Abstract
There has been great interest in understanding how human islets differ from rodent islets. Three major issues about human islet morphology have remained controversial over recent decades: 1) the proportion of the islet made up of β-cells; 2) whether islet cell types have a non-random mantle-core pattern, as seen in rodents, or are randomly scattered throughout the islet; 3) the relation of the different cell types to the blood vessels within the islet, which has implications for intraislet function. We re-examined these issues on immunostained sections of non-diabetic adult human pancreas. The composition of the islets can vary by the analysis method (number vs volume) and by the sampling of islets by size. The majority of adult human islets have clear, non-random clustering of β-cells and blood vessels that penetrate into the β-cell cores. We conclude that although there is far more variability in islet composition both within each human pancreas and among different human pancreas than in rodent pancreas, the islet architecture is not so different between the species. The intrapancreatic variability raises important questions about how islets evolve and function throughout life and how this might relate to the pathogenesis of diabetes.
Keywords: human islets, islet composition, islet architecture, mantle-core
Introduction
As the incidence of both type 1 and type 2 diabetes has increased, there has been great interest in the structure and function of human islets and how they differ from rodent islets, which have provided so much of our knowledge about islet biology. The importance of this understanding for human disease is obvious. One would think that there would be general agreement about human islet morphology; however, this is not the case. Three major issues have remained controversial over recent decades. The first is the proportion of the islet that is β-cells; the second is whether there is a variation of the non-random mantle-core pattern seen in rodents or whether different cell types are simply scattered throughout the islet; the third is the relation of the different cell types to the blood vessels within the islet, which has implications on intraislet function. In this paper, we have revisited these issues by providing both a review of the relevant studies and presenting our own data.
The adult human islet is far more variable than that of rodents: occasional large islets (greater than 200 um diameter) are seen with a majority of glucagon-positive cells (Bonner-Weir et al. 2014; Rahier et al. 1983; Yoon et al. 2003), and islets from the pancreatic polypeptide (PP)-rich uncinate process are mainly PP cells, with β-cells making up only 32.7% ± 7.8% as compared with 65.5% ± 4.9% in the rest of pancreas (Sakuraba et al. 2002). Islets from adult non-diabetic humans have been reported to be between 52%–75% β-cell, with some of the discrepancy due to whether the percentage was based on cell number or cell volume (Pisania et al. 2010). With brightfield analysis assessing cell volume, islets have been determined to comprise 52.0% ± 4.1% (Butler et al. 2003), 59% ± 10.3% (Yoon et al. 2003) or 74.8% (Maclean and Ogilvie 1955) β-cells, but, more recently, using laser-scanning confocal microscopy, the β-cell proportion of islets was estimated to be 53.9% ± 2.5% (cell volume, 32 islets isolated from 6 pancreas; Brissova et al. 2005) or 55% (cell number with visible nucleus, 2–5 islets/section, 3 sections/region, 4 regions/5 pancreas; Cabrera et al. 2006). In our studies using ultrastructural analyses to determine cell type and cell boundaries, we found 73.6% ± 1.7% β-cells by cell number in islets isolated from 33 pancreases (Pisania et al. 2010).
In most mammalian species, the different islet endocrine cells have a non-random pattern, with a core of β-cells surrounded by a discontinuous mantle of non-β-cells that are one to three cells thick (Erlandsen et al. 1976; Orci and Unger 1975). However, humans and some primates have a more complex arrangement, which has led to some reports of a random distribution. Four decades ago, it was suggested that human islets could be considered as composites of several mantle-core subunits or as lobulated with mantle-core lobules (Erlandsen et al. 1976; Orci and Unger 1975). Most non-β-cells are found along blood vessels that penetrate the islet and at the islet periphery (Erlandsen et al. 1976; Grube et al. 1983), thus maintaining a mantle-core arrangement. More recently, studies using confocal microscopy have both agreed and disagreed with the concept of human islets having a mantle-core architecture (Bosco et al. 2010; Brissova et al. 2005; Cabrera et al. 2006; Kilimnik et al. 2012; Wang et al. 2013). Brissova et al. (2005) stated, “…[human islets] lack the typical core/mantle…[α/β-] cells were dispersed throughout the islet. This pattern of cell distribution was present in all human islet preparations and islets of various sizes and was also seen in histological sections of human pancreas.” Agreeing with them, Cabrera et al. (2006) stated, “Contrary to descriptions of prototypical islets in textbooks and in the literature, human islets did not show anatomical subdivisions. Insulin-immunoreactive cells, glucagon-immunoreactive cells, and somatostatin-containing cells were found scattered throughout the human islet. Human β-cells were not clustered….” However, Bosco et al. (2010) found that, “In small human islets (40–60 μm in diameter), β-cells had a core position, α-cells had a mantle position, and vessels laid at their periphery. In bigger islets, α-cells had a similar mantle position but were found also along vessels that penetrate and branch inside the islets.” Hara’s group reported, “In human islets, while small islets show similar cellular composition with mice, in larger islets (>60 μm in diameter), the fraction of α- and δ-cells increases…” and while, “more abundant α-cells are intermingled with β-cells in the islet core as well as periphery, they are clearly not a random mixture of α- and β-cells” (Kilimnik et al. 2012; Wang et al. 2013).
