Abstract
Wake-up stroke, defined as the situation where a patient awakens with stroke symptoms that were not present prior to falling asleep, represents roughly 1 in 5 acute ischemic strokes and remains a therapeutic dilemma. Patients with wake-up stroke were excluded from most ischemic stroke treatment trials and are often not eligible for acute reperfusion therapy in clinical practice, leading to poor outcomes. Studies of neuroimaging with standard noncontrast computed tomography (CT), magnetic resonance imaging (MRI), and multimodal perfusion-based CT and MRI suggest wake-up stroke may occur shortly before awakening and may assist in selecting patients for acute reperfusion therapies. Pilot studies of wake-up stroke treatment based on these neuroimaging features are promising but have limited generalizability. Ongoing randomized treatment trials using neuroimaging-based patient selection may identify a subset of patients with wake-up stroke that can safely benefit from acute reperfusion therapies.
Keywords: acute stroke, wake-up stroke, tPA, thrombolysis, hemorrhage, outcome
Introduction
Definition
Acute stroke evaluation and management is fundamentally predicated on time from symptom onset.1,2 Intravenous tissue plasminogen activator (tPA) remains the only Food and Drug Administration (FDA)-approved nonsurgical reperfusion therapy for acute stroke with evidence-based efficacy, and well-designed, adequately powered studies have consistently shown that efficacy is exquisitely time sensitive.3–7 That being the case, knowledge of the exact time of symptom onset, or at least the time at which the patient was last known to be normal, is paramount.
Patients who go to sleep normal and awaken with stroke symptoms, a phenomenon known as “wake-up stroke,” present a management dilemma for acute stroke providers. Sometimes the period of sleep is short and a patient can still be eligible for tPA based on standard time-based criteria; however, when the time at which the patient was last known to be normal is the night prior to a morning presentation, which is often the case, the acute stroke provider is left without the key time-based data by which one typically makes safe therapeutic decisions for tPA candidacy. This makes for a diagnostic and therapeutic “gray area” in acute stroke practice.
Epidemiology
The wake-up stroke phenomenon is common. Numerous studies of various size and methodological strength through the years have given a sense of actual incidence of wake-up stroke as compared to other stroke presentations. These mostly stroke-registry–based studies range in estimation of wake-up stroke incidence from 8% in California8 to 33% in a region of France9 to nearly 39% in Ohio10 but most typically suggest somewhere in between 15% and 25%.11–24 Clinical and radiographic characteristics distinguishing wake-up stroke from other modes of stroke onset have been sought, but results have been conflicting. Some older studies have suggested that wake-up strokes seem to be more severe at onset14,19 and portend a worse outcome overall,17,19 while others suggested there are no appreciable clinical or radiographic differences between wake-up and “while awake” strokes.16,22,25–27
The best estimate of wake-up stroke prevalence comes from a retrospective population-based study of 1854 acute ischemic strokes in the Greater Cincinnati/Northern Kentucky region. In this representative biracial sample, 273 (14.3%) of acute strokes were wake-up strokes, resulting in an adjusted event rate of 26.0/100 000. No clinically significant differences in baseline characteristics were observed between wake-up and nonwake-up strokes; however, patients with wake-up stroke were older (72.3 vs 70.0 years, P = .01) and had higher baseline retrospectively calculated National Institutes of Health Stroke Scale (NIHSS) scores (4 vs 3, P = .004). Importantly, 98 (35%) patients were otherwise eligible for tPA if time was not a factor.27
Overall, in spite of the methodological heterogeneity and different focus of published studies, the common theme is that wake-up stroke is not rare and the clinical features suggest that there is a place for therapeutic optimism; although, no definitive clinical or radiographic paradigm has yet been established to select wake-up stroke candidates for safe and efficacious reperfusion therapy (Table 1).
Table 1.
Wake-Up Stroke Characteristics by Study.
| Authors | Study Design | Total Patients | Wake-Up Stroke #, % | Clinical Differences vs While-Awake Stroke | Outcome Differences vs While-Awake Stroke | Imaging |
|---|---|---|---|---|---|---|
| CASPR group8 | Retrospective, prospectively collected data, US state registry | 374 | 30 (8) | — | — | — |
| Michel et al9 | Retrospective, prospectively collected data, hospital registry | 1633 | 568 (33.1) | — | — | — |
| Tanimoto et al10 | Retrospective, prospectively collected data, hospital registry | 72 | 28 (38.9) | WUS: tended to be African American, younger, small vessel mechanism, less severe NIHSS, worse lipid profile | — | — |
| Marler et al11 | Retrospective, prospectively collected data, hospital registry | 1167 | 331 (28) | — | — | — |
| Ricci et al12 | Retrospective, prospectively collected data, regional registry | 375 | 68 (18.1) | — | — | — |
| Lago et al13 | Retrospective, prospectively collected data, hospital registry | 1223 | 309 (25.2) | — | — | — |
| Bornstein et al14 | Retrospective, prospectively collected data, national registry | 1671 | 311 (18.6) | WUS more severe | — | — |
| Chaturvedi et al15 | Subanalysis of prospective RCT | 1272 | 323 (25.4) | — | — | — |
| Serena et al16 | Retrospective, prospectively collected data, national registry | 1248 | 301 (24.1) | None | — | WUS: CT head normal in 39.4% of patients seen within 6 hours of symptom recognition (60% in stroke while awake) |
| Nadeau et al17 | Retrospective, prospectively collected data, national registry | 2585 | 349 (13.5) | WUS had higher BP and ischemic stroke subtype | WUS less likely to return home | — |
| Boode et al18 | Retrospective, hospital registry | 263 | 48 (18.3) | — | — | — |
| Jiménez-Conde et al19 | Retrospective, prospectively collected data, hospital registry | 813 | 127 (15.6) | WUS had more obesity, less AF, and higher initial stroke severity | WUS had worse 3-month outcome | — |
| Silva et al20 | Prospective cohort study, hospital registry | 676 | 131 (19.4) | None | None | Similar prevalence of CTP mismatch and arterial occlusion in WUS and known onset groups |
| Turin et al21 | Retrospective, prospectively collected data, national registry | 897 | 87 (9.7) | WUS more hypertension and increased initial severity | None | — |
| Fink et al22 | Retrospective, prospectively collected data, hospital registry | 364 | 100 (27) | None | — | Similar prevalence of MRI DWI/PWI mismatch |
| Moradiya et al23 | Subanalysis of a prospective RCT | 17 398 | 5152 (29.6) | WUS initially less severe | None | — |
| Koton et al24 | Retrospective, prospectively collected data, national registry | 4408 | 820 (18.6) | None | None | 20%-40% prevalence of penumbra |
| Todo et al25 | Retrospective, prospectively collected data, hospital registry | 158 | 17 (10.8) | — | — | CT findings in WUS similar to patients within 3 hours of known symptom onset |
| Huisa et al26 | Prospective cohort study, hospital registry | 96 | 28 (29.6) | None | Trend toward favorable (0-1) 90 d mRS in WUS vs 4 hours from symptoms controls (73% vs 45%) | Favorable CT ASPECTS (8-10) similar in WUS and known 4 h from symptoms (89.3% vs 95.6%) |
| Mackey et al27 | Population-based registry | 1854 | 273 (14.7) | “Minor differences” in age and rNIHSS (WUS older, higher rNIHSS) | None | — |
| Roveri et al28 | Retrospective, prospectively collected data, hospital registry | 1531 | 190 (12.4) | None | Outcome better in controls (patients treated with tPA within 3 hours of symptoms) | Baseline ASPECTS similar in WUS and controls within 3 hours of symptoms and treated with tPA |
| Manawadu et al29 | Retrospective, prospectively collected data, hospital registry | 1836 | 193 (10.5) | — | Outcome better in thrombolyzed WUS vs nonthrombolyzed WUS | CT ASPECTS and CTP to select patients for IV tPA |
Abbreviations: WUS, wake-up stroke; RCT, randomized controlled trial; CT, computed tomography; CTP, CT perfusion; MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging; PWI, perfusion-weighted imaging; ASPECTS, Alberta Stroke Program Early CT Score; rNIHSS, retrospective National Institutes of Health Stroke Scale; BP, blood pressure; AF, atrial fibrillation.
