Abstract
Data from randomized clinical trials have supported the safety and efficacy of intravenous tissue-type plasminogen activator (IV tPA) for acute ischemic stroke when administered within 3 hours of symptom onset, and regulatory approvals for this indication have been in place for almost 20 years. However, recent clinical trials have provided evidence that IV tPA may be safe and effective in selected patients up to 4.5 hours after symptom onset, thereby increasing the proportion of patients that may be eligible for treatment. Although professional organizations in the United States and many regulatory agencies internationally have supported this expanded time window for IV tPA, the US Food and Drug Administration has declined to approve this expanded indication and so this use of IV tPA has remained off-label in the United States. Here we review the current evidence for IV tPA in the standard and the expanded time windows and the data on current clinical practice in the United States as it relates to IV tPA treatment for acute stroke within 3 to 4.5 hours of symptom onset.
Keywords: stroke and cerebrovascular disease, clinical specialty, stroke, cerebrovascular disorders, clinical trials, techniques
Introduction
Thrombolysis with intravenous tissue-type plasminogen activator (IV tPA) is the only approved treatment for patients with acute ischemic stroke (AIS). Although the proportion of patients with AIS treated with IV tPA has steadily increased since it was first approved in 1996, the treatment rate is still low—only 3.4% to 5.2% of all patients with AIS in the United States receive IV tPA.1 Delays in activating emergency medical services, suboptimal hospital infrastructure and processes, medical contraindications, and the narrow therapeutic time window all contribute to this low treatment rate.2 Recent efforts to increase the treatment rate for IV tPA include expanding access to stroke centers through telestroke networks and developing quality metrics and reimbursement incentives to encourage administering IV tPA to all eligible patients with stroke who present within 2 hours of symptom onset.3 Single-center studies have shown that the development of telestroke networks is associated with increased IV tPA treatment rates (14%-15.5%)4,5 and fewer hospital transfers (from 44% to 19%, P < .001).4 As of 2012, there were at least 56 telestroke programs in the United States.6
Another potential target for increasing treatment rates would be to expand the therapeutic time window. The US Food and Drug Administration (FDA) approved IV tPA for treating AIS within 3 hours of symptom onset in 1996. However, numerous studies have been conducted to evaluate the efficacy and safety of extending the time window beyond 3 hours, including the pivotal European Cooperative Acute Stroke Study III (ECASS-III),7 which directly led to regulatory approvals for an expanded treatment time window of <4.5 hours in Europe and many other countries (Figure 1). The US FDA did not approve expanding the labeled indication for IV tPA to include this extended time window, though major professional organizations, such as the American Heart Association/American Stroke Association (AHA/ASA) and American Academy of Neurology, have issued scientific advisories that endorse this extended time window.8
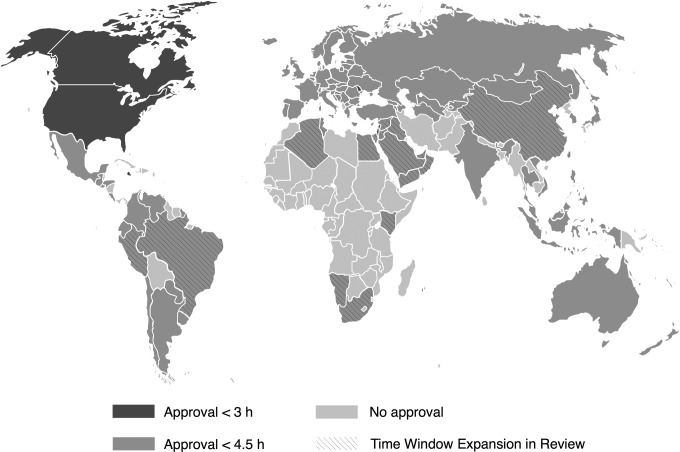
Figure 1.
World map of countries with IV tPA approval in the 3- to 4.5-hour window as of January 20, 2014 (courtesy of Peter Schillinger and Boeringer-Ingelheim).
Here we review the study design and the results of the major clinical trials that established the original 3-hour time window for IV tPA as well as the state of current evidence for the use of IV tPA within an extended time window of up to 4.5 hours after symptom onset (Figure 2; Table 1).
Figure 2.
