Abstract
Objectives
To compare risks of hypernatraemia on admission to hospital in persons who were with those who were not identified as care home residents and evaluate the association of hypernatraemia with in-hospital mortality.
Design
Retrospective observational study.
Setting
A National Health Service Trust in London.
Participants
A total of 21,610 patients aged over 65 years whose first admission to the Trust was between 1 January 2011 and 31 December 2013.
Main outcome measures
Hypernatraemia on admission (plasma Na > 145 mmol/L) and in-hospital death.
Results
Patients admitted from care homes had 10-fold higher prevalence of hypernatraemia than those from their own homes (12.0% versus 1.3%, respectively; odds ratio [OR]: 10.5, 95% confidence interval [CI]: 8.43–13.0). Of those with hypernatraemia, nine in 10 cases were associated with nursing home ECOHOST residency (attributable fraction exposure: 90.5%), and the population attributable fraction of hypernatraemia on admission associated with care homes was 36.0%. After correcting for age, gender, mode of admission and dementia, care home residents were significantly more likely to be admitted with hypernatraemia than were own-home residents (adjusted odds ratio [AOR]: 5.32, 95% CI: 3.85–7.37). Compared with own-home residents, care home residents were also at about a two-fold higher risk of in-hospital mortality compared with non-care home residents (AOR: 1.97, 95% CI: 1.59–2.45). Consistent with evidence that hypernatraemia is implicated in higher mortality, the association of nursing homes with in-hospital mortality was attenuated after adjustment for it (AOR: 1.61, 95% CI: 1.26–2.06).
Conclusions
Patients admitted to hospital from care homes are commonly dehydrated on admission and, as a result, appear to experience significantly greater risks of in-hospital mortality.
Keywords: care homes, hypernatraemia, older people, dehydration
Introduction
As the population ages, a growing number of patients are admitted to hospital from elderly care homes. There have long been concerns about the quality of care of residents in such homes in the UK.1 In some cases, this has involved actual abuse, in the form of violence, but others have focused on neglect, especially in relation to help with basic activities such as drinking, feeding and toileting. There is a particular concern that persons living in care homes have not benefited to the same extent as those living in their own homes from initiatives such as the Quality and Outcomes Framework.2
Old and infirm people, wherever they are, are at increased risk of dehydration.3 Some may intentionally restrict their intake because of difficulties in going to the toilet.4 However, those receiving care, in residential homes or hospital may be especially at risk as they may require assistance with drinking and, left to themselves may fail to achieve an adequate intake. The now notorious accounts to the Francis Inquiry of patients in the Mid Staffs hospital drinking water from flower vases represent gross neglect.5 While this was, hopefully, an extreme example, one study of carers of people with dementia living in their own homes noted how ‘A common strategy reported to prevent nocturia was the restriction of fluids later in the day’.6
Although published evidence is lacking, it is at least plausible that this may occur in settings such as care homes. Yet, as this practice is unlikely to be revealed voluntarily to researchers, it is very difficult to study. One newspaper report, based on freedom of information requests, raised concerns about deaths from dehydration and related causes,7 but official figures would suggest that any problem is small, with under 200 patients per year admitted to hospital from residential care in England with a diagnosis of dehydration.8 However, this seems implausibly low.
Mild to moderate dehydration in older people can be easily missed, 9 although growing recognition of its importance has stimulated research to develop new means of detecting it.10,11 Often it is only detected once individuals are admitted to hospital and have their electrolytes measured, revealing hypernatraemia.
Hypernatraemia, by definition, has two causes, sodium excess and water depletion. Sodium excess is relatively rare, associated with excessive administration of intravenous solutions containing high concentrations of sodium or with oral administration of large amounts of salt. Water deficiency is much more common. This occurs either due to an inadequate intake in the face of excessive losses, for instance in diarrhoea, or as a result of an absolute reduction in intake. In the ideal 70 kg man, total body water is taken to be 42 L, and so a one litre deficiency of water will result in an increase in concentration of all dissolved substances by 42/41. For plasma sodium, this represents a rise from 138 mmol/L to 141 mmol/L. The upper limit for plasma sodium is 145 mmol/L; a value greater than this represents a deficit of at least 5%.