Pathophysiologic Hypotheses
The wake-up stroke phenomenon is incompletely understood pathophysiologically. What seems clear, though, is that wake-up strokes are not actuarial quirks of evenly spread stroke risk through the course of a day but likely the result of circadian changes in coagulability, serum catecholamine levels, and autonomic tone. Much like cardiac events,30 there is a preponderance of strokes of all subtypes in the morning as compared to evening onset.11,31 Several homeostatic and structural factors may contribute to this phenomenon. Proposed factors include sleep-disordered breathing with or without patent foramen ovale,32–34 overnight changes in autonomic tone affecting blood pressure with morning surges,35,36 morning increases in platelet aggregation37,38 relatively refractory to clopidogrel,39 endothelial dysfunction,40 blood viscosity,41 and fluctuating prothrombotic/fibrinolytic factor level balance.42–44 The circadian blood pressure-related changes behind the morning “surges,” which essentially mirror stroke incidence through the course of a day, are a tempting therapeutic target. Given the preponderance of strokes of all types between 0600 and 1200,31 a treatment trial targeting morning blood pressure changes did not change the distribution of strokes through the course of a day.45 Lending credence to the contribution of overnight paroxysms of atrial fibrillation,46 a recent study demonstrated a significant association between wake-up stroke and a new diagnosis of atrial fibrillation.47 Given the heterogeneity of wake-up stroke subtypes, it is likely that no one factor underlies wake-up stroke but some combination of the aforementioned and other yet undiscovered contributors (Table 2).
Table 2.
Proposed Pathophysiologic Mechanisms of Wake-Up Stroke.
| Structural | |
| Homeostatic | |
| Serological |
Abbreviation: PFO, patent foramen ovale.
Neuroimaging and Wake-Up Stroke
The key feature of wake-up stroke that makes it a therapeutic dilemma is the absence of distinct time of symptom onset, which limits the ability to establish eligibility for acute reperfusion therapies. Diagnostic neuroimaging in a patient with wake-up stroke thereby plays an even stronger role than usual in acute stroke evaluations. Several studies have been conducted to evaluate neuroimaging modalities as a surrogate marker of cerebral ischemia and the so-called “tissue clock” to supplant the absent time of onset that starts the strictly clinical “time clock.”
Several noncontrast computed tomography (CT)-based studies compared early ischemic changes on CT between wake-up stroke and stroke of known onset. Overall, there was no significant difference in early CT changes between wake-up stroke and stroke of known onset within 3 hours25,28 or 6 hours.26 These results suggest that the ischemic insult may occur shortly before or at the time of awakening in the absence of early ischemic change.
Perfusion and volume-based imaging with magnetic resonance (MR) or CT-based studies provide more granular physiologic data than noncontrast CT for acute stroke (see Table 3). These advanced neuroimaging techniques estimate the volume of brain tissue potentially at risk for progression to infarction (ie, ischemic penumbra) if recanalization does not occur. The volumetric difference between a surrogate for established infarction and penumbra, if present, is referred to as a “mismatch” and represents a rational biomarker for treatment selection (see Figure 1). Studies of advanced neuroimaging techniques have been conducted in patients with wake-up stroke. An MR-based study of diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI), imaging surrogates of ischemic, and “at-risk” tissue, respectively, found that patients with wake-up stroke and stroke of known onset had similar DWI and PWI lesion volumes as well as a similarly high proportion of DWI-PWI mismatch.52 A similarly designed CT-based study of mismatch between cerebral blood volume (like DWI, the estimate of infarcted tissue) and cerebral blood flow (like PWI, the at-risk tissue) found no difference between the percentage of mismatch between patients with wake-up stroke and stroke of known onset within a therapeutic window.20
Table 3.
Wake-Up Stroke Imaging Modalities.
| Parenchymal imaging | CT | Noncontrast CT | Screen for early ischemic changes | Time to ischemic change: variable; with MCA occlusion as little as 1 hour48 but often 3+ hours |
| Perfusion CT CBV—total volume of blood in a given volume of brain (mL/100 g) CBF—total volume of blood moving through a given volume of brain (mL/100 g) MTT—the average transit time of blood through a brain region TTP—time from contrast arrival-to-peak-intensity flow through a brain region | Screen for infarct/penumbra mismatch (see Figure 1) | Time to ischemic change: variable, depends on patient hemodynamics and CBV threshold for “infarct,” but is typically abnormal before clear signs on noncontrast CT | ||
| MRI | Routine MRI | Screen for infarct volume, DWI/FLAIR mismatch (see Figure 2) | Time to ischemic change: DWI—3 minutes49; T2WI—1-4 hours50; FLAIR—3-6 hours51 | |
| Perfusion (PWI) MRI CBV, CBF, and MTT as defined previously are the basic sequences of a PWI protocol | Screen for infarct/penumbra mismatch | |||
| Vascular imaging | CT | CT angiography | Screen for large artery occlusion | |
| MRI | MR angiography | |||
| Ultrasound | Complete neurosonology (carotid duplex ultrasonography plus transcranial Doppler) |
Abbreviations: CT, computed tomography; CBV, cerebral blood volume; CBF, cerebral blood flow; MTT, mean transit time; TTP, time to peak; MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging; PWI, perfusion-weighted imaging; FLAIR, fluid attenuated inversion recovery.