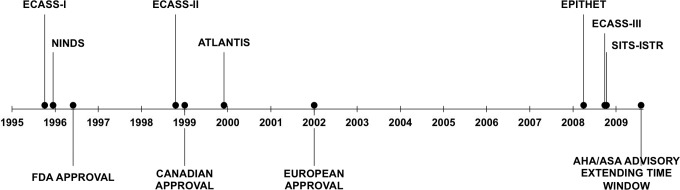
Time line of major IV tPA trials and regulatory approvals. ECASS indicates European Cooperative Acute Stroke Study; NINDS, National Institute of Neurological Disorders and Stroke tPA Trial; ATLANTIS, Alteplase ThromboLysis for Acute Noninterventional Therapy in Ischemic Stroke; EPITHET, Echoplanar Imaging Thrombolytic Evaluation Trial; SITS-ISTR, Safe Implementation of Thrombolysis in Stroke—International Stroke Treatment Registry; FDA, Food and Drug Administration; AHA/ASA, American Heart Association/American Stroke Association.
Table 1.
Summary of Major Clinical Trials of IV tPA for Acute Ischemic Stroke.
| Trial (Year) | tPA Dose | Time Window, h | Outcome Measures | Number of Patients | Results | Notes |
|---|---|---|---|---|---|---|
| ECASS I (1995)9 | 1.1 mg/kg | ≤6 | Barthel index and mRS at 90 days | 620 | No significant difference in ITT analysis. Significant increase in large ICH in tPA group. | High rate of protocol violations (17.4% of patients) |
| NINDS tPA Trial (1995)10 | 0.9 mg/kg | ≤3 | Part 1—improvement in NIHSS by ≥4 points or resolution of symptoms within 24 hours of onset Part 2—Barthel index, mRS, GCS, and NIHSS at 3 months | 624 | Part 1—no significant difference between placebo and tPA Part 2—significant improvement in BI, mRS, GOS, and NIHSS for tPA group 6.4% vs 0.6% rate of symptomatic ICH in tPA vs placebo. No difference in mortality | First trial demonstrating the efficacy of IV tPA in improving neurologic outcome with an acceptable safety profile |
| ECASS II (1998)11 | 0.9 mg/kg | ≤6 | Favorable outcome (mRS 0 or 1) at 90 days | 800 | Stratified analyses of primary and secondary outcomes: no significant difference between IV tPA vs placebo in the 3-6-hour time window | Treatment effect attenuated since the median NIHSS was 11 compared to 14 in NINDS trial |
| ATLANTIS-part B (1999)12 | 0.9 mg/kg | 3-5 | Excellent neurologic recovery (NIHSS 0-1) at 90 days | 613 | No significant difference between tPA and placebo | Study stopped early due to slow recruitment. Almost 80% of patients were enrolled in the 4 to 5 hours interval |
| ECASS III (2008)7 | 0.9 mg/kg | 3-4.5 | Favorable outcome (mRS 0-1) at 90 days | 821 | tPA group had significant likelihood of favorable outcome (OR 1.42 [1.02-1.98]). Significantly higher rate of sICH in tPA group but no difference in mortality | Strength: enrollment was spread evenly over time window, large sample size |
| IST-3 (2012)13 | 0.9 mg/kg | ≤6 | Alive and independent (OHS 0-2) at 6 months | 3035 | No significant difference in primary outcome | Demonstrated possible benefit and safety of tPA in patients age >80 |
Abbreviations: ITT, intention to treat; mRS, modified Rankin scale; NIHSS, National Institute of Health Stroke Scale; GCS, Glasgow Coma Scale; OHS, Oxford Handicap Score; IST-3, third International Stroke Trial; BI, Barthel index; tPA, tissue-type plasminogen activator; ECASS-III, European Cooperative Acute Stroke Study III; NINDS, National Institute of Neurological Disorders and Stroke; ATLANTIS, Alteplase ThromboLysis for Acute Noninterventional Therapy in Ischemic Stroke; OR, odds ratio.
