Diabetes mellitus is a heterogeneous group of disorders characterized by high blood glucose levels. It is projected that by 2025 there will be 300 million people afflicted with diabetes worldwide (Kaul et al. 2012). There are numbers of complications caused by diabetes, including increased heart disease, stroke, blindness, kidney failure, and delayed wound healing. Diabetes affects the oral cavity by increasing the prevalence of caries, gingivitis, periodontal disease, implantitis, fibromas, and traumatic ulcers (Guggenheimer et al. 2000). Chronic diabetic wounds affect up to one-fourth of diabetic patients (Eming et al. 2014), and oral soft tissue lesions are twice as prevalent (Guggenheimer et al. 2000). A number of factors may contribute to impaired healing in diabetics, such as increased blood glucose levels that cause metabolic distress at the cellular level, greater formation of advanced glycation endproducts (AGEs) that form as a result of increased glucose, increased expression of inflammatory cytokines, and elevated oxidative stress (Hameedaldeen et al. 2014).
Wound healing is a complex and highly orchestrated biological process that includes inflammation, re-epithelialization, granulation tissue formation, and remodeling. The proliferation and migration of epidermal keratinocytes into the wound site is required for normal healing. Recent evidence indicates that the forkhead box O1 (FOXO1) transcription factor plays a critical role in orchestrating the events that promote a healing response in keratinocytes. FOXO1 belongs to a family of forkhead transcription factors that participate in a wide range of cellular processes, including cell cycle arrest, DNA repair, apoptosis, and oxidative stress resistance. Ponugoti et al. (2013) showed that FOXO1 nuclear localization increased 4-fold in wound-healing keratinocytes. FOXO1 promotes keratinocytes migration by upregulating transforming growth factor β1 (TGF-β1) and its downstream target genes: integrin α3 and ß6 and matrix metalloproteinase 3 and 9. FOXO1 activation is also important for resisting oxidative stress that would otherwise interfere with keratinocyte migration and stimulate apoptosis (Ponugoti et al. 2013). Similarly, in oral mucosa, deletion of FOXO1 in keratinocytes results in reduced TGF-β1 expression, diminished mucosal epithelial migration, and impaired re-epithelialization (Xu et al. 2015). Thus, in normal wounds, FOXO1 represents an important transcription factor that promotes a healing response.
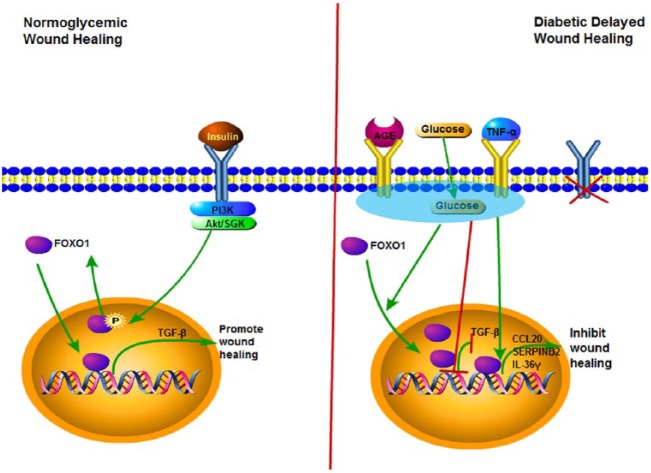
Conditional deletion of FOXO1 in keratinocytes of diabetic wounds has the opposite effect compared with its role in normal healing (Xu et al. 2015; Zhang et al. 2015). Deleting FOXO1 in keratinocytes in oral mucosal wounds or in skin wounds significantly improves re-epithelialization. This result indicates that FOXO1 switches from promoting healing under normal conditions to inhibiting it in diabetic wounds. To better understand this differential effect, mRNA profiling was carried out, which identified genes that were upregulated by high glucose in a FOXO1-dependent manner. These genes include chemokine (C-C motif) ligand 20 (CCL20), serpin peptidase inhibitor clade B2 (SERPINB2), and interleukin-36γ (IL-36γ), and their expression is elevated in diabetic wounds in a FOXO1-dependent manner in vivo. Furthermore, conditions present in diabetes, such as high levels of glucose, AGEs, and TNF, stimulate FOXO1 binding to the promoters of these genes to increase their expression (Zhang et al. 2015). This is detrimental to healing since high levels of CCL20, SERPINB2, and IL-36γ interfere with keratinocyte migration in vitro and are associated with reduced re-epithelialization in vivo (Xu et al. 2015; Zhang et al. 2015). Interestingly, high concentrations of glucose, AGEs, and TNF also reduce binding of FOXO1 to the promoter region of TGF-β1, preventing FOXO1 from inducing TGF-β1 transcription. This finding provides a mechanistic basis for how diabetes may cause a reduction in TGF-β1 to contribute to an impaired healing response. Thus, factors that are elevated in diabetic wounds cause a switch in FOXO1 downstream gene targets to reduce FOXO1 induction of TGF-β1 and increase FOXO1 binding to the promoter regions of factors that may inhibit re-epithelialization, as summarized in the Figure.
Figure.
