Abstract
Human and rodent islets differ substantially in several features, including architecture, cell composition, gene expression and some aspects of insulin secretion. Mouse pancreatic islets are highly vascularized with interactions between islet endothelial and endocrine cells being important for islet cell differentiation and function. To determine whether human islets have a similar high degree of vascularization and whether this is altered with diabetes, we examined the vascularization of islets from normal human subjects, subjects with type 2 diabetes (T2D), and normal mice. Using an integrated morphometry approach to quantify intra-islet capillary density in human and mouse pancreatic sections, we found that human islets have five-fold fewer vessels per islet area than mouse islets. Islets in pancreatic sections from T2D subjects showed capillary thickening, some capillary fragmentation and had increased vessel density as compared with non-diabetic controls. These changes in islet vasculature in T2D islets appeared to be associated with amyloid deposition, which was noted in islets from 8/9 T2D subjects (and occupied 14% ± 4% of islet area), especially around the intra-islet capillaries. The physiological implications of the differences in the angioarchitecture of mouse and human islets are not known. Islet vascular changes in T2D may exacerbate β cell/islet dysfunction and β cell loss.
Keywords: amyloid, diabetes, islet, pancreas, vasculature
Introduction
Pancreatic islets in rodents have a rich capillary network (Bonner-Weir and Orci 1982; Brissova et al. 2006; Nyman et al. 2008) and endothelial cells provide signals for early pancreas endoderm specification and endocrine cell differentiation (Lammert et al. 2001; Magenheim et al. 2011; Sand et al. 2011; Yoshitomi and Zaret 2004). Although islet endocrine cells produce several angiogenic factors, the development and maintenance of pancreatic islet vasculature is primarily controlled by vascular endothelial growth factor-A (VEGF-A) and VEGF receptor 2 (VEGFR2) signaling, where VEGF-A produced by islet endocrine cells interacts with VEGFR2 expressed on intra-islet endothelial cells (Brissova et al. 2006). A series of recent studies has demonstrated that the precise control of VEGF-A production in developing and adult β cells is essential for normal islet vascularization, which, in return, regulates several important aspects of islet biology, including β cell mass, function, regeneration and islet innervation (Brissova et al. 2006; Lammert et al. 2003; Reinert et al. 2013; Reinert et al. 2014). When VEGF-A is inactivated either in the early pancreas or newly formed β cells, the intra-islet capillary plexus fails to fully mature, resulting in substantial defects in β cell proliferation, insulin secretion and glucose homeostasis (Brissova et al. 2006; Lammert et al. 2003; Reinert et al. 2013). In contrast, overexpression of VEGF-A in developing and adult β cells induces endothelial cell expansion and hyper-vascularization, which are detrimental to islet formation and result in β cell loss (Brissova et al. 2014; Cai et al. 2012). Interestingly, islet VEGF-A expression remains tightly controlled even during insulin resistance when islets become progressively hyperplastic due to β cell hyperplasia. We recently showed that the vascular remodeling in ob/ob mice is surprisingly not mediated by angiogenesis but rather through dilation of preexisting islet capillaries (Dai et al. 2013). Similar alterations in islet capillary morphology, to those seen in ob/ob mice, have been described in other rodent models of insulin resistance and diabetes (Li et al. 2006; Like and Chick 1970; Nakamura et al. 1995; Mizuno et al. 1999; Shao et al. 2006; Tikellis et al. 2004). How the islet vasculature and its response to insulin resistance and diabetes compare in human and rodent pancreas is not known.
Human islet morphology is substantially different from that of rodent islets (Brissova et al. 2005; Cabrera et al. 2006). Additionally, human and mouse islets differ in their expression of antioxidant enzymes (Dai et al. 2012; Kondegowda et al. 2012; Vernier et al. 2012), glucose transporters (GLUT1 vs. GLUT2) (De Vos et al. 1995; Ferrer et al. 1995), islet-enriched transcription factors (Dai et al. 2012; Guo et al. 2013), β cell proliferative capacity (Avrahami et al. 2014,; Fiaschi-Taesch et al. 2013), epigenetics (Avrahami et al. 2014; Kameswaran et al. 2014), and regulation of insulin secretion (Dai et al. 2012; Henquin et al. 2006a, 2006b; Ling and Pipeleers 1996). Moreover, Butler and colleagues showed that islets are not hyperplastic in obese human subjects with insulin resistance (Butler et al. 2003) unlike those in mouse models.