Establishing the 3-Hour Time Window
Development of tPA
The first study of thrombolysis for ischemic stroke was published in 1958.14 Experimental studies using fibrinolysin (also known as plasmin), streptokinase, and urokinase that were conducted in the 1960s and 1970s raised concerns about the risk of intracranial hemorrhage (ICH).15 In the mid-1980s, recombinant DNA technology was used to develop tPA, which was initially applied, as with other thrombolytics, to treat myocardial infarction. However, the development of tPA also reinvigorated interest in thrombolytic treatment of AIS. An initial phase I dose-escalating study enrolled patients in 2 arms based on time to treatment: within 90 minutes of symptom onset and between 91 and 180 minutes of symptom onset.16,17 These time windows were based on animal studies that demonstrated evidence of ischemic brain injury when arterial occlusion persisted beyond 2 to 3 hours.18 This study established that doses greater than 0.85 mg/kg increased the risk of ICH and that acute stroke evaluation and treatment could be accomplished within 90 minutes.16,17 These studies ultimately led to a phase III study to assess clinical efficacy and safety.
ECASS-I and National Institute of Neurological Disorders and Stroke tPA Trials
The first large randomized, multicenter, double-blind, placebo-controlled trial of thrombolysis for stroke was the first ECASS trial, published in 1995.9 This study was designed to evaluate the efficacy of IV tPA within 6 hours of symptom onset when compared to placebo in patients presenting with acute hemispheric ischemic stroke with moderate to severe neurologic deficits and, of note, without major early signs of infarct on computed tomography (CT). There was no significant difference in the 2 groups for the primary end point of functional outcome as measured by Barthel index (BI) and modified Rankin scale (mRS) at 90 days. Notably, there was a significantly higher rate of large intraparenchymal hemorrhage in the IV tPA group (6.3% vs 2.4% in placebo group). The ECASS authors pointed out that the failure to demonstrate a treatment effect could be attributed to major protocol violations that resulted in the incorporation of patients who did not meet the study’s CT inclusion criteria into the final analysis—a group that comprised 17.4% of the entire cohort. The study results were impacted by these widespread protocol violations and the inclusion of patients treated up to 6 hours after symptom onset.19,20
Two months after the ECASS study was published, the landmark National Institute of Neurological Disorders and Stroke (NINDS) tPA trial results were published10—the first randomized, double-blinded, controlled trial that demonstrated the clinical efficacy and safety of IV tPA for treating AIS. Patients were enrolled from 1991 to 1994. Part 1 of the study evaluated for evidence of clinical activity in the first 24 hours of symptom onset. The primary outcome was an improvement of 4 or more points in the National Institute of Health Stroke Scale (NIHSS) score or the resolution of the neurologic deficit. This was the first study to use improvement within 24 hours as a primary outcome measure, which some criticized as a premature measure of ultimate neurologic outcome.21 The primary outcome for part 2 was recovery with minimal or no deficit using a composite of BI, mRS, Glasgow outcome scale (GOS), and NIHSS at 90 days. The study demonstrated a significant difference for each outcome measure in favor of the IV tPA group. In terms of adverse outcomes, the rate of symptomatic ICH (sICH) was significantly higher in the IV tPA group (6.4% vs 0.6% in placebo group, P < .001), but there was no significant difference in 90-day mortality.10 Six months after the NINDS results were published, the FDA approved the use of IV tPA for AIS within 3 hours of symptom onset.
