive cells were detected in the tooth germs of the different genotypes except that the control, Osr2-/-, and Osr2-/-Runx2-/- mutant tooth germs all showed specific TUNEL-positive cells in the primary enamel knot, whereas the Runx2-/- mutant mandibular molar tooth germs did not have the primary enamel knot structure (data not shown). The BrdU-labeling assay showed that the cell proliferation index for the epithelium was similar in the control and Osr2-/- tooth germs but reduced in Runx2-/- and Osr2-/-Runx2-/- mutants (Fig. 4A–E). The deletion of Osr2 did not significantly change the dental epithelial proliferation compared with Runx2-/- single mutants. In the mesenchyme, the proliferation index from lowest to highest was as follows: Runx2-/-, Osr2-/-Runx2-/-, control, and Osr2-/- tooth germs (Fig. 4A–D, F). Thus, Osr2 deletion significantly improved cell proliferation in the Runx2-deficient mesenchyme, although the growth of the Osr2-/-Runx2-/- mutant tooth germs was still significantly retarded compared with the control tooth germs. These results suggest that Runx2 is required for activation of important growth factors in the tooth mesenchyme even in the absence of Osr2-mediated suppression.
Figure 4.
Comparative analysis of dental cell proliferation in the control, Osr2-/-, Runx2-/-, and Osr2-/-Runx2-/- mutant mouse embryos at E14.5. (A–D) Representative immunofluorescent images of the mandibular molar tooth germs in control (A), Osr2-/- (B), Runx2-/- (C), and Osr2-/-Runx2-/- (D) embryos. The white dotted line outlines the basement membrane in between the dental epithelium and mesenchyme. The yellow dotted line marks the outer margin of the tooth mesenchyme. The primary enamel knot is circled by the green dotted line in each of the control (A), Osr2-/- (B), and Osr2-/-Runx2-/- (D) tooth germs. (E, F) Percentage of BrdU-labeled cells in the dental epithelium (E) and mesenchyme (F). Error bar represents SD. ***P < 0.001; *P < 0.05; NS, not significant.
It has been shown that the tooth bud developmental arrest in the Runx2-/- mutant mouse embryos was accompanied by failure of activation of Fgf3 expression in the developing tooth mesenchyme (Aberg, Wang, et al. 2004). We found that both Runx2-/- and Osr2-/-Runx2-/- mutant molar tooth germs failed to activate Fgf3 expression at E15.5, in comparison with the robust Fgf3 expression in the dental papilla in the control and Osr2-/- mutant embryos (Fig. 5A–D). In contrast, whereas the Msx1-/- mutant molar tooth germs failed to activate Fgf3 expression, the Msx1-/-Osr2-/- double mutant molar tooth germs showed partially restored Fgf3 expression in comparison with the control and Osr2-/- tooth germs (Fig. 5E–H). Fgf3 mutations in mice or humans were associated with abnormalities in tooth crown size or cusp patterning (Wang et al. 2007; Charles et al. 2009). However, the differences in Fgf3 expression alone could not account for the differences in molar tooth morphogenesis between the Osr2-/-Runx2-/- and Msx1-/-Osr2-/- mutant embryos since mice lacking Fgf3 were able to form the full mouse dentition (Mansour et al. 1993; Wang et al. 2007). A related fibroblast growth factor, Fgf10, is weakly expressed in the developing tooth mesenchyme at the bud stage but is strongly up-regulated in the dental papilla by the cap stage during tooth development (Aberg, Wang, et al. 2004). Although mice lacking Fgf10 also had apparently normal prenatal tooth development, mice deficient in both Fgf3 and Fgf10 had tooth developmental arrest at the bud stage (Harada et al. 2002; Wang et al. 2007). We found that both Runx2-/- and Osr2-/-Runx2-/- embryos failed to activate Fgf10 expression in the tooth mesenchyme, in contrast to the robust Fgf10 expression in the dental papilla in the control and Osr2-/- littermates (Fig. 5I–L). Since Fgf3 and Fgf10 have been shown to act synergistically to regulate molar tooth size (Wang et al. 2007), the lack of activation of both Fgf3 and Fgf10 in the tooth germs accounts for the significantly reduced rate of dental cell proliferation and small tooth germ size in the Osr2-/-Runx2-/- embryos.
Figure 5.
