Abstract
Chronic hepatitis C (CHC) affects over 185 million individuals worldwide, approximately 3% of the world’s population. CHC can lead to quality of life impairment, cirrhosis, hepatocellular carcinoma (HCC), liver failure and liver-related death. While CHC has been associated with increases in HCC, liver-related mortality and all-cause mortality, being cured of CHC is associated with improvement in these outcomes. Older interferon-based regimens were complex and toxic and required 6–12 months of therapy, with cure rates averaging around 40–45% for HCV genotype 1. Newer interferon-free regimens are now available in the US, Europe, Japan and in other countries. These regimens have short durations, minimal side effects, low pill burden and efficacy approaching 90–100%. We may eventually see single-tablet regimens lasting no more than 4–6 weeks. This review will summarize the data regarding these interferon-free regimens, including Gilead’s Harvoni (sofosbuvir/ledipasvir), AbbVie’s Viekira Pak (paritaprevir/ritonavir/ombitasvir with dasabuvir), and Janssen’s Olysio (simeprevir) with sofosbuvir. Some practical considerations as we move into an interferon-free era will also be discussed, such as patient adherence and drug–drug interactions.
Keywords: direct-acting antiviral agents, Harvoni, ledipasvir, paritaprevir, sofosbuvir, Viekira
Introduction
Over the last several years, the management of chronic hepatitis C (CHC) has been revolutionized by the development of cell-mediated targeted therapies [direct-acting antiviral agents (DAAs)] against hepatitis C virus (HCV). Indeed, we are at the beginning of a new era of HCV management, which is a boon to patients and clinicians alike. Left behind is a treatment regimen fraught with side effects, quality of life (QOL) impairment and high treatment failure rates [Hézode et al. 2014; McHutchison and Sulkowski, 2008; McHutchison et al. 2002]. The new regimens are simple, safe, effective regimens of short duration with minimal side effects.
Although numerous agents have completed phase II and III clinical trials, this review will focus on the interferon-free regimens currently available and the implications they present in the day to day management of patients with CHC. Therefore, the following interferon-free regimens will be reviewed: sofosbuvir/ledipasvir (SOF/LDV), simeprevir (SIM) with SOF, and paritaprevir/ritonavir/ombitasvir with dasabuvir (PTV/r/OBV with DSV).
Epidemiology and natural history
CHC is a worldwide cause of liver-related morbidity and mortality. It affects over 185 million people, approximately 2–3% of the world’s population. While a prevalence of 2–3% may be relatively low overall, prevalence varies by age group and is typically much higher in cohorts between the ages of 45 and 75. For example, in Central and East Asia, the prevalence peaks at 8.8–8.9% for those aged 55–64. Similarly, in Europe, prevalence peaks at 3.9–5.2% for those aged 55–64 [Mohd Hanafiah et al. 2013].
In the US, it is estimated that HCV affects about 2.7–3.9 million people. These estimates are based on the data from the National Health and Nutrition Examination Survey, which excludes the homeless and incarcerated [Smith et al. 2012; Armstrong et al. 2006]. If these and other high-risk groups (people who inject drugs, those with mental illness, nursing home residents, active military duty) are factored in, the true prevalence in the US may be closer to 2–3% or 5.2–7.1 million individuals [Chak et al. 2011]. Similar to worldwide trends, HCV is disproportionally more common in the ‘Baby Boomer’ cohort, those born between 1945 and 1965, nearing 5% by some estimates [Smith et al. 2012].
Six different genotypes of HCV (genotypes 1, 2, 3, 4, 5, 6) have been identified [Messina et al. 2014]. Genotype 1, specifically 1b, is the most common subtype worldwide affecting 42% of HCV-infected individuals [Messina et al. 2014]. This is followed by genotype 3 (26%), most commonly found in Pakistan and India, and genotype 4 (14%) which is most common in North Africa and the Middle East. In the US, genotype 1a is the most common, accounting for 58% of HCV-infected individuals. Genotype 1b accounts for 21%, genotype 2 accounts for 15% and genotype 3 accounts for 5% [Messina et al. 2014]. The genotype is clinically relevant given that the majority of current DAAs do not have pangenotypic efficacy, as will be discussed below. In addition, in the past, each genotype was associated with a different sustained virologic response (SVR) rate. In the future, the effect of genotype will become less prominent as more potent, pangenotypic DAA regimens become available.
HCV is transmitted most often through exposure to infected blood. Sexual or vertical transmission is rare. The most common route of transmission, before blood donor testing was instituted in 1992, was through blood transfusions. Currently, the most common route of transmission is intravenous drug use, a result of a new epidemic of illicit heroin and prescription narcotic abuse has surfaced among the young [Suryaprasad et al. 2014]. Other risk factors include intranasal cocaine use, tattoos, piercings, incarceration, hemodialysis and needlesticks in health care workers. Once exposed, 75–80% of individuals will progress to chronic infection. Of these, approximately 10–20% will develop cirrhosis over two to three decades and become at risk for hepatic decompensation and primary liver cancer (HCC). Progression to cirrhosis is accelerated by certain risk factors such as alcohol use, male sex and comorbid diabetes, obesity or fatty liver, or coinfection with HIV or hepatitis B [Chen and Morgan, 2006]. The 5- and 10-year survival rates for patients with compensated cirrhosis are relatively high at 91% and 79%; however, after the initial episode of hepatic decompensation, survival decreases dramatically, falling to 50% at 5 years. In addition, as the Baby Boomer cohort continues to age, health-related costs to society will continue to rise [Davis et al. 2010; Rein et al. 2011]. Given the worldwide burden associated with CHC, it is fortunate and timely that potentially curative treatment regimens have been discovered.
It is now accepted that HCV infection can truly be ‘cured’. HCV replicates in the cytoplasm of the hepatocyte without a reservoir or archived copies in the nucleus. Because of this, effective treatment results in lasting viral eradication. A number of studies [Rutter et al. 2013; Ng and Saab, 2011] have shown that SVR is durable. SVR, most commonly measured as SVR12, is defined as having undetectable viral replication in the blood 12 weeks after completion of therapy. The obtainment of SVR has been significantly associated with a decrease in all-cause mortality, liver-related mortality, the incidence of hepatocellular carcinoma (HCC) as well as fibrosis regression [Ng and Saab, 2011] and cirrhosis resolution in 50–60% of patients with CHC [D’Ambrosio et al. 2012; Shiffman et al. 2014]. The benefits of being cured also improve worker productivity and health-related QOL [as measured by patient-reported outcomes (PROs)] in patients who achieve SVR [Younossi et al. 2014a, 2014c, 2014d].