Extending the Time Window
European Cooperative Acute Stroke Study II
Since ECASS-I used a higher dose of IV tPA than the NINDS tPA trial and had numerous protocol violations, ECASS-II was designed to evaluate whether a lower tPA dose of 0.9 mg/kg would be efficacious in an extended time window of up to 6 hours.11 The basis of the study was that the per-protocol analysis of ECASS-I data did suggest that there was an improvement in functional outcomes in the IV tPA group with effect sizes that were comparable to the NINDS tPA trial. In addition to using a lower tPA dose, ECASS-II also used slightly modified CT exclusion criteria compared to ECASS-I: swelling exceeding 33% of the middle cerebral artery territory excluded patients from ECASS-II, whereas diffuse swelling of one entire brain hemisphere was required for exclusion in ECASS-I. The primary outcome was favorable outcome (mRS of 0 or 1) at 90 days. The results revealed a nonsignificant odds ratio (OR) of 1.2 (95% confidence interval [CI] 0.8-1.6) favoring the 3- to 6-hour window tPA group. There was a significantly higher rate of sICH in the IV tPA group overall (8.8% vs 3.4%), but no significant difference in the mortality rate between the treatment and the placebo groups for the 3- to 6-hour time window.11 Some have suggested that the observed treatment effect in ECASS-II was diminished because the strokes were less severe with a median NIHSS of 11 compared to 14 in the NINDS tPA trial.12
Alteplase ThromboLysis for Acute Noninterventional Therapy in Ischemic Stroke
Several studies to explore the possibility of extending the treatment time window followed (Table 1). The Alteplase ThromboLysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS) trial was a randomized, placebo-controlled, double-blind study that was originally designed to evaluate the efficacy and safety of IV tPA within 6 hours of symptom onset.12 Two years into enrollment, the study was suspended due to safety concerns for the 5- to 6-hour time window. The trial was restarted as part B, which was restricted to patients who received treatment between 3 and 5 hours of symptom onset. The primary outcome measure was excellent neurologic recovery (NIHSS 0 or 1) at 90 days. Secondary outcomes were excellent functional recovery (mRS, BI, and GOS) at 30 and 90 days. There was no significant difference in the primary outcome nor was there any significant treatment effect for any of the secondary outcomes. Of note, as was seen in the NINDS tPA trial, there was a significantly higher rate of both asymptomatic ICH and sICH in the tPA group but no difference in mortality rates at 30 or 90 days.12 The authors pointed out that overall milder stroke severity (median NIHSS 10 compared to 14 in the NINDS tPA trial) as well as later treatment times (nearly 80% of patients were enrolled in the 4- to 5-hour time interval) would serve to attenuate a potential treatment effect.
A pooled analysis of ATLANTIS, ECASS-I, and NINDS was published in 2004.22 This study showed that while the benefit of tPA, expressed as the OR of a favorable outcome, diminishes over time, a potential treatment benefit could be still seen through the 3- to 4.5-hour window (OR 1.4, 95% CI 1.1-1.9). Furthermore, the adjusted hazard ratio for death was not significantly worse in the extended time window compared to the standard 3-hour time window. These results suggested that extending the time window could safely provide a clinical benefit.
Echoplanar Imaging Thrombolytic Evaluation Trial
In 2008, a phase II randomized, double-blind, placebo-controlled trial that evaluated the use of IV tPA in the 3- to 6-hour window after symptom onset in select patients with perfusion–diffusion mismatch on magnetic resonance imaging (MRI) was published.23 The primary end point was infarct growth between the baseline diffusion-weighted MRI lesion and the MRI T2 lesion at day 90. The secondary end points were reperfusion, good neurological outcome (NIHSS 0 or 1 at 90 days or improvement of at least 8 points from baseline), and good functional outcome (mRS of 0-2 at 90 days). Mean infarct growth trended lower in the treatment group and reperfusion was significantly higher in the IV tPA group. Although there was a nonsignificant trend toward good neurological and functional outcomes in the treatment group, achieving reperfusion was associated with good neurological and functional outcomes.23 Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) suggested that in carefully selected patients, reperfusion after 3 hours might offer good neurological and functional outcomes, but the authors noted that as a phase II study it was relatively underpowered for establishing clinical efficacy.
European Cooperative Acute Stroke Study III
The third ECASS trial aimed to definitively answer the question of whether IV tPA in the 3- to 4.5-hour window was safe and effective. Like the previous 2 trials, ECASS-III was a phase III, randomized, placebo-controlled, double-blinded trial.7 A total of 821 patients were enrolled in the study. The initial study design involved a 3- to 4-hour time window, but after publication of the pooled analysis of data from ATLANTIS, ECASS-I, and NINDS which suggested that treatment up to 4.5 hours was safe,22 the trial protocol was extended by half an hour. Notably, major exclusion criteria were age >80, NIHSS >25, any oral anticoagulant use regardless of coagulation test results, and a history of prior stroke and diabetes. The primary end point was similar to ECASS-II: favorable outcome (mRS 0-1) at 90 days. In the adjusted analysis, the OR for favorable outcome was 1.42 (95% CI 1.02-1.98, P = .04), which is consistent with the notion that IV tPA in the extended time window is efficacious. The rate of sICH was significantly higher in the IV tPA group even when all commonly used definitions of sICH were applied. However, again there was no difference in the mortality rate, which suggested that IV tPA in the extended time window was safe (Figure 3).7
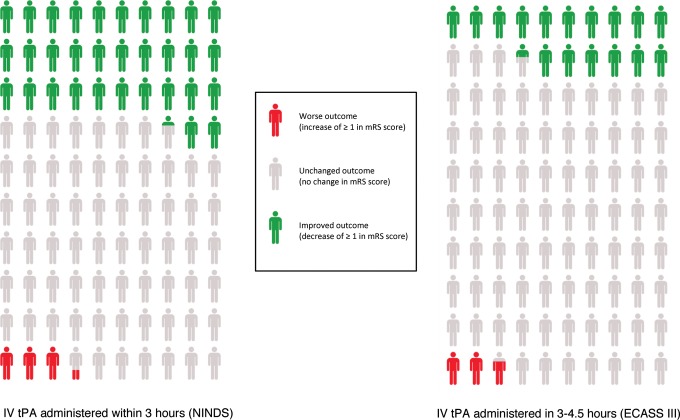
Figure 3.
