Abstract
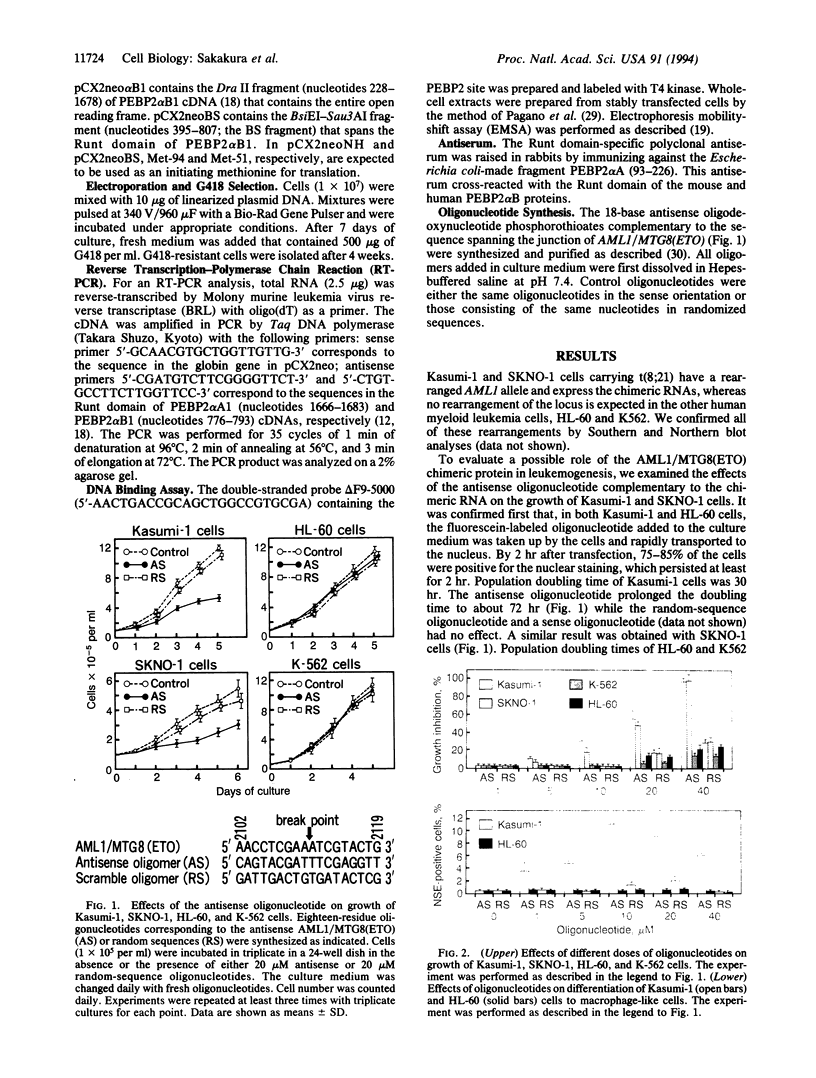
The translocation from chromosome 8 to chromosome 21, t(8;21), associated with acute myeloid leukemia results in production of an AML1/MTG8(ETO) fusion transcript. The product of the AML1 gene contains an evolutionarily conserved 128-amino acid region referred to as the "Runt domain," which is necessary for binding to DNA at the PEBP2 site. A fragment of the AML1 protein containing mainly the Runt domain and the antisense oligonucleotide complementary to the fusion transcript strongly inhibited the growth and induced differentiation of cell lines derived from acute myeloid leukemia containing t(8;21). These results indicate that the transcriptional regulation through the PEBP2 site is critically important for growth and differentiation of t(8;21) leukemic cells and that the product of the chimeric gene is responsible for the maintenance of the leukemic phenotype.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asou H., Tashiro S., Hamamoto K., Otsuji A., Kita K., Kamada N. Establishment of a human acute myeloid leukemia cell line (Kasumi-1) with 8;21 chromosome translocation. Blood. 1991 May 1;77(9):2031–2036. [PubMed] [Google Scholar]
- Bae S. C., Ogawa E., Maruyama M., Oka H., Satake M., Shigesada K., Jenkins N. A., Gilbert D. J., Copeland N. G., Ito Y. PEBP2 alpha B/mouse AML1 consists of multiple isoforms that possess differential transactivation potentials. Mol Cell Biol. 1994 May;14(5):3242–3252. doi: 10.1128/mcb.14.5.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S. C., Yamaguchi-Iwai Y., Ogawa E., Maruyama M., Inuzuka M., Kagoshima H., Shigesada K., Satake M., Ito Y. Isolation of PEBP2 alpha B cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993 Mar;8(3):809–814. [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L. Oncogenic conversion of transcription factors by chromosomal translocations. Cell. 1991 Aug 23;66(4):619–622. doi: 10.1016/0092-8674(91)90105-8. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Erickson P., Gao J., Chang K. S., Look T., Whisenant E., Raimondi S., Lasher R., Trujillo J., Rowley J., Drabkin H. Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood. 1992 Oct 1;80(7):1825–1831. [PubMed] [Google Scholar]
- Grignani F., Ferrucci P. F., Testa U., Talamo G., Fagioli M., Alcalay M., Mencarelli A., Grignani F., Peschle C., Nicoletti I. The acute promyelocytic leukemia-specific PML-RAR alpha fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell. 1993 Aug 13;74(3):423–431. doi: 10.1016/0092-8674(93)80044-f. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Kagoshima H., Shigesada K., Satake M., Ito Y., Miyoshi H., Ohki M., Pepling M., Gergen P. The Runt domain identifies a new family of heteromeric transcriptional regulators. Trends Genet. 1993 Oct;9(10):338–341. doi: 10.1016/0168-9525(93)90026-e. [DOI] [PubMed] [Google Scholar]
- Kamachi Y., Ogawa E., Asano M., Ishida S., Murakami Y., Satake M., Ito Y., Shigesada K. Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J Virol. 1990 Oct;64(10):4808–4819. doi: 10.1128/jvi.64.10.4808-4819.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Baltimore D. E2A-Pbx1, the t(1;19) translocation protein of human pre-B-cell acute lymphocytic leukemia, causes acute myeloid leukemia in mice. Mol Cell Biol. 1993 Jan;13(1):351–357. doi: 10.1128/mcb.13.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Look A. T., Baltimore D. The human t(1;19) translocation in pre-B ALL produces multiple nuclear E2A-Pbx1 fusion proteins with differing transforming potentials. Genes Dev. 1991 Mar;5(3):358–368. doi: 10.1101/gad.5.3.358. [DOI] [PubMed] [Google Scholar]