In rodents we have previously reported that the islet microvasculature imposed a directional flow of blood through the islet (Bonner-Weir and Orci 1982; Samols et al. 1986) and that the polarity of the β-cells in relation to the capillaries provided an anatomical basis for a functional compartmentalization of the islet (Bonner-Weir 1988). With passive neutralization experiments Samols et al. (1988) showed that directional blood flow was important for regulation of the intraislet interactions, not only in the rat but also in dog, monkey and human (Stagner and Samols 1992; Stagner et al. 1988; Stagner et al. 1992). Yet, more recent studies have suggested that the relation of the blood vessels and islet endocrine cells was different in the human islet than in rodent (Bosco et al. 2010; Cabrera et al. 2006). Cabrera et al. (2006) stated, “Most β-, α- and δ-cells were aligned along blood vessels with no particular order or arrangement, indicating that islet microcirculation likely does not determine the order of paracrine interactions…Thus, an anatomical basis for an order in paracrine signaling as determined by the direction of blood flow likely does not exist for human islets.” Moreover, Bosco et al. (2010) reported that, “after a triple immunofluorescence for insulin, glucagon, and CD34, we confirmed that no vascular channel penetrated the core of 40 to 60 µm-diameter islets or the clusters of β-cells in bigger islets.”
These three major issues about the architecture of human islets have remained controversial over several decades. We have reexamined the in situ islet in the normal human pancreas with these issues in mind. The first is the proportion of the islet occupied by β-cells; the second is whether there is a variation of the mantle-core pattern seen in rodents; the third is the relation of the different cell types to the blood vessels within the islet.
Materials & Methods
Tissue
Pancreases from brain-dead donors for clinical islet transplantation were obtained by the Harvard/Joslin Islet Resource Center from the New England Organ Bank following informed consent from the donors’ families. Research consent had been obtained for all donors used in this study; the tissues were de-identified and were considered exempt by the Joslin IRB. Only pancreases with cold ischemia at times of 12 hr or less were processed for clinical islet transplantation. For each pancreas, a small piece from the body of the pancreas near the cannulated duct was fixed in 4% (para)-formaldyde overnight and embedded in paraffin for quality control assessment; sections from these blocks have been used for the current study.
Immunostaining
Sections were immunostained for insulin (guinea pig anti- porcine insulin, 1:200, Linco or 1:1000, Dako; Carpinteria, CA), glucagon (rabbit anti-bovine glucagon; 1:3000, gift from Dr. M. Appel, UMass Medical Center, Worcester, MA) and somatostatin (rabbit anti-somatostatin, 1:200, EMD Milllipore; Billerica, MA). The sections were incuba-ted overnight at 4°C, washed with Tris buffer, pH 7.4, sequentially incubated with goat anti-rabbit IgG and rabbit peroxidase anti-peroxidase (PAP) (Cappel Labo-ratories; Cochranville, PA), reacted with chromagen 3,3’-diaminobenzidine (DAB, Sigma-Aldrich; St Louis, MO) and counterstained with hematoxylin. Alternatively, sections were incubated with a mixture of the anti-glucagon and anti-somatostatin antibodies overnight and then anti-insulin antibodies for 1 hr at room temperature, followed by biotinylated secondary antibodies (biotinylated anti-rabbit IgG, AlexaFluor488-streptavidin; Alexa-Fluor594 anti-guinea pig IgG, all at 1:200 and all from Jackson Immunoresearch Laboratories; West Grove, PA) for 1 hr at room temperature. Since the tissues were from the body and pancreatic polypeptide (PP) poor areas, we did not include anti-PP antibody staining. To visualize vasculature relationships with the endocrine cells, endocrine cells were stained with insulin and glucagon antibodies as above and blood vessels with a mixture of mouse antibodies against smooth muscle actin (1:4000; Sigma-Aldrich) and CD34 (1:500, Biogenex; Freemont, CA) (Cabrera et al. 2006) with an amplification by tyramide kit (Perkin-Elmer; Freemont, CA). Slides were mounted with Vectashield with DAPI (Vector Laboratories; Burlingame, CA) for nuclear staining. Immunostained sections were examined using an Olympus BH-2 microscope (Olympus,: Tokyo, Japan) or in confocal mode on a Zeiss LSM 710 microscope (Zeiss Microimaging; Thornwood, NY). Figures were compiled using Adobe Photoshop (Adobe Systems, Inc.; San Jose, CA).