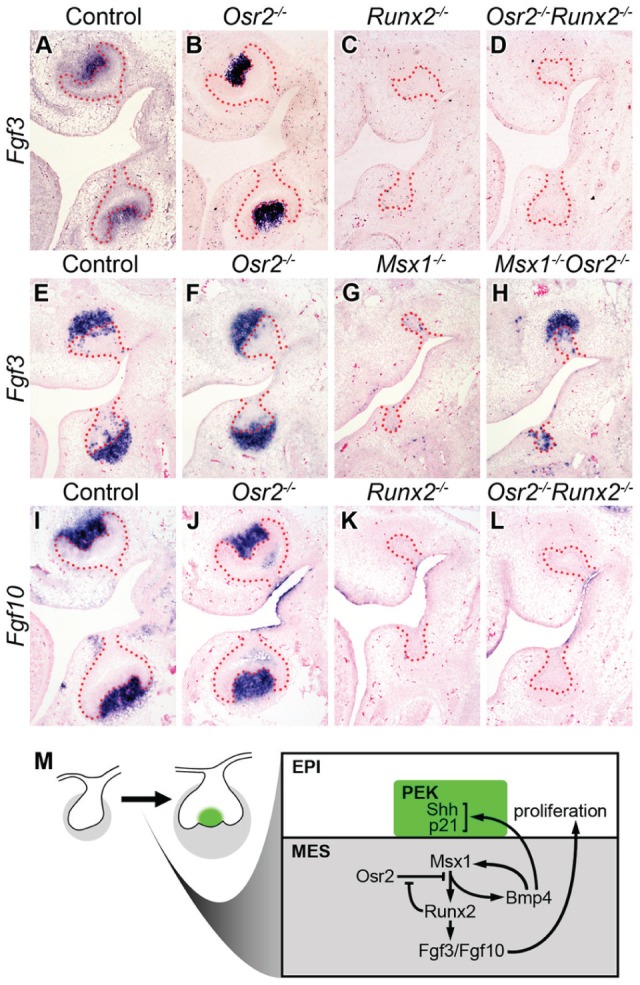
Expression of Fgf3 and Fgf10 in control, Osr2-/-, Runx2-/-, Osr2-/-Runx2-/-, Msx1-/-, and Msx1-/-Osr2-/- mutant mouse embryos at E14.5–E15.5. (A–D) Fgf3 was present in the dental mesenchyme in controls and Osr2-/- mutants at E15.5 (A, B). Absence of Fgf3 expression in the Runx2-/- mutants (C) was not rescued in the Osr2-/-Runx2-/- mutants (D). (E–H) Fgf3 was present in the dental mesenchyme in controls and Osr2-/- mutants at E14.5 (E, F). Absence of Fgf3 in the Msx1-/- mutants (G) was rescued in the Msx1-/-Osr2-/- mutants (H). (I–L) Fgf10 was present in the dental mesenchyme in controls and Osr2-/- mutants at E15.5 (I, J). Absence of Fgf10 expression in the Runx2-/- mutants (K) was not rescued in the Osr2-/-Runx2-/- mutants (L). (M) Schematic diagram illustrating the bud and cap stage tooth germs, with the deduced molecular regulatory network involving Runx2, Msx1, Osr2, Bmp4, Fgf3, and Fgf10, during the bud-to-cap transition. EPI, dental epithelium; MES, dental mesenchyme (marked in gray); PEK, primary enamel knot (marked in green).
Discussion
Classic tissue recombination studies more than 40 y ago showed that the developing tooth mesenchyme from the bud to bell stages was able to induce and instruct complete tooth morphogenesis even when recombined with embryonic nondental epithelium (Kollar and Baird 1970a, 1970b). The molecular nature and mechanisms of regulation of this “mesenchymal odontogenic potential” are still not completely understood, however. Mutant mouse studies showed that mice lacking either Msx1 or Runx2 had tooth developmental arrest at early to late bud stages and that neither Msx1-/- nor Runx2-/- mutant tooth bud mesenchyme could support tooth organogenesis when recombined with wild-type embryonic dental epithelium (Satokata and Maas 1994; Bei and Maas 1998; D’Souza et al. 1999; Aberg, Cavender, et al. 2004; Aberg, Wang, et al. 2004), indicating that both Msx1 and Runx2 are important for activation of the mesenchymal odontogenic potential. Aberg, Wang, et al. (2004) showed that Runx2 mRNA expression was significantly reduced in the molar tooth mesenchyme in E14 Msx1-/- embryos and suggested that Runx2 acts downstream of Msx1 to activate Fgf3 expression during the bud-to-cap transition. However, the reduction of Fgf3 expression alone in the tooth mesenchyme could not account for the bud-stage developmental arrest in the Runx2-/- mice since Fgf3-null mice had full dentition with only minor abnormalities in tooth crown size (Wang et al. 2007). Extensive molecular marker studies showed that most of the important tooth mesenchyme factors were expressed normally in the Runx2-/- mutant tooth germs at the bud stage (Aberg, Wang, et al. 2004). Thus, the molecular mechanism of Runx2-mediated tooth development remains to be elucidated. In this study, we show that deletion of Osr2 partially rescued Runx2-/- molar tooth morphogenesis to the early bell stage. Our data provide new insights into the role of Runx2 in tooth development.