Figure 1.
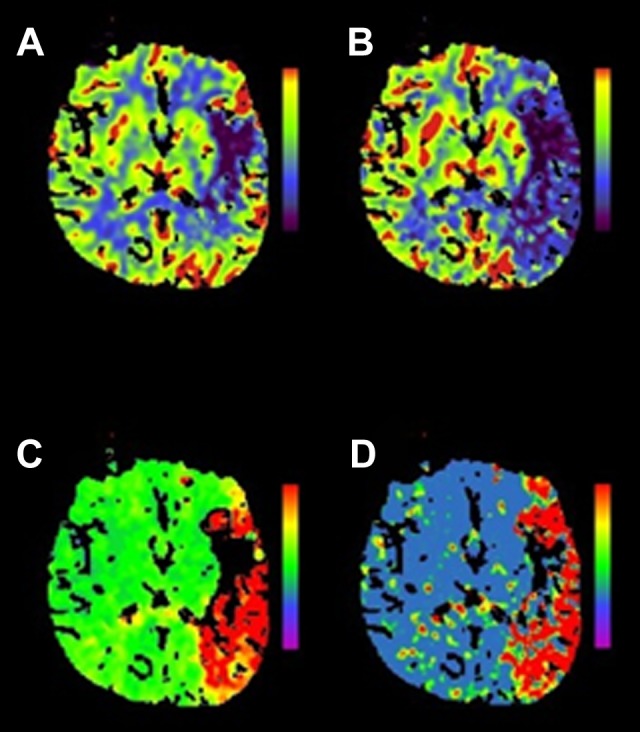
Multimodal CT mismatch (or “penumbra”). Panel (A) is a cerebral blood volume (CBV) map. The dark area noted in the left frontal operculum suggests low contrast volume in the region and is considered a surrogate for infarcted tissue, or the “infarct core.” The other maps—cerebral blood flow (CBF) in panel (B), time to peak (TTP) in panel (C), and mean transit time (MTT) in panel (D)—are different measures of contrast movement through cerebral vasculature (see Table 3) and clearly involve much more of the left hemisphere than the CBV map. This discordance is referred to as a multimodal CT mismatch or “penumbra” and may represent tissue at risk of infarction but potentially salvageable by reperfusion therapy. Siemens SOMATOM, syngo perfusion software. CT indicates computed tomography.
Another interesting mismatch approach utilizes DWI and fluid attenuated inversion recovery (FLAIR) sequences of magnetic resonance imaging (MRI) to identify infarcted versus at-risk tissue. Both sequences detect cerebral water changes but in different time sequences (see Figure 2). The DWI sequence is very sensitive to early cerebral water changes; however, the abnormalities noted do not change once they appear after the first several minutes of ischemia, so exact timing of an ischemic injury cannot be made with DWI. The T2-based FLAIR sequence measures the accumulation of cerebral edema as the infarction process proceeds. Thus, in principle, the presence of a DWI lesion and absence of a matched FLAIR abnormality should represent a relatively early infarct. This idea has been studied in several single-center51,53–56 pilots and a multicenter investigation.57 Overall, the DWI-FLAIR mismatch was found to very accurately identify ischemic tissue beyond 3 to 6 hours and can identify ischemia within the 3- to 4.5-hour window with excellent specificity. The large multicenter study of 543 patients supported the findings of the single-center studies, with DWI-FLAIR mismatch identified in patients within 4.5 hours of stroke onset with a sensitivity of only 62% but a good specificity of 81%, although interrater agreement was less than ideal (κ = .569). A recent publication sought to increase interrater reliability of DWI-FLAIR mismatch identification by color-coding FLAIR intensity and did just that with a roughly 10% increase in positive predictive value for both observers (85%-95% in one and 72%-82% in the other) once color coding was introduced.58 A recent small observational study noted a DWI-FLAIR mismatch in 44% of their patients with wake-up stroke.52 These findings supported the initiation of thrombolysis treatment trials based on the identification of a DWI-FLAIR mismatch, which are ongoing and will be discussed further in a subsequent section.
Figure 2.
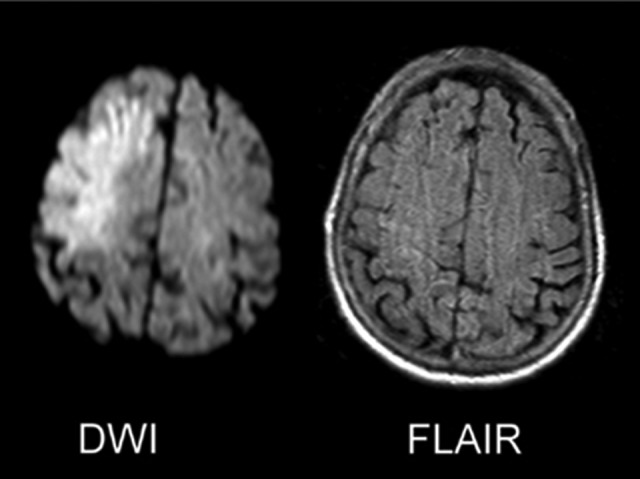
The DWI/FLAIR mismatch. These 2 axial images of the brain at a level just above the lateral ventricles represent the so-called DWI/FLAIR mismatch that can be seen in the early hours after symptom onset when DWI (left) hyperintensity—which can arise in minutes from symptom onset—occurs in the absence of T2-based FLAIR (right) hyperintensity, which takes 3 to 6 hours to develop. DWI indicates diffusion-weighted imaging; FLAIR, fluid attenuated inversion recovery.
Emergent neuroimaging is of heightened importance in the setting of wake-up stroke, given the absence of a clear time of symptom onset. Imaging modalities as simple and rapid as noncontrast CT and as sophisticated as multimodal CT and MRI show promise in identifying patients with acute ischemic stroke who may benefit from systemic thrombolysis. Ongoing randomized treatment trials based on these neuroimaging findings have practice-changing implications.
Treatment Evidence
Currently, there is a lack of a high-level evidence base to support any acute treatment in the setting of wake-up stroke. In the absence of high-level data, there are now many small studies of off-label use of acute reperfusion therapies for wake-up stroke employing various clinical and neuroimaging criteria for inclusion.
The majority of studies conducted so far are small, single-center observational studies of off-label intravenous tPA with or without endovascular reperfusion therapies.29,59–75 Largermulticenter studies,76 organized phase II studies,77 and randomized placebo-controlled treatment trials78–80 are fewer in number. Of note, the only large, multicenter, randomized placebo-controlled trial studied a glycoprotein IIb/IIIa inhibitor (abciximab) as an adjunct to intravenous tPA and patients with wake-up stroke made up only a small subgroup of the cohort. The individual characteristics and levels of evidence are detailed in Table 4.