Another important difference between human and rodent islets is that human, unlike rodent, β cells produce amyloidogenic islet amyloid polypeptide (IAPP, also known as amylin). IAPP aggregation and amyloid formation is present in the majority of islets of humans with type 2 diabetes (T2D) (Clark et al. 1988; Jurgens et al. 2011; Westermark 1972) but does not occur in models of rodent diabetes (Westermark et al. 1990). Islet amyloid formation is associated with β cell toxicity (Lorenzo et al. 1994), increased β cell apoptosis and decreased β cell mass in human diabetes (Jurgens et al. 2011).
In T2D, decreased insulin secretion is a critical contributor to the pathogenesis of the disease. This is due to both functional abnormalities in the β cell, which manifests as impaired insulin release (Porte 1991), and decreased β cell mass (Butler et al. 2003; Jurgens et al. 2011; Klöppel et al. 1985; Rahier et al. 2008). The contribution of genetic and environmental factors (e.g., nutrient excess) to β cell loss and dysfunction has been widely studied (Halban et al. 2014). However, our understanding of the mechanisms of β cell loss and dysfunction in diabetes remain incompletely understood. Islet amyloid, similar to collagen deposition (Hayden et al. 2008), is found in the extracellular matrix that lies between β cells and islet capillaries (de Koning et al. 1994; Narita et al. 1992; Schneider et al. 1980,). Thus, in addition to exerting toxic effects on the β cell, islet amyloid deposition may also be associated with loss or disruption of islet capillaries.
In the present study, we determined islet capillary density and morphology in autopsy pancreas specimens from humans with and without T2D, and investigated the association of islet amyloid deposition with the islet vasculature.
Materials & Methods
Human Autopsy Pancreas Specimens
Formalin-fixed, paraffin-embedded pancreas specimens from nine patients with T2D and eight non-diabetic control subjects were identified as part of a study approved by the institutional review boards at the University of Washington and the VA Puget Sound Health Care System. These subjects form a subset of those described in a previously published study, which includes a detailed description of the inclusion/exclusion criteria (Jurgens et al. 2011). An additional five subjects without T2D were also included in this study. These were obtained from a de-identified repository at Vanderbilt University Medical School and for whom demographic information is not available.
Mice
Animal studies were performed in accordance with guidelines of the Vanderbilt University IACUC. Pancreata were obtained from six mice on the C57BL/6J background (Jackson Laboratory; Bar Harbor, ME) and processed as described previously (Brissova et al. 2006).
Immunohistochemical Procedures and Islet Imaging
Islet capillaries in mouse pancreata were labeled with FITC-conjugated endothelium-binding tomato lectin (Brissova et al. 2006). To visualize islet capillaries, human pancreas tissue sections first underwent antigen retrieval in EDTA, pH 9.0, at 104°C for 20 min using a pressure cooker. Slides were cooled for 10 min at room temperature and all subsequent steps were also performed at room temperature. Endogenous peroxidase activity was quenched with 0.03% H2O2 containing sodium azide for 5 min. Sections were blocked in a serum-free protein block (X0909; Dako, Carpinteria, CA) for 20 min and then stained with anti-CD34 mouse monoclonal antibody (M7165, Dako; 1:100) for 30 min. CD34 staining was visualized with EnVision+ detection system (K4007, Dako) for 30 min and DAB chromogen for 5 min. Insulin and islet amyloid were visualized as follows. Sections were deparaffinized and rehydrated through a graded alcohol series. Non-specific binding was blocked by incubation of slides in 1×PBS containing 5% (v/v) normal donkey serum. Sections were incubated with anti-insulin antibody (IS002, Dako; 1:2000) followed by Cy3-conjugated donkey anti-guinea pig IgG (Jackson ImmunoResearch, West Grove, PA; 1:500) prepared in 1% (v/v) bovine serum albumin/0.1% (v/v) Triton X-100/1× PBS. Sections were then counterstained with thioflavin S (0.5% w/v) to visualize amyloid deposits. Pancreatic sections were imaged at 20× magnification with either an Olympus BX41 microscope or using whole-slide imaging devices ScanScope CS and ScanScope FL (Aperio Technologies, Inc.; Vista, CA).
Quantitative Morphometric Analyses
Vascular morphometry was performed using MetaMorph version 7.0 software (Universal Imaging Inc., Bedford Hills, NY). Integrated morphometry analysis was applied to 20–30 islets per tissue block to determine islet capillary density expressed as the number of capillaries per islet area (Brissova et al. 2006). Capillaries were counted using a technique described by Weidner and colleagues (Weidner 1995) where any fluorescently or chromogen-labeled endothelial cell or endothelial cluster clearly separate from adjacent microvessels was considered a single, countable microvessel. Islet amyloid deposition was computed as Σ thioflavin S-positive area / Σ islet area, as previously described (Jurgens et al. 2011).