Quantification
For islet composition, glucagon immunoperoxidase-stained sections from seven consecutive donor pancreases with research consent were systematically scanned under 40× objective. For composition, at least 25 islets with 30 or more cells in cross section were counted at a final magnification of 420× for each donor; this comprised at least 2400 cells (with at least 800 in islets between 30–99 cells). After quantification, the islets were binned as medium (30–99 cells, 50–175 µm in diameter) and large (greater than 100 cells or 175 µm in diameter).
Using the same immunostained slides, all islets on the section were assessed for the clustering of β-cells that resembled the mantle-core subunit pattern found in rodents. After being categorized as having or not having clumps of β-cells, the islets were categorized by the number of cells/cross section: small (20–30 cells), medium (30–99 cells) and large (greater than 100 cells).
Cell composition by histological brightfield determination of the quality-controlled pancreatic sections (as above) was compared to that of ultrastructural analysis of the isolated islet preparation (Pisania et al. 2010) for an additional seven donors that were consecutively used for clinical transplants. With electron microscopy (EM), acinar cells, islet cells (β-, and the non-β cells α, δ and PP), and duct cells can be definitively identified, and cell composition determined by counting cells to give the proportion of the different cell types (Pisania et al. 2010).
Statistical Analysis
Two tailed student’s t-test was used for statistical analysis with p<0.05 considered significant.
Results
Islet Composition
Human islet composition by cell type has been reported numerous times but with major differences in the reported values. These differences could be due to methodological and/or sampling differences. We have previously reported a mean of 73.6% ± 1.7% β-cells/islet from 33 pancreases for clinical islet transplantations in the Harvard program (Pisania et al. 2010) using numeric quantification of ultrastructurally identified islet cell types. Here, we compared the composition of seven consecutive isolations that were used for clinical transplants with two different methods to quantify the number of cells; first, at the ultrastructural level and second, using immunoperoxidase staining on quality-controlled section (Table 1). By ultrastructure, β-cells accounted for 72.2% ± 3.5% of the islet cells and, by immunostaining, 70.3% ± 3.0%. By the ultrastructural analysis, all islet cells were counted without regard to whether they were in large islets or as single cells, whereas in the immunostained sections, we arbitrarily considered 40 µm as the minimum diameter for a cluster of islet cells to be considered an islet. Such an islet was typically 20–30 endocrine cells in cross section. Although the above mean values do not differ, the values for the same donor were variable with the two methods, suggesting that there was a difference due to sampling, since only 500–700 islet cells were counted for the ultrastructural analysis.
Table 1.
Comparison of Proportion of β-cells in Human Islets of Same Donors using Two Different Cell Counting Methods.