Number needed to treat to benefit and harm per 100 patients treated with intravenous recombinant tissue-type plasminogen activator (IV tPA) for acute ischemic stroke in the <3-hour versus 3- to 4.5-hour time windows.24 mRS indicates modified Rankin scale; NINDS, National Institute of Neurologic Disorders and Stroke; ECASS-III, European Cooperative Acute Stroke Study-III.
Safe Implementation of Thrombolysis in Stroke-International Stroke Treatment Registry
After the approval of IV tPA in Europe, the European Medicines Evaluation Agency (EMEA) required the creation of a registry to monitor treatment outcomes. This was termed the Safe Implementation of Thrombolysis in Stroke (SITS) Monitoring Study. This data registry was analyzed as part of a prospective multinational study, the SITS-International Stroke Treatment Registry (SITS-ISTR).25 The authors hypothesized that there was no significant difference in outcomes for those treated with IV tPA within 3 to 4.5 hours after symptom onset and those treated within 3 hours. Nearly 2 weeks after the publication of ECASS-III, the SITS-ISTR group published their results: They reported no significant difference in rates of sICH, mortality, and functional independence (mRS < 2) at 3 months in patients treated within the 3-hour and the 3- to 4.5-hour time windows.
Updated Pooled Analysis
In 2010, the pooled analysis of ATLANTIS, ECASS-I, and NINDS conducted in 2004 was updated with data from ECASS-II, ECASS-III, and EPITHET.26 This analysis included a total of 3,670 patients and evaluated end points similar to those used in the original studies, including favorable outcome (mRS 0-1 or global outcome combining mRS, BI, and NIHSS score), sICH, and overall mortality. The treatment effect size decreased as the time from stroke onset increased: the odds of a favorable outcome were 1.34 (95% CI 1.06-1.68) for the 3- to 4.5-hour window and there was no benefit seen after 4.5 hours. Although the IV tPA group was significantly more likely to have sICH, there was no difference in mortality between the control and IV tPA groups for the 3- to 4.5-hour time window. This analysis suggested that there was some benefit of IV tPA in the extended time window but likely not past 4.5 hours.
Third International Stroke Trial
In response to the exclusion of older patients in prior stroke trials, the third International Stroke Trial (IST-3) was conducted to determine the benefits and harms of treating adults of all ages within the 6-hour time window. This international, multicenter, randomized, masked placebo trial was the largest stroke thrombolysis trial ever published with more than 3 times the number of participants as any prior trial. The primary outcome was defined as independence at 6 months as measured by an Oxford Handicap Score of 0 to 2. Of the 3035 patients enrolled, over half (53%) were older than 80 years of age. The study demonstrated a greater benefit for IV tPA in patients older than 80.13
Cochrane Review
In July 2014, a Cochrane review evaluated evidence regarding efficacy and safety of IV tPA, as well as other thrombolytics (70% of participants were in trials evaluating IV tPA), for patients with AIS. This review included data from 27 trials involving 10 187 patients. Thrombolytic therapy given up to 6 hours after onset of symptoms significantly reduced the likelihood of death or dependency (mRS 3-6) at 3 to 6 months after stroke (OR 0.85, 95% CI 0.78-0.93) while also significantly increasing the risk of sICH (OR 3.75, 95% CI 3.11-4.51) and death at 3 to 6 months (OR 1.18, 95% CI 1.06-1.30).27 Based on data from the EPITHET, IST-3, and NINDS trials, the authors found that patients older than 80 years of age benefited just as much as younger patients, with similar odds of death or dependency at the end of follow-up period for the elderly (OR 0.80, 95% CI 0.64-0.99) and younger groups (OR 0.85, 95% CI 0.76-0.95).