Historical treatment of HCV
While a ‘cure’, defined as SVR, has been available over the past two decades, achieving this outcome with old regimens was quite rare. The mainstay of therapy over the last two decades involved a combination of interferon α and ribavirin (RBV). Both have indirect antiviral activity against HCV while the mechanisms of each are still not completely understood. Interferons are proteins produced by the host in response to infection, resulting in antiviral effects and immunomodulation [Feld and Hoofnagle, 2005]. Well documented side effects of interferon α include myelosuppression (anemia, neutropenia and thrombocytopenia), flu-like symptoms and neuropsychiatric side effects (irritability, depression, anxiety and fatigue). Interferon also lowers the seizure threshold and exacerbates immune-mediated diseases. Given the numerous side effects associated with interferon, many patients have historically been ‘interferon ineligible or intolerant’ due to pre-existing health conditions, such as mental illness or autoimmune disease. Treating patients with a history of recent substance abuse is also problematic, as interferon use in this group is often associated with recidivism. In addition, SVR rates with pegylated interferon and RBV were very low, averaging between 40% and 50%.
RBV is a guanosine analogue that has efficacy against a number of viruses [Feld and Hoofnagle, 2005]. Adding RBV to interferon improves SVR, but it also adds the side effects of hemolytic anemia, occasional rash and more insomnia. Further, the treatment duration required with pegylated interferon and RBV is long, ranging from 24 to 48 weeks, depending on genotype. Essentially, in the past, most patients were asked to take on a year of this regimen with significant side effects and less than 50% chance at being cured [McHutchison and Sulkowski, 2008; McHutchison et al. 2002].
First-generation direct-acting antivirals: protease inhibitors
The advent of the first-generation DAAs shifted the treatment focus to protease inhibitor (PI)-based therapy. The first PIs, telaprevir and boceprevir, became widely available in 2011, increasing SVR rates to about 70%. However, these PIs still had to be given with pegylated interferon and RBV and were used only for patients with genotype 1 HCV. For patients who responded well to treatment and had an appropriate decrease in viremia, the duration of therapy could be shortened to 24 weeks with the telaprevir-based regimen and 28 weeks with the boceprevir-based regimen. Patients who were treatment experienced or those with cirrhosis had to endure almost a year of therapy.
The use of these initial DAA regimens was complicated by response-guided management and a large pill burden, requiring thrice daily dosing [Hézode et al 2014]. In addition to the pegylated interferon and RBV side effects, these PIs carried a large number of additional side effects. Telaprevir has been associated with nausea, rectal burning, diarrhea, and in some cases severe rash and anemia. Recently, telaprevir-based treatment was found to be associated with decreased renal function (measured by estimated glomerular filtration rate), which led to decreased renal elimination of RBV, and subsequently, a greater degree of hemolytic anemia [Tempestilli et al. 2014]. Boceprevir treatment has been associated with dysgeusia, anemia, nausea and headache. Furthermore, these regimens had significant drug–drug interactions (DDIs). Therefore, while SVR rates improved with these first-generation PIs, so did the complexity of the regimen and the incidence and severity of side effects. In fact, these regimens were associated with substantially lower SVR rates with high rates of adverse events [Hézode et al. 2014].
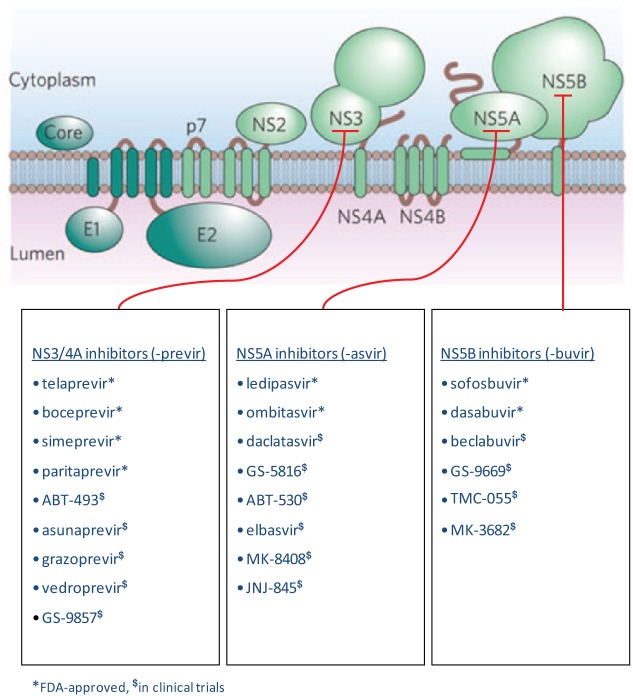
The next generation of DAAs focused on different targets. The first new targeted site has focused on HCV viral replication specifically in the cytoplasm, where HCV viral RNA is translated into a polypeptide, which then undergoes cleavage into 10 distinct proteins. Three of these are structural, consisting of a core protein and the envelope proteins E1 and E2, leaving the remaining seven as nonstructural proteins. The DAAs currently approved or closest to market inhibit the activity of one of three nonstructural proteins involved in HCV replication, as detailed in Table 1 and Figure 1.
Table 1.
Direct acting antivirals.
| Drug manufacturer | NS3/4A ‘-previr’ | NS5A ‘-asvir’ | NS5B ‘-buvir’ |
|
|---|---|---|---|---|
| Nucleos(t)ide analogues | Non-nucleoside inhibitors | |||
| Gilead | GS-9857$ | Ledipasvir* | Sofosbuvir* | GS-9669$ |
| Vedroprevir$ | GS-5816$ | |||
| Janssen | Simeprevir* | JNJ-845$ | TMC-055$ | |
| AbbVie | Paritaprevir* | Ombitasvir* | Dasabuvir* | |
| ABT-493$ | ABT-530$ | |||
| BMS | Asunaprevir$ | Daclatasvir$ | Beclabuvir$ | |
| Merck | Grazoprevir$ | Elbasvir$ | MK-3682$ | |
| MK-8408$ | ||||
US Food and Drug Administration approved.
In clinical trials.
Figure 1.
Targets for inhibition of hepatitis C virus (HCV) viral replication.
An essential step in the HCV life cycle is the replication of viral RNA. This is mediated by the NS3/4A, NS5A and NS5B nonstructural proteins, which are currently the main targets of therapeutic intervention.
Currently approved DAA treatment regimens
The possibility of interrupting HCV replication at three distinct sites has led to the rapid development of new drug regimens for the treatment and potential cure of HCV. The following will provide the reader with an overview of the three drug regimens that are currently available in the US.
Ledipasvir/sofosbuvir
In late 2014, Gilead’s single tablet regimen of LDV/SOF was approved by the US Food and Drug Administration (FDA) for the treatment of genotype 1 CHC. It is commonly marketed in the US under the brand name Harvoni, Gilead Sciences, Inc., Foster City, CA. SOF, a uridine nucleotide analog prodrug, was approved by the US FDA in December 2013 and marketed in the US under the trade name Sovaldi, Gilead Sciences, Inc., Foster City, CA. GS-461203, the active metabolite of SOF, is incorporated by the NS5b polymerase into HCV RNA, resulting in chain termination. For patients with genotype 2 HCV, 12 weeks of SOF and RBV is indicated, yielding an overall SVR rate of 93%. For patients with genotype 3 HCV, 24 weeks of SOF and RBV is indicated, yielding an overall SVR rate of 84%. SOF-based regimens are also indicated for use in patients with human immunodeficiency virus (HIV)/HCV coinfection and in patients with HCC who are on the waitlist for liver transplant. Further information about the current indications and use of SOF can be found in detail elsewhere [Lam et al. 2014].