Table 4.
Wake-Up Stroke Treatment Studies and Level of Evidence.
| Authors | Study Design | Clinical Inclusion Criteria | Imaging Inclusion Criteria | # Patients With Wake- Up Stroke (Treated) | # Control Patients | Treatment Type | Mean Door to Treatment in WUS | Mean/Median NIHSS of Treated Patients (Control) | Mean Age of Treated Patients (Control) | sICH in WUS, % (Control) | mRS 0-1, % (Control) | mRS 0-2, % (Control) | Level of Evidencea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iosif et al60 | Case report | “Admitted to hospital rapidly after waking up with stroke” | MRI DWI/PWI and DWI/FLAIR mismatch, MRA occlusion | 2 (2) | 0 | IAT | – | 15.5 | 45 | 50 | At d/c: 100 | – | Class IIb level C |
| Kuruvilla et al71 | Case report | – | CT | 2 (2) | – | IAT | – | 16 | 33 | 0 | – | – | Class IIa level C |
| Bracco et al74 | Case report | – | Multimodal CT | 1 (1) | – | IAT | – | 12 | 74 | 0 | – | – | Class IIb level C |
| Sung and Lee75 | Retrospective review | – | – | 10 | – | IAT | 168 min | 19 | – | 0 | – | 90 d: 20 | Class IIb level C |
| Stampfl et al69 | Retrospective review | WUS, NIHSS ≥10 | CTA occlusion, multimodal MRI mismatch (DWI/PWI) | 19 | – | IAT | – | 11 | 73.7 | 21.1 | – | 10.5 | Class IIb level C |
| Jung et al70 | Retrospective review | NIHSS >4, symptoms <24 h but >6 h from last normal | Multimodal MRI mismatch (DWI/PWI) | 55 (55) | 804 | IAT | – | 16.8 (16.8) | 61.9 (62.6) | 3.7 (6) | 90 d: 16.7 (23.3) | 90 d: 37 (39.7) | Class IIa level C |
| Natarajan et al68 | Retrospective review | 7-23 h from last normal, WUS, mRS ≤1, NIHSS 5-22 | CT ASPECTS, multimodal CT mismatch | 30 (30) | – | IAT | – | 13 | 72 | 10 | – | 20 | Class IIb level C |
| Natarajan et al72 | Retrospective review | WUS within 12 h of noticing symptoms, NIHSS >8 | Multimodal CT mismatch, CTA occlusion | 25 (25) | – | IAT | – | – | – | 14.3b | – | 42.9b | Class IIb level C |
| Barreto et al59 , c | Retrospective review | Major deficit from stroke, neurologically normal prior to stroke | No EIC in >1/3 of vascular territory | 80 (46) | 34/174 | IV tPA, IAT, or both | 2.4 h/1.2 h | 16 (10.5/11) | 62 (64/65) | 4.3 (0/2.9) | At d/c: 14 (6/48) | At d/c: 28 (13/48) | Class IIb level C |
| Cho et al61 | Retrospective review | Present within 6 hours of symptom recognition | Multimodal CT or MRI mismatch | 26 (26) | 223 | IV tPA, IAT or both | 154 min (90 min) | 14.5 (13) | 67.1 (65.8) | 6.3 (5.8) | 90 d: 37.5 (35) | 90 d: 50 (49.3) | Class IIa level C |
| Breuer et al62 | Retrospective review | Present within 6 hours of symptom recognition | Multimodal MRI mismatch | 45 (10) | 35 | IV tPA | – | – | – | 0 (0) | 90 d: 30 (31) | 90 d: 50 (60) | Class IIb level C |
| Kim et al63 | Retrospective review | Present within 3 hours of symptom recognition | CT ASPECTS, multimodal MRI mismatch | 26 (-) | 49 | IV tPA, IAT or both | – | 13 (12) | 67 (72) | 10.3 (8.2) | 90 d: 27.6 (4.1) | 90 d: 44.8 (14.3) | Class IIa level C |
| Manawadu et al73 | Retrospective case-control | WUS, last normal <12 hours but >4.5 hours | CT ASPECTS, multimodal CT mismatch | 122 (68) | 54 | IV tPA | – | 11.5 (9) | 73.9 (70.6) | 2.9 (0) | 90 d: 16.2 (9.3) | 90 d: 36.8 (25.9) | Class IIa level C |
| Aoki et al64 | Prospective cohort | Unknown onset stroke, “last known normal not consistent with first found abnormal” | MRI DWI/FLAIR mismatch | 4 (4) | – | IV tPA | 1 hour | 15.5 | 73.25 | 0 | 90 d: 25 | 90 d: 25 | Class IIb level C |
| Ebinger et al54,65 | Trial substudy, observational cohort | European guideline, “disregarding the contraindication of unknown time of onset” | Multimodal MRI mismatch | 13 (13) | 131 | IV tPA | 86 mind (60 min) | 13d | 81d | 0 (3.1) | 90 d: 29.4d (38.9) | 90 d: 35.3d (49.6) | Class IIb level C |
| Manawadu et al66 | Prospective case-control | WUS, last normal <12 hours but >4.5 hours vs controls within 4.5 hours symptom onset | CT ASPECTS | 68 (68) | 326 | IV tPA | 73 min (60 min) | 12 (13) | 73.9 (72.8) | 2.9 (3.4) | 90 d: 16 (24) | 90 d: 37 (38) | Class IIa level C |
| Bai et al67 | Prospective case—control | WUS and all patients with ischemic stroke within 12 h of symptom onset | Multimodal MRI mismatch (DWI/FLAIR) | 68 (48) | 172 | IV tPA | – | – | – | 2 (2) | 90 d: 77 (76) | – | Class IIa level C |
| Kang et al76 | Prospective multicenter observational | Last normal and symptom awareness times discordant, in emergency department within 6 hours of symptom awareness | Multimodal MRI mismatch (DWI/PWI and DWI/FLAIR) | 63 (63) | 156 | IV tPA, IAT or both | 155 min | 14 (12) | 67 (70) | 3.2 (-) | 90 d: 28.6 (-) | 90 d: 46 (-) | Class IIa level B |
| Hill et al77 | Prospective observational | Last normal <12 hours, WUS, “disabling stroke” | Arterial occlusion, ASPECTS >5 | 89 (20) | – | IV tPA | 148 min (awakening to treatment) | 13 | 75 | 0 | 45 | – | Class IIa level C |
| Adams et al78,79 | Subanalysis of RCT | WUS within 3 hours of symptom awareness | CT | 43 (43; 22 tPA + abciximab, 21 tPA only) | 758 | IV tPA + abciximab or placebo | – | 10 (8) | 69.5 (68.9) | 18.2 (4.8) | – | 90 d: 9.3 (29.2) | Class III harm level B |
| Michel et al9,80 | Pilot RCT | Supratentorial stroke, too late for standard tPA but not more than 24 hours or WUS | Multimodal CT | 9 (4) | 5 | IV tPA | 109.5 min (113 min) | 17 (14.5) | 69.5 (49) | 0 (0) | -e | -e | Class IIa level C |
Abbreviations: sICH, symptomatic intracranial hemorrhage; mRS, modified Rankin Scale; CT, computed tomography; MRI, magnetic resonance imaging; CTP, CT perfusion; DWI, diffusion-weighted imaging; PWI, perfusion-weighted imaging; FLAIR, fluid attenuated inversion recovery; IAT, intra-arterial therapy; IV tPA, intravenous tissue plasminogen activator; d/c, discharge; ASPECTS, Alberta Stroke Program Early CT Score; WUS, wake-up stroke; EIC, early ischemic change; NIHSS, National Institutes of Health Stroke Scale; d, day.
