Abstract
We report the results of a long-term follow-up of subjects in a phase 1 study of AAV2-hAADC (adeno-associated virus type 2–human aromatic L-amino acid decarboxylase) gene therapy for the treatment of Parkinson's disease (PD). Ten patients with moderately advanced PD received bilateral putaminal infusions of either a low or a high dose of AAV2-hAADC vector. An annual positron emission tomography (PET) imaging with [18F]fluoro-L-m-tyrosine tracer was used for evaluation of AADC expression, and a standard clinical rating scale [Unified Parkinson's Disease Rating Scale (UPDRS)] was used to assess effect. Our previous analysis of the 6-month data suggested that this treatment was acutely safe and well tolerated. We found that the elevated PET signal observed in the first 12 months persisted over 4 years in both dose groups. A significantly increased PET value compared with the presurgery baseline was maintained over the 4-year monitoring period. The UPDRS in all patients off medication for 12 hr improved in the first 12 months, but displayed a slow deterioration in subsequent years. This analysis demonstrates that apparent efficacy continues through later years with an acceptable safety profile. These data indicate stable transgene expression over 4 years after vector delivery and continued safety, but emphasize the need for a controlled efficacy trial and the use of a higher vector dose.
Mittermeyer and colleagues report results from a phase 1 study of AAV2-hAADC (human aromatic l-amino acid decarboxylase) gene therapy for the treatment of Parkinson's disease. The study shows that AAV2-hAADC treatment was safe and that AADC gene expression was maintained at least 4 years after administration of therapy. Positron emission tomography analysis showed that a similar number of neurons continued expressing the transgene throughout the study.
Introduction
As Parkinson's disease (PD) progresses, the effectiveness of most pharmacotherapies wanes. The most serious problem is the inexorable loss of levodopa (L-Dopa; Sinemet) response, because it is the main and most effective drug available. PD patients, over the course of a number of years, must take more drug more frequently to maintain therapeutic efficacy, and this dose escalation is associated with the appearance of dyskinesia, freezing of gait, and other complications. The suggestion that L-Dopa becomes less effective over time due to declining levels of aromatic amino acid decarboxylase (AADC) (Nagatsu and Sawada, 2007) has led to efforts to increase AADC expression with gene therapy. AADC is the rate-limiting enzyme for the conversion of L-Dopa to dopamine (DA), and its loss (Ichinose et al., 1994) may be responsible for the decreasing effect of long-term L-Dopa therapy. Increasing AADC expression in putaminal neurons should, therefore, provide the necessary catalytic activity for efficient metabolism of medically administered L-Dopa to DA. Numerous nonhuman primate (NHP) studies have supported this theory by showing that infusion of an adeno-associated virus (AAV) vector containing the cDNA encoding human AADC (hAADC) to the putamen of PD models has the potential to restore DA in the striatum (Eberling et al., 1997; Bankiewicz et al., 2000) and provide functional correction of the motor deficit (Bankiewicz et al., 2006).
PET imaging during the first 6 months of a phase 1 study of AAV2-hAADC gene therapy demonstrated a significant elevation in AADC expression, a good safety profile, and preliminary indications of clinical benefit (Christine et al., 2009). It has not yet been established whether AADC gene therapy has an effect on patients in the OFF state, the clinical state when the benefits of dopaminergic medications are minimal in patients with fluctuating motor symptoms. The OFF state typically occurs a number of hours after the last of L-Dopa. The 6-month analysis (Christine et al., 2009) suggested an improvement in scores of both total and part III Unified Parkinson's Disease Rating Scale (UPDRS) scores in the OFF state, which could be attributed either to a direct effect of AADC gene therapy or to a placebo effect. This report presents the long-term results of the phase 1 study. Ten patients received bilateral convection-enhanced delivery (CED) of AAV2-hAADC gene therapy. Our intent was to evaluate the safety and tolerability of the therapy, to evaluate the long-term level of gene expression, and to help in the design of future clinical studies.