Statistical Analysis
Student’s t-test was used for comparisons of two groups; this is with the exception of categorical data, which were compared using a Fisher’s exact test. All data are presented as the mean ± SEM and a p<0.05 is considered significant.
Results
Islet Capillary Density Differs in Mouse and Human Pancreata
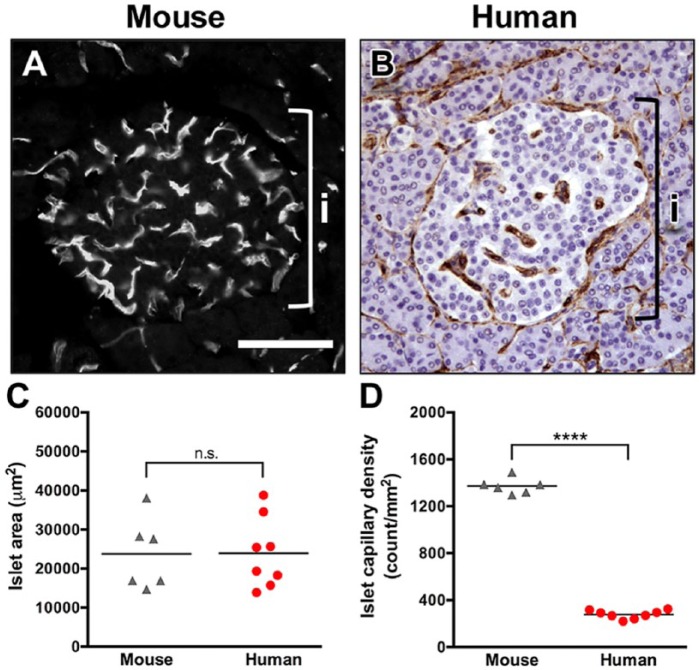
Several studies have previously demonstrated that mouse and rat islets are extensively vascularized (Bonner-Weir and Orci 1982; Brissova et al. 2006; Nyman et al. 2008). To compare islet vascularization in mouse and human islets, pancreatic sections were labeled with lectin-FITC or CD34 vascular markers, and islet capillary density was analyzed by morphometry (Fig. 1A, 1B). Whereas islet cross-sectional area was similar in both species (Fig. 1C), islet capillary density was nearly five-fold lower in the human compared with mouse islets (Fig. 1D). In addition to lower density, the capillaries in human islets appeared to be significantly less tortuous than those in the mouse. Our data indicate that the angioarchitecture differs dramatically between mouse and human islets. This may have important physiological implications for islet function.
Figure 1.
Human islets are less vascularized than mouse islets. (A) Vasculature in mouse islets was visualized by endothelium-binding lectin-FITC. Brackets in A and B denote an islet (i). (B) Human pancreatic sections from normal autopsy specimens were labeled with endothelial cell marker CD34. (C) Area of individual islets was similar in the mouse (n=6) and human (n=8). p=0.97. (D) Human islets (n=8) had a lower capillary density as compared with that of the mouse (n=6). ****p<0.0001. Scale, 100 μm.
Islet Capillary Density in Type 2 Diabetes
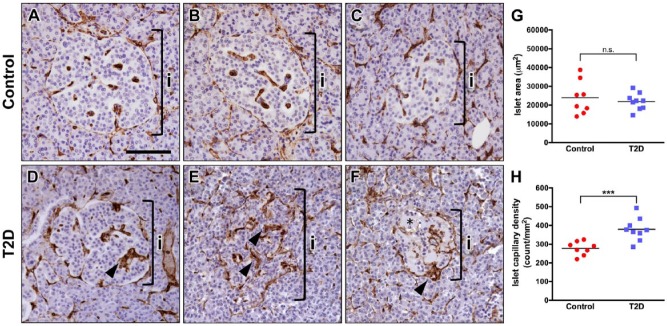
Next, we analyzed the vascular phenotype of islets in autopsy specimens from subjects with T2D and compared it with non-diabetic control samples. The group characteristics for these subjects, where available, are provided in Table 1. Representative examples of islet morphology and CD34 capillary labeling in control and T2D subjects are displayed in Figure 2. In a small subset of T2D islets, CD34+ capillaries were contiguous and similar to the controls (Fig. 2A–2D). However, the vast majority of T2D islets had capillaries of irregular appearance (Fig. 2E, 2F), suggesting that they are morphologically altered. In addition, intra-islet capillaries in T2D islets were frequently thicker, but the thickness of peri-islet capillaries was similar to that of control islets (Fig. 2D–2F; arrowheads). Furthermore, we found that some T2D islets displayed partial or near complete loss of hematoxylin-positive nuclei (Figs. 2F and 3F), which correlated with amyloid deposits (Fig. 3C). Morphometric analysis showed that the average islet cross-sectional area did not differ between T2D and control groups (Fig. 2G). In contrast, islet capillary density was 36% greater in subjects with T2D compared with controls (Fig. 2H).