| β-Cell/Islet (%) |
||||||
|---|---|---|---|---|---|---|
| Donor | Age(Years) | Sex | BMI | Total Cells(Using EM) | Using EM | Using LM |
| H0424 | 54 | M | 25.4 | 621 | 83.9 | 66.5 |
| H0415 | 56 | F | 36.9 | 629 | 57.1 | 76.5 |
| H0411 | 57 | F | 21.4 | 558 | 73.5 | 73.9 |
| H0410 | 58 | M | 25.2 | 548 | 65.5 | 56.3 |
| H0407 | 50 | F | 47.6 | 669 | 71.9 | 66.9 |
| H0406 | 54 | M | 28.3 | 662 | 82.7 | 79.9 |
| H0401 | 38 | F | 20.8 | 791 | 70.8 | 72.3 |
| Mean (SEM) | 72.2 (3.5) | 70.3 (3.0) | ||||
BMI, body mass index; M, male; F, female; EM, electron microscopy; LM, light microscopy
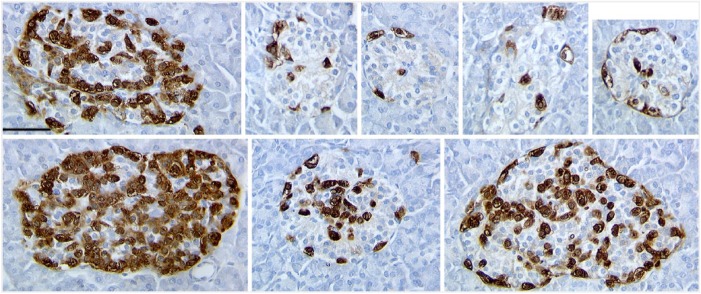
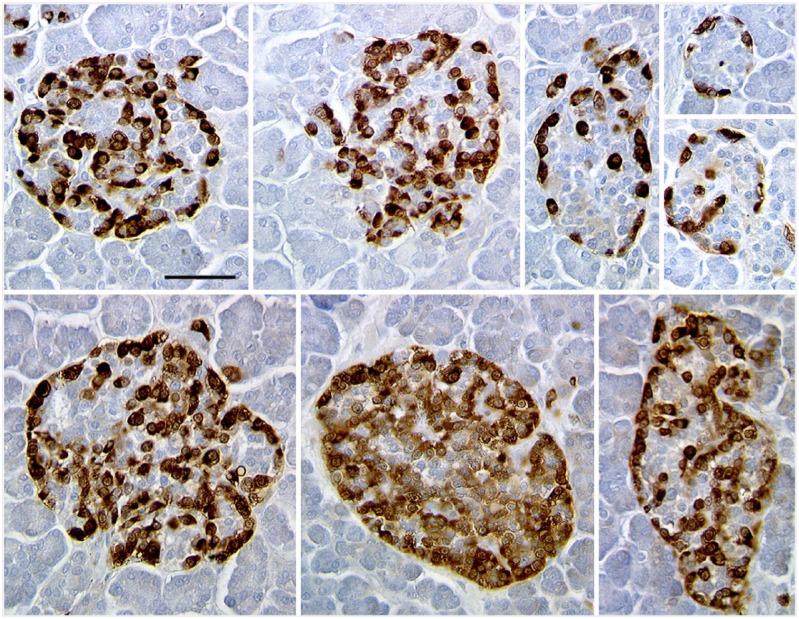
We then assessed the cell composition by islet size in another subset of donor pancreases using immunoperoxidase staining (Table 2); this cohort consisted of seven consecutive donors with research consent. With systematic scanning of the sections, all islets (at least 50 µm in diameter) were counted until at least 25 islets or 2500 cells were assessed (with the exception of H0015, in which counting all the islets greater than 30 cells on the section did not quite reach these cutoffs). In all but one case (H0019), the islets greater than 100 cells in cross-section (greater than 175 µm in diameter) had a significantly lower proportion of β-cells/islet than the medium-sized islets. The large islets (mean, 177 ±10 cells) had a mean of 59.9% ± 2.8% β-cells/islet, and the medium-sized islets (mean, 57.5 ± 2.1 cells) had 74.2% ± 1.5% β-cells/islet (two-tailed paired t-test, p<0.003). Strikingly, as has been previously reported (Bonner-Weir et al. 2014; Butler et al. 2013; Yoon et al. 2003), there were some large islets that had only a small proportion of β-cells, and were instead mainly composed of glucagon-staining cells [see Fig. 1 (H0021) and Fig. 2 (H015)].
Table 2.