LDV is an inhibitor of the NS5A replication complex, and LDV is coformulated with SOF into a fixed dose combination (FDC) tablet, which is given once daily with or without food. The indicated use and durations are outlined in Table 2 and based on the phase III ION trials. These open-label, multicenter trials examined the use of LDV/SOF with or without RBV in patients with genotype 1 HCV. The treatment population, regimen, durations and SVR rates for each cohort are detailed in Tables 3 and 4.
Table 2.
Currently available therapies for genotype 1 with US Food and Drug Administration (FDA) approved indications.
| Name of regimen | Trial name | Group* | Regimen | Duration of treatment (weeks) | Dosing considerations | Potential for cure | FDA approval date |
|---|---|---|---|---|---|---|---|
| LDV/SOF | ION-3 | TN without cirrhosis HCV RNA < 6 million iu | HAR | 8 | Adding RBV did not confer any significant benefit. | 97% | 10 October 2014 |
| ION-1, 3 | TN with or without cirrhosis | HAR | 12 | 96–99% | |||
| ION-2 | TE without cirrhosis | HAR | 12 | This is the only regimen indicated for PI failures | 95% | ||
| ION-2 | TE with cirrhosis | HAR | 24 | Adding RBV did not confer any significant benefit | 100% | ||
| SIM + SOF | COSMOS OPTIMIST-1$ |
TN and TE without cirrhosis | OLY + SOV | 12 | Adding RBV did not confer any significant benefit | 95% | 6 November 2014 |
| COSMOS OPTIMIST-2$ |
TN and TE with cirrhosis | OLY + SOV | 24 | 100% | |||
| OBV/PTV/r + DSV‡ | SAPPHIRE-I, II PEARL-IV | GT1a§, TN and TE without cirrhosis | VIE + RBV | 12 | 96–97% | 19 December 2014 | |
| TURQUOISE-II | GT1a§, TN and TE with cirrhosis | VIE + RBV | 24¶ | See comments below¶ | TN: 95% Rel: 100% Part: 100% NR: 93% |
||
| SAPPHIRE-I, II PEARL-II, III |
GT1b, TN and TE without cirrhosis | VIE | 12 | 96.7–100% | |||
| TURQUOISE-II | GT1b, TN and TE with cirrhosis | VIE + RBV | 12 | 100% |
No patients with decompensated cirrhosis were enrolled in these trials. Direct comparison across studies should not be made due to differing study designs.
At the time of publication, results from the phase III OPTIMIST trials of SIM + SOF are yet to be publicly released.
Dosing for patients coinfected with HIV/HCV is the same as for patients only infected with HCV. Twenty-four weeks of VIE + RBV is indicated for certain liver transplant recipients.
For mixed genotype 1 or unknown genotype 1 subtype, follow dosing recommendations for genotype 1a.
VIE + RBV for 12 weeks may be considered for some patients based on prior treatment history (see text or VIE package insert section 14.3).
GT, genotype; HAR, Harvoni; HCV, hepatitis C virus; LDV, ledipasvir; NR, prior null response; OLY, Olysio; OBV/PTV/r + DSV, ombitasvir/paritaprevir/ritonavir + dasabuvir; Part, prior partial response; PI failures, previous non-SVR with pegylated interferon; RBV, ribavirin; Rel, prior relapse; SIM, simeprevir; SOF, sofosbuvir; TE, treatment experienced; TN, treatment naïve; VIE, Viekira Pak.
Table 3.
Ledipasvir/sofosbuvir FDC for genotype 1.
| Study | Population | Regimen | Overall SVR12 | Cirrhotic SVR12 |
|---|---|---|---|---|
| ION-1 | GT1 TN (N = 865) | SOF/LDV, 12 weeks | 99% | 94% |
| (211/214) | (32/34) | |||
| SOF/LDV/RBV, 12 weeks | 97% | 100% | ||
| (211/217) | (33/33) | |||
| SOF/LDV, 24 weeks | 98% | 94% | ||
| (212/217) | (31/33) | |||
| SOF/LDV/RBV, 24 weeks | 99% | 100% | ||
| (215/217) | (36/36) | |||
| ION-2 | GT1 TE (N = 440) | SOF/LDV, 12 weeks | 94% | 86% |
| (102/109) | (19/22) | |||
| SOF/LDV/RBV, 12 weeks | 96% | 82% | ||
| (107/111) | (18/22) | |||
| SOF/LDV, 24 weeks | 99% | 100% | ||
| (108/109) | (22/22) | |||
| SOF/LDV/RBV, 24 weeks | 99% | 100% | ||
| (110/111) | (22/22) | |||
| ION-3 | GT1 TN (N = 647) | SOF/LDV, 8 weeks | 94% | n/a |
| (202/215) | ||||
| SOF/LDV/RBV, 8 weeks | 93% | n/a | ||
| (201/216) | ||||
| SOF/LDV, 12 weeks | 95% | n/a | ||
| (206/216) |
FDC, fixed dose combination tablet; GT, genotype; LDV, ledipasvir; n/a, not applicable; RBV, ribavirin; SOF, sofosbuvir; TE, treatment experienced; TN, treatment naïve.
Table 4.
Ledipasvir/sofosbuvir FDC for patients whose condition failed to respond to treatment with a protease inhibitor.
| Study | Population | Regimen | SVR12 PEG/RBV failures | SVR12 PI/PEG/RBV failures |
|---|---|---|---|---|
| ION-2 | GT1 TE (N = 440) | SOF/LDV, 12 weeks | 93% | 94% |
| (40/43) | (62/66) | |||
| SOF/LDV/RBV, 12 weeks | 96% | 97% | ||
| (45/47) | (62/64) | |||
| SOF/LDV, 24 weeks | 100% | 98% | ||
| (58/58) | (49/50) | |||
| SOF/LDV/RBV, 24 weeks | 98% | 100% | ||
| (58/59) | (51/51) |
FDC, fixed dose combination tablet; GT, genotype; LDV, ledipasvir; PEG, pegylated interferon; PI, protease inhibitor; RBV, ribavirin, SOF, sofosbuvir; TE, treatment experienced.