- Kania M. A., Bonner A. S., Duffy J. B., Gergen J. P. The Drosophila segmentation gene runt encodes a novel nuclear regulatory protein that is also expressed in the developing nervous system. Genes Dev. 1990 Oct;4(10):1701–1713. doi: 10.1101/gad.4.10.1701. [DOI] [PubMed] [Google Scholar]
- Liu P., Tarlé S. A., Hajra A., Claxton D. F., Marlton P., Freedman M., Siciliano M. J., Collins F. S. Fusion between transcription factor CBF beta/PEBP2 beta and a myosin heavy chain in acute myeloid leukemia. Science. 1993 Aug 20;261(5124):1041–1044. doi: 10.1126/science.8351518. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Mitelman F., Heim S. Quantitative acute leukemia cytogenetics. Genes Chromosomes Cancer. 1992 Jul;5(1):57–66. doi: 10.1002/gcc.2870050109. [DOI] [PubMed] [Google Scholar]
- Miyoshi H., Kozu T., Shimizu K., Enomoto K., Maseki N., Kaneko Y., Kamada N., Ohki M. The t(8;21) translocation in acute myeloid leukemia results in production of an AML1-MTG8 fusion transcript. EMBO J. 1993 Jul;12(7):2715–2721. doi: 10.1002/j.1460-2075.1993.tb05933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Shimizu K., Kozu T., Maseki N., Kaneko Y., Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., Nimer S. D. Transcription factors, translocations, and leukemia. Blood. 1992 Dec 15;80(12):2953–2963. [PubMed] [Google Scholar]
- Nisson P. E., Watkins P. C., Sacchi N. Transcriptionally active chimeric gene derived from the fusion of the AML1 gene and a novel gene on chromosome 8 in t(8;21) leukemic cells. Cancer Genet Cytogenet. 1992 Oct 15;63(2):81–88. doi: 10.1016/0165-4608(92)90384-k. [DOI] [PubMed] [Google Scholar]
- Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991 Dec 15;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Ogawa E., Inuzuka M., Maruyama M., Satake M., Naito-Fujimoto M., Ito Y., Shigesada K. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993 May;194(1):314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- Ogawa E., Maruyama M., Kagoshima H., Inuzuka M., Lu J., Satake M., Shigesada K., Ito Y. PEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Dürst M., Joswig S., Draetta G., Jansen-Dürr P. Binding of the human E2F transcription factor to the retinoblastoma protein but not to cyclin A is abolished in HPV-16-immortalized cells. Oncogene. 1992 Sep;7(9):1681–1686. [PubMed] [Google Scholar]
- Rabbitts T. H. Translocations, master genes, and differences between the origins of acute and chronic leukemias. Cell. 1991 Nov 15;67(4):641–644. doi: 10.1016/0092-8674(91)90057-6. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Identificaton of a translocation with quinacrine fluorescence in a patient with acute leukemia. Ann Genet. 1973 Jun;16(2):109–112. [PubMed] [Google Scholar]
- Satake M., Ibaraki T., Yamaguchi Y., Ito Y. Loss of responsiveness of an AP1-related factor, PEBP1, to 12-O-tetradecanoylphorbol-13-acetate after transformation of NIH 3T3 cells by the Ha-ras oncogene. J Virol. 1989 Sep;63(9):3669–3677. doi: 10.1128/jvi.63.9.3669-3677.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczylik C., Skorski T., Nicolaides N. C., Manzella L., Malaguarnera L., Venturelli D., Gewirtz A. M., Calabretta B. Selective inhibition of leukemia cell proliferation by BCR-ABL antisense oligodeoxynucleotides. Science. 1991 Aug 2;253(5019):562–565. doi: 10.1126/science.1857987. [DOI] [PubMed] [Google Scholar]
- Wang S., Wang Q., Crute B. E., Melnikova I. N., Keller S. R., Speck N. A. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol Cell Biol. 1993 Jun;13(6):3324–3339. doi: 10.1128/mcb.13.6.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]