Table 1.
Subject Characteristics.
| Type 2 Diabetes (n=9) | Controls (n=8) | p value | |
|---|---|---|---|
| Age (years) | 57 ± 6 | 65 ± 8 | 0.5 |
| Gender (F/M) | 3/6 | 2/6 | 1.0 |
| Body mass index (kg/m2) | 32.2 ± 2.7 | 27.3 ± 2.1 | 0.2 |
| Blood glucose (mmol/L) | 8.7 ± 0.8 | 5.9 ± 0.3 | 0.005 |
| Diabetes duration (years; n=5 only) | 11 ± 5 | N/A | - |
| Diabetes medication (n) | |||
| Unknown | 2 | N/A | - |
| Oral hypoglycemic | 4 | N/A | - |
| Insulin | 3 | N/A | - |
Figure 2.
Human type 2 diabetes (T2D) islets have abnormal capillary morphology. (A–C) Representative images of normal human islets with uniformly labeled CD34+ capillaries. (D–F) Representative images of CD34 staining in T2D human islets. (D) A small fraction of T2D islets had uniform CD34 staining, similar to the controls. (E) The majority of T2D islets had capillaries of irregular appearance, with a diffuse CD34 staining pattern. (F) Some T2D islets displayed partial or near complete loss of hematoxylin-positive nuclei (area marked by asterisk). Brackets in A–F denote an islet (i). Intra-islet capillaries in T2D islets were frequently thicker as compared with control islets (arrowheads). (G) Area of individual islets was similar in normal (n=8) and T2D pancreatic tissues (n=9). p=0.55. (H) Islets in T2D subjects (n=9) had higher capillary density as compared with controls (n=8). ***p<0.001. Scale, 100 μm.
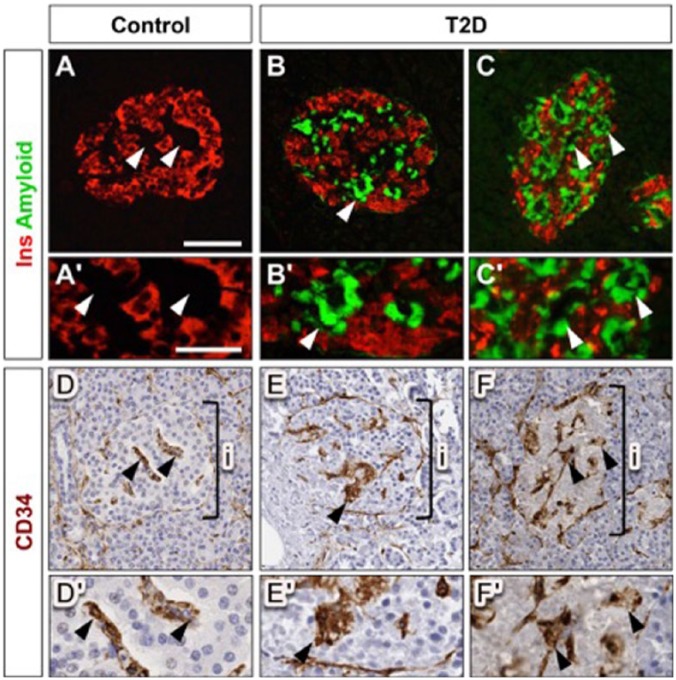
Figure 3.
Alterations in type 2 diabetes (T2D) islet capillary morphology are associated with amyloid deposition. (A–C) β cells and islet amyloid deposition in normal and T2DM pancreas are visualized by fluorescence labeling for insulin (Ins; red) and thioflavin S (green), respectively. (Aʹ–Cʹ) Insets show higher magnification images of vascular regions in panels A–C, as marked by arrowheads. (D–F) Islet capillaries in adjacent sections are visualized by CD34 labeling (brown). (Dʹ–Fʹ) Insets show higher magnification images of vascular regions in panels D–F, as marked by arrowheads. Staining in panels B, C, E and F reveals that amyloid deposits in T2D islets coincide with perivascular location (arrowheads). Brackets in D–F denote an islet (i). Scale (A–F), 100 μm (A’-F’), 50 µm.