The ION-1 and ION-3 studies enrolled patients who were naïve to treatment. ION-1 randomized patients to durations of 12 or 24 weeks [Afdhal et al. 2014a; Kowdley et al. 2014]. ION-3 studied a shorter duration of 8 weeks versus 12 weeks [Afdhal et al. 2014b]. ION-2 randomized treatment-experienced patients to durations of 12 or 24 weeks. Patients whose condition had previously failed to respond to PI regimens were also included in ION-2. Patients with compensated cirrhosis were included in ION-1 and ION-2. A total of 1952 patients were treated in the ION trials with 12% (n = 224) having compensated cirrhosis and 52% (n = 231) in the ION-2 trial having disease that had previously failed to respond to a PI regimen. The overall SVR12 rate for all three trials was 97%. In ION-1, in the 12-week RBV-free arm, SVR rates for treatment-naive patients with cirrhosis were fairly comparable to those of treatment-naïve patients without cirrhosis (94% versus 99%). However, in ION-2, treatment-experienced patients with cirrhosis had a lower SVR rate in the 12-week RBV-free arm (86%) compared with the 24-week RBV-free arm (100%); this is reflected in the prescribing information for Harvoni as detailed in Table 2. The SVR rates of all treatment arms are delineated in Table 3. Notably, as detailed in Table 4, patients who had previously failed to achieve SVR with telaprevir or boceprevir had similar SVR rates to patients whose prior regimen only included pegylated interferon and RBV.
Overall, SOF and LDV were well tolerated with very few treatment discontinuations due to adverse effects (13 total, 1%) and no deaths. The most common side effects included headache, fatigue, nausea, insomnia and diarrhea. Adverse effects were more common in the treatment arms that included RBV. The addition of RBV did not significantly increase SVR rates, but the comparison between RBV-containing arms and the corresponding RBV-free arms is limited by smaller cohort sizes in certain harder-to-cure subgroups, such as treatment-experienced patients with cirrhosis. Nevertheless, SVR rates were very high in RBV-free arms, and any additional benefit gained by adding RBV would in all likelihood be marginal.
While small cohorts of patients with genotype 3, 4 and 6 CHC have been treated with LDV/SOF (www.hcvguidelines.org), in the US, Harvoni (LDV/SOF) is only FDA approved for genotype 1 CHC. As detailed in Table 2, a 12-week course of Harvoni is indicated in treatment-naive patients, regardless of genotype 1 subtype and regardless of fibrosis stage (including compensated cirrhosis). For treatment-naive patients without cirrhosis and baseline HCV RNA less than 6 million iu/ml, 8 weeks of Harvoni can be considered. Twelve weeks of Harvoni is also indicated in treatment-experienced patients without cirrhosis, including patients whose disease previously failed to respond to treatment with a PI. For treatment-experienced patients (including those whose disease failed to respond to PI treatment) with compensated cirrhosis, 24 weeks of Harvoni is indicated.
Harvoni cannot currently be recommended in patients with severe renal disease, as defined by having an estimated glomerular filtration rate (eGFR) less than 30 ml/min/1.73 m2 and in patients with end-stage renal disease, given that the main metabolite of SOF is increased in patients with eGFR less than 30. As with all the DAA regimens, DDIs must be carefully considered before therapy is considered. Harvoni should not be administered concomitantly with strong P-glycoprotein inducers, such as rifampin and St John’s wort, or with acid-reducing therapies, certain anticonvulsants, antiarrhythmics, SOF, SIM, certain HIV antiretrovirals or rosuvastatin.
Simeprevir with sofosbuvir
Again in late 2014, the US FDA approved Janssen’s supplemental NDA (sNDA) for the use of its NS3/4A PI SIM (marketed in the US as Olysio, Janssen Therapeutics, Titusville, NJ) in an interferon-free regimen with Gilead’s SOF for treatment of patients with genotype 1. This interferon-free regimen, commonly referred to as ‘the COSMOS regimen’ or ‘SIM/SOF’, is based primarily on the phase II COSMOS trial [Lawitz et al. 2014]. The phase III OPTIMIST trials have been completed and results should be released soon. COSMOS was a randomized, open-label trial which studied the use of SIM and SOF with or without RBV for 12- or 24-week treatment periods in patients with genotype 1 HCV. The ‘null responders’ in the COSMOS study were those who during previous treatment had not achieved a two-log decrease in HCV RNA levels after 12 weeks of pegylated interferon and RBV. A total of 167 patients with or without cirrhosis were randomized to 12 or 24 weeks of SIM with SOF with or without RBV. Forty-one participants (25%) had compensated cirrhosis. In patients without cirrhosis, SVR12 was achieved in 154 patients (92%), as detailed in Table 5. In the compensated cirrhosis group, the SVR12 rate was 95% (37 of the 39 that completed treatment). The most common side effects experienced across all groups were fatigue, headache and nausea. Anemia and increased levels of bilirubin were also observed in the arms containing RBV. Discontinuation due to adverse events was seen inly in the 24-week arms at a low rate of 2%.
Table 5.
Simeprevir with sofosbuvir for genotype 1.
| Study | Population | Regimen | SVR12 |
|---|---|---|---|
| COSMOS (Cohort 1) | GT1 TE METAVIR F0–F2 (N = 80) | SIM/SOF, 12 weeks | 93% |
| (13/14) | |||
| SIM/SOF/RBV, 12 weeks | 96% | ||
| (26/27) | |||
| SIM/SOF, 24 weeks | 93% | ||
| (14/15) | |||
| SIM/SOF/RBV, 24 weeks | 79% | ||
| (19/24) | |||
| COSMOS (Cohort 2) | GT1 TN or TE METAVIR F3–F4 (N = 87) | SIM/SOF, 12 weeks | 93% |
| (13/14) | |||
| SIM/SOF/RBV, 12 weeks | 93% | ||
| (25/27) | |||
| SIM/SOF, 24 weeks | 100% | ||
| (16/16) | |||
| SIM/SOF/RBV, 24 weeks | 93% | ||
| (28/30) |
GT, genotype; RBV, ribavirin; SIM, simeprevir; SOF, sofosbuvir; TE, treatment experienced; TN, treatment naïve.
As reported at the annual Liver Meeting of the American Association for the Study of Liver Diseases (AASLD) in 2014, the TRIO and HCV-TARGET studies examined the use of SIM and SOF in the real world. Overall SVR rates were found to be 83–89%, slightly lower than the SVR rates reported in the COSMOS trial. The slight decrease in the overall SVR rates may be partially explained by the types of patients receiving this medication regimen, which included patients previously exposed to PIs, patients with decompensated cirrhosis, history of liver transplant, and other comorbidities typically excluded from controlled clinical trials. Fatigue and headache were the most commonly reported adverse events. Overall rates of serious adverse events (4–8% in the HCV-TARGET cohort) and adverse events leading to treatment discontinuation (1.4% in the Trio cohort) were relatively low. In the HCV-TARGET cohort, adding RBV to SIM and SOF did not result in a statistically significant increase in SVR in the overall cohort or in any subgroup (cirrhotic, noncirrhotic, 1a, 1b, treatment naïve, treatment experienced) [Dieterich et al. 2014; Jensen et al. 2014].
Phase III trials of SIM with SOF are currently underway. OPTIMIST-1 is a randomization of patients with genotype 1 HCV without cirrhosis to either 8 or 12 weeks of SIM with SOF in an open-label fashion [ClinicalTrials.gov identifier: NCT02114177]. OPTIMIST-2 is a randomization of patients with genotype 1 HCV and cirrhosis to 12 weeks of SIM with SOF. Both trials include treatment-naive and treatment-experienced patients. Neither OPTIMIST-1 nor OPTIMIST-2 have RBV-containing arms, so the question of whether RBV may afford a small increase in SVR rate in certain subgroups will not be answered; however, the close hematologic monitoring that RBV requires will not be required, thus decreasing the treatment regimen burden.