The consequences of hypernatraemia can be severe. A few studies have looked at the consequences of a high sodium level in hospitalised patients, typically in those admitted to intensive care units (ICU), finding significantly worse outcomes. This includes between a 1.5 - and 10-fold greater risk of dying,12–17 increased risk of acute coronary events, pneumonia and thromboembolism,18 worse outcomes of patients with cancer,19 with chronic kidney disease20 and stroke,21 as well as of unselected admissions.22 However, most of these studies have focused either on hypernatraemia arising during admission, increasingly viewed as a marker of quality of hospital or ICU care, or have not differentiated that present on admission from that arising later.
Our study arose from the recent concerns over the incidence of dehydration observed anecdotally among UK care homes residents. To our knowledge for the first time, our analysis links hospital admission records with care home residency status that make it possible to test the hypothesis that patients admitted from care homes experienced increased risk of hypernatraemia and, as a result, have higher in-hospital mortality risks.
Methods
Data sources
We linked data from two sources operated by the Barnet and Chase Farm Hospitals National Health Service (NHS) Trust. The first is the Patient Administration System, containing sociodemographic data, such as date of birth, gender and address, and administrative data such as dates and modes of admission and discharge. The second is the laboratory system, containing dates and times of tests and their results. These data are stored in an Oracle database on a secure server managed by the hospitals’ Information Technology department.
We performed a retrospective analysis of all individuals admitted to Trust hospitals over a two-year period who were aged over 65 years between 1 January 2011 and 31 December 2013. Taking only first admissions in this period, they numbered 27,603. Of these 21,833 had plasma sodium measured within the first 24 h of admission. One hundred forty-three records did not have a valid postcode. Ninety did not have a valid admission code. These were excluded from analysis leaving 21,610 admissions with a complete set of data. In order to ensure independence between admissions, only an individual’s first admission was included in the analysis.
For each admission we extracted: the age and gender of the patient, the patient’s home postcode, the type of admission (planned or emergency) and (if measured) the first plasma sodium concentration following admission. Finally whether the patient was discharged alive was recorded. Following standard classifications,23 patients were coded as hypernatraemic if the first plasma sodium measured within the first 24 h of admission exceeded 145 mmol/L. The frequency of hypernatraemia on admission varied with age, and whether the admission was as an emergency. The final analytical sample included complete data on 21,610 admissions, including 432 cases of hypernatraemia and 1413 hospital deaths.
Linking hospital records to care home residency
To identify patients admitted from care homes, we used the website www.carehome.co.uk to identify 90 care homes serving the Barnet and Chase Farm NHS Trust hospitals. We excluded homes that did not provide nursing care and were described in terms of number of flats as opposed to number of rooms, which were judged to be residential homes. Although the Patient Administration System includes information on the source of admission, its coverage was inadequate (it was recorded for only 76 patients, less than 5% of those identified using the aforementioned method).
We then matched these care homes with patient admissions data by screening those postcodes which exceeded 20 admissions of patients aged 65 years and over covering the study period of 2011–2013. These high volume postcodes were easily distinguished from normal residential postcode sectors, which typically generate 1–2 admissions per year in this age group. This identified 53 care homes. Although a few postcode sectors also generated high admission numbers, on further inspection we found that these contained complexes of sheltered dwellings, so these were categorised as normal residential postcode sectors.
This coding approach is likely to include a small number of patients who live in their own homes but in the same postcode sector as a care home. However, as 80% of individual postcodes produced only one admission in this age group in the three-year period and 97% of postcodes had five or less admissions, it was assumed that a patient who had a postcode that included a care home was resident in that home.
