Abstract
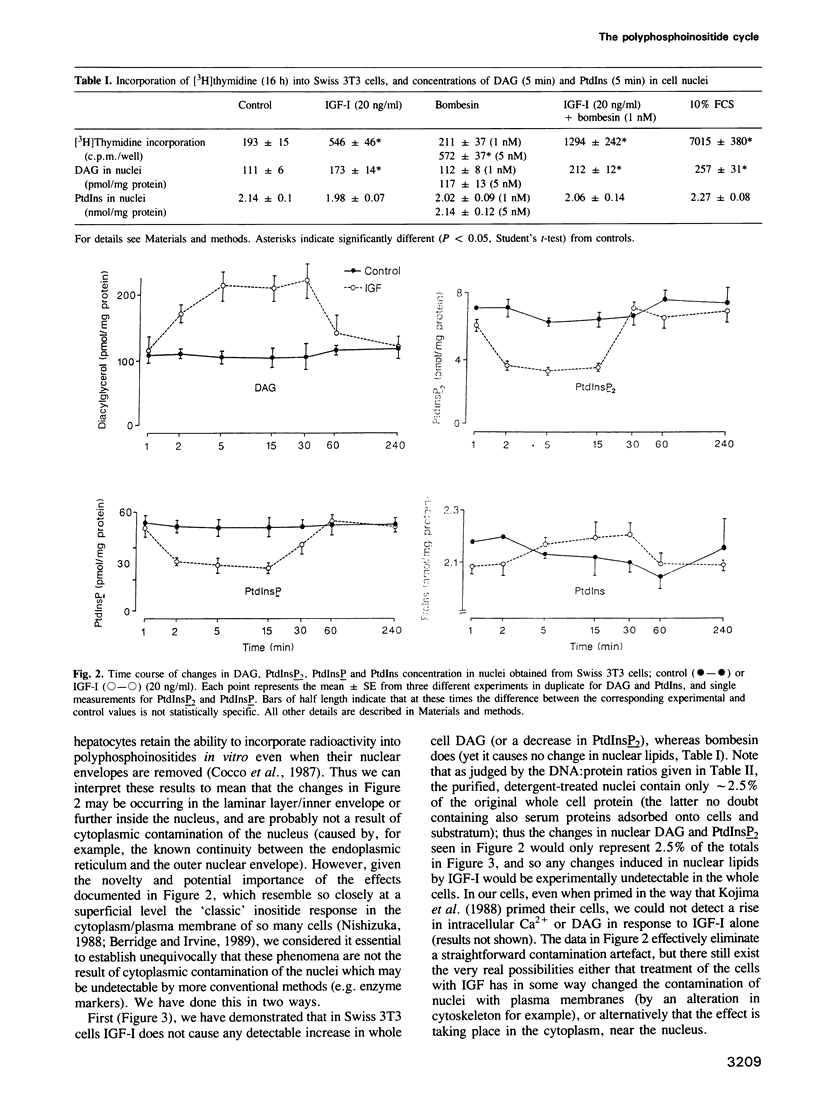
When Swiss 3T3 cells are treated with Insulin-like Growth Factor I, a rapid decrease in the mass of polyphosphoinositol lipids (phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate) occurs within the nuclei, with a concomitant increase in nuclear diacylglycerol and translocation of protein kinase C to the nuclear region. This is in contrast to the effects of the regulatory peptide, bombesin, which causes similar inositol lipid changes in the plasma membrane, has no effect on nuclear inositide levels and causes a translocation of protein kinase C to post-nuclear membranes. These results suggest the existence of a discrete nuclear polyphosphoinositide signalling system entirely distinct from the well-known plasma membrane-located system, which is under regulatory control by cell surface-located receptors.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H., Müller R., Húbsch D., Almendral J. M. Bombesin induces c-fos and c-myc expression in quiescent Swiss 3T3 cells. Comparative study with other mitogens. Exp Cell Res. 1987 May;170(1):103–115. doi: 10.1016/0014-4827(87)90120-0. [DOI] [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco L., Gilmour R. S., Ognibene A., Letcher A. J., Manzoli F. A., Irvine R. F. Synthesis of polyphosphoinositides in nuclei of Friend cells. Evidence for polyphosphoinositide metabolism inside the nucleus which changes with cell differentiation. Biochem J. 1987 Dec 15;248(3):765–770. doi: 10.1042/bj2480765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco L., Martelli A. M., Gilmour R. S., Ognibene A., Manzoli F. A., Irvine R. F. Changes in nuclear inositol phospholipids induced in intact cells by insulin-like growth factor I. Biochem Biophys Res Commun. 1989 Mar 15;159(2):720–725. doi: 10.1016/0006-291x(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Cocco L., Martelli A. M., Gilmour R. S., Ognibene A., Manzoli F. A., Irvine R. F. Rapid changes in phospholipid metabolism in the nuclei of Swiss 3T3 cells induced by treatment of the cells with insulin-like growth factor I. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1266–1272. doi: 10.1016/0006-291x(88)90276-8. [DOI] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Analysis of the water-soluble products of phosphatidylcholine breakdown by ion-exchange chromatography. Bombesin and TPA (12-O-tetradecanoylphorbol 13-acetate) stimulate choline generation in Swiss 3T3 cells by a common mechanism. Biochem J. 1989 Oct 15;263(2):581–587. doi: 10.1042/bj2630581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corps A. N., Rees L. H., Brown K. D. A peptide that inhibits the mitogenic stimulation of Swiss 3T3 cells by bombesin or vasopressin. Biochem J. 1985 Nov 1;231(3):781–784. doi: 10.1042/bj2310781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fields A. P., Pincus S. M., Kraft A. S., May W. S. Interleukin-3 and bryostatin 1 mediate rapid nuclear envelope protein phosphorylation in growth factor-dependent FDC-P1 hematopoietic cells. A possible role for nuclear protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21896–21901. [PubMed] [Google Scholar]
- Fields A. P., Tyler G., Kraft A. S., May W. S. Role of nuclear protein kinase C in the mitogenic response to platelet-derived growth factor. J Cell Sci. 1990 May;96(Pt 1):107–114. doi: 10.1242/jcs.96.1.107. [DOI] [PubMed] [Google Scholar]