aClassification schema from the American Heart Association/American Stroke Association.
bAnterior circulation WUS only.
cTwo control groups: control 1 = untreated WUS and control 2 = treated patients within 3 hours of known symptom onset (control 1/control 2).
dInvestigators combined WUS with unknown onset strokes, so exact proportions of WUS alone is unknown.
eMean mRS at 90 d was 1.5 in the treatment group, 3 in placebo.
Their inherently biased study designs, small numbers, and marked heterogeneity in inclusion criteria and reported results do not allow for much to be said about acute reperfusion therapy for wake-up stroke other than there seems to be a case for therapeutic optimism and large, randomized, placebo- and sham-controlled studies are justified. Ongoing trials include Efficacy and Safety of MRI-based thrombolysis in Wake Up Stroke (WAKE-UP),81,82 THrombolysis for Acute Wake-up and Unclear-onset Strokes With Alteplase at 0.6 mg/kg Trial (THAWS),83 Extending the Time for Thrombolysis in Emergency Neurological Deficits (EXTEND),84 A Phase IIa Safety Stusdy of Intravenous Thrombolysis With Alteplase in MRI-Selected Patients (MR WITNESS),85 Safety of Intravenous Thrombolysis for Wake Up Stroke,86 Diffusion-Weighted Imaging or Computerized Tomography Perfusion Assessment With Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention (DAWN),87 Safety of Intravenous Thrombolytics in Stroke on Awakening (SAIL-ON),88 and Wake Up Symptomatic Stroke in Acute Brain Ischemia (WASSABI; Table 5).89
Table 5.
Ongoing Wake-Up Stroke Treatment Trials.
Abbreviations: DAWN, Diffusion-Weighted Imaging or Computerized Tomography Perfusion Assessment With Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention; EXTEND, Extending the Time for Thrombolysis in Emergency Neurological Deficits; MR WITNESS, A Phase IIa Safety Study of Intravenous Thrombolysis With Alteplase in MRI-Selected Patients; SAIL-ON, Safety of Intravenous Thrombolytics in Stroke on Awakening; WASSABI, Wake Up Symptomatic Stroke in Acute Brain Ischemia; THAWS, THrombolysis for Acute Wake-up and Unclear-onset Strokes With Alteplase at 0.6 mg/kg Trial.
What to do With Wake-Up Stroke
Evaluation
Based on the studies mentioned previously and our own clinical experience, we recommend a wake-up stroke evaluation proceeds as any other acute stroke assessment, with as much clinical detail as possible, an NIHSS examination and basic laboratory studies according to guidelines. This should be followed by multiparametric CT or MRI with noninvasive angiography or fast-track neurosonology (eg, transcranial Doppler and carotid duplex ultrasonography)90–94 to screen for an infarction/at-risk mismatch and large arterial occlusion, respectively. Even if one is uncomfortable providing treatment recommendations based on the results given the lack of high-level supporting evidence, the diagnostics can serve to inform prognostication.
Treatment
Regarding treatment of wake-up stroke, the authors agree with the sentiment of the recent systematic review of treatment strategies in wake-up stroke95 that routine treatment of wake-up stroke cannot be offered based on available evidence. Readers are encouraged to participate in clinical trials so that we as stroke providers may know how best to treat our patients with wake-up stroke (Table 6).
Table 6.
Wake-Up Stroke Recommendations.
| Evaluation |
|
| Treatment |
|
Abbreviations: NIHSS, National Institutes of Health Stroke Scale; CT, computed tomography; AHA/ASA, American Heart Association/American Stroke Association; MRI, magnetic resonance imaging.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Saver JL. Time is brain—quantified. Stroke J Cereb Circ. 2006;37 (1):263–266. [DOI] [PubMed] [Google Scholar]
- 2. Meretoja A, Keshtkaran M, Saver JL, et al. Stroke thrombolysis: save a minute, save a day. Stroke J Cereb Circ. 2014;45 (4):1053–1058. [DOI] [PubMed] [Google Scholar]
- 3. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333 (24):1581–1588. [DOI] [PubMed] [Google Scholar]
- 4. Albers GW, Clark WM, Madden KP, Hamilton SA, Davis SM, Donnan GA. ATLANTIS trial: results for patients treated within 3 hours of stroke onset * editorial comment: results for patients treated within 3 hours of stroke onset. Stroke. 2002;33 (2):493–496. [DOI] [PubMed] [Google Scholar]
- 5. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359 (13):1317–1329. [DOI] [PubMed] [Google Scholar]
- 6. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363 (9411):768–774. [DOI] [PubMed] [Google Scholar]
- 7. Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA J Am Med Assoc. 2013;309 (23):2480–2488. [DOI] [PubMed] [Google Scholar]
- 8. California Acute Stroke Pilot Registry (CASPR) Investigators. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64 (4):654–659. [DOI] [PubMed] [Google Scholar]
- 9. Michel P, Odier C, Rutgers M, et al. The Acute STroke Registry and Analysis of Lausanne (ASTRAL): design and baseline analysis of an ischemic stroke registry including acute multimodal imaging. Stroke J Cereb Circ. 2010;41 (11):2491–2498. [DOI] [PubMed] [Google Scholar]
- 10. Tanimoto A, Mehndiratta P, Koo BB. Characteristics of wake-up stroke. J Stroke Cerebrovasc Dis. 2014;23 (6):1296–1299. [DOI] [PubMed] [Google Scholar]
- 11. Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke J Cereb Circ. 1989;20 (4):473–476. [DOI] [PubMed] [Google Scholar]
- 12. Ricci S, Celani MG, Vitali R, La Rosa F, Righetti E, Duca E. Diurnal and seasonal variations in the occurrence of stroke: a community-based study. Neuroepidemiology. 1992;11 (2):59–64. [DOI] [PubMed] [Google Scholar]