Cell Composition of Human Islets in Islets of Different Sizes.
| More Than 100 |
30–99 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Donor | Age(Years) | Sex | BMI | Counted Cells | Islets | Cells/Islet | β-Cells (%) | Cells/islet | β-Cells (%) |
| H0015 | 15 | M | 25.1 | 2402 | 23 | 225 | 45.5 | 51.5 | 74.1 |
| H0017 | 23 | M | 26.0 | 3354 | 35 | 159 | 65.5 | 62.8 | 76.6 |
| H0018 | 48 | F | 17.9 | 3137 | 30 | 161 | 56.1 | 66.7 | 72.3 |
| H0019 | 64 | F | 20.8 | 4146 | 36 | 189 | 64.2 | 56.0 | 68.2 |
| H0020 | 16 | M | 32.5 | 2624 | 27 | 164 | 59.7 | 51.4 | 70.2 |
| H0021 | 39 | F | 22.5 | 3108 | 34 | 154 | 60.6 | 57.1 | 77.4 |
| H0022 | 53 | F | 23.5 | 3537 | 35 | 186 | 67.7 | 56.9 | 80.6 |
| Mean ± SEM | 31.4 ± 1.9 | 176.9 ± 9.5 | 59.9 ± 2.8* | 57.5 ± 2.1 | 74.2 ± 1.6* | ||||
Two-tailed unpaired t-test; significance; p<0.0019. BMI, body mass index; M, male; F, female; 30–99 cells represents islets that are 60–175 µm in diameter; 100+ cells represents islets that are <175 µm in diameter.
Figure 1.
Representative islets in pancreas from donor H0015 immunostained for glucagon (brown) showed great variability in islet composition, both in the proportion of cell types and the clarity of β-cell core arrangement. The smaller islets resemble those of rodents with a clear mantle-core pattern. However, as shown here, even the larger islets with fewer β-cells have a discernible clustering of β-cells. Scale, 50 µm.
Figure 2.
Representative islets in pancreas from donor H0021 immunostained for glucagon (brown) showed greater variability in islet composition, both in the proportion of cell types and the clarity of β-cell core arrangement. Some of the large islets have mainly glucagon staining cells, with few β-cells (here unstained). Scale, 50 µm.
Organization of Islets
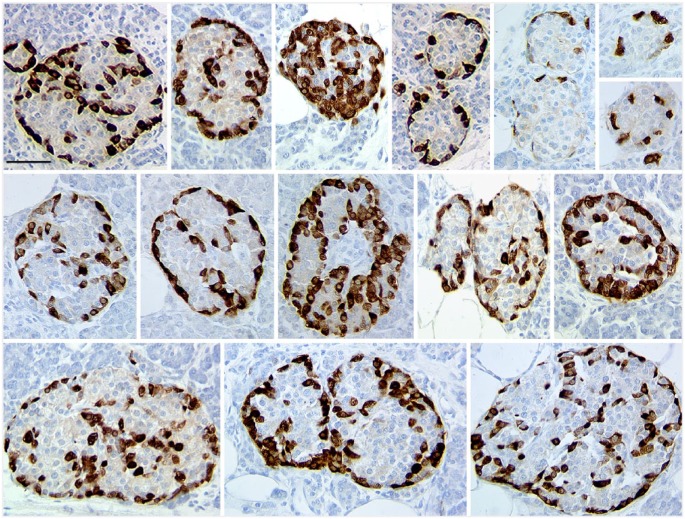
Whereas others have done complex analyses of cell-to-cell contacts to conclude that human islets were “clearly not a random mixture of α- and β-cells” (Kilimnik et al. 2012; Wang et al. 2013), we have been struck by the clear patterns on glucagon-stained sections as illustrated in Figure 3. Larger islets usually consist of multiple subunits, whereas smaller islets resemble the classic rodent pattern. In the set of seven consecutive donors shown in Table 2, we analyzed all islets (40 µm or greater diameter) as to whether they had a clear pattern of mantle-core with sizable clusters of β-cells partially surrounded by non-β-cells. Table 3 shows the proportion of islets that lacked an obvious clustering of β-cells surrounded by non-β-cells. In five pancreases, less than 10% of the islets lacked an obvious mantle-core pattern, and only in 2/7 (28.5%) of the pancreases were there a large number of islets without the obvious mantle-core architecture. Even those islets classified as lacking mantle-core architecture did indeed have small clusters of β-cells and tended to have a higher proportion of non-β-cells than the others.
Figure 3.
Representative islets in pancreas from donor H0019 immunostained for glucagon (brown) showed a clear pattern of small subunits resembling that of the rodent mantle-core islet organization in most of the islets of all sizes. Scale, 50 µm.
Table 3.