As detailed in Table 2 and the prescribing information for Olysio, 12 weeks of Olysio with SOF is indicated for patients without cirrhosis, whether treatment naive or treatment experienced. Twenty-four weeks of Olysio with SOF is indicated for patients with cirrhosis, both treatment naive and treatment experienced. Olysio with SOF is approved for the treatment of genotype 4 in Europe, but not in the US. Olysio with SOF is not recommended for patients whose disease has previously failed to respond to treatment with a PI, or in patients with severe liver dysfunction (Child’s class C). Caution needs to be exercised when Olysio is coadministered with moderate and strong cytochrome P450 3A (CYP3A) inducers or inhibitors. Significant DDIs occur with milk thistle, St John’s wort, statins, calcium channel blockers, certain macrolide antibiotics, antiarrhythmics, anticonvulsants, antifungals, antimycobacterials, immunosuppressants and certain HIV antiretrovirals. Patients taking Olysio will also need to avoid grapefruit juice, as it affects CYP3A4 metabolism. As with all the DAA regimens, DDIs need to be considered before Olysio is prescribed.
Paritaprevir/ritonavir/ombitasvir with dasabuvir
In 2014, AbbVie’s 3-DAA (3D) regimen of PTV boosted by ritonavir (PTV/r) coformulated with OBV and given with DSV with or without RBV received approval by the US FDA for use in patients with genotype 1. It is marketed in the US under the brand name Viekira Pak AbbVie Inc., North Chicago, IL (PTV/r/OBV tablets with DSV tablets) and in Europe as Viekirax (PTV/r/OBV), AbbVie Inc., Maidenhead, UK and Exviera (DSV) AbbVie Inc., Maidenhead, UK. In Europe, Viekirax without Exviera received the indication for use in genotype 4 and in Japan, Viekirax without Exviera is approved for genotype 1b. In the US, Viekira Pak is also indicated for treatment of HCV/HIV-1 coinfection and liver transplant recipients with mild fibrosis [AbbVie Inc., 2015a, 2015b, 2015c]. PTV (formerly known as ABT-450) is a PI; OBV (previously known as ABT-267) inhibits the NS5A replication complex while DSV (previously known as ABT-333) is a non-nucleotide inhibitor of the NS5B polymerase. OBV is coformulated in a single tablet with PTV and ritonavir, which increases the overall exposure of PTV and allows for once daily dosing of PTV. The indicated use and duration are detailed in Table 2.
As detailed in Table 6, six phase III trials and two phase II trials enrolled a total of 2405 patients with genotype 1 HCV [Andreone et al. 2014; Feld et al. 2014; Ferenci et al. 2014; Zeuzem et al. 2014], including 380 patients with cirrhosis in the TURQUOISE-II trial [Poordad et al. 2014], 63 patients with HIV-1/HCV coinfection, and 34 liver transplant recipients. The 3D regimen was studied extensively in both treatment-naive and treatment-experienced patients. The PEARL trials (PEARL-II, PEARL-III, PEARL-IV) examined the effect of adding RBV to the 3D regimen [Andreone et al. 2014; Feld et al. 2014; Ferenci et al. 2014]. For patients with compensated cirrhosis, TURQUOISE-II examined whether 24 weeks of 3D with RBV were better than 12 weeks.
Table 6.
AbbVie 3D (ombitasvir/paritaprevir/ritonavir FDC with dasabuvir) for genotype 1.
| Study | Population | Regimen | Overall SVR12 | Cirrhotic SVR12 |
|---|---|---|---|---|
| SAPPHIRE-I (12 weeks) | GT1 TN (N = 631) | 3D + RBV | 96% | n/a |
| (455/473)* | ||||
| SAPPHIRE-II (12 weeks) | GT1 TE (N = 394) | 3D + RBV | 96% | n/a |
| (286/297)* | ||||
| PEARL-II (12 weeks) | GT1b TE (N = 179) | 3D | 100% | n/a |
| (91/91) | ||||
| 3D + RBV | 97% | |||
| (85/88) | ||||
| PEARL-III (12 weeks) | GT1b TN (N = 419) | 3D | 99% | n/a |
| (207/209) | ||||
| 3D + RBV | 99% | |||
| (209/210) | ||||
| PEARL-IV (12 weeks) | GT1a TN (N = 305) | 3D | 90% | n/a |
| (185/205) | ||||
| 3D + RBV | 97% | |||
| (97/100) | ||||
| TURQUOISE-II (12 or 24 weeks) | GT1 TN and TE with compensated cirrhosis (N = 380) | 3D + RBV, 12 weeks | 94% | 92% |
| (356/380) | (191/208) | |||
| 3D + RBV, 24 weeks | 96% | |||
| (165/172) | ||||
| TURQUOISE-I (12 or 24 weeks) | GT1 TN and TE with HIV-1 coinfection (N = 63) | 3D + RBV, 12 weeks | 94% | n/a |
| (29/31) | ||||
| 3D + RBV, 24 weeks | 91% | |||
| (29/62) | ||||
| CORAL-I (24 weeks) | GT1 post OLT (N = 34) | 3D + RBV, 24 weeks | 97%(33/34) | n/av |
3D, paritaprevir/ritonavir/ombitasvir + dasabuvir; FDC, fixed dose combination tablets; GT, genotype; HIV, human immunodeficiency virus; n/a, not applicable; n/av, not available; OLT, orthotopic liver transplant; RBV, ribavirin; SVR, sustained virologic response; TE, treatment experienced; TN, treatment naïve.
Available SVR12 data from primary analysis.
Overall, the SVR12 rates were very high, no lower than 96% for all cohorts except for patients with genotype 1a HCV who did not receive RBV in the PEARL-IV trial (SVR12: 90%), patients with HIV/HCV coinfection in the TURQUOISE-I trial (SVR12: ⩽ 94%), and patients with genotype 1a HCV and cirrhosis in the TURQUOISE-II trial (SVR12: 88.6% for 12 weeks, 94.2% for 24 weeks). SVR rates are detailed in Table 6. It is worthwhile to note that aside from liver transplant recipients, the main group which may benefit from extending therapy to 24 weeks would be patients with cirrhosis and genotype 1a HCV who were null responders (80.0% with 12 weeks versus 92.9% with 24 weeks). In comparison, patients with cirrhosis and genotype 1a HCV who were treatment naive had equally high SVR rates regardless of duration (92.2% with 12 weeks versus 92.9% with 24 weeks). Patients with cirrhosis, genotype 1a HCV and a history of partial response and relapse had SVR rates of 93.3–100.0%, but small cohort sizes make it difficult to say whether 24 weeks was significantly better than 12 weeks. Twelve weeks of treatment was sufficient for all patients with genotype 1b; SVR12 rates were 97–100% regardless of prior treatment history or the presence of cirrhosis. Twelve weeks of 3D with RBV was also sufficient for patients without cirrhosis with genotype 1a HCV, whether treatment naive or treatment experienced.