- 13. Lago A, Geffner D, Tembl J, Landete L, Valero C, Baquero M. Circadian variation in acute ischemic stroke: a hospital-based study. Stroke J Cereb Circ. 1998;29 (9):1873–1875. [DOI] [PubMed] [Google Scholar]
- 14. Bornstein NM, Gur AY, Fainshtein P, Korczyn AD. Stroke during sleep: epidemiological and clinical features. Cerebrovasc Dis Basel Switz. 1999;9 (6):320–322. [DOI] [PubMed] [Google Scholar]
- 15. Chaturvedi S, Adams HP, Woolson RF. Circadian variation in ischemic stroke subtypes. Stroke J Cereb Circ. 1999;30 (9):1792–1795. [DOI] [PubMed] [Google Scholar]
- 16. Serena J, Dávalos A, Segura T, Mostacero E, Castillo J. Stroke on awakening: looking for a more rational management. Cerebrovasc Dis Basel Switz. 2003;16 (2):128–133. [DOI] [PubMed] [Google Scholar]
- 17. Nadeau JO, Fang J, Kapral MK, Silver FL, Hill MD; Registry of the Canadian stroke network. Outcome after stroke upon awakening. Can J Neurol Sci J Can Sci Neurol. 2005;32 (2):232–236. [DOI] [PubMed] [Google Scholar]
- 18. Boode B, Welzen V, Franke C, van Oostenbrugge R. Estimating the number of stroke patients eligible for thrombolytic treatment if delay could be avoided. Cerebrovasc Dis Basel Switz. 2007;23 (4):294–298. [DOI] [PubMed] [Google Scholar]
- 19. Jiménez-Conde J, Ois A, Rodríguez-Campello A, Gomis M, Roquer J. Does sleep protect against ischemic stroke? Less frequent ischemic strokes but more severe ones. J Neurol. 2007;254 (6):782–788. [DOI] [PubMed] [Google Scholar]
- 20. Silva GS, Lima FO, Camargo ECS, et al. Wake-up stroke: clinical and neuroimaging characteristics. Cerebrovasc Dis Basel Switz. 2010;29 (4):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turin TC, Kita Y, Rumana N, et al. Wake-up stroke: incidence, risk factors and outcome of acute stroke during sleep in a Japanese population. Takashima Stroke Registry. 1988-2003. Eur Neurol. 2013;69 (6):354–359. [DOI] [PubMed] [Google Scholar]
- 22. Fink JN, Kumar S, Horkan C, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke J Cereb Circ. 2002;33 (4):988–993. [DOI] [PubMed] [Google Scholar]
- 23. Moradiya Y, Janjua N. Presentation and outcomes of “wake-up strokes” in a large randomized stroke trial: analysis of data from the international stroke trial. J Stroke Cerebrovasc Dis. 2013;22 (8):e286–e292. [DOI] [PubMed] [Google Scholar]
- 24. Koton S, Tanne D, Bornstein NM; NASIS Investigators. Ischemic stroke on awakening: patients’ characteristics, outcomes and potential for reperfusion therapy. Neuroepidemiology. 2012;39 (3-4):149–153. [DOI] [PubMed] [Google Scholar]
- 25. Todo K, Moriwaki H, Saito K, Tanaka M, Oe H, Naritomi H. Early CT findings in unknown-onset and wake-up strokes. Cerebrovasc Dis Basel Switz. 2006;21 (5-6):367–371. [DOI] [PubMed] [Google Scholar]
- 26. Huisa BN, Raman R, Ernstrom K, et al. Alberta Stroke Program Early CT Score (ASPECTS) in patients with wake-up stroke. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2010;19 (6):475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mackey J, Kleindorfer D, Sucharew H, et al. Population-based study of wake-up strokes. Neurology. 2011;76 (19):1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roveri L, La Gioia S, Ghidinelli C, Anzalone N, De Filippis C, Comi G. Wake-up stroke within 3 hours of symptom awareness: imaging and clinical features compared to standard recombinant tissue plasminogen activator treated stroke. J Stroke Cerebrovasc Dis. 2013;22 (6):703–708. [DOI] [PubMed] [Google Scholar]
- 29. Manawadu D, Bodla S, Keep J, Jarosz J, Kalra L. An observational study of thrombolysis outcomes in wake-up ischemic stroke patients. Stroke. 2013;44 (2):427–431. [DOI] [PubMed] [Google Scholar]
- 30. Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79 (11):1512–1516. [DOI] [PubMed] [Google Scholar]
- 31. Elliott WJ. Circadian Variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29 (5):992–996. [DOI] [PubMed] [Google Scholar]
- 32. Pizza F, Biallas M, Kallweit U, Wolf M, Bassetti CL. Cerebral hemodynamic changes in stroke during sleep-disordered breathing. Stroke J Cereb Circ. 2012;43 (7):1951–1953. [DOI] [PubMed] [Google Scholar]
- 33. Ozdemir O, Beletsky V, Hachinski V, Spence JD. Cerebrovascular events on awakening, patent foramen ovale and obstructive sleep apnea syndrome. J Neurol Sci. 2008;268 (1-2):193–194. [DOI] [PubMed] [Google Scholar]
- 34. Ciccone A, Proserpio P, Roccatagliata DV, et al. Wake-up stroke and TIA due to paradoxical embolism during long obstructive sleep apnoeas: a cross-sectional study. Thorax. 2013;68 (1):97–104. [DOI] [PubMed] [Google Scholar]
- 35. Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328 (5):303–307. [DOI] [PubMed] [Google Scholar]
- 36. Redon J. The normal circadian pattern of blood pressure: implications for treatment. Int J Clin Pract Suppl. 2004;(145):3–8. [DOI] [PubMed] [Google Scholar]
- 37. Musumeci V, Rosa S, Caruso A, Zuppi C, Zappacosta B, Tutinelli F. Abnormal diurnal changes in in-vivo platelet activation in patients with atherosclerotic diseases. Atherosclerosis. 1986;60 (3):231–236. [DOI] [PubMed] [Google Scholar]
- 38. Andrews NP, Gralnick HR, Merryman P, Vail M, Quyyumi AA. Mechanisms underlying the morning increase in platelet aggregation: a flow cytometry study. J Am Coll Cardiol. 1996;28 (7):1789–1795. [DOI] [PubMed] [Google Scholar]
- 39. Kozinski M, Bielis L, Wisniewska-Szmyt J, et al. Diurnal variation in platelet inhibition by clopidogrel. Platelets. 2011;22 (8):579–587. [DOI] [PubMed] [Google Scholar]
- 40. Otto ME. Early morning attenuation of endothelial function in healthy humans. Circulation. 2004;109 (21):2507–2510. [DOI] [PubMed] [Google Scholar]
- 41. Ehrly AM, Jung G. Circadian rhythm of human blood viscosity. Biorheology. 1973;10 (4):577–583. [DOI] [PubMed] [Google Scholar]