Forkhead box O1 (FOXO1) differentially regulates wound healing in normoglycemic and diabetic conditions. Wounding stimulates FOXO1 translocation to the nucleus. Left panel: In normal wounds, FOXO1 binds to the transforming growth factor β1 (TGF-β1) promoter to increase TGF-β1 transcription and expression. FOXO1-induced TGF-β1 enhances keratinocyte migration, which promotes re-epithelialization and wound closure. Insulin signaling through Akt prevents hyperactivation of FOXO1 by driving it out of the nucleus. Right panel: In diabetic wounds, high glucose, advanced glycation endproducts (AGEs), and TNF-α stimulate FOXO nuclear localization but modulate FOXO1 so that it does not bind to TGF-β1 promoter but instead exhibits increased binding to chemokine (C-C motif) ligand 20 (CCL20), serpin peptidase inhibitor, clade B2 (SERPINB2), and interleukin-36γ (IL-36γ) promoters. High levels of CCL20, SERPINB2, and IL-36γ interfere with keratinocyte migration and impair re-epithelialization. Insulin, which would normally act as a break on FOXO1 activity, is reduced, compounding the effect of high glucose, AGE, and TNF stimulation. Green arrows indicate stimulation of a cellular event; red lines represent inhibition.
The precise mechanisms that cause this shift in FOXO1 downstream targets have not yet been clarified. Two possibilities are that FOXO1 activity is modulated by (1) posttranslational modification or (2) a change in proteins that interact with FOXO1, which function as coactivators or corepressors (Ponugoti et al. 2012). FOXO1 undergoes posttranslational modification near its DNA-binding domain by acetylation or phosphorylation, which may increase or decrease FOXO1 binding to target gene promoters. In addition, FOXO1 forms complexes with other proteins that help to enhance or reduce FOXO1’s transcriptional activity. For example, FOXO1 interacts with another transcription factor, specificity factor-1 (Sp1), to bind to the promoter region of Tbx21 and suppress transcription, an important control point in limiting natural killer cell activity (Deng et al. 2015). Thus, FOXO1 expressed in keratinocytes may have posttranslational modifications induced by glucose, AGEs, or TNF, or these factors may cause FOXO1 to interact with different proteins to alter its binding to DNA of specific gene promoters. Lastly, insulin signaling phosphorylates FOXO1 and excludes it from the nucleus. Zhang et al. (2015) found that insulin reversed the negative effect of FOXO1 on keratinocytes incubated in media with high glucose, AGE, or TNF. Thus, insulin may be protective by moving FOXO1 out of the nucleus under normal conditions. But in diabetic wounds, low insulin signaling may compound the effect of high glucose, AGEs, and TNF. This finding may also explain how topical insulin treatment overcomes delayed wound healing in diabetic patients (Paul 1966). It is also possible that FOXO1 activity in keratinocytes affects connective tissue healing due to the influence of keratinocytes on the formation of granulation tissue, but this question remains to be explored (Hameedaldeen et al. 2014). Moreover, diabetes affects many different factors and pathways that modulate healing. While FOXO1 has been shown to play an important role in repair, there are many aspects of wound healing that are likely to be independent of FOXO1. Thus, the specific benefits of inhibiting FOXO1 in diabetic healing need to be experimentally examined.
Author Contributions
E. Xiao, contributed to conception, drafted the manuscript. D.T. Graves, contributed to conception and design, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
The authors have given this perspective based on research supported by the National Institutes of Health (DE-019108).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Deng Y, Kerdiles Y, Chu J, Yuan S, Wang Y, Chen X, Mao H, Zhang L, Zhang J, Hughes T, et al. 2015. Transcription factor Foxo1 is a negative regulator of natural killer cell maturation and function. Immunity. 42(3):457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. 2014. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 6(265):265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheimer J, Moore PA, Rossie K, Myers D, Mongelluzzo MB, Block HM, Weyant R, Orchard T. 2000. Insulin-dependent diabetes mellitus and oral soft tissue pathologies: II. Prevalence and characteristics of Candida and Candidal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 89(5):570–576. [DOI] [PubMed] [Google Scholar]
- Hameedaldeen A, Liu J, Batres A, Graves GS, Graves DT. 2014. FOXO1, TGF-beta regulation and wound healing. Int J Mol Sci. 15(9):16257–16269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul K, Tarr JM, Ahmad SI, Kohner EM, Chibber R. 2012. Introduction to diabetes mellitus. Adv Exp Med Biol. 771:1–11. [DOI] [PubMed] [Google Scholar]
- Paul TN. 1966. Treatment by local application of insulin of an infected wound in a diabetic. Lancet. 2(7463):574–576. [DOI] [PubMed] [Google Scholar]
- Ponugoti B, Dong G, Graves DT. 2012. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res. 2012;2012:939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponugoti B, Xu F, Zhang C, Tian C, Pacios S, Graves DT. 2013. FOXO1 promotes wound healing through the up-regulation of TGF-beta1 and prevention of oxidative stress. J Cell Biol. 203(2):327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Othman B, Lim J, Batres A, Ponugoti B, Zhang C, Yi L, Liu J, Tian C, Hameedaldeen A, et al. 2015. Foxo1 inhibits diabetic mucosal wound healing but enhances healing of normoglycemic wounds. Diabetes. 64(1):243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ponugoti B, Tian C, Xu F, Tarapore R, Batres A, Alsadun S, Lim J, Dong G, Graves DT. 2015. FOXO1 differentially regulates both normal and diabetic wound healing. J Cell Biol. 209(2):289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]