Statistical modelling
In the first stage of the analysis, we evaluated whether patients from care homes had greater risks of hypernatraemia on admission. Multivariate logistic regression models were used to adjust for potential confounding factors, including age-bands, mode of admission (emergency/routine), sex, and compared with patients not admitted from care homes using multivariate regression. It is also necessary to take account of the likelihood that patients from care homes are sicker and frailer. Although there are case-mix adjustment models available, such as certain commercial products used for calculating standardised mortality rates, these are subject to major limitations associated with variations in coding.24 Moreover, they are calibrated to adjust for risk of death, not hypernatraemia. Thus, the models also included an adjustment for whether the patients had been diagnosed with dementia, which a priori is likely to be the most common risk factor for inadequate drinking. In the subsequent stage, we quantified the association of hypernatraemia with in-hospital mortality risk, including care residency status as a dummy variable to perform a mediation analysis. All models were performed using STATA v13.1. Standard errors were clustered by place of residence to adjust for non-independence of sampling.
Results
Association of care homes with hypernatraemia
Table 1 shows the characteristics of the patients admitted from a care home and those admitted from their own home. Care home residents were older (mean age 85.5 years in care home residents versus 79.8 years in own-home residents), more likely to suffer dementia (61.9% versus 14.7%) and had higher rates of hypernatraemia (12.0% versus 1.3%). Figure 1 shows the probability that a patient with a given level of sodium on admission will have been admitted from a care home. Thus, over 70% of those with plasma sodium over 160 mmol/L will have come from these settings.
Table 1.
Characteristics of patients admitted from their care homes and own homes.
| Care home residents n = 1430 | Residents of own homes n = 20,180 | |
|---|---|---|
| Mean age | 85.5 | 79.8 |
| Male | 32.4% | 45.0% |
| Emergency admission | 99.6% | 96.1% |
| Hypernatraemic | 12.0% | 1.3% |
| Dementia | 61.9% | 14.7% |
| In-hospital mortality | 14.2% | 6.0% |
Figure 1.
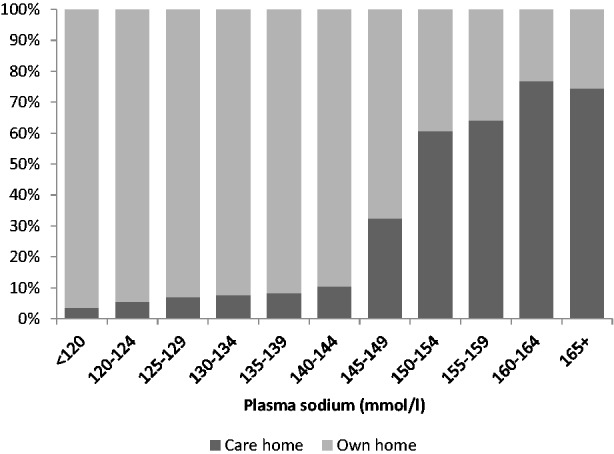
Probability that a patient with a given sodium level will have been admitted from a care home.
The odds of hypernatraemia were 10-fold greater in nursing home residents than in own-home residents, before adjusting for potential confounding factors (odds ratio [OR] 10.5, 95% confidence interval [CI]: 8.43–13.0). Based on this estimated association, the fraction attributable to care home residency was 90.5%, and the associated population attributable fraction of hypernatraemia was 36.0%. Table 2 presents the results of multivariate logistic regression models addressing potential confounding factors for hypernatraemia. As shown in the table, older age persons had greater risks of hypernatraemia (adjusted odds ratio [AOR]: 1.02, 95% CI: 1.01–1.03), as did persons with a diagnosis of dementia (AOR: 3.43, 95% CI: 2.18–5.39) and patients whose admissions were emergencies (AOR: 2.89, 95% CI: 2.11–3.96). The association of care home residency was attenuated but remained strongly statistically significant (AOR: 5.32, 95% CI: 3.85–7.37).
Table 2.