- 42. Soulban G, Labrecque G. Circadian rhythms of blood clotting time and coagulation factors II, VII, IX and X in rats. Life Sci. 1989;45 (25):2485–2489. [DOI] [PubMed] [Google Scholar]
- 43. Andreotti F, Davies GJ, Hackett DR, et al. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am J Cardiol. 1988;62 (9):635–257. [DOI] [PubMed] [Google Scholar]
- 44. Bremner WF, Sothern RB, Kanabrocki EL, et al. Relation between circadian patterns in levels of circulating lipoprotein(a), fibrinogen, platelets, and related lipid variables in men. Am Heart J. 2000;139 (1):164–173. [DOI] [PubMed] [Google Scholar]
- 45. Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289 (16):2073–2082. [DOI] [PubMed] [Google Scholar]
- 46. Deguchi Y, Amino M, Adachi K, et al. Circadian distribution of paroxysmal atrial fibrillation in patients with and without structural heart disease in untreated state. Ann Noninvasive Electrocardiol. 2009;14 (3):280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riccio PM, Klein FR, Pagani Cassara F, et al. Newly diagnosed atrial fibrillation linked to wake-up stroke and TIA: hypothetical implications. Neurology. 2013;80 (20):1834–1840. [DOI] [PubMed] [Google Scholar]
- 48. Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Correlation of early CT signs in the deep middle cerebral artery territories with angiographically confirmed site of arterial occlusion. AJNR Am J Neuroradiol. 2001;22 (4):654–659. [PMC free article] [PubMed] [Google Scholar]
- 49. Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol. 1990;11 (3):423–429. [PMC free article] [PubMed] [Google Scholar]
- 50. Venkatesan R, Lin W, Gurleyik K, et al. Absolute measurements of water content using magnetic resonance imaging: preliminary findings in an in vivo focal ischemic rat model. Magn Reson Med. 2000;43 (1):146–150. [DOI] [PubMed] [Google Scholar]
- 51. Thomalla G, Rossbach P, Rosenkranz M, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65 (6):724–732. [DOI] [PubMed] [Google Scholar]
- 52. Huisa BN, Liebeskind DS, Raman R, et al. Diffusion-weighted imaging-fluid attenuated inversion recovery mismatch in nocturnal stroke patients with unknown time of onset. J Stroke Cerebrovasc Dis. 2013;22 (7):972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aoki J, Kimura K, Iguchi Y, Shibazaki K, Sakai K, Iwanaga T. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293 (1-2):39–44. [DOI] [PubMed] [Google Scholar]
- 54. Ebinger M, Galinovic I, Rozanski M, Brunecker P, Endres M, Fiebach JB. Fluid-attenuated inversion recovery evolution within 12 hours from stroke onset: a reliable tissue clock? Stroke. 2010;41 (2):250–255. [DOI] [PubMed] [Google Scholar]
- 55. Petkova M, Rodrigo S, Lamy C, et al. MR imaging helps predict time from symptom onset in patients with acute stroke: implications for patients with unknown onset time. Radiology. 2010;257 (3):782–92. [DOI] [PubMed] [Google Scholar]
- 56. Emeriau S, Serre I, Toubas O, Pombourcq F, Oppenheim C, Pierot L. Can diffusion-weighted imaging-fluid-attenuated inversion recovery mismatch (positive diffusion-weighted imaging/negative fluid-attenuated inversion recovery) at 3 tesla identify patients with stroke at <4.5 hours? Stroke. 2013;44 (6):1647–1651. [DOI] [PubMed] [Google Scholar]
- 57. Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10 (11):978–986. [DOI] [PubMed] [Google Scholar]
- 58. Kim BJ, Kim Y-H, Kim Y-J, et al. Color-coded fluid-attenuated inversion recovery images improve inter-rater reliability of fluid-attenuated inversion recovery signal changes within acute diffusion-weighted image lesions. Stroke. 2014;45 (9):2801–2804. [DOI] [PubMed] [Google Scholar]
- 59. Barreto AD, Martin-Schild S, Hallevi H, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40 (3):827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iosif C, Oppenheim C, Trystram D, Domigo V, Meder J-F. MR Imaging-based decision in thrombolytic therapy for stroke on awakening: report of 2 cases. Am J Neuroradiol. 2008;29 (7):1314–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cho A-H, Sohn S-I, Han M-K, et al. Safety and efficacy of MRI-based thrombolysis in unclear-onset stroke. Cerebrovasc Dis. 2008;25 (6):572–579. [DOI] [PubMed] [Google Scholar]
- 62. Breuer L, Schellinger PD, Huttner HB, et al. Feasibility and safety of magnetic resonance imaging-based thrombolysis in patients with stroke on awakening: initial single-centre experience. Int J Stroke. 2010;5 (2):68–73. [DOI] [PubMed] [Google Scholar]
- 63. Kim J-T, Park M-S, Nam T-S, et al. Thrombolysis as a factor associated with favorable outcomes in patients with unclear-onset stroke: thrombolysis in unclear-onset stroke. Eur J Neurol. 2011;18 (7):988–994. [DOI] [PubMed] [Google Scholar]
- 64. Aoki J, Kimura K, Iguchi Y, et al. Intravenous thrombolysis based on diffusion-weighted imaging and fluid-attenuated inversion recovery mismatch in acute stroke patients with unknown onset time. Cerebrovasc Dis. 2011;31 (5):435–441. [DOI] [PubMed] [Google Scholar]
- 65. Ebinger M, Scheitz JF, Kufner A, Endres M, Fiebach JB, Nolte CH. MRI-based intravenous thrombolysis in stroke patients with unknown time of symptom onset: MRI-based thrombolysis in stroke of unknown onset. Eur J Neurol. 2012;19 (2):348–350. [DOI] [PubMed] [Google Scholar]
- 66. Manawadu D, Bodla S, Jarosz J, Keep J, Kalra L. A case-controlled comparison of thrombolysis outcomes between wake-up and known time of onset ischemic stroke patients. Stroke. 2013;44 (8):2226–2231. [DOI] [PubMed] [Google Scholar]
- 67. Bai Q, Zhao Z, Fu P, et al. Clinical outcomes of fast MRI-based thrombolysis in wake-up strokes compared to superacute ischemic strokes within 12 hours. Neurol Res. 2013;35 (5):492–497. [DOI] [PubMed] [Google Scholar]