Association between Fragmentation of Islet Capillaries and Amyloid Deposition
Amyloid deposition in T2D islets is localized to the perivascular space (de Koning et al. 1994; Narita et al. 1992; Schneider et al. 1980) and is associated with increased β cell apoptosis and β cell loss (Jurgens et al. 2011). In this study, islet amyloid was present in 8/9 diabetic subjects (comprising, on average, 14% ± 4% of islet area). To determine whether islet amyloid deposits are also associated with abnormal islet capillary morphology, co-registration of digital islet images from consecutive pancreatic sections stained for CD34 and insulin/thioflavin S, respectively, was performed. This analysis revealed extensive islet amyloid deposition especially in regions with thicker islet capillaries (Fig. 3B, 3C, 3E, 3F). Among these subjects, a modest positive correlation (r = 0.53; p=0.1) between islet amyloid severity and capillary density was observed.
Discussion
We have determined, for the first time, pancreatic islet capillary density in human autopsy specimens and have made the following two key observations. First, relative to mouse (and rat) islets, capillary density is substantially lower in human islets. This further underscores the difference in islet morphology that exists between rodents and humans. Second, we observed that islet capillary density was significantly increased, by 36%, in islets from subjects with T2D relative to age-matched non-diabetic controls. This increase in capillary density was associated with capillary thickening and fragmentation, suggesting that abnormalities in islet capillary morphology occur in human T2D.
Islet vasculature, among other functions, provides a scaffold for extension of nerve fibers into islets and is essential for islet autonomic innervation (Reinert et al. 2014). The five-fold lower islet capillary density observed in the present study is in line with reports of innervation of human islets, which found an approximate 50% decrease in sympathetic innervation between human and mouse islets (Rodriguez-Diaz et al. 2011). This observation suggests that there may be a different spatial relationship between capillaries (and nerves) and endocrine cells in humans versus rodents; namely, that each endocrine cell in rodent islets is in contact with a capillary (Kragl and Lammert 2010). Based on the data from the present study, it is not clear whether such is the case in human islets. Aside from changes in vascular/nerve fiber density, the capillary-associated extracellular matrix (basement membrane) is also thicker in human than rodent islets (Otonkoski et al. 2008; Virtanen et al. 2008). Islet endothelial cells and extracellular matrix molecules have both been suggested to be critical for maintaining islet viability and function (Kragl and Lammert 2010). The manner in which islet endothelial cells and the associated extracellular matrix interact with endocrine cells in human islets to modulate their function and survival may be different from that which occurs in rodent islets. This needs to be further evaluated, in light of the present findings.
Although the difference in islet capillary density between rodent and human islets is substantial, it is less clear whether non-human primate (NHP) and human islet vascular density are more similar. A recent report stated that islets in normal human primates are “highly vascularized”, based on a greater degree of CD31 (platelet endothelial cell adhesion molecule 1) immunoreactivity in endocrine versus exocrine pancreata (Pound et al. 2014). The methods of quantitation and units for data presentation differ between this study and the present one, making it difficult to directly compare islet capillary density per se. However, the appearance of the islet vasculature in the NHP study is similar to that seen in human islets in the present study and appears quite different from that of rodent islets. In rodent islets, there is a clear increase in islet vascular and nerve density relative to that of the exocrine pancreas (Dai et al. 2013; Rodriguez-Diaz et al. 2011). However, this difference does not exist in humans, with vascular (data not shown) and nerve fiber density [(Rodriguez-Diaz et al. 2011) and personal communication with G.J. Taborsky Jr, University of Washington] being similar in the human endocrine and exocrine pancreata.
A limitation of this study is that it is not possible to discern whether the increase in CD34+ capillaries equates to an increase in functional blood vessels (McDonald and Choyke 2003). In fact, our examination of CD34 labeling in T2D islets suggests that a breakdown of normal capillary morphology has occurred. Thus, we believe it is likely that the seeming increase in islet capillary density could result from vascular thickening, which would lead to vessel instability and fragment formation, and therefore overall greater vessel count in T2D islets. Islet capillary dilation/thickening has been described in rodent models of insulin resistance and diabetes (Li et al. 2006; Mizuno et al. 1999; Nakamura et al. 1995). In these models, β cell hyperplasia and islet hyperplasticity were accompanied by a decrease in islet capillary density; yet, capillaries compensated for the lack of angiogenesis by increasing thickness/dilation. Dilated capillaries were frequently associated with hypertrophied supporting pericytes, and occasional hemorrhaging was noted (Dai et al. 2013; Nakamura et al. 1995).