First, our data indicate that Runx2 regulates tooth morphogenesis through the bud-to-cap transition by modulating the antagonistic interactions of the Msx1 and Osr2 transcription factors. A critical step in tooth morphogenesis during the bud-to-cap transition is formation of the primary enamel knot in the tooth bud epithelium, which occurs at about E13.5 in mouse embryos. The primary enamel knot cells express multiple signaling molecules, including Shh, Fgf4, Bmp4, Wnt10a, and Wnt10b, which drive growth and morphogenesis of both the dental epithelium and mesenchyme to and through the cap stage (Jernvall and Thesleff 2000). In contrast to the Msx1-/- mutant mice, which exhibit tooth bud arrest and lack of primary enamel knot formation in all tooth germs, Runx2-/- mutant embryos showed expression of several enamel knot markers, including Shh, p21, Fgf4, Edar, and Bmp4, in the maxillary first molars but not in the mandibular molar tooth buds (Chen et al. 1996; Bei and Maas 1998; Aberg, Wang, et al. 2004). This difference in maxillary and mandibular molar tooth phenotypes was initially explained by possible partial complementation of Runx2 function by Runx3 since Runx3 expression was shown to be up-regulated in the maxillary but not mandibular molar mesenchyme in Runx2-/- embryos (Aberg, Wang, et al. 2004). However, subsequent studies showed that the primary enamel knot also formed in the maxillary molar tooth germs in Runx2-/-Runx3-/- double mutants (Wang et al. 2005), leaving the molecular basis of tooth developmental arrest in Runx2-/- embryos unresolved. We recently showed that mice with neural crest–specific inactivation of the Bmp4 gene had mandibular molar bud arrest while their maxillary molars and incisors continued morphogenesis (Jia et al. 2013). Remarkably, deletion of one Osr2 allele rescued mandibular molar tooth morphogenesis, whereas a reduction of Msx1 by 50% neutralized this effect, in the neural crest–specific Bmp4-mutant mice (Jia et al. 2013; Lan et al. 2014). In this study, we found that, whereas Runx2-/- mutant molar tooth mesenchyme exhibited reduced expression of Msx1 and Bmp4 mRNAs, both Osr2-/- and Osr2-/-Runx2-/- mutant embryos had increased expression of Msx1 and Bmp4 mRNAs in the tooth mesenchyme compared with the control embryos (Fig. 3A–H). Since Osr2 physically interacts with Msx1 and antagonizes Msx1-mediated activation of the mesenchymal odontogenic factors, including Bmp4 (Zhang et al. 2009; Zhou et al. 2011), and since Bmp4 is able to induce Msx1 expression in the dental mesenchyme through a positive feedback loop (Chen et al. 1996; Bei et al. 2000) as well as to induce expression of Shh and p21 in the dental epithelium (Jernvall et al. 1998; Chen et al. 2000), these results suggest that Runx2 normally acts to attenuate Osr2-mediated suppression of Msx1 function during the bud-to-cap transition (Fig. 5M). In the absence of Runx2 function, stronger than the normal level of Osr2-mediated repression of Msx1 function causes reduction in Bmp4 and other Msx1-dependent odontogenic factors in the developing tooth mesenchyme, leading to tooth developmental arrest in bud-to-cap transition in Runx2-/- mutant mice. In the Osr2-/-Runx2-/- mutant embryos, the lack of Osr2-mediated repression causes increased expression of Msx1-dependent mesenchymal odontogenic factors, including Bmp4, which induces primary enamel knot formation and drives successful transition of the molar tooth germs from the bud to cap stage.