Odds ratio of admission hypernatraemia.
| Covariate | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Age | 1.02 | 1.01–1.03 | 0.006 |
| Male | 1.09 | 0.94–1.25 | 0.254 |
| Emergency admission | 2.89 | 2.11–3.96 | <0.001 |
| Dementia | 3.43 | 2.18–5.39 | <0.001 |
| Care home resident | 5.32 | 3.85–7.37 | <0.001 |
| Number of patients | 21,610 | ||
| Pseudo-R2 | 0.13 |
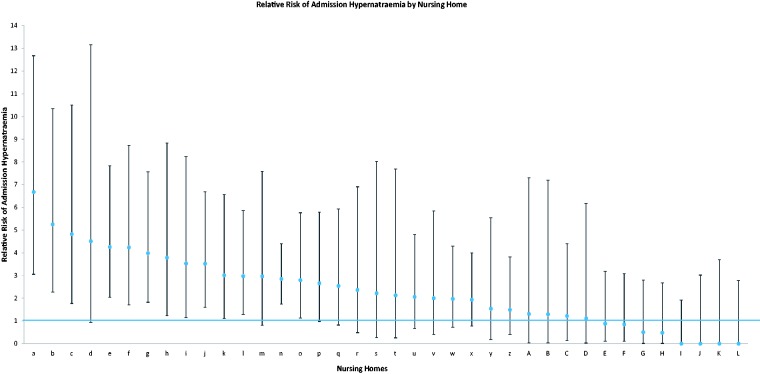
We performed a series of robustness tests. First, we re-estimated controls for age using five-year age intervals, finding that none of the results was significantly altered. Then, we removed potential outliers based on standardised residuals greater than |2|. Again none of the results was changed. We then disaggregated the risk of hypernatraemia on admission for the 53 individual care homes included in the study to identify whether cases arose from a few clusters. Figure 2 shows the odds of having hypernatraemia on admission for each care home (signified by a letter) compared to those in their own homes. This shows that, admission from a care home is not inevitably associated with hypernatraemia, but the probability is significantly increased in about one-third of care homes, after adjustment for age, gender, type of admission and presence of dementia. Finally to further adjust for potential unobserved frailty, we included a control for whether the patient died in hospital as a proxy for other unobserved frailty and the results did not qualitatively differ.
Figure 2.
Odds ratio of admission hypernatraemia for each care home (95% confidence intervals).
Next in order to put these findings into clinical perspective, we quantified the probability of hypernatraemia of four scenarios for a representative age 80-year-old patient, as follows: own-home residency/care home residency/no dementia/dementia. The lowest probability of admission hypernatraemia was observed for persons who were admitted from their own home and had no dementia, at 0.9%, followed by those who had dementia (3.3%). This was followed by persons from care homes who did not have dementia (5.6%) and finally by those who were in care homes and had dementia (14.7%).
Association of in-hospital mortality with hypernatraemia
Mortality among those admitted from care homes was higher than among those admitted from their own homes (14.2% versus 6.0%). Unadjusted, the attributable fraction of deaths associated with hypernatraemia was 85.2%, and the population attributable fraction was 7.9%. That associated with care home residency was estimated at 61.4% with a corresponding population attributable fraction of 8.8%.
Again we performed multivariate logistic regression models to evaluate the contribution of care home residency to in hospital mortality, shown in Table 3. As anticipated, age, male sex and emergency admission were significant and positive correlates of in-hospital mortality. Even after correcting for these potential confounders, care home residency was estimated to confer about a two-fold higher risk of in-hospital mortality (AOR: 1.97, 95% CI: 1.59–2.45).
Table 3.