A weak association between the presence of “fragmented” capillaries (measured as increased capillary density) and islet amyloid deposition was noted in the present study. The investigation of a greater number of subjects is required in order to better understand this relationship. Islet amyloid accumulates in the peri-capillary islet extracellular matrix. Although its unique amyloidogenic component is the β cell-derived peptide islet amyloid polypeptide (IAPP or amylin), islet amyloid deposits also contain extracellular matrix molecules (e.g., heparan sulfate proteoglycans) that are chiefly derived from islet endothelial cells. Based on data from the Alzheimer’s disease-related peptide Aβ, endothelial cells are susceptible to toxicity by amyloidogenic peptides (Preston et al. 1998; Thomas et al. 1996), and amyloid deposition in the brain of subjects with Alzheimer’s disease is associated with altered endothelial structure (Thomas et al. 1996). Additionally, it has been shown that amyloid peptide toxicity to endothelial cells varies markedly with the state of the cell; namely, confluent monolayers of cells are much more resistant to Aβ toxicity than subconfluent cells (Balcells et al. 2008). Thus, islet amyloid deposition could contribute to the disturbance of islet capillary morphology in T2D, with fragmented capillaries in islets from subjects with T2D having increased susceptibility to amyloid toxicity, regardless of the mechanism by which fragmentation occurs.
In summary, we have identified major differences in islet angioarchitecture between rodents and humans, with a five-fold lower vascular density occurring in human islets. In human T2D, islet capillary density is significantly increased and appears to be associated with capillary dilation and fragmentation. Thus, disturbances in islet capillary integrity may contribute to a deterioration of β cell/islet function and exacerbate β cell loss in T2D.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Veterans Affairs, VA Tennessee Valley and Puget Sound Health Care Systems and grant support from the Department of Veterans Affairs (Merit Review), the NIH (DK69603, DK88082, DK89572, DK89538), the Vanderbilt Diabetes Research and Training Center (DK20593) and the University of Washington Diabetes Research Center (Cellular and Molecular Imaging Core; DK17047).
References
- Avrahami D, Li C, Yu M, Jiao Y, Zhang J, Naji A, Ziaie S, Glaser B, Kaestner KH. (2014). Targeting the cell cycle inhibitor p57Kip2 promotes adult human beta cell replication. J Clin Invest 124:670-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcells M, Wallins JS, Edelman ER. (2008). Amyloid beta toxicity dependent upon endothelial cell state. Neurosci Lett 441:319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Orci L. (1982). New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31:883-889. [DOI] [PubMed] [Google Scholar]
- Brissova M, Aamodt K, Brahmachary P, Prasad N, Hong JY, Dai C, Mellati M, Shostak A, Poffenberger G, Aramandla R, Levy SE, Powers AC. (2014). Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes beta cell regeneration. Cell Metab 19:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. (2005). Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53:1087-1097. [DOI] [PubMed] [Google Scholar]
- Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC. (2006). Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes 55:2974-2985. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. (2003). Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102-110. [DOI] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. (2006). The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 103:2334-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Brissova M, Reinert RB, Pan FC, Brahmachary P, Jeansson M, Shostak A, Radhika A, Poffenberger G, Quaggin SE, Jerome WG, Dumont DJ, Powers AC. (2012). Enhanced expression of VEGF-A in beta cells increases endothelial cell number but impairs islet morphogenesis and beta cell proliferation. Dev Biol 367:40-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC. (1988). Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis - quantitative changes in the pancreas in type-2 diabetes. Diab Res Clin Exptl 9:151-159. [PubMed] [Google Scholar]
- Dai C, Brissova M, Hang Y, Thompson C, Poffenberger G, Shostak A, Chen Z, Stein R, Powers AC. (2012). Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia 55:707-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Brissova M, Reinert RB, Nyman L, Liu EH, Thompson C, Shostak A, Shiota M, Takahashi T, Powers AC. (2013). Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes 62:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning EJ, Höppener JW, Verbeek JS, Oosterwijk C, van Hulst KL, Baker CA, Lips CJ, Morris JF, Clark A. (1994). Human islet amyloid polypeptide accumulates at similar sites in islets of transgenic mice and humans. Diabetes 43:640-644. [DOI] [PubMed] [Google Scholar]