- Friedman D. L., Ken R. Insulin stimulates incorporation of 32Pi into nuclear lamins A and C in quiescent BHK-21 cells. J Biol Chem. 1988 Jan 25;263(3):1103–1106. [PubMed] [Google Scholar]
- Hornbeck P., Huang K. P., Paul W. E. Lamin B is rapidly phosphorylated in lymphocytes after activation of protein kinase C. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2279–2283. doi: 10.1073/pnas.85.7.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss Z., Deli E., Kuo J. F. Temporal changes in intracellular distribution of protein kinase C during differentiation of human leukemia HL60 cells induced by phorbol ester. FEBS Lett. 1988 Apr 11;231(1):41–46. doi: 10.1016/0014-5793(88)80698-7. [DOI] [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Cordera R., Olefsky J. M. Substrate specificities of insulin and epidermal growth factor receptor kinases. Biochem Biophys Res Commun. 1985 Feb 28;127(1):254–263. doi: 10.1016/s0006-291x(85)80152-2. [DOI] [PubMed] [Google Scholar]
- Kojima I., Matsunaga H., Kurokawa K., Ogata E., Nishimoto I. Calcium influx: an intracellular message of the mitogenic action of insulin-like growth factor-I. J Biol Chem. 1988 Nov 15;263(32):16561–16567. [PubMed] [Google Scholar]
- Leach K. L., Powers E. A., Ruff V. A., Jaken S., Kaufmann S. Type 3 protein kinase C localization to the nuclear envelope of phorbol ester-treated NIH 3T3 cells. J Cell Biol. 1989 Aug;109(2):685–695. doi: 10.1083/jcb.109.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Orrenius S., Nilsson T., Berggren P. O. An inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in liver nuclei. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6858–6862. doi: 10.1073/pnas.87.17.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Regazzi R., Li G., Ullrich S., Jaggi C., Wollheim C. B. Different requirements for protein kinase C activation and Ca2+-independent insulin secretion in response to guanine nucleotides. Endogenously generated diacylglycerol requires elevated Ca2+ for kinase C insertion into membranes. J Biol Chem. 1989 Jun 15;264(17):9939–9944. [PubMed] [Google Scholar]
- Schröder H. C., Rottmann M., Wenger R., Bachmann M., Dorn A., Müller W. E. Studies on protein kinases involved in regulation of nucleocytoplasmic mRNA transport. Biochem J. 1988 Jun 15;252(3):777–790. doi: 10.1042/bj2520777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia V., Curtin G., Norman J., Stec J., Busbee D. Activation of a low specific activity form of DNA polymerase alpha by inositol-1,4-bisphosphate. Cell. 1988 Aug 26;54(5):651–658. doi: 10.1016/s0092-8674(88)80009-6. [DOI] [PubMed] [Google Scholar]
- Thomas G., Siegmann M., Bowman P. D., Gordon J. The isolation and analysis of polysomes and ribosomal RNA from cells growing in monolayer culture. Exp Cell Res. 1977 Sep;108(2):253–258. doi: 10.1016/s0014-4827(77)80032-3. [DOI] [PubMed] [Google Scholar]
- Trilivas I., Brown J. H. Increases in intracellular Ca2+ regulate the binding of [3H]phorbol 12,13-dibutyrate to intact 1321N1 astrocytoma cells. J Biol Chem. 1989 Feb 25;264(6):3102–3107. [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Whitman M., Cantley L. Phosphoinositide metabolism and the control of cell proliferation. Biochim Biophys Acta. 1989 Feb;948(3):327–344. doi: 10.1016/0304-419x(89)90005-x. [DOI] [PubMed] [Google Scholar]