Odds ratio of mortality.
| Covariate | Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value |
|---|---|---|---|---|---|---|
| Age | 1.06 | 1.06–1.07 | <0.001 | 1.06 | 1.06–1.07 | <0.001 |
| Male | 1.29 | 1.23–1.34 | <0.001 | 1.28 | 1.23–1.34 | <0.001 |
| Emergency admission | 2.59 | 2.26–2.96 | <0.001 | 2.51 | 2.19–2.87 | <0.001 |
| Dementia | 0.94 | 0.75–1.18 | 0.600 | 0.85 | 0.68–1.06 | 0.138 |
| Care home resident | 1.97 | 1.59–2.45 | <0.001 | 1.61 | 1.26–2.06 | <0.001 |
| Hypernatraemic | – | – | – | 5.10 | 3.71–7.00 | <0.001 |
| Number of patients | 21,610 | 21,610 | ||||
| Pseudo-R2 | 0.044 | 0.059 |
Consistent with evidence of a mechanism, we found that the inclusion of hypernatraemia into the model attenuated the association of care home residence with increased mortality by about 50% (AOR: 1.61, 95% CI: 1.26–2.06). Hypernatraemia itself was associated with a five-fold greater risk of in-hospital mortality (AOR: 5.10, 95% CI: 3.71–7.00).
Discussion
Our study establishes that patients admitted from a substantial number of care homes are at a greatly increased risk of hypernatraemia. This association persists even after adjusting for significant differences between the characteristics of care home patients and patients admitted from their own home, including age, dementia diagnosis and whether the admission was an emergency. We further found evidence that is consistent with a mechanism that living in certain care homes increases the risk of hypernatraemia and that hypernatraemia on admission to hospital is an independent predictor of in-hospital mortality.
As with all statistical analyses, our study has several limitations. First, it is difficult to separate association from causation, even with adjustment for potential confounders. However, irrespective of the causality of nursing homes in driving hypernatraemia risks, it reveals that there is a clear and highly prevalent problem in these institutions. Nonetheless, our models do fulfil several of Bradford-Hill’s criteria of causality,25 including analogy, strength of association and coherence, among others. Additionally, even when dementia as a proxy for general poor medical condition is included as a determinant of admission hypernatraemia, care home residence remains as the single most important determinant. We did not adjust for other indicators of severity because, while there are case-mix adjustment models available, including various commercial products used for calculating standardised mortality rates, these are subject to major limitations associated with variations in coding,24 and they have been calibrated to adjust for risk of death, not hypernatraemia. Consequently, these findings suggest that frailty or poor general health is not confounding the observed relationship of care home residency and the elevated risk of hypernatraemia. Second, there is potential risk of mis-specification of a patient to a care home who is actually in a domestic residence. However, the numbers affected are likely to be very small. Third, our study investigated a single NHS Trust and, as a result, it is not possible to generalise these findings with certainty beyond the individual hospitals concerned and the care homes in the study population. These hospitals operate in a suburban area where there are many care homes. In contrast, inner city hospitals, especially London, characterised by high property prices, will receive few patients from care homes so the magnitude of hypernatraemia may be considerably smaller than in the studied population. However, higher property prices may also negatively impact on the ability of hospitals to attract and retain high-quality care staff who could prevent hypernatraemia.
Our findings are consistent with concerns about the quality of care in care homes. However, this analysis moves beyond prior work to evaluate outcome once patients are admitted to hospital. If care home residents are dehydrated, there are a few possible reasons why. One is that the residents themselves choose to drink less. Another is that care home staff do not offer water in sufficient amounts, either as an act of omission or more perversely, as has been suggested (personal communication), as an active decision in an attempt to reduce incontinence and frequent requests for assistance. The balance of these possibilities is not well understood. So far, there seems to have been relatively little research seeking to bridge the interface between hospitals and care homes, a finding symptomatic of a larger problem of disciplinary and other research-related silos.26 American research has identified inadequate staffing and staff unable to speak English as important risk factors for dehydration among residents.27,28 In a comparison of six countries, England, along with the USA, Canada and Germany, but not Norway and Sweden, had care home staffing levels below those recommended by experts.29
Dehydration is clearly common in care homes. In itself, our observation that hypernatraemia is significantly more prevalent in care home residents is disturbing, as it represents a state of being among patients which in itself is distressing. Further concern is evident from the clear link of hypernatraemia with in-hospital mortality, as suggested in prior reviews.