- De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F. (1995). Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 96:2489-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J, Benito C, Gomis R. (1995). Pancreatic islet GLUT2 glucose transporter mRNA and protein expression in humans with and without NIDDM. Diabetes 44:1369-1374. [DOI] [PubMed] [Google Scholar]
- Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, Casinelli G, Cox AE, Takane KK, Scott DK, Stewart AF. (2013). Human pancreatic beta-cell G1/S molecule cell cycle atlas. Diabetes 62:2450-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. (2013). Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 123:3305-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L, Weir GC. (2014). β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 37:1751-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MR, Patel K, Habibi J, Gupta D, Tekwani SS, Whaley-Connell A, Sowers JR. (2008). Attenuation of endocrine-exocrine pancreatic communication in type 2 diabetes: pancreatic extracellular matrix ultrastructural abnormalities. J Cardiometab Syndr 3:234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin JC, Dufrane D, Nenquin M. (2006a). Nutrient control of insulin secretion in isolated normal human islets. Diabetes 55:3470-3477. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Nenquin M, Stiernet P, Ahren B. (2006b). In vivo and in vitro glucose-induced biphasic insulin secretion in the mouse: pattern and role of cytoplasmic Ca2+ and amplification signals in beta-cells. Diabetes 55:441-451. [DOI] [PubMed] [Google Scholar]
- Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Aston-Mourney K, Carr DB, Westermark P, Westermark GT, Kahn SE, Hull RL. (2011). Beta-cell loss and beta-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol 178:2632-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameswaran V, Bramswig NC, McKenna LB, Penn M, Schug J, Hand NJ, Chen Y, Choi I, Vourekas A, Won KJ, Liu C, Vivek K, Naji A, Friedman JR, Kaestner KH. (2014). Epigenetic regulation of the DLK1-MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab 19 135-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. (1985). Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 4:110-125. [DOI] [PubMed] [Google Scholar]
- Kondegowda NG, Mozar A, Chin C, Otero A, Garcia-Ocaña A, Vasavada RC. (2012). Lactogens protect rodent and human beta cells against glucolipotoxicity-induced cell death through Janus kinase-2 (JAK2)/signal transducer and activator of transcription-5 (STAT5) signalling. Diabetologia 55:1721-1732. [DOI] [PubMed] [Google Scholar]
- Kragl M, Lammert E. (2010). Basement membrane in pancreatic islet function. Adv Exp Med Biol 654:217-234. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. (2001). Induction of pancreatic differentiation by signals from blood vessels. Science 294 564-567. [DOI] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. (2003). Role of VEGF-A in vascularization of pancreatic islets. Curr Biol 13:1070-1074. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang L, Meshinchi S, Dias-Leme C, Raffin D, Johnson JD, Treutelaar MK, Burant CF. (2006). Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes 55:2965-2973. [DOI] [PubMed] [Google Scholar]
- Like AA, Chick WL. (1970). Studies in the diabetic mutant mouse. II. Electron microscopy of pancreatic islets. Diabetologia 6:216-242. [DOI] [PubMed] [Google Scholar]
- Ling Z, Pipeleers DG. (1996). Prolonged exposure of human beta cells to elevated glucose levels results in sustained cellular activation leading to a loss of glucose regulation. J Clin Invest 98:2805-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Razzaboni B, Weir GC, Yankner BA. (1994). Pancreatic islet cell toxicity of amylin associated with type 2 diabetes mellitus. Nature 368:756-760. [DOI] [PubMed] [Google Scholar]
- Magenheim J, Ilovich O, Lazarus A, Klochendler A, Ziv O, Werman R, Hija A, Cleaver O, Mishani E, Keshet E, Dor Y. (2011). Blood vessels restrain pancreas branching, differentiation and growth. Development 138:4743-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM, Choyke PL. (2003). Imaging of angiogenesis: from microscope to clinic. Nat Med 9:713-725. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Noma Y, Kuwajima M, Murakami T, Zhu M, Shima K. (1999). Changes in islet capillary angioarchitecture coincide with impaired B-cell function but not with insulin resistance in male Otsuka-Long-Evans-Tokushima fatty rats: dimorphism of the diabetic phenotype at an advanced age. Metabolism 48:477-483. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kitamura H, Konishi S, Nishimura M, Ono J, Ina K, Shimada T, Takaki R. (1995). The endocrine pancreas of spontaneously diabetic db/db mice: microangiopathy as revealed by transmission electron microscopy. Diabetes Res Clin Pract 30:89-100. [DOI] [PubMed] [Google Scholar]