Percentage of Human Islets within a Pancreatic Section without Obvious β-cell Cored Subunits.
| Donor | Large | Medium | Small | Total Islets Counted | Large (%) | Medium (%) | Small (%) |
|---|---|---|---|---|---|---|---|
| H0015 | 9/15 | 7/25 | 5/10 | 50 | 60.0 | 28.0 | 50.0 |
| H0017 | 5/30 | 16/47 | 9/37 | 104 | 16.7 | 34.0 | 24.3 |
| H0018 | 3/42 | 2/34 | 1/18 | 94 | 7.1 | 6.0 | 5.6 |
| H0019 | 0/35 | 7/154 | 6/85 | 274 | 0.0 | 5.9 | 7.1 |
| H0020 | 0/12 | 2/22 | 1/43 | 77 | 0.0 | 4.5 | 2.3 |
| H0021 | 1/18 | 6/66 | 1/44 | 128 | 5.5 | 9.1 | 2.3 |
| H0022 | 0/26 | 1/34 | 1/20 | 80 | 5.0 | 2.9 | 5.0 |
| Mean ± SEM | 115 ± 28 | 13.5 ± 7.4 | 12.9 ± 4.4 | 13.8 ± 6.2 | |||
The pattern seemed inherent to the individual pancreas, as islets of different size categories had similar values within a donor pancreas yet there were marked differences among the donors. Another striking variable was the distribution of islet sizes among the different donors, as noted in Table 3, since all islets per section were assessed.
Relationship to Intra-islet Blood Vessels and Its Implications
The islet microvasculature in rodents is thought to provide a functional compartmentalization for the interactions of islet cells (Weir and Bonner-Weir 1990). However, the random pattern of islet cell types in human islets purported to be present by some groups would preclude such regulated local paracrine effects. It is of interest that Bosco et al. (2010) defined a 3-dimensional visualization of the human islets based on “ trilaminar epithelial plates [42 µm thick], folded with different degrees of complexity and bordered by vessels on both sides.” Yet they reported that “after a triple immunofluorescence for insulin, glucagon, and CD34, … no vascular channel penetrated the core of 40 to 60 µm-diameter islets or the clusters of β-cells in bigger islets.” This finding seemed odd to us based on rodent islets, so we examined whether human islets about 50 µm in diameter had internal blood vessels and whether there were blood vessels penetrating into the β-cell cores of larger islets.
In many of the brightfield images of immunostained sections (Figs. 2 and 3), vascular channels or vessels can be seen coursing in larger islets. In immunofluorescence images stained for islet hormones (Fig. 4), these vascular channels appear as black spaces between cords of cells. However, to clearly visualize the blood vessels (CD34+) and their pericyte/smooth muscle cells (smooth muscle actin+, SMA) that fill these vascular channels, we immunostained pancreas sections using antibodies against insulin, glucagon and a mixture of antibodies against CD34 and SMA. As shown in Figure 5, blood vessels clearly penetrate into the clusters of β-cells and into the centers of small 50-µm diameter islets. Indeed, the pattern of β-cells adjacent to the blood vessels is often in the rosette pattern we described in rat islets (Bonner-Weir 1988).
Figure 4.
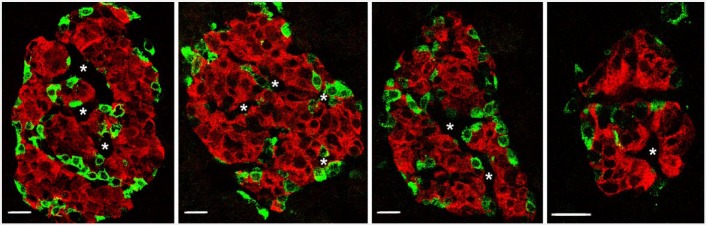
Islets in pancreas from donor H0021 immunofluorescently stained for insulin (red) and non-β-cells (glucagon and somatostatin; green) show clear clustering of β-cells with surrounding non-β-cells. Additionally, apparent vascular channels (white asterisks) are seen penetrating in the β-cell clusters. Scale, 20 µm.
Figure 5.
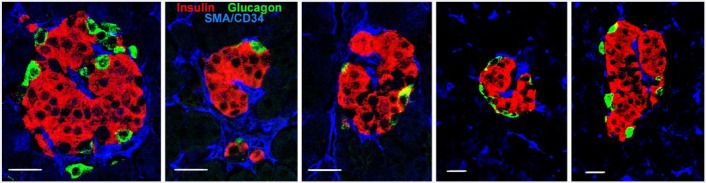
Even small islets of pancreas from donor H0021 show blood vessels, here visualized by immunostaining for smooth muscle actin and CD34 (blue), coursing into clumps of β-cells (red) with no glucagon cells (green) nearby. Scale, 20 µm.