Response to ECASS-III and Impact of the Extended Time Window
The EMEA approved IV tPA for the 3- to 4.5-hour window in 2010. In contrast, the EMEA’s American counterpart, the FDA, denied Genentech application to extend the indication for IV tPA to include the extended time window. The FDA did not release a statement detailing its reasoning behind this decision. Some have speculated that the data from ECASS-III were not sufficiently robust for FDA approval.28 Others have also raised concerns about baseline differences in stroke severity favoring the IV tPA group and a disproportionately higher rate of prior stroke in the placebo group which would also favor the IV tPA group.29 Furthermore, ECASS-III may reflect a selected group of patients who did particularly well overall, and the results may not generalize as well to those with worse prognoses, since the control group had better outcomes compared to the control patients of prior IV tPA trials.
However, since its publication in 2008, the ECASS-III study has influenced the acute management of patients with stroke in the United States. The AHA/ASA issued a science advisory in August 2009 recommending the use of IV tPA in the extended time window. This class I, level of evidence B recommendation included additional exclusion criteria based on ECASS-III including age >80 years, use of oral anticoagulants regardless of international normalized ratio, NIHSS >25, or history of stroke and diabetes.8 Although professional organizations have endorsed the view that IV tPA may be administered within the 3-hour window under the principle of implied consent,30 an informed consent process may be particularly salient for treatment in the extended time window, given it remains technically an off-label use in the United States, particularly when administered to patients with these ECASS-III exclusion criteria.
The AHA/ASA scientific statement suggested that the efficacy of IV tPA within the extended time window for patients with ECASS-III exclusion criteria was not well established (class IIb, level of evidence C). Two single-center retrospective studies found no significant difference in rates of sICH in patients treated in the extended window when comparing those who met ECASS-III exclusion criteria versus those who did not.31,32 The authors of the first study suggest that the exclusion criteria used in ECASS-III and other major IV tPA trials were based on a conservative judgment about those patients deemed most likely to develop sICH or have poorer outcomes. A recent large meta-analysis of 9 major IV tPA trials found that the odds of a good outcome (mRS 0-1) were not significantly different among older patients (age >80) or patients with more severe strokes (NIHSS ≥22),33 supporting the notion that strict application of ECASS-III criteria for IV tPA within the extended time window may be overly conservative. Accordingly, an analysis of Get with the Guidelines-Stroke data from 2009 to 2012 (GWTG-Stroke, a national registry for patients with stroke treated at participating hospitals in the United States) revealed that 31.5% of patients treated in the 3- to 4.5-hour window had at least one of the ECASS-III exclusion criteria, the most common being age >80.34
Some have questioned whether extending the time window from 3 to 4.5 hours would have an appreciable impact on IV tPA treatment rates overall. Despite the classification of IV tPA within the extended time window as off-label use in the United States, since the publication of ECASS-III, IV tPA treatment rates for patients in the extended time window have continued to increase from 1.2% to 3.5%.35 One retrospective single-center study found that if IV tPA administration was restricted to a 3-hour rather than a 4.5-hour time window, 19% of their treated patients would not have received treatment.36 Another similar study conducted at 17 sites demonstrated a much smaller impact: Only 0.5% of patients with AIS would have been excluded from treatment.37 A third multicenter prospective study suggested that extending the time window from 3 to 6 hours would result in only a modest increase (from 4.3% to 8.3%) in IV tPA treatment rates.2
In addition, observational studies suggest that in broad clinical practice, extending the time window may have resulted in similar clinical outcomes and safety compared to the standard time window. Analysis of GWTG data from 32 019 patients who were treated with IV tPA for AIS revealed that patients treated in the extended window were significantly more likely to be ambulatory at discharge and to be discharged home without a significant difference in mortality or sICH when compared to patients treated in the standard time window.34 Several studies have also demonstrated that treatment in the extended time window may also be cost effective.38,39
Ongoing Investigations and Future Directions
Additional data on the efficacy of IV tPA in the extended time window may aid in the development of additional tools that may help clinicians to quickly, safely, and effectively stratify patients by risk and by predicted treatment response that go beyond the information provided by knowing the time after symptom onset. Currently, the Extending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND/ECASS-IV) trial is enrolling patients presenting within 3 to 9 hours of stroke onset or with wake-up stroke within 9 hours from midpoint of sleep duration who have significant penumbral mismatch on either MRI or CT imaging.40 These studies are a response to the imperfect but often readily available information provided by the symptom onset time. However with further refinement and validation of penumbral imaging or other diagnostic techniques, it is conceivable that we may be able to further tailor management by identifying patients for whom IV tPA in the extended window and beyond would be safe and effective.