- 68. Natarajan SK, Snyder KV, Siddiqui AH, Ionita CC, Hopkins LN, Levy EI. Safety and effectiveness of endovascular therapy after 8 hours of acute ischemic stroke onset and wake-up strokes. Stroke. 2009;40 (10):3269–3274. [DOI] [PubMed] [Google Scholar]
- 69. Stampfl S, Ringleb PA, Haehnel S, et al. Recanalization with stent-retriever devices in patients with wake-up stroke. Am J Neuroradiol. 2013;34 (5):1040–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jung S, Gralla J, Fischer U, et al. Safety of endovascular treatment beyond the 6-h time window in 205 patients. Eur J Neurol. 2013;20 (6):865–871. [DOI] [PubMed] [Google Scholar]
- 71. Kuruvilla A, Norris GM, Xavier AR. Acute endovascular recanalization therapy in wake-up stroke. J Neurol Sci. 2011;300 (1-2):148–150. [DOI] [PubMed] [Google Scholar]
- 72. Natarajan SK, Karmon Y, Snyder KV, et al. Prospective acute ischemic stroke outcomes after endovascular therapy: a real-world experience. World Neurosurg. 2010;74 (4-5):455–464. [DOI] [PubMed] [Google Scholar]
- 73. Manawadu D, Bodla S, Keep J, Kalra L. Influence of age on thrombolysis outcome in wake-up stroke. Stroke. 2013;44 (10):2898–2900. [DOI] [PubMed] [Google Scholar]
- 74. Bracco S, Tassi R, Gennari P, et al. Wake-up (or wake-up for) stroke: a treatable stroke. Neuroradiol J. 2013;26 (5):573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sung S, Lee T. E-070 acute recanalization with stenting for wake-up stroke. J Neurointerv Surg. 2014;6 (suppl 1):A71–A71. [Google Scholar]
- 76. Kang D-W, Sohn S-I, Hong K-S, et al. Reperfusion Therapy in Unclear-Onset Stroke Based on MRI Evaluation (RESTORE): a prospective multicenter study. Stroke. 2012;43 (12):3278–3283. [DOI] [PubMed] [Google Scholar]
- 77. Hill MD, Kenney C, Dzialowski I, et al. Tissue Window in Stroke Thrombolysis study (TWIST): a safety study. Can J Neurol Sci J Can Sci Neurol. 2013;40 (1):17–20. [DOI] [PubMed] [Google Scholar]
- 78. Adams HP, Effron MB, Torner J, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: abciximab in Emergency Treatment of Stroke Trial (AbESTT-II). Stroke J Cereb Circ. 2008;39 (1):87–99. [DOI] [PubMed] [Google Scholar]
- 79. Adams HP, Leira EC, Torner JC, et al. Treating patients with “wake-up” stroke: the experience of the AbESTT-II trial. Stroke. 2008;39 (12):3277–3282. [DOI] [PubMed] [Google Scholar]
- 80. Michel P, Ntaios G, Reichhart M, et al. Perfusion-CT guided intravenous thrombolysis in patients with unknown-onset stroke: a randomized, double-blind, placebo-controlled, pilot feasibility trial. Neuroradiology. 2012;54 (6):579–588. [DOI] [PubMed] [Google Scholar]
- 81. Thomalla G, Fiebach JB, Østergaard L, et al. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP): protocols. Int J Stroke. 2014;9 (6):829–836. [DOI] [PubMed] [Google Scholar]
- 82. Thomalla G, Ebinger M, Fiehler J, Fiebach JB, Endres M, Gerloff C. EU-funded treatment study: WAKE-UP: a randomized, placebo-controlled MRI-based trial of thrombolysis in wake-up stroke [in German]. Nervenarzt. 2012;83 (10):1241–1251. [DOI] [PubMed] [Google Scholar]
- 83. Koga M, Toyoda K, Kimura K, et al. THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0·6 mg/kg (THAWS) trial. Int J Stroke. 2014;9 (8):1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ma H, Parsons MW, Christensen S, et al. A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND). Int J Stroke. 2012;7 (1):74–80. [DOI] [PubMed] [Google Scholar]
- 85. MR WITNESS: a study of intravenous thrombolysis with alteplase in MRI-selected patients [Internet]; 2014. Web site http://www.clinicaltrials.gov/show/NCT01282242. Accessed October 9, 2014.
- 86. Safety of Intravenous Thrombolysis for Wake-up Stroke [Internet]; 2014. Web site http://clinicaltrials.gov/ct2/show/NCT01183533. Accessed October 9, 2014.
- 87. Trevo and Medical Management Versus Medical Management Alone in Wake Up and Late Presenting Strokes (DAWN) [Internet]; 2014. Web site http://clinicaltrials.gov/ct2/show/NCT02142283.
- 88. Safety of Intravenous Thrombolytics in Stroke on Awakening (SAIL-ON) [Internet]; 2014. Web site http://clinicaltrials.gov/ct2/show/NCT01643902.
- 89. Wake up Symptomatic Stroke in Acute Brain Ischemia (WASSABI) Trial [Internet]; 2014. Web site http://clinicaltrials.gov/ct2/show/NCT01455935.
- 90. Chernyshev OY, Garami Z, Calleja S, et al. Yield and accuracy of urgent combined carotid/transcranial ultrasound testing in acute cerebral ischemia. Stroke. 2005;36 (1):32–37. [DOI] [PubMed] [Google Scholar]
- 91. Chambers BR, Merory JR, Smidt V. Doppler diagnosis in cases of acute stroke. Med J Aust. 1989;150 (7):382–384. [DOI] [PubMed] [Google Scholar]
- 92. Camerlingo M, Casto L, Censori B, Ferraro B, Gazzaniga GC, Mamoli A. Transcranial Doppler in acute ischemic stroke of the middle cerebral artery territories. Acta Neurol Scand. 1993;88 (2):108–111. [DOI] [PubMed] [Google Scholar]
- 93. Lee DH, Gao FQ, Rankin RN, Pelz DM, Fox AJ. Duplex and color Doppler flow sonography of occlusion and near occlusion of the carotid artery. AJNR Am J Neuroradiol. 1996;17 (7):1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 94. Alexandrov AV, Demchuk AM, Wein TH, Grotta JC. Yield of transcranial Doppler in acute cerebral ischemia. Stroke J Cereb Circ. 1999;30 (8):1604–1609. [DOI] [PubMed] [Google Scholar]
- 95. Buck D, Shaw LC, Price CI, Ford GA. Reperfusion therapies for wake-up stroke: systematic review. Stroke. 2014;45 (6):1869–1875. [DOI] [PubMed] [Google Scholar]
- 96. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44 (3):870–947. [DOI] [PubMed] [Google Scholar]