The most commonly reported side effects were headache, fatigue and nausea. The rate of discontinuation due to adverse effects was very low, ranging from 0 to 2%. Elevations of alanine aminotransferase (ALT) greater than five times the upper limit of normal were noted in approximately 1% of subjects. These elevations were typically asymptomatic and typically occurred and resolved without cessation of therapy within the first 2 months of treatment. Elevation of ALT occurred more frequently in patients using ethinyl estradiol. Elevation of bilirubin above twice the upper limit of normal, not in association with ALT elevation, was also noted in 2% of subjects in the RBV-free arms. Hyperbilirubinemia was noted in 15% of subjects in the RBV-containing arms. Bilirubin elevations typically peaked after one week and resolved without cessation of therapy.
As detailed in Table 2 and the prescribing information for Viekira Pak, 12 weeks of Viekira Pak alone is indicated in patients without cirrhosis with genotype 1b HCV. Twelve weeks of Viekira Pak with RBV is indicated in patients without cirrhosis with genotype 1a HCV and those with cirrhosis and genotype 1b HCV. Twenty-four weeks of Viekira Pak with RBV is recommended for transplant recipients and patients with cirrhosis and genotype 1a HCV, although 12 weeks may be considered for treatment-naive patients with cirrhosis and genotype 1a HCV. The indications above apply for monoinfected patients with HCV and those coinfected with HCV/HIV-1 alike.
DDIs are particularly important to assess with Viekira Pak, given that ritonavir is coformulated in the FDC tablets. Viekira Pak interacts with drugs metabolized by CYP2C8 and CYP3A. Viekira Pak is not recommended for patients with moderate liver dysfunction (Child’s B) and contraindicated in patients with severe liver dysfunction (Child’s C). Viekira Pak can be given without dose adjustment to patients with mild, moderate and severe renal impairment, though caution must be used if RBV is used. Viekira Pak should not be coadministered with ethinyl estradiol containing products, salmeterol, certain HIV antiretrovirals, rifamipin, St John’s wort, lovastatin, simvastatin, certain anticonvulsants and oral midazolam or triazolam. Caution must be used with a number of other medications, and these are detailed in the prescribing information.
Future regimens
While this review focused on currently available interferon-free regimens, it is expected that a number of other regimens will be moving towards approval over the next two years. Especially notable among these are the regimens which are investigating a shorter duration of therapy (Table 7). Results were presented at the Conference on Retroviruses and Opportunistic Infections in March of 2014 from the SYNERGY trial, which examined a 6-week regimen of SOF, LDV given with either GS-9669 (a non-nucleoside thumb site 2 NS5B inhibitor) or vedroprevir (a NS3 PI formerly called GS-9451) [Kohli et al. 2014]. Although only 20 patients were randomized to each arm, SVR rates were very high in both 6-week arms and comparable to the 12-week comparator arm of SOF and LDV. All patients were treatment naïve and patients with cirrhosis were only enrolled in the 12-week SOF and LDV arm. Seventy percent of patients were infected with genotype 1a. Compared with SOF and LDV alone, the decrease in viral load was significantly more rapid in the 3D regimens. Short-duration trials of SOF/LDV with vedroprevir are ongoing in treatment experienced patients and patients with cirrhosis [ClinicalTrials.gov identifier: NCT02226549].
Table 7.
Short duration regimens.
| Study | Population | Regimen | Overall SVR12 | Cirrhotic SVR12 |
|---|---|---|---|---|
| NIAID SYNERGY (6 or 12 weeks) | GT1, TN, cirrhosis allowed | SOF/LDV, 12 weeks | 100% | 100% |
| (20/20) | (3/3) | |||
| GT1, TN, noncirrhotic | SOF/LDV/GS-9669, 6 weeks | 95% | n/a | |
| (19/20) | ||||
| GT1, TN, noncirrhotic | SOF/LDV/VDR, 6 weeks | 100% | n/a | |
| (20/20) | ||||
| BMS FOURWARD (4 or 6 weeks) | GT1, TN, noncirrhotic | DCV/ASV/BMS-791325 + SOF, 4 weeks | Pending | n/a |
| GT1, TN, noncirrhotic | DCV/ASV/BMS-791325 + SOF, 6 weeks | Pending | n/a |
ASV, asunaprevir; DCV, daclatasvir; GT, genotype, SOF, sofosbuvir; TN, treatment naïve; LDV, ledipasvir; n/a, not applicable; VDR, vedroprevir.
In June 2014, Bristol-Myers Squibb announced a bold pilot study examining 4 versus 6 weeks of daclatasvir/asunaprevir/beclabuvir plus SOF in 30 treatment-naïve patients without cirrhosis with genotype 1 HCV [ClinicalTrials.gov identifier: NCT02175966]. Merck has also announced plans for its C-CREST studies, which will examine short-duration three-drug regimens, using their nucleotide analogue MK-3682 with their PI grazoprevir and one of their NS5a inhibitors, elbasvir or MK-8408. If the efficacy is high in these trials, the treatment regimens of 2016 may be no more than a month in duration for certain patients.
Patient-reported outcomes
The availability of new DAA-only regimens comes with the potential for substantial gains, both on the level of the individual patient and on the societal level. CHC infection is known to impair important PROs such as health-related QOL (HRQOL) and work productivity [Younossi et al. 2014b, 2015b, 2015d]. This impairment becomes more severe with more advanced liver disease (cirrhosis) and coinfection with HIV [Younossi et al. 2015b, 2015d]. Treatment of CHC with regimens containing interferon and RBV are associated with further decline in PRO scores [McHutchison et al. 2001; Younossi et al. 2014b]. The addition of first-generation PIs only further compounded this impairment because of significant side effects [Hézode et al 2014]. With the newer DAAs, the PRO profile is substantially better. Most of the currently published PRO data come from SOF-based regimens. When pegylated interferon/RBV regimens were compared with SOF/RBV regimens, PRO analysis showed that the PRO profile of interferon-free regimens (SOF/RBV) was significantly better than that of pegylated interferon/RBV regimens [Younossi et al. 2014b]. However, some PRO impairment was still noted in RBV-containing regimens, possibly due to anemia and mental health side effects of RBV [Younossi et al. 2014b, 2015b, 2015c, 2015d]. When both RBV and interferon are removed from the regimen, even more substantial improvements are noted in QOL and work productivity, as was noted in the ION trials. In fact, improvements in HRQOL, work productivity and other PROs were actually noted as early as 2 weeks after starting SOF/LDV without RBV. This was the first evidence that regimens without substantial side effects which suppress the virus early can lead to early improvement of PROs [Younossi et al. 2015b, 2015c]. Finally, regardless of the regimen, significant improvements in PRO scores are noted after achieving SVR. Nevertheless, these improvements are more prominent in patients who achieve SVR12 with LDV/SOF. It is important to note that improvements in PROs are not only important to patients but can also lead to a substantial societal benefit in terms of cost savings from improved work productivity [Younossi et al. 2015a, 2015b].