Second, our data demonstrate that Runx2 function is required for activation of expression of both Fgf3 and Fgf10 in the dental papilla. Previous studies showed that Runx2 is required for Fgf4 induction of Fgf3 expression in the tooth mesenchyme (D’Souza et al. 1999; Aberg, Wang, et al. 2004). Since Fgf10 is only weakly expressed in the tooth mesenchyme at the bud stage and since the developmentally arrested Runx2-/- tooth germs also had weak Fgf10 expression at E14, initial Fgf10 expression in the tooth bud mesenchyme is not Runx2-dependent (Aberg, Wang, et al. 2004). However, Fgf10 expression is dramatically up-regulated in the tooth mesenchyme by the cap stage during normal tooth development (Aberg, Wang, et al. 2004b), but it was not detected in the E15.5 Osr2-/-Runx2-/- mutant tooth germs that have progressed to the cap stage (Fig. 5L). The lack of Fgf10 expression in the E15.5 Osr2-/-Runx2-/- mutant tooth germs when the control and Osr2-/-mutant dental mesenchyme had robust Fgf10 expression strongly suggests that Runx2 mediates the up-regulation of Fgf10 expression during the bud-to-cap transition. It has been shown that Fgf10 significantly stimulated tooth epithelial proliferation (Kettunen et al. 2000). Cell proliferation was significantly reduced in the Runx2-/- and Osr2-/-Runx2-/- tooth germs by E14.5, in comparison with wild-type littermates (Wang et al. 2005; Fig. 4). Moreover, Fgf3 and Fgf10 have been shown to act partly redundantly to regulate molar tooth size (Wang et al. 2007). Thus, the inability of the tooth mesenchyme to up-regulate either Fgf3 or Fgf10 expression is likely a major contributor to the growth retardation of the molar tooth germs in the Osr2-/-Runx2-/- mutant embryos.
Taken together, our data indicate that Runx2 acts downstream of and interacts with the Msx1-Osr2 antagonistic pair of transcription factors to regulate the mesenchymal odontogenic activity and that Runx2 plays an additional role in mediating up-regulation of mesenchymal Fgf signals to control tooth germ growth and morphogenesis beyond the cap stage.
Author Contributions
H.J.E. Kwon, R. Jiang, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; E.K. Park, Y. Lan, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; S. Jia, H. Liu, contributed to data acquisition, analysis, and interpretation, drafted the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
Acknowledgments
We thank Dr. Rena D’Souza for providing the Runx2+/- mice.
Footnotes
This work was supported by National Institutes of Health National Institute of Dental and Craniofacial Research grant R01DE018401 to R.J. E.K.P. was in part supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2014R1A1A4 A01006935).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aberg T, Cavender A, Gaikwad JS, Bronckers AL, Wang X, Waltimo-Sirén J, Thesleff I, D’Souza RN. 2004. Phenotypic changes in dentition of Runx2 homozygote-null mutant mice. J Histochem Cytochem. 52(1):131–139. [DOI] [PubMed] [Google Scholar]
- Aberg T, Wang XP, Kim JH, Yamashiro T, Bei M, Rice R, Ryoo HM, Thesleff I. 2004. Runx2 mediates FGF signaling from epithelium to mesenchyme during tooth morphogenesis. Dev Biol. 270(1):76–93. [DOI] [PubMed] [Google Scholar]
- Bei M, Kratochwil K, Maas RL. 2000. BMP4 rescues a non-cell-autonomous function of Msx1 in tooth development. Development. 127(21):4711–4718. [DOI] [PubMed] [Google Scholar]
- Bei M, Maas R. 1998. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development. 125(21):4325–4333. [DOI] [PubMed] [Google Scholar]
- Charles C, Lazzari V, Tafforeau P, Schimmang T, Tekin M, Klein O, Viriot L. 2009. Modulation of Fgf3 dosage in mouse and men mirrors evolution of mammalian dentition. Proc Natl Acad Sci USA. 106(52):22364–22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. 1996. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 122(10):3035–3044. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Jiang TX, Barlow AJ, St Amand TR, Hu Y, Heaney S, Francis-West P, Chuong CM, Maas R. 2000. Conservation of early odontogenic signaling pathways in Aves. Proc Natl Acad Sci USA. 97(18):10044–10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza RN, Aberg T, Gaikwad J, Cavender A, Owen M, Karsenty G, Thesleff I. 1999. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development. 126(13):2911–2920. [DOI] [PubMed] [Google Scholar]
- Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, Sekine K, Ohuchi H. 2002. FGF10 maintains stem cell compartment in developing mouse incisors. Development. 129(6):1533–1541. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Aberg T, Kettunen P, Keränen S, Thesleff I. 1998. The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development. 125(2):161–169. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. 2000. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 92(1):19–29. [DOI] [PubMed] [Google Scholar]
- Jia S, Zhou J, Gao Y, Baek JA, Martin JF, Lan Y, Jiang R. 2013. Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development. 140(2):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussila M, Thesleff I. 2012. Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages. Cold Spring Harb Perspect Biol. 4(4):a008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen P, Laurikkala J, Itäranta P, Vainio S, Itoh N, Thesleff I. 2000. Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Dev Dyn. 219(3):322–332. [DOI] [PubMed] [Google Scholar]
- Kollar EJ, Baird GR. 1970a. Tissue interactions in embryonic mouse tooth germs. I. Reorganization of the dental epithelium during tooth-germ reconstruction. J Embryol Exp Morphol. 24(1):159–171. [PubMed] [Google Scholar]
- Kollar EJ, Baird GR. 1970b. Tissue interactions in embryonic mouse tooth germs. II. The inductive role of the dental papilla. J Embryol Exp Morphol. 24(1):173–186. [PubMed] [Google Scholar]
- Lan Y, Jia S, Jiang R. 2014. Molecular patterning of the mammalian dentition. Semin Cell Dev Biol. 25–26:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Ovitt CE, Cho ES, Maltby KM, Wang Q, Jiang R. 2004. Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development. 131(13):3207–3216. [DOI] [PubMed] [Google Scholar]
- Maas R, Bei M. 1997. The genetic control of early tooth development. Crit Rev Oral Biol Med. 8(1):4–39. [DOI] [PubMed] [Google Scholar]
- Mandler M, Neubüser A. 2001. FGF signaling is necessary for the specification of the odontogenic mesenchyme. Dev Biol. 240(2):548–559. [DOI] [PubMed] [Google Scholar]
- Mansour SL, Goddard JM, Capecchi MR. 1993. Mice homozygous for a targeted disruption of the proto-oncogene int-2 have developmental defects in the tail and inner ear. Development. 117(1):13–28. [DOI] [PubMed] [Google Scholar]
- Neubüser A, Peters H, Balling R, Martin GR. 1997. Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell. 90(2):247–255. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 89(5):765–771. [DOI] [PubMed] [Google Scholar]
- Peters H, Neubüser A, Kratochwil K, Balling R. 1998. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 12(17):2735–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pispa J, Thesleff I. 2003. Mechanisms of ectodermal organogenesis. Dev Biol. 262(2):195–205. [DOI] [PubMed] [Google Scholar]
- Ryoo HM, Wang XP. 2006. Control of tooth morphogenesis by Runx2. Crit Rev Eukaryot Gene Expr. 16(2):143–154. [DOI] [PubMed] [Google Scholar]
- Satokata I, Maas R. 1994. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 6(4):348–356. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Sharpe P. 1997. Signalling networks regulating dental development. Mech Dev. 67(2):111–123. [DOI] [PubMed] [Google Scholar]
- Tucker A, Sharpe P. 2004. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 5(7):499–508. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Matthews KL, Sharpe PT. 1998. Transformation of tooth type induced by inhibition of BMP signaling. Science. 282(5391):1136–1138. [DOI] [PubMed] [Google Scholar]
- Vainio S, Karavanova I, Jowett A, Thesleff I. 1993. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 75(1):45–58. [PubMed] [Google Scholar]
- Wang XP, Aberg T, James MJ, Levanon D, Groner Y, Thesleff I. 2005. Runx2 (Cbfa1) inhibits Shh signaling in the lower but not upper molars of mouse embryos and prevents the budding of putative successional teeth. J Dent Res. 84(2):138–143. [DOI] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, Maas RL, Chuong CM, Schimmang T, Thesleff I. 2007. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 5(6):e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lan Y, Chai Y, Jiang R. 2009. Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science. 323(5918):1232–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Gao Y, Zhang Z, Zhang Y, Maltby KM, Liu Z, Lan Y, Jiang R. 2011. Osr2 acts downstream of Pax9 and interacts with both Msx1 and Pax9 to pattern the tooth developmental field. Dev Biol. 353(2):344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]