Materials and Methods
Subjects
A detailed description of the human study methods has been published (Eberling et al., 2008; Christine et al., 2009) and is summarized here only briefly. Ten patients (five men and five women) with a mean age of 64 years (range 57–71) were entered into the study. Two dose cohorts (five patients each) were evaluated. The low-dose cohort received a total dose of 9×1010 vector genomes, and the high-dose cohort received 3×1011 vector genomes. The entry criteria included the following: PD with intractable motor fluctuations despite optimized medical treatment; Hoehn and Yahr Stage III to IV off medication; a history and screening examinations showing improvement with dopaminergic therapy; and optimized and stable anti-PD medication for at least 2 months prior to screening.
Putaminal AAV2-hAADC infusion
On the morning of the operation, subjects were admitted to the neurosurgical service and antiparkinsonian medications were withheld. The subjects were fitted with a Leksell stereotactic head frame (Elekta, Norcross, GA) with local anesthesia at the pin sites and intravenous sedation with midazolam. The target in the postcommissural putamen was localized with gadolinium-enhanced volumetric T1 and either T2 or inversion recovery slab magnetic resonance (MR) acquisitions of the brain. Two parallel cannulae were placed 6 mm apart in the center of the postcommissural putamen, one anterior and one posterior. Trajectories to these targets were planned so as to avoid traversing the lateral ventricle, sulci, and cortical veins. Subjects were infused bilaterally with a total volume of 200 μl over the four injection sites (50 μl/site) with CED at a flow rate of 1 μl/min (van Hilten et al., 1994; Bankiewicz et al., 2000). Customized infusion cannulae were designed to minimize potential reflux up the injection tract, and a “waiting period” of 10 min after cessation of active infusion was observed before the cannulae were removed.
PET scanning
Subjects underwent positron emission tomography (PET) scans with the AADC-specific tracer [18F]fluoro-L-m-tyrosine (FMT) 1–10 days before surgery, at 1 and 6 months after surgery, and annually for up to 5 years. PET studies were performed on a Siemens ECAT EXACT HR+ PET scanner in 3D acquisition mode. All subjects were studied approximately 60–90 min after an oral dose of 2.5 mg/kg carbidopa. Prior to the emission scan, a 5-min transmission scan was obtained for attenuation correction. Subsequently, 1–2 mCi of FMT was injected as a bolus in an antecubital vein, and a 90-min dynamic acquisition sequence was obtained. Data were quantified as previously described (Eberling et al., 2008), yielding the term KiC to describe tracer uptake. The superscript letter denotes the use of cerebellum as a reference region to correct for variations in tracer decay, etc.
UPDRS evaluation
Subjects were evaluated clinically at baseline and then monthly for 6 months postoperatively. Standardized evaluations with the UPDRS (van Hilten et al., 1994) and the stand–walk–sit test (O'Sullivan et al., 1998) were performed at baseline and 6 months and then annually in the off- and on-medication states. The UPDRS is a widely used rating scale that evaluates cognitive, functional, motor deficits (UPDRS, part III) and medication-related complications. UPDRS scores indicate worse disease as the score increases. It was administered in the morning in the OFF state, 12 hr after the last dose of dopaminergic medication, and in the fully ON state, as judged by the patient and clinician. In most cases, this occurred 1 hr after the usual morning medications were taken. However, if the subject was not fully “on,” an additional 12.5/50 or 25/100 of carbidopa/L-Dopa was administered and the patient evaluated when fully “on.”
Statistics
Comparisons between groups were made with Student's t test, with a threshold of p<0.05 considered significant.