Caveats and final considerations
In summary, when considering what regimen is optimal for the individual patient, only a few key baseline variables need to be considered: prior treatment history, presence of fibrosis, and for certain patients or regimens, baseline HCV RNA level and genotype 1 subtype. Overall, all three regimens detailed above are associated with very high SVR rates and good safety and tolerability. As new regimens become available, the optimal option(s) for each subgroup will change, and the reader is referred to the AASLD/Infectious Diseases Society of America (IDSA)/ International Antiviral Society-USA (IAS) guidance for updated recommendations and fuller coverage of which options are most suitable for each subgroup (www.hcvguidelines.org).
While the benefits of these new interferon-free regimens are many, a few caveats are warranted: treatment adherence and DDIs. As these regimens move from the closely monitored clinical trials environment to everyday office settings, a small drop in SVR rates may arise as issues of adherence and DDIs are encountered [Dieterich et al. 2014; Jensen et al. 2014].
Treatment adherence is an important issue to consider when prescribing the new DAAs. As a result, clinicians must move away from the accepted 80/80/80 rule (which states that efficacy will not be adversely affected as long as patients take 80% of the pegylated interferon and 80% of the RBV for at least 80% of the planned duration of therapy) [McHutchison et al. 2002]. With the new interferon-free, DAA-only regimens, suboptimal adherence may lead to on-treatment viral breakthrough [Afdhal et al. 2014a, 2014b]. Suboptimal adherence may also contribute to the selection of resistance-associated variants, which may decrease the chance of SVR and limit options for ‘rescue therapy’ or retreatment. Reviewing the need for drug adherence will be a very important part of treating patients with DAAs.
In addition, the issue of DDIs will need to be considered with the upcoming regimens. A number of these newer DAAs are either inhibitors or inducers of CYP3A4 or the P-glycoprotein transporter. Some regimens, notably AbbVie’s regimen (OBV/PTV/r), also require boosting with ritonavir, which strongly inhibits CYP3A4 and CYP2D6. It is essential for clinicians to check for DDIs before prescribing these newer DAA regimens, and for patients to be educated to inform prescribers if they start new medications, over the counter drugs, herbs or supplements. Enlisting the help of a good specialty pharmacy will be very beneficial.
A number of special populations also require additional detailed study. These groups include high-risk groups, such as patients with decompensated cirrhosis, post-liver transplant patients, patients with end-stage renal disease and patients on chronic immunosuppressive therapies. Other important groups to consider are patients coinfected with HCV and HIV, and patients on opioid replacement therapy. Better therapies for patients who have non-genotype 1 HCV, especially those who have genotype 3 HCV, and pangenotypic regimens are also needed. Many of the DAAs reviewed above have data in these populations of special interest, however reviewing all these data is beyond the scope of this introductory review. These data are publicly available in the joint AASLD/IDSA/IAS guidance (www.hcvguidelines.org). The role of short-course DAA regimens in acute HCV infection has also yet to be defined. It is possible that a few weeks of a DAA regimen during acute infection may prevent more individuals from progressing to CHC.
Lastly, as these newer DAAs are gaining wider use by prescription and clinical trials, there are an increasing number of patients whose disease has failed to respond to treatment with one or a combination DAAs. The possibility of cross resistance between DAAs and the impact of postfailure resistance-associated variants warrants further study. Appropriate ‘salvage regimens’ will need to be studied and clarified. It may be that for these ‘unfortunate 5%’, interferon and RBV may not be off the table entirely.
With shorter, safer and highly efficacious treatment regimens, a unique opportunity exists to significantly impact the impending public health burden posed by CHC. Keeping in mind the mentioned caveats and considerations, treatment with DAA-only regimens should be relatively simple to adopt and prescribe, which will be welcomed by patients and practitioners alike. As more regimens reach the market, pricing issues may become less of a barrier, creating more access to medications for most if not all patients with HCV. In the West and around the world, the upcoming decade will present a remarkable opportunity to decrease the scourge of CHC and limit its effects on long-term health-related QOL, work productivity, morbidity and mortality.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Brian P. Lam, Center for Liver Diseases, Department of Medicine, Inova Fairfax Hospital, Falls Church, VA, USA
Thomas Jeffers, Center for Liver Diseases, Department of Medicine, Inova Fairfax Hospital, Falls Church, VA, USA.
Zahra Younoszai, Center for Liver Diseases, Department of Medicine, Inova Fairfax Hospital, Falls Church, VA, USA.
Yousef Fazel, Center for Liver Diseases, Department of Medicine, Inova Fairfax Hospital, Falls Church, VA, USA.
Zobair M. Younossi, Betty and Guy Beatty Center for Integrated Research, Claude Moore Health Education and Research Building, 3300 Gallows Road, Falls Church, VA 22042, USA
References
- AbbVie Inc. (2015a) Viekira Pak (prescribing information). North Chicago, Illinois: AbbVie Inc. [Google Scholar]
- AbbVie Ltd (2015b) Exviera (prescribing information). Maidenhead, UK: AbbVie Ltd. [Google Scholar]
- AbbVie Ltd (2015c) Viekirax (prescribing information). Maidenhead, UK: AbbVie Ltd. [Google Scholar]
- Afdhal N., Reddy K., Nelson D., Lawitz E., Gordon S., Schiff E., et al. ION-2 Investigators (2014a) Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 370: 1483–1493. [DOI] [PubMed] [Google Scholar]
- Afdhal N., Zeuzem S., Kwo P., Chojkier M., Gitlin N., Puoti M., et al. ION-1 Investigators (2014b) Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 370: 1889–1898. [DOI] [PubMed] [Google Scholar]
- Andreone P., Colombo M., Enejosa J., Koksal I., Ferenci P., Maieron A., et al. (2014) ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology 147: 359–365. [DOI] [PubMed] [Google Scholar]
- Armstrong G., Wasley A., Simard E., McQuillan G., Kuhnert W., Alter M. (2006) The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 144: 705–714. [DOI] [PubMed] [Google Scholar]
- Chak E., Talal A., Sherman K., Schiff R., Saab S. (2011) Hepatitis C virus infection in USA. Liver Int 31: 1090–1101. [DOI] [PubMed] [Google Scholar]
- Chen S., Morgan T. (2006) The natural history of hepatitis C virus (HCV) infection. Int J Med Sci 3: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R., Aghemo A., Rumi M., Ronchi G., Donato M., Paradis V., et al. (2012) A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 56: 532–543. [DOI] [PubMed] [Google Scholar]
- Davis G., Alter M., El-Serag H., Poynard T., Jennings L. (2010) Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 138: 513–521. [DOI] [PubMed] [Google Scholar]