Discussion
After a re-examination of the architecture of the human islet, we conclude that the human islet is similar to the rodent islet in terms of the three controversial issues investigated. Even so, there is far more variability in composition within each human pancreas as well as among different human pancreas than seen in rodent pancreas. This variability necessitates care in sampling islets both as to the number and size for both morphological and functional studies. Importantly, it raises questions about how human islets evolve and function throughout life and how this might relate to the pathogenesis of diabetes.
The issue of islet cell composition is particularly sensitive to the method of determination, (whether by volume or number) and to the size of the islets. We previously discussed the issue of volume versus number (Pisania et al. 2010). The large difference in size/volume of the different cell types results in the discrepancies obtained by assessments of volume and cell number. Most notably, the β-cells are larger and so are more likely to be cut in a sectioned islet than would be the case for the smaller α- or δ-cells. There are additional issues including whether the volume only comprises hormone-positive cells or the whole islet, including vascular channels, and whether only cells with clear nuclei are counted. Thus, some caution is needed when comparing the proportions of cell types between different studies. In the current study, we found that cell composition varied with islet size. As seen in Table 2, larger islets had a lower percentage of β-cells being only 59.9% ± 2.8%, whereas medium-sized islets were 74.2% ± 1.6% even in the same sections. These latter islets were not the tiny clusters often seen that would not contribute much to the total β-cell mass but rather the bulk of the islets within the pancreas, with a mean of 57.5 cells in cross section or about 120 µm in diameter. Moreover, the size distribution of islets varied among different adult human pancreases.
As far as the presence of non-random organization of the human islets, we conclude that the majority of islets do have the pattern of composites of modular subunits that resemble the rodent mantle-core arrangement. It is particularly striking that, in all the pancreases studied, the small islets closely resembled the rodent mantle-core pattern as previously noted (Bosco et al. 2010; Kilimnik et al. 2012; Wang et al. 2013). Most of the larger islets consisted of subunits of cell aggregates with a mantle-core arrangement similar to rodent islets. However, some of the larger islets with a lower proportion of β-cells gave the suggestion of random organization. Yet even in these larger islets, there was clear clustering of β-cells, just smaller clusters.
The third issue was the relation of the islet cells and the blood vessels. It is clear to us that blood vessels penetrate both into the β-cell clusters in larger islets and into centers of small islets, unlike the conclusions drawn in the study of Bosco et al. (2010). Their lack of finding such vessels may be due to a technical problem. When we used only immunostaining for CD34 to visualize the vessels—as they did—it was difficult to see any vessels within the islets, even in putative vascular channels, whereas the vessels in the exocrine pancreas were stained. This difficulty may be due to lower CD34 expression in the fenestrated islet capillaries than in non-fenestrated capillaries in the surrounding exocrine tissue. However, using a mixture of antibodies against smooth muscle actin and CD34, as used by Cabrera et al (2006), we could see staining along the putative vascular channels since the pericytes or smooth muscle cells surrounding the vessels were also stained. A clear intraislet vascularization, even in the small islets, makes sense since our understanding is that β-cells have high oxygen needs for proper function. In fact, with hypoxia β-cells secrete VEGF to enhance their vascularization (Vasir et al. 1998; Vasir et al. 2000). Together, the mantle-core subunit arrangement and the intraislet vessels penetrating the β-cell cores support the physiological studies of Stagner and Samols (1992) on intraislet interactions in the human islet being similar to that in rodents. In conclusion, whereas human and rodent islets may have some functional differences—many to still be determined—their fundamental architectural arrangement is similar.
Acknowledgments
The authors thank the Harvard-Joslin Human Islet Isolation team, particularly Jack O’Neill, Abdulkadir Omer, Ji Lei, and Vaja Tchipashvili, for the processing of human pancreases.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This current study was supported by grants from the National Institutes of Health (NIH R01 DK093909 (SBW), P30 DK036836 Joslin Diabetes Research Center (DRC), NCRR ICR U4ZRR016606 (GCW), the Diabetes Research and Wellness Foundation and an important group of private donors.