- Narita R, Toshimori H, Nakazato M, Kuribayashi T, Toshimori T, Kawabata K, Takahashi K, Masukura S. (1992). Islet amyloid polypeptide (IAPP) and pancreatic-islet amyloid deposition in diabetic and nondiabetic patients. Diab Res Clin Pract 15:3-14. [DOI] [PubMed] [Google Scholar]
- Nyman LR, Wells KS, Head WS, McCaughey M, Ford E, Brissova M, Piston DW, Powers AC. (2008). Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest 118:3790-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. (2008). Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes Metab 10(Suppl 4):119-127. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr (1991). Beta-cells in type II diabetes mellitus. Diabetes 40:166-180. [DOI] [PubMed] [Google Scholar]
- Pound LD, Comstock SM, Grove KL. (2014). Consumption of a Western-style diet during pregnancy impairs offspring islet vascularization in a Japanese macaque model. Am J Physiol Endocrinol Metab 307:E115-E123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JE, Hipkiss AR, Himsworth DT, Romero IA, Abbott JN. (1998). Toxic effects of beta-amyloid(25-35) on immortalised rat brain endothelial cell: protection by carnosine, homocarnosine and beta-alanine. Neurosci Lett 242:105-108. [DOI] [PubMed] [Google Scholar]
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. (2008). Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 10(Suppl 4):32-42. [DOI] [PubMed] [Google Scholar]
- Reinert RB, Brissova M, Shostak A, Pan FC, Poffenberger G, Cai Q, Hundemer GL, Kantz J, Thompson CS, Dai C, McGuinness OP, Powers AC. (2013). Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes 62:4154-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert RB, Cai Q, Hong JY, Plank JL, Aamodt K, Prasad N, Aramandla R, Dai C, Levy SE, Pozzi A, Labosky PA, Wright CV, Brissova M, Powers AC. (2014). Vascular endothelial growth factor coordinates islet innervation via vascular scaffolding. Development 141:1480-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. (2011). Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 14:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand FW, Hörnblad A, Johansson JK, Lorén C, Edsbagge J, Ståhlberg A, Magenheim J, Ilovich O, Mishani E, Dor Y, Ahlgren U, Semb H. (2011). Growth-limiting role of endothelial cells in endoderm development. Dev Biol 352:267-277. [DOI] [PubMed] [Google Scholar]
- Schneider HM, Storkel S, Will W. (1980). Das amyloid der Langerhansschen Inseln und seine Beziehung zum Diabetes mellitus. Deutsche Medizinische Wochenschrift 105:1143-1147. [DOI] [PubMed] [Google Scholar]
- Shao J, Iwashita N, Ikeda F, Ogihara T, Uchida T, Shimizu T, Uchino H, Hirose T, Kawamori R, Watada H. (2006). Beneficial effects of candesartan, an angiotensin II type 1 receptor blocker, on beta-cell function and morphology in db/db mice. Biochem Biophys Res Commun 344:1224-1233. [DOI] [PubMed] [Google Scholar]
- Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. (1996). Beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature 380:168-171. [DOI] [PubMed] [Google Scholar]
- Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. (2004). Improved islet morphology after blockade of the renin- angiotensin system in the ZDF rat. Diabetes 53:989-997. [DOI] [PubMed] [Google Scholar]
- Vernier S, Chiu A, Schober J, Weber T, Nguyen P, Luer M, McPherson T, Wanda PE, Marshall CA, Rohatgi N, McDaniel ML, Greenberg AS, Kwon G. (2012). Beta-cell metabolic alterations under chronic nutrient overload in rat and human islets. Islets 4:379-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius A, Thornell LE, Kikkawa Y, Sekiguchi K, Hukkanen M, Konttinen YT, Otonkoski T. (2008). Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia 51:1181-1191. [DOI] [PubMed] [Google Scholar]
- Weidner N. (1995). Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 36:169-180. [DOI] [PubMed] [Google Scholar]
- Westermark P. (1972). Quantitative studies on amyloid in the islets of Langerhans. Ups J Med Sci, 77, 91-94. [DOI] [PubMed] [Google Scholar]
- Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. (1990). Islet amyloid polypeptide - pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A 87:5036-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi H, Zaret KS. (2004). Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development 131:807-817. [DOI] [PubMed] [Google Scholar]