Although extended time windows for thrombolysis may allow more patients to receive acute thrombolysis therapy, the goal of achieving revascularization as soon as safely possible remains a central tenet of acute stroke care. A 2013 analysis of GWTG data on 58 353 patients demonstrated that faster onset to treatment times was associated with a reduced rate of sICH, a higher rate of independent ambulation at discharge, and a greater likelihood of discharge to home.41 Therefore, clinicians should remain vigilant to not allow the availability of extended time windows to contribute to delays in treatment. Instead, continued efforts to define the outer frontiers of thrombolysis for patients with acute stroke presenting during extended time windows and to further individualize the treatment approaches should occur in parallel with continued efforts to treat patients in timely fashion.42
Footnotes
Declaration of Conflicting Interests: ASK receives research grant support from NIH/NCATS for unrelated work, NIH/NINDS for stroke research and from SanBio Inc. for stroke research unrelated to this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42(7):1952–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Investigators CASPR (CASPR). Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64(4):654–659. [DOI] [PubMed] [Google Scholar]
- 3. Katzan IL. Improvement in stroke performance measures: are we moving forward or in circles? Circ Cardiovasc Qual Outcomes. 2011;4(5):493–495. [DOI] [PubMed] [Google Scholar]
- 4. Chalouhi N, Dressler JA, Kunkel ESI, et al. Intravenous tissue plasminogen activator administration in community hospitals facilitated by telestroke service. Neurosurgery. 2013;73(4):667–671; discussion 671-672. [DOI] [PubMed] [Google Scholar]
- 5. Hess DC, Wang S, Hamilton W, et al. REACH: clinical feasibility of a rural telestroke network. Stroke. 2005;36(9):2018–2020. [DOI] [PubMed] [Google Scholar]
- 6. Silva GS, Farrell S, Shandra E, Viswanathan A, Schwamm LH. The status of telestroke in the United States: a survey of currently active stroke telemedicine programs. Stroke. 2012;43(8):2078–2085. [DOI] [PubMed] [Google Scholar]
- 7. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. [DOI] [PubMed] [Google Scholar]
- 8. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274(13):1017–1025. [PubMed] [Google Scholar]
- 10. Group NS. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med. 1995;333(24):1581–1587. [DOI] [PubMed] [Google Scholar]
- 11. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–1251. [DOI] [PubMed] [Google Scholar]
- 12. Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282(21):2019–2026. [DOI] [PubMed] [Google Scholar]
- 13. Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379(9834):2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sussman BJ, Fitch TS. Thrombolysis with fibrinolysin in cerebral arterial occlusion. JAMA. 1958;167(14):1705–1709. [DOI] [PubMed] [Google Scholar]
- 15. Barreto AD. Intravenous thrombolytics for ischemic stroke. Neurotherapeutics. 2011;8(3):388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brott TG, Haley EC, Levy DE, et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke. 1992;23(5):632–640. [DOI] [PubMed] [Google Scholar]
- 17. Haley EC, Levy DE, Brott TG, et al. Urgent therapy for stroke. Part II. Pilot study of tissue plasminogen activator administered 91-180 minutes from onset. Stroke. 1992;23(5):641–645. [DOI] [PubMed] [Google Scholar]
- 18. Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J. Neurosurg. 1981;54(6):773–782. [DOI] [PubMed] [Google Scholar]
- 19. Wood L. Thrombolysis for acute stroke. JAMA. 1996;275(13):983; author reply 984-985. [DOI] [PubMed] [Google Scholar]
- 20. Von Kummer R, Bozzao L. Thrombolysis for acute stroke. JAMA. 1996;275(13):984–985. [DOI] [PubMed] [Google Scholar]
- 21. Stemer A, Lyden P. Evolution of the thrombolytic treatment window for acute ischemic stroke. Curr Neurol Neurosci Rep. 2010;10(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–774. [DOI] [PubMed] [Google Scholar]