As our results demonstrate, hypernatraemia is uncommon in patients admitted from their own homes occurring in less than 1% of admissions. A patient with dementia would have a much lower risk in their own home than a patient without dementia in a care home. Therefore, it would seem reasonable that on admission, sodium above 145 mmol/L in a patient from a care home should trigger concern, perhaps expressed in the discharge summary to the general practitioner responsible for medical care in that care home. A second admission with hypernatraemia, within a given period may well be indicative of a systemic problem at the care home and the issue should be raised more formally with, potentially, the Care Quality Commission. Taken together, our evidence calls for a follow-up interventional study involving education and the active encouragement of water intake in care homes should now be undertaken.
Declarations
Competing interest
None declared
Funding
DS is funded by a Wellcome Trust Investigator Award
Ethical approval
This study was submitted to Barnet and Chase Farm NHS Trust, which issued a waiver certifying that ethics approval was not required.
Guarantor
AW
Contributorship
AW conceived the study and conducted initial analysis of the data. DS conducted additional analyses. MM advised on the analysis and drafted the manuscript, which AW and DS revised. All authors contributed to the interpretation of the data.
Acknowledgements
None
Provenance
Not commissioned; peer-reviewed by Daniel Gibbon.
References
- 1.Fahey T, Montgomery AA, Barnes J, Protheroe J. Quality of care for elderly residents in nursing homes and elderly people living at home: controlled observational study. BMJ 2003; 326: 580–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah SM, Carey IM, Harris T, Dewilde S, Cook DG. Quality of chronic disease care for older people in care homes and the community in a primary care pay for performance system: retrospective study. BMJ 2011; 342: d912–d912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell N. Dehydration: why is it still a problem? Nurs Times 2011; 107: 12–15. [PubMed] [Google Scholar]
- 4.Dowd TT, Campbell JM, Jones JA. Fluid intake and urinary incontinence in older community-dwelling women. J Community Health Nurs 1996; 13: 179–186. [DOI] [PubMed] [Google Scholar]
- 5.Daily Telegraph. Mid Staffordshire Trust inquiry: how the care scandal unfolded. Daily Telegraph 2013. See http://www.telegraph.co.uk/health/healthnews/9851763/Mid-Staffordshire-Trust-inquiry-how-the-care-scandal-unfolded.html (last checked 11 December 2014).
- 6.Drennan VM, Cole L, Iliffe S. A taboo within a stigma? A qualitative study of managing incontinence with people with dementia living at home. BMC Geriatr 2011; 11: 75–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley-Smith B. More than a thousand care home residents die thirsty. Daily Telegraph 2013. See http://www.telegraph.co.uk/health/healthnews/10487305/More-than-a-thousand-care-home-residents-die-thirsty.html (last checked 11 December 2014).
- 8.Lamb N. Health services: older people. Reply to question by Chris Skidmore MP. Hansard 2012;12 December, Column 355W.