References
- Bonner-Weir S. (1988). Morphological evidence for pancreatic polarity of B-cell within the islets of Langerhans. Diabetes 37:616-621. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, In’t Veld PA, Weir GC. (2014). Reanalysis of study of pancreatic effects of incretin therapy: methodological deficiencies. Diabetes Obes Metab 16:661-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Orci L. (1982). New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31:883-939. [DOI] [PubMed] [Google Scholar]
- Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. (2010). Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 59:1202-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. (2005). Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53:1087-1097. [DOI] [PubMed] [Google Scholar]
- Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. (2013). Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 62:2595-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. (2003). Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102-110. [DOI] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. (2006). The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 103:2334-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsen SL, Hegre OD, Parsons JA, McEvoy RC, Elde RP. (1976). Pancreatic islet cell hormones distribution of cell types in the islet and evidence for the presence of somatostatin and gastrin within the D cell. J Histochem Cytochem 24:883-897. [DOI] [PubMed] [Google Scholar]
- Grube D, Eckert I, Speck PT, Wagner HJ. (1983) Immuno-histochemistry and microanatomy of the islets of Langerhans. BiomedRes 4(Suppl):25-36. [Google Scholar]
- Kilimnik G, Jo J, Periwal V, Zielinski MC, Hara M. (2012). Quantification of islet size and architecture. Islets 4:167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean N, Ogilvie RF. (1955). Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes 4:367-376. [DOI] [PubMed] [Google Scholar]
- Orci L, Unger RH. (1975). Functional subdivision of islets of Langerhans and possible role of D-cells. Lancet 2:1243-1244. [DOI] [PubMed] [Google Scholar]
- Pisania A, Weir GC, O’Neil JJ, Omer A, Tchipashvili V, Lei J, Colton CK, Bonner-Weir S. (2010). Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest 90:1661-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier J, Goebbels RM, Henquin JC. (1983). Cellular Composition of the Human Diabetic Pancreas. Diabetologia 24:366-371. [DOI] [PubMed] [Google Scholar]
- Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. (2002). Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 45:85-96. [DOI] [PubMed] [Google Scholar]
- Samols E, Stagner JI, Ewart RB, Marks V. (1988). The order of islet microvascular cellular perfusion is B–A–D in the perfused rat pancreas. J Clin Invest 82:350-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols E, Weir GC, Bonner-Weir S. (1986). Intra-islet insulin-glucagon-somatostatin relationships. Clin Endocrinol Metab 15:33-58. [DOI] [PubMed] [Google Scholar]
- Stagner JI, Samols E. (1992). The vascular order of islet cellular perfusion in the human pancreas. Diabetes 41:93-97. [DOI] [PubMed] [Google Scholar]
- Stagner JI, Samols E, Bonner-Weir S. (1988). B - A - D pancreatic islet cellular perfusion in dogs. Diabetes 37:1715-1721. [DOI] [PubMed] [Google Scholar]
- Stagner JI, Samols E, Koerker DJ, Goodner CJ. (1992). Perfusion with anti-insulin gamma globulin indicates a B to A to D cellular perfusion sequence in the pancreas of the Rhesus monkey. Macaca mulatta. Pancreas 7:26-29. [DOI] [PubMed] [Google Scholar]
- Vasir B, Aiello LP, Yoon KH, Quickel RR, Bonner-Weir S, Weir GC. (1998). Hypoxia induces vascular endothelial growth factor gene and protein expression in cultured rat islet cells. Diabetes 47:1894-1903. [DOI] [PubMed] [Google Scholar]
- Vasir B, Reitz P, Xu G, Sharma A, Bonner-Weir S, Weir GC. (2000). Effects of diabetes and hypoxia on gene markers of angiogenesis (HGF, cMET, uPA and uPAR, TGF-alpha, TGF-beta, bFGF and Vimentin) in cultured and transplanted rat islets. Diabetologia 43:763-762. [DOI] [PubMed] [Google Scholar]
- Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, Ricordi C, Khan A, Szust J, Shen J, Millis JM, Witkowski P, Hara M. (2013). Regional differences in islet distribution in the human pancreas–preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One 8:e67454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S. (1990). Islets of Langerhans: The puzzle of intraislet interactions and their relevance to diabetes. J Clin Invest 85:983-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim DG, Lee IK, Bonner-Weir S. (2003). Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endoc Metab 88:2300-2308. [DOI] [PubMed] [Google Scholar]