Results
FMT uptake
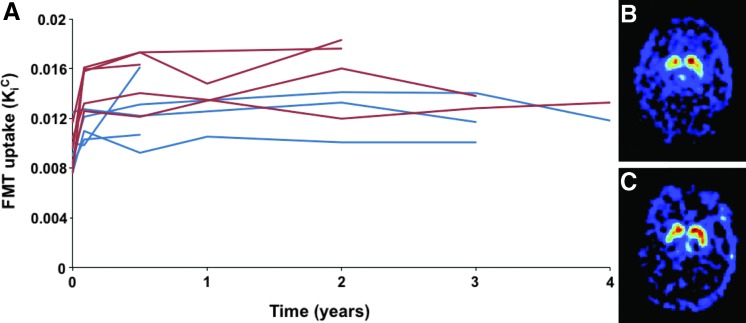
The KiC values showed a significant increase in the first 6 months that was maintained throughout the 4-year duration of the study (Fig. 1). After an initial increase of 25% and 65% in the low- and high-dose groups, respectively, there was no significant change in KiC in either group.
FIG. 1.
Time course of FMT-PET. (A) Time course of PET signal in low-dose (blue) or high-dose (red) subjects over time. (B, C) Scans of high-dose patient at baseline (B) and 4 years after treatment (C). Color images available online at www.liebertonline.com/hum
Clinical safety and efficacy
Six and one half years after infusion of the first subject, all subjects are alive and ambulatory. One patient did not return for follow-up UPDRS and PET evaluations after the first year follow-up. Four subjects underwent treatment with deep brain stimulation (DBS) after gene therapy (at 10, 18, 30, and 50 months), and data obtained after DBS surgery were removed from the UPDRS analysis. Table 1 lists adverse events that have occurred so far. Mild worsening of dyskinesias occurred in four subjects; this was transient and responded to adjustments of L-Dopa dosage with the addition of amantadine in one subject and an increase of the frequency of amantadine dosing in another.
Table 1.
Adverse Events
| Subjects affected | aRelationship To surgery | aRelationship to Infusion | |
|---|---|---|---|
| Incisional tenderness | 8 | 8 | |
| Transient headache | 6 | 6 | |
| Facial edema | 5 | 5 | |
| Scalp numbness or paresthesia | 4 | 4 | |
| Anxiety | 4 | ||
| Transient increase in dyskinesia | 4 | 4 | |
| Transient nasal congestion | 3 | 3 | |
| Falling | 10 | ||
| Intracranial hemorrhageb | 3 | 3 | |
| Insomnia | 2 | ||
| Lower extremity numbness | 2 | ||
| Seizure disorderc | 1 | ||
| Prostatitis and urinary retention | 1 | ||
| Syncope | 1 | ||
| Upper respiratory infection | 1 |
Designation includes adverse events that were possibly, probably, or definitely related to the intervention.
Subject 9 suffered a hemorrhagic infarct (attributed to an arterial rupture). The other two events were asymptomatic and discovered incidentally on postoperative MRI. One subject had a small subdural and subarachnoid hemorrhage, and the other an intra-cerebral hemorrhage thought to be secondary to venous infarction. All hemorrhages were dorsal to the infusion site and were attributed to the surgical procedure.
Partial seizure disorder developed 4 months after surgery in the subject with an intra-cerebral arterial hemorrhage and resolved on treatment with phenytoin.