- Dieterich D., Bacon B., Flamm S., Kowdley K., Milligan S., Tsai N., et al. (2014) Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. Hepatology 60: S-220A. [Google Scholar]
- Feld J., Kowdley K., Coakley E., Sigal S., Nelson D., Crawford D., et al. (2014) Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 370: 1594–1603. [DOI] [PubMed] [Google Scholar]
- Feld J., Hoofnagle J. (2005) Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 436: 967–972. [DOI] [PubMed] [Google Scholar]
- Ferenci P., Bernstein D., Lalezari J., Cohen D., Luo Y., Cooper C., et al. (2014) ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 370: 1983–1992. [DOI] [PubMed] [Google Scholar]
- Hézode C., Fontaine H., Dorival C., Zoulim F., Larrey D., Canva V., et al. CUPIC Study Group (2014) Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology 147: 132–142. [DOI] [PubMed] [Google Scholar]
- Jensen D., O’Leary J., Pockros P., Sherman K., Kwo P., Mailliard M., et al. (2014) Safety and efficacy of sofosbuvir-containing regimens for hepatitis C: real-world experience in a diverse, longitudinal observational cohort. Hepatology 60: S-220A. [Google Scholar]
- Kohli A., Sims Z., Nelson A., Osinusi A., Tefari G., Pang P., et al. (2014) Combination oral, hepatitis C antiviral therapy for 6 or 12 weeks: final results of the SYNERGY trial (CROI abstract 27LB). Special Issue: Abstracts From the 2014 Conference on Retroviruses and Opportunistic Infections Top Antivir Med 22: 14–15. [Google Scholar]
- Kowdley K., Gordon S., Reddy K., Rossaro L., Bernstein D., Lawitz E., et al. ION-3 Investigators (2014) Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 370: 1879–1888. [DOI] [PubMed] [Google Scholar]
- Lam B., Henry L., Younossi Z. (2014) Sofosbuvir (Sovaldi) for the treatment of hepatitis C. Expert Rev Clin Pharmacol 7: 555–566. [DOI] [PubMed] [Google Scholar]
- Lawitz E., Sulkowski M., Ghalib R., Rodriguez-Torres M., Younossi Z., Corregidor A., et al. (2014) Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 384: 1756–1765. [DOI] [PubMed] [Google Scholar]
- McHutchison J., Manns M., Patel K., Poynard T., Lindsay K., Trepo C., et al. International Hepatitis Interventional Therapy Group (2002) Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 123: 1061–1069. [DOI] [PubMed] [Google Scholar]
- McHutchison J., Ware J., Jr, Bayliss M., Pianko S., Albrecht J., Cort S., et al. (2001) The effects of interferon alpha-2b in combination with ribavirin on health related quality of life and work productivity. J Hepatol 34: 140–147. [DOI] [PubMed] [Google Scholar]
- McHutchison J., Sulkowski M. (2008) Scientific rationale and study design of the individualized dosing efficacy vs flat dosing to assess optimal pegylated interferon therapy (IDEAL) trial: determining optimal dosing in patients with genotype 1 chronic hepatitis C. J Viral Hepat 15: 475–481. [DOI] [PubMed] [Google Scholar]
- Messina J., Humphreys I., Flaxman A., Brown A., Cooke G., Pybus O., et al. (2014) Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. Epub ahead of print 28 July 2014. DOI: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd Hanafiah K., Groeger J., Flaxman A., Wiersma S. (2013) Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57: 1333–1342. [DOI] [PubMed] [Google Scholar]
- Ng V., Saab S. (2011) Effects of a sustained virologic response on outcomes of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 9: 923–930. [DOI] [PubMed] [Google Scholar]
- Poordad F., Hezode C., Trinh R., Kowdley K., Zeuzem S., Agarwal K., et al. (2014) ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 370: 1973–1982. [DOI] [PubMed] [Google Scholar]
- Rein D., Wittenborn J., Weinbaum C., Sabin M., Smith B., Lesesne S. (2011) Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis 43: 66–72. [DOI] [PubMed] [Google Scholar]
- Rutter K., Hofer H., Beinhardt S., Dulic M., Gschwantler M., Maieron A., et al. (2013) Durability of SVR in chronic hepatitis C patients treated with peginterferon-α2a/ribavirin in combination with a direct-acting anti-viral. Aliment Pharmacol Ther 38: 118–123. [DOI] [PubMed] [Google Scholar]
- Shiffman M., Sterling R., Contos M., Hubbard S., Long A., Luketic V., et al. (2014) Long term changes in liver histology following treatment of chronic hepatitis C virus. Ann Hepatol 13: 340–349. [PubMed] [Google Scholar]
- Smith B., Morgan R., Beckett G., Falck-Ytter Y., Holtzman D., Ward J. (2012) Hepatitis C virus testing of persons born during 1945–1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med 157: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryaprasad A., White J., Xu F., et al. (2014) Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis 59: 1411–1419. [DOI] [PubMed] [Google Scholar]
- Tempestilli M., Lionetti R., D’Offizi G., Montalbano M., Giaffreda A., Fazio S., et al. (2014) Increased plasma concentration of ribavirin as a result of renal dysfunction in hepatitis C virus patients treated with telaprevir. Hepatology 60: 1109–1110. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Jiang Y., Smith N., Smith N., Stepanova M., Beckerman R. (2015a) Ledipasvir/sofosbuvir regimens for chronic hepatitis C infection: insights from a work productivity economic model from the United States. Hepatology 64: 1471–1478. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Kanwal F., Saab S., Brown K., El-Serag H., Kim W., et al. (2014a) The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther 39: 518–531. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Stepanova M., Afdhal N., Kowdley K., Zeuzem S., Henry L., et al. (2015b) Improvement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvir. J Hepatol. Epub ahead of print 17 March 2015. DOI: 10.1016/j.jhep.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Stepanova M., Henry L., Gane E., Jacobson I., Lawitz E., et al. (2014b) Effects of sofosbuvir-based treatment, with and without interferon, on outcome and productivity of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 12: 1349–1359. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Stepanova M., Marcellin P., Afdhal N., Kowdley K., Zeuzem S., et al. (2015c) Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: results from the ION-1, -2, and -3 clinical trials. Hepatology. Epub ahead of print 27 January 2015. DOI: 10.1002/hep.27724. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Stepanova M., Nader F., Jacobson I., Gane E., Nelson D., et al. (2014c) Patient-reported outcomes in chronic hepatitis C patients with cirrhosis treated with sofosbuvir-containing regimens. Hepatology 59: 2161–2169. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Stepanova M., Sulkowski M., Naggie S., Puoti M., Orkin C., et al. (2015d) Sofosbuvir and ribavirin for treatment of chronic hepatitis C in patients coinfected with hepatitis C virus and HIV: the impact on patient-reported outcomes. J Infect Dis. Epub ahead of print 12 January 2015. PII: jiv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi Z., Stepanova M., Zeuzem S., Dusheiko G., Esteban R., Hezode C., et al. (2014d) Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: The VALENCE study. J Hepatol 61: 228–234. [DOI] [PubMed] [Google Scholar]
- Zeuzem S., Jacobson I., Baykal T., Marinho R., Poordad F., Bourlière M., et al. (2014) Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 370: 1604–1614. [DOI] [PubMed] [Google Scholar]