- 23. Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7(4):299–309. [DOI] [PubMed] [Google Scholar]
- 24. Saver JL, Gornbein J, Grotta J, et al. Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3- to 4.5-hour window: joint outcome table analysis of the ECASS 3 trial. Stroke. 2009;40(7):2433–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase 3-4.5h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372(9646):1303–1309. [DOI] [PubMed] [Google Scholar]
- 26. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695–1703. [DOI] [PubMed] [Google Scholar]
- 27. Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;7(7):CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wechsler LR. The 4.5-hour time window for intravenous thrombolysis with intravenous tissue-type plasminogen activator is not firmly established. Stroke. 2014;45(3):914–915. [DOI] [PubMed] [Google Scholar]
- 29. Selim MH, Molina CA. Thrombolysis in the 3- to 4.5-hour window: what do ECASSkeptics want? Stroke. 2014;45(3):916–917. [DOI] [PubMed] [Google Scholar]
- 30. American Academy of Neurology. Policy on Consent Issues for the Administration of IV TPA (AAN Policy 2011-21). Approved by the Ethics, Law and Humanities Committee, a joint committee of the AAN, ANA, and CNS, April 11, 2011. Approved by the AAN Board of Directors August 4, 2011. Available at: https://www.aan.com/uploadedFiles/Website_Library_Assets/Documents/6.Public_Policy/1.Stay_Informed/2.Position_Statements/3.PDFs_of_all_Position_Statements/IV.pdf. Accessed April 10, 2015.
- 31. Cronin CA, Shah N, Morovati T, Hermann LD, Sheth KN. No increased risk of symptomatic intracerebral hemorrhage after thrombolysis in patients with European Cooperative Acute Stroke Study (ECASS) exclusion criteria. Stroke. 2012;43(6):1684–1686. [DOI] [PubMed] [Google Scholar]
- 32. Montaño A, Staff I, McCullough LD, Fortunato G. Community implementation of intravenous thrombolysis for acute ischemic stroke in the 3- to 4.5-hour window. Am J Emerg Med. 2013;31(12):1707–1709. [DOI] [PubMed] [Google Scholar]
- 33. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;6736(14):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cronin CA, Sheth KN, Zhao X, et al. Adherence to third European cooperative acute stroke study 3- to 4.5-hour exclusions and association with outcome: data from get with the guidelines-stroke. Stroke. 2014;45(9):2745–2749. [DOI] [PubMed] [Google Scholar]
- 35. Messé SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With The Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321–326. [DOI] [PubMed] [Google Scholar]
- 36. Lyerly MJ, Albright KC, Boehme AK, et al. The potential impact of maintaining a 3-Hour IV thrombolysis window: how many more patients can we safely treat? J Neurol Disord Stroke. 2013;1(2):1015. [PMC free article] [PubMed] [Google Scholar]
- 37. De Los Ríos la Rosa F, Khoury J, Kissela BM, et al. Eligibility for intravenous recombinant tissue-type plasminogen activator within a population: the effect of the European Cooperative Acute Stroke Study (ECASS) III Trial. Stroke. 2012;43(6):1591–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan Tanny SP, Busija L, Liew D, Teo S, Davis SM, Yan B. Cost-effectiveness of thrombolysis within 4.5 hours of acute ischemic stroke: experience from Australian stroke center. Stroke. 2013;44(8):2269–2274. [DOI] [PubMed] [Google Scholar]
- 39. Tung CE, Win SS, Lansberg MG. Cost-effectiveness of tissue-type plasminogen activator in the 3- to 4.5-hour time window for acute ischemic stroke. Stroke. 2011;42(8):2257–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma H, Parsons MW, Christensen S, et al. A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND). Int J Stroke. 2012;7(1):74–80. [DOI] [PubMed] [Google Scholar]
- 41. Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480–2488. [DOI] [PubMed] [Google Scholar]
- 42. Walter S, Kostpopoulos P, Haass A, et al. Bringing the hospital to the patient: first treatment of stroke patients at the emergency site. PLoS One. 2010;5(10):e13758. [DOI] [PMC free article] [PubMed] [Google Scholar]