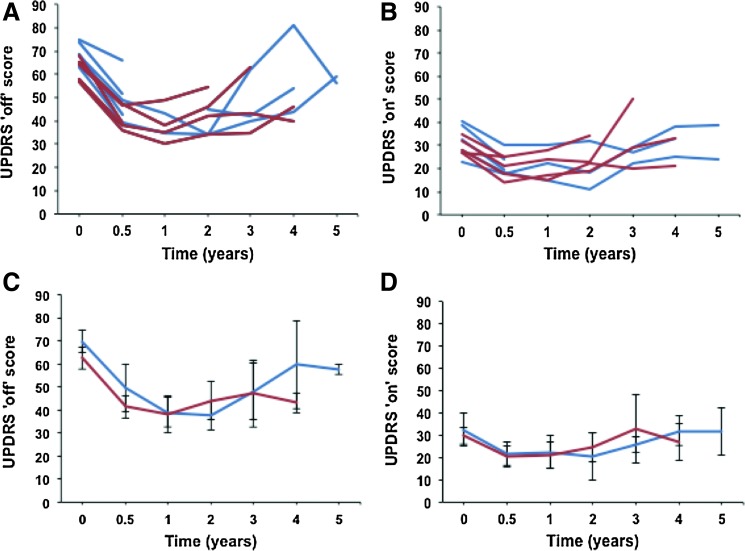
Temporal analyses of the UPDRS scores in the ON and OFF states for all subjects are summarized in Fig. 2. Both ON and OFF scores showed a significant improvement in the first 12 months in all patients, followed by a slow deterioration over the following years. There were no significant differences between the high- and low-dose patients in either the ON or OFF score.
FIG. 2.
Time course of UPDRS ON and OFF scores. (A, B) OFF (A) and ON (B) scores for individual study subjects over time for low dose (blue) and high dose (red). (C, D) Aggregate OFF (C) and ON (D) scores for low-dose (blue) and high-dose (red) subjects. Color images available online at www.liebertonline.com/hum
Discussion
This study showed that AAV2-hAADC treatment was safe and that AADC gene expression was maintained at least 4 years after administration of therapy. The PET analysis showed that FMT uptake increased initially and remained elevated throughout the study duration, indicating an improvement in comparison with other studies that have documented a decrease in FMT uptake over time in PD patients (Asari et al., 2011). The persistent elevation of FMT suggested that a similar number of neurons continued expressing the transgene throughout the study, consistent with our previous observations in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)–lesioned rhesus macaques (NHPs) (Hadaczek et al., 2010).
These results demonstrate the dose dependence of the treatment and support nonclinical data indicating that vector dose determines the number of neurons expressing the transgene (Cunningham et al., 2008). However, a pivotal dose-ranging study in NHPs (Forsayeth et al., 2006) showed that, as vector dose is increased, there is a modest positive linear correlation between vector dose and FMT-PET that is followed by a sharp transition to a much higher PET signal. We found that the low signal was bound by the ranges of PET values found in MPTP-lesioned animals, and the high signal by the range of values found in unlesioned animals. This striking transition suggests that expression of AADC activity in putamen is either rate-limiting or not. The fact that the two doses of vector in the phase 1 study display a modest positive correlation, but a low overall intensity, suggests that a higher vector dose will be required to maximize the efficacy of L-Dopa conversion in a phase 2 study. The data showed a trend toward decline of KiC at 3 and 4 years in the high-dose group that possibly reflects ongoing degeneration of dopaminergic neurons. It should be kept in mind that AAV2-hAADC therapy results in expression of AADC in putaminal medium spiny neurons that do not degenerate, in contrast to nigral neurons that express endogenous AADC. MPTP-lesioned monkeys that were treated with AAV2-hAADC and studied for 8 years showed consistent FMT signal and robust AADC expression in the striatal interneurons (Hadaczek et al., 2010). As there is no progressive degeneration of dopaminergic neurons in the MPTP model beyond that occurring with the acute intoxication with MPTP, we believe that reduction of FMT-PET signal in patients treated with AAV2-hAADC indeed reflects ongoing neurodegeneration rather than loss of AADC expression.
Aside from the three intracranial hemorrhages (two were asymptomatic and were detected by postoperative scans), adverse effects during the course of the study were no more than would be expected in any long-term study of PD subjects, namely, fractures due to falls and infections of lung and bladder. The increased anxiety and depression that occurred in one patient are also common in PD.
The UPDRS showed a dramatic improvement within the first 12 months. This is most likely due, at least partly, to a placebo effect, which has been well documented for PD (de la Fuente-Fernández and Stoessl, 2002). As this was an open-label study, both patients and physicians were susceptible to a placebo effect, as we acknowledged in our original publication.