- 9.Weinberg AD, Minaker KL. Dehydration. Evaluation and management in older adults. Council on Scientific Affairs, American Medical Association. JAMA 1995; 274: 1552–1556. [DOI] [PubMed] [Google Scholar]
- 10.Wotton K, Crannitch K, Munt R. Prevalence, risk factors and strategies to prevent dehydration in older adults. Contemp Nurse 2008; 31: 44–56. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita K, Hattori K, Ota Y, Kanai T, Shimizu M, Kobayashi H, et al. The measurement of axillary moisture for the assessment of dehydration among older patients: a pilot study. Exp Gerontol 2013; 48: 255–258. [DOI] [PubMed] [Google Scholar]
- 12.Polderman KH, Schreuder WO, Strack van Schijndel RJ, Thijs LG. Hypernatremia in the intensive care unit: an indicator of quality of care? Crit Care Med 1999; 27: 1105–1108. [DOI] [PubMed] [Google Scholar]
- 13.Stelfox HT, Ahmed SB, Zygun D, Khandwala F, Laupland K. Characterization of intensive care unit acquired hyponatremia and hypernatremia following cardiac surgery. Can J Anaesth 2010; 57: 650–658. [DOI] [PubMed] [Google Scholar]
- 14.Stelfox HT, Ahmed SB, Khandwala F, Zygun D, Shahpori R, Laupland K. The epidemiology of intensive care unit-acquired hyponatraemia and hypernatraemia in medical-surgical intensive care units. Crit Care 2008; 12: R162–R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darmon M, Timsit JF, Francais A, Nguile-Makao M, Adrie C, Cohen Y, et al. Association between hypernatraemia acquired in the ICU and mortality: a cohort study. Nephrol Dial Transplant 2010; 25: 2510–2515. [DOI] [PubMed] [Google Scholar]
- 16.Lindner G, Funk GC, Schwarz C, Kneidinger N, Kaider A, Schneeweiss B, et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis 2007; 50: 952–957. [DOI] [PubMed] [Google Scholar]
- 17.Vandergheynst F, Sakr Y, Felleiter P, Hering R, Groeneveld J, Vanhems P, et al. Incidence and prognosis of dysnatraemia in critically ill patients: analysis of a large prevalence study. Eur J Clin Invest 2013; 43: 933–948. [DOI] [PubMed] [Google Scholar]
- 18.Leung AA, McAlister FA, Finlayson SR, Bates DW. Preoperative hypernatremia predicts increased perioperative morbidity and mortality. Am J Med 2013; 126: 877–886. [DOI] [PubMed] [Google Scholar]
- 19.Salahudeen AK, Doshi SM, Shah P. The frequency, cost, and clinical outcomes of hypernatremia in patients hospitalized to a comprehensive cancer center. Support Care Cancer 2013; 21: 1871–1878. [DOI] [PubMed] [Google Scholar]
- 20.Jovanovich A, Berl T. Mortality and serum sodium in CKD–yet another U shaped curve. Nat Rev Nephrol 2012; 8: 261–263. [DOI] [PubMed] [Google Scholar]
- 21.Fofi L, Dall'armi V, Durastanti L, Valenza A, Lorenzano S, Prencipe M, et al. An observational study on electrolyte disorders in the acute phase of ischemic stroke and their prognostic value. J Clin Neurosci 2012; 19: 513–516. [DOI] [PubMed] [Google Scholar]
- 22.Erasmus RT, Matsua TE. Frequency, aetiology and outcome of hypernatraemia in hospitalised patients in Umtata, Transkei, South Africa. East Afr Med J 1999; 76: 85–88. [PubMed] [Google Scholar]
- 23.Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med 2000; 342: 1493–1499. [DOI] [PubMed] [Google Scholar]
- 24.Spiegelhalter D. Statistics behind the headlines. Have there been 13,000 needless deaths at 14 NHS trusts? BMJ 2013; 347: f4893–f4893. [DOI] [PubMed] [Google Scholar]
- 25.Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965; 58: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyke S, Adamson J, Dixon D, Hunt K. Consultation and illness behaviour in response to symptoms: a comparison of models from different disciplinary frameworks and suggestions for future research directions. Soc Sci Med 2013; 86: 79–87. [DOI] [PubMed] [Google Scholar]
- 27.Kayser-Jones J, Schell ES, Porter C, Barbaccia JC, Shaw H. Factors contributing to dehydration in nursing homes: inadequate staffing and lack of professional supervision. J Am Geriatr Soc 1999; 47: 1187–1194. [DOI] [PubMed] [Google Scholar]
- 28.Kayser-Jones J. Malnutrition, dehydration, and starvation in the midst of plenty: the political impact of qualitative inquiry. Qual Health Res 2002; 12: 1391–1405. [DOI] [PubMed] [Google Scholar]
- 29.Harrington C, Choiniere J, Goldmann M, Jacobsen FF, Lloyd L, McGregor M, et al. Nursing home staffing standards and staffing levels in six countries. J Nurs Scholarsh 2012; 44: 88–98. [DOI] [PubMed] [Google Scholar]