In comparable sham-controlled studies in which striatal implantation of DA-secreting cells (Gross et al., 2011) or AAV2 containing neurturin cDNA (Marks et al., 2010), improvements of 7–10 points on the UPDRS motor score (on or off medication) were seen in both sham and active arms of the two studies (for review, see Forsayeth et al., 2010). This is very similar to the degree of improvement seen in the present study in the first 12 months. The fact that the two cited studies showed no clinical benefit shows the importance of a sham surgery control group and limitation in using the UPDRS as a primary outcome measure in the first 12 months.
After the first 12 months, there was a long period during which patients' responsiveness to L-Dopa (as measured in the ON state) was effectively maintained despite worsening disease severity (as measured by the OFF state). Data in MPTP-treated NHPs, in which there is no progressive loss of dopaminergic neurons, revealed constant OFF scores (Hadaczek et al., 2010), suggesting that the worsening of the UPDRS OFF score in this trial is due to ongoing neurodegeneration. During this period, the difference between the ON and OFF scores may therefore provide a valuable measure of the effectiveness of AAV2-hAADC therapy.
There are several important implications of these clinical data. AAV2-mediated gene transfer appears to be permanent, and it is altered little by the ongoing neurodegenerative process. Because AAV2 targets striatal interneurons that do not degenerate in idiopathic PD, we conclude that gene transfer in PD patients is permanent. The results of this trial do not exclude a likely placebo effect most dramatically in evidence in the first 12 months, and this should be borne in mind in the design of any phase 2 trials. Furthermore, measuring treatment effects by the difference between ON and OFF scores may be a reasonable approach to evaluating the efficacy of L-Dopa response after AAV2-hAADC therapy. Finally, analysis of FMT-PET in low- and high-dose subjects (Valles et al., 2010) indicated that distribution of AADC expression was confined to less than 25% of the putamen and 45% of postcommissural putamen. The contrast in behavioral outcome in parkinsonian NHPs (Bankiewicz et al., 2006; Forsayeth et al., 2006) and the present clinical trial may be attributed, in part, to the approximate fivefold difference in size between NHP and human putamen (Yin et al., 2009). Distribution of AADC immunoreactivity in parkinsonian NHPs (Cunningham et al., 2008) correlated with strong behavioral improvements in L-Dopa response. We concluded that, whereas infusion of 0.1 ml of AAV2-hAADC into NHP putamen is clearly sufficient for full restoration of L-Dopa response, such a volume is likely inadequate in humans. Accordingly, we have developed an MRI-guided infusion procedure in which distribution of vector may be monitored in real time (Richardson et al., 2011a,b). We are currently planning a follow-up phase 1 study in which larger volumes and doses of AAV2-hAADC will be infused under conditions in which more than 60% of the putamen should be transduced.
Acknowledgments
This study was supported by Genzyme Corporation, which was also the study sponsor.
Author Disclosure Statement
Dr. Kaplan is an employee of Genzyme Corporation, the study sponsor. No other authors have any financial disclosure to make.
References
- Asari S. Fujimoto K. Miyauchi A., et al. Subregional 6-[18F]fluoro-L-m-tyrosine uptake in the striatum in Parkinson's disease. BMC Neurol. 2011;11:35. doi: 10.1186/1471-2377-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz K.S. Eberling J.L. Kohutnicka M., et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp. Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Bankiewicz K.S. Forsayeth J. Eberling J.L., et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol. Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Christine C.W. Starr P.A. Larson P.S., et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J. Pivirotto P. Bringas J., et al. Biodistribution of adeno-associated virus type-2 in nonhuman primates after convection-enhanced delivery to brain. Mol. Ther. 2008;16:1267–1275. doi: 10.1038/mt.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Fernández R. Stoessl A.J. The placebo effect in Parkinson's disease. Trends Neurosci. 2002;25:302–306. doi: 10.1016/s0166-2236(02)02181-1. [DOI] [PubMed] [Google Scholar]
- Eberling J.L. Bankiewicz K.S. Jordan S., et al. PET studies of functional compensation in a primate model of Parkinson's disease. Neuroreport. 1997;8:2727–2733. doi: 10.1097/00001756-199708180-00017. [DOI] [PubMed] [Google Scholar]
- Eberling J.L. Jagust W.J. Christine C.W., et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- Forsayeth J.R. Eberling J.L. Sanftner L.M., et al. A dose-ranging study of AAV-hAADC therapy in Parkinsonian monkeys. Mol. Ther. 2006;14:571–577. doi: 10.1016/j.ymthe.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsayeth J. Bankiewicz K.S. Aminoff M.J. Gene therapy for Parkinson's disease: where are we now and where are we going? Expert Rev. Neurother. 2010;10:1839–1845. doi: 10.1586/ern.10.161. [DOI] [PubMed] [Google Scholar]
- Gross R.E. Watts R.L. Hauser R.A., et al. Intrastriatal transplantation of microcarrier-bound human retinal pigment epithelial cells versus sham surgery in patients with advanced Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2011;10:509–519. doi: 10.1016/S1474-4422(11)70097-7. [DOI] [PubMed] [Google Scholar]
- Hadaczek P. Eberling J.L. Pivirotto P., et al. Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC. Mol. Ther. 2010;18:1458–1461. doi: 10.1038/mt.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose H. Ohye T. Fujita K., et al. Quantification of mRNA of tyrosine hydroxylase and aromatic L-amino acid decarboxylase in the substantia nigra in Parkinson's disease and schizophrenia. J. Neural Transm. Park. Dis. Dement. Sect. 1994;8:149–158. doi: 10.1007/BF02250926. [DOI] [PubMed] [Google Scholar]
- Marks W.J., Jr. Bartus R.T. Siffert J., et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- Nagatsu T. Sawada M. Biochemistry of postmortem brains in Parkinson's disease: historical overview and future prospects. J. Neural Transm. Suppl. 2007:113–120. doi: 10.1007/978-3-211-73574-9_14. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J.D. Said C.M. Dillon L.C., et al. Gait analysis in patients with Parkinson's disease and motor fluctuations: influence of levodopa and comparison with other measures of motor function. Mov. Disord. 1998;13:900–906. doi: 10.1002/mds.870130607. [DOI] [PubMed] [Google Scholar]
- Richardson R.M. Gimenez F. Salegio E.A., et al. T2 imaging in monitoring of intraparenchymal real-time convection enhanced delivery. Neurosurgery. 2011a;69:154–163. doi: 10.1227/NEU.0b013e318217217e. [DOI] [PubMed] [Google Scholar]
- Richardson R.M. Kells A.P. Martin A.J., et al. Novel platform for MRI-guided convection-enhanced delivery of therapeutics: preclinical validation in nonhuman primate brain. Stereotact. Funct. Neurosurg. 2011b;89:141–151. doi: 10.1159/000323544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles F. Fiandaca M.S. Eberling J.L., et al. Qualitative imaging of adeno-associated virus serotype 2-human aromatic L-amino acid decarboxylase gene therapy in a phase I study for the treatment of Parkinson disease. Neurosurgery. 2010;67:1377–1385. doi: 10.1227/NEU.0b013e3181f53a5c. [DOI] [PubMed] [Google Scholar]
- van Hilten J.J. van der Zwan A.D. Zwinderman A.H. Roos RA. Rating impairment and disability in Parkinson's disease: evaluation of the Unified Parkinson's Disease Rating Scale. Mov. Dis. 1994;9:84–88. doi: 10.1002/mds.870090113. [DOI] [PubMed] [Google Scholar]
- Yin D. Valles F.E. Fiandaca M.S., et al. Striatal volume differences between non-human and human primates. J. Neurosci. Methods. 2009;176:200–205. doi: 10.1016/j.